TO THE EDITOR
In recent years, a regulatory role has emerged for the glycan-binding protein galectin-9 (Gal-9) in normal physiology and pathology (reviewed by Wiersma et al. (2011). In melanoma and other malignancies, the available data suggest that Gal-9 has a tumor-suppressor function, with loss of Gal-9 being closely associated with metastatic progression (Kageshita et al., 2002; Irie et al., 2005; Yamauchi et al., 2006; Liang et al., 2008). In particular, melanocytic nevi and primary melanoma lesions highly express Gal-9, whereas metastatic melanoma lesions have no or minimal expression of Gal-9 (Kageshita et al., 2002). Furthermore, ectopic expression of Gal-9 abrogates the formation of metastases by Gal-9-deficient B16F10 murine melanoma cells (Nobumoto et al., 2008). Similarly, treatment of Gal-9-deficient B16F10 cells with a recombinant form of Gal-9, designated Gal-9(0), strongly reduced metastasis formation (Nobumoto et al., 2008).
This anti-metastatic activity of Gal-9(0) on B16F10 has been attributed mainly to inhibition of melanoma cell adhesion to endothelial cells and/or extracellular matrix components, such as collagen type I (collagen-I; Nobumoto et al., 2008). The data presented in the current letter suggest that within the 1-h time frame of adhesion-type assays, treatment with Gal-9(0) triggers early apoptotic cellular changes. In line with earlier findings, Gal-9(0) inhibits the adhesion of B16F10 and 7 human melanoma cell lines to collagen-I (Figure 1a). However, the morphology of Gal-9(0)-treated cells that had adhered to collagen-I-coated wells resembled that of dying cells (Figure 1b; illustrated for B16F10). Subsequent analysis of this melanoma cell line panel, as well as primary patient–derived malignant melanoma cells for the early apoptotic marker phosphatidyl serine (PS), revealed that treatment with Gal-9(0) induced ∼90% cell death within the time frame used in the adhesion assay (Figure 1c). Early apoptotic PS exposure was followed by apoptotic cell death within 24 h of treatment, as evidenced by loss of viability (Supplementary Figure S1a online), the presence of late apoptotic Annexin-V/PI double-positive cells (Supplementary Figure S1b and c online), and an increase in DNA fragmentation (Supplementary Figure S1d online). In primary human melanocytes, Gal-9(0) also triggered PS exposure, albeit to a lesser extent (Figure 1c; ∼55%). More importantly, the viability of these normal cells was not negatively affected after 24 h (Supplementary Figure S1a online). Thus, Gal-9(0) induces rapid apoptotic cell death in melanoma cells, but not in normal human melanocytes. PS exposure induced by Gal-9(0) was fully dependent on the glycan-binding specificity of Gal-9(0), as it was selectively blocked by the competitive Gal-9 inhibitor alpha-lactose but not by the irrelevant carbohydrate sucrose (Figure 1d). Sensitivity to Gal-9(0) did not or only weakly correlated with expression of endogenous Gal-9 (Supplementary Figure S2 online; r2=0.250). Time-course analysis in five of the human melanoma cell lines demonstrated that treatment with Gal-9(0) induced PS exposure in >60% of melanoma cells within 5 minutes of treatment (Figure 1e). Furthermore, the extent of PS exposure closely correlated with the inhibitory effect of Gal-9(0) on collagen I binding (Figure 1f, MM-RU; r2=0.693). Together, these data suggest that the biological effect of Gal-9(0) in adhesion assays is mediated, at least partly, through the induction of cell death. It is noteworthy that pan-caspase inhibition failed to block the anti-adhesive and apoptotic activity of Gal-9(0; Supplementary Figure S1e and f online). Thus, Gal-9(0)-mediated melanoma apoptosis does not require caspase activation, which is in line with e.g. reports on gal-1-mediated cell death of T cells (Hahn et al., 2004).
Figure 1.
Galectin-9 (Gal-9(0)) rapidly induces apoptosis in serum-free conditions. (a). Adhesion of B16F10 and a panel of human melanoma cell lines to collagen-I-coated wells is inhibited by recombinant Gal-9. In brief, 3 × 104 melanoma cells were added to collagen-I-coated plates and incubated for 1 h at 37 °C in the presence or absence of 100 nM Gal-9(0). Subsequently, wells were washed twice with phosphate-buffered saline, and the number of adhered cells was counted in three wells per condition. Experiments were performed in triplicates. (b) Light microscopic pictures of B16F10 cells adhered to collagen-I-coated wells in untreated conditions and upon treatment with 100 nM Gal-9(0). Bar=100 μm. (c) Cells were treated for 1 h with 100 nM Gal-9(0) and subsequently analyzed for the early apoptotic event of phosphatidyl serine (PS) exposure using flow cytometric Annexin-V-FITC staining. The following cells were analyzed: B16F10, A2058, Sk-MEL-28, MM-RU, MM-WK, MM-AN, MM-EP, A375M, primary (prim.) patient–derived melanoma cells (n=1), and primary human melanocytes (n=1). (d) B16F10, Sk-MEL-28, A2058, and MM-RU were treated with Gal-9(0) with or without alpha-lactose or sucrose. (e) Sk-MEL-28, A2058, A375M, MM-AN, and MM-RU were treated with 100 nM Gal-9(0) for 5, 10, 15, and 20 minutes. Graph represents the average PS exposure detected in all five cell lines from three separate experiments. (f) MM-RU cells were treated with various Gal-9(0) concentrations (ranging from 5 to 200 nM), after which cells were simultaneously assessed for collagen-I adhesion and PS exposure. Statistical analysis was performed by linear regression, yielding an r2 of 0.6497. In all cell death assays, cell death was assessed by flow cytometry using Annexin-V-FITC, which measures the early apoptotic exposure of PS on the outer leaflet of the cell membrane.
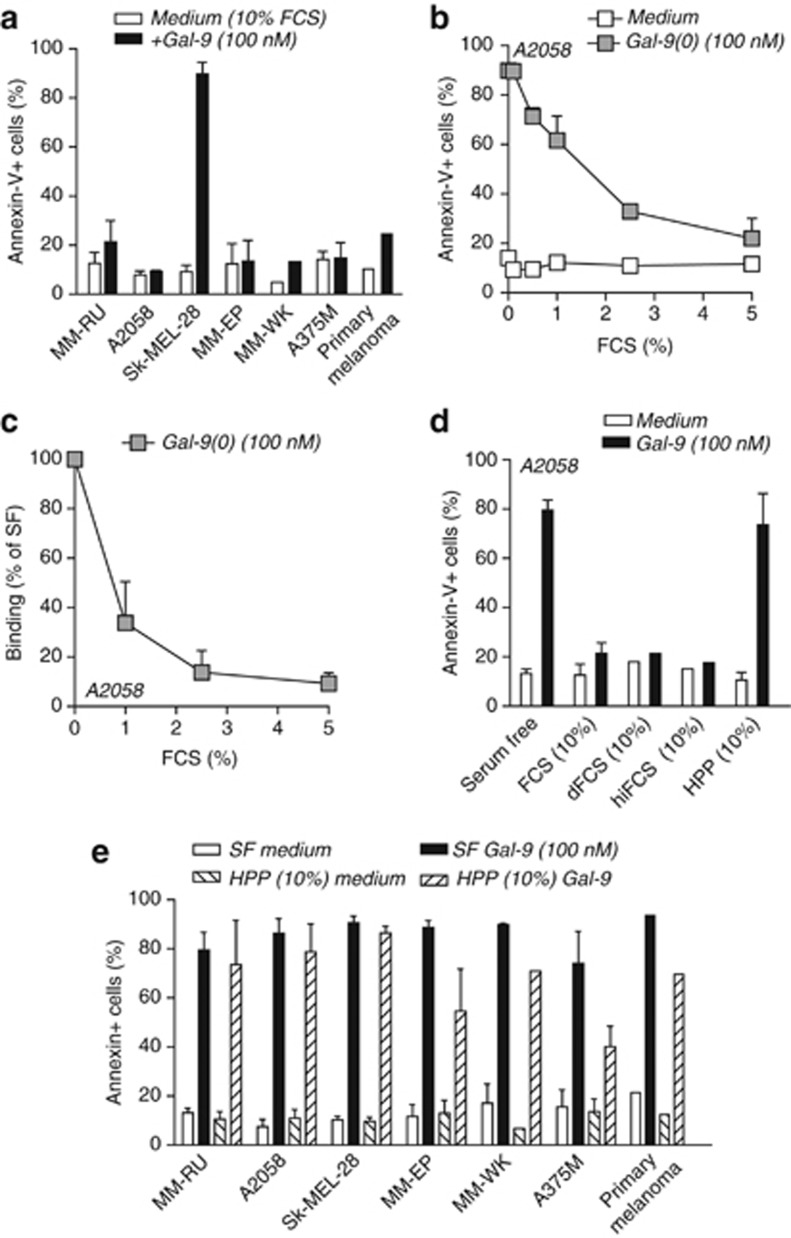
Adhesion assays are typically performed in serum-free conditions, whereas in normal physiological situations the presence of serum and/or plasma components may affect the biological activity of Gal-9. Indeed, it is well established that Gal-9 can interact with serum components (Cederfur et al., 2008). Therefore, the biological activity of Gal-9(0) was next evaluated in the presence of 10% fetal calf serum (FCS), the standard serum additive in cell death assays. The inclusion of 10% FCS in these apoptosis experiments completely abrogated the morphological changes in melanoma cells, with induction of PS exposure by Gal-9(0) being abrogated in six of the seven cell lines tested (Figure 2a). Similarly, PS exposure by Gal-9(0) was also strongly inhibited in the primary melanoma cells (Figure 2a). Indeed, FCS dose dependently inhibited PS exposure induced by Gal-9(0) (illustrated for cell line A2058 in Figure 2b) and blocked the binding of Gal-9(0) to A2058 cells (Figure 2c). When using dialysed FCS or heat-inactivated FCS, the activity of Gal-9(0) was still inhibited (Figure 2d). Thus, the inhibitory component present in FCS is not heat labile (i.e., complement factors) and is >10 kDa in size. Notably, FCS did not inhibit Gal-9(0) activity toward SK-MEL-28 cells (Figure 2a), which suggests that the inhibitory effect of FCS is not merely due to binding of a serum component to Gal-9(0). Possibly, a serum component may shield the receptor(s) of Gal-9 on most melanoma cells. On Sk-MEL-28 cells, Gal-9(0) may interact with an alternative receptor not subject to binding/inhibition by FCS.
Figure 2.
Cell death induction by galectin-9 (Gal-9(0)) is blocked by fetal calf serum (FCS) but not by human pooled plasma. (a) A panel of melanoma cells and primary patient–derived melanoma cells (n=1) were treated for 1 h with Gal-9(0) in standard cell death conditions (with 10% FCS). (b) A2058 cells were treated with 100 nM Gal-9(0) in the presence of increasing concentrations of FCS and analyzed for the percentage of cells with phosphatidyl serine (PS) exposure. (c) A2058 cells were incubated on ice with 100 nM biotinylated Gal-9(0) in the presence of increasing concentrations of FCS. Cell surface binding of Gal-9(0)-biotin was evaluated using Streptavidin-Alexa488 by flow cytometry. (d) A2058 cells were treated with 100 nM Gal-9(0) in serum-free (SF) conditions or in the presence of 10% FCS, 10% dialyzed FCS (dFCS), heat-inactivated FCS (hiFCS), or human pooled plasma (HPP). (e) A panel of melanoma cell lines and primary patient–derived melanoma cells (n=1) were treated with 100 nM Gal-9(0) in SF conditions or in the presence of 10% HPP. In all assays, cell death was assessed by flow cytometry using Annexin-V (AnneV)-FITC. All experiments were performed in triplicates.
Although FCS is the standard additive in in vitro cell death assays, a better approximation of physiological settings is the addition of human plasma. Importantly, 10% human pooled plasma did not abrogate Gal-9(0)-induced PS exposure in A2058 cells, with only a slight reduction in PS exposure compared with serum-free conditions (Figure 2d). Similar results were obtained in six melanoma cell lines and in primary patient–derived melanoma cells (Figure 2e).
These experiments suggest that an important biological effect of Gal-9(0) on human melanoma cells is the induction of apoptosis. This biological effect of Gal-9(0) is masked by as yet unidentified components present in FCS, but is unmasked in serum-free conditions or when human plasma is used. Indeed, in standard FCS-containing culture conditions, apoptotic cell death of the melanoma cell line MM-RU was only detected after 72 h of treatment with Gal-9 (Kageshita et al., 2002). The use of FCS may similarly mask the biological activity of other gal family members. In this respect, gal-2, -3, -4, and -8 interact with various serum components (Cederfur et al., 2008). Notably, plasma levels of several of the gal family members are increased in malignancy (Barrow et al., 2011). The use of human pooled serum/plasma instead of FCS for in vitro biological assays with members of the Gal family therefore appears prudent.
In conclusion, recombinant Gal-9 has a hitherto unrecognized cytotoxic effect toward human melanoma cells, which further highlights its potential therapeutic applicability for the treatment of human metastatic melanoma.
Acknowledgments
This work was supported by the Dutch Cancer Society grants RUG 2009-4355 (EB), RUG2009-4542 (to EB/WH), RUG2011-5206 (to EB/WH), and RUG2007-3784 (to WH), the Netherlands Organization for Scientific Research (EB), the Melanoma Research Alliance (EB), and the Alexander von Humboldt Foundation (EB).
Glossary
- collagen-I
collagen type I
- Gal-9
galectin-9
- PS
phosphatidyl serine
The authors state no conflict of interest.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Barrow H, Guo X, Wandall HH, et al. Serum galectin-2, -4, and -8 Are greatly increased in colon and breast cancer patients and promote cancer cell adhesion to blood vascular endothelium. Clin Cancer Res. 2011;17:7035–7046. doi: 10.1158/1078-0432.CCR-11-1462. [DOI] [PubMed] [Google Scholar]
- Cederfur C, Salomonsson E, Nilsson J, et al. Different affinity of galectins for human serum glycoproteins: galectin-3 binds many protease inhibitors and acute phase proteins. Glycobiology. 2008;18:384–394. doi: 10.1093/glycob/cwn015. [DOI] [PubMed] [Google Scholar]
- Hahn HP, Pang M, He J, et al. Galectin-1 induces nuclear translocation of endonuclease G in caspase- and cytochrome c-independent T cell death. Cell Death Differ. 2004;11:1277–1286. doi: 10.1038/sj.cdd.4401485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie A, Yamauchi A, Kontani K, et al. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin Cancer Res. 2005;11:2962–2968. doi: 10.1158/1078-0432.CCR-04-0861. [DOI] [PubMed] [Google Scholar]
- Kageshita T, Kashio Y, Yamauchi A, et al. Possible role of galectin-9 in cell aggregation and apoptosis of human melanoma cell lines and its clinical significance. Int J Cancer. 2002;99:809–816. doi: 10.1002/ijc.10436. [DOI] [PubMed] [Google Scholar]
- Liang M, Ueno M, Oomizu S, et al. Galectin-9 expression links to malignant potential of cervical squamous cell carcinoma. J Cancer Res Clin Oncol. 2008;134:899–907. doi: 10.1007/s00432-008-0352-z. [DOI] [PubMed] [Google Scholar]
- Nobumoto A, Nagahara K, Oomizu S, et al. Galectin-9 suppresses tumor metastasis by blocking adhesion to endothelium and extracellular matrices. Glycobiology. 2008;18:735–744. doi: 10.1093/glycob/cwn062. [DOI] [PubMed] [Google Scholar]
- Wiersma VR, de Bruyn M, Helfrich W, et al. 2011Therapeutic potential of Galectin-9 in human disease Med Res Reve-pub ahead of print 26 July 2011 [DOI] [PubMed]
- Yamauchi A, Kontani K, Kihara M, et al. Galectin-9, a novel prognostic factor with antimetastatic potential in breast cancer. Breast J. 2006;12:S196–S200. doi: 10.1111/j.1075-122X.2006.00334.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.