Abstract
Metabotropic glutamate receptors (mGluRs) were thought until recently to function mainly as stable homodimers, but recent work suggests that heteromerization is possible. Despite the growth in available compounds targeting mGluRs, little is known about the pharmacological profile of mGluR heterodimers. Here, this question was addressed for the mGluR2/4 heterodimer, examined by coexpressing both receptors in isolated sympathetic neurons from the rat superior cervical ganglion (SCG), a native neuronal system with a null mGluR background. Under conditions that favor mGluR2/4 heterodimer formation, activation of the receptor was not evident with the mGluR2-selective agonist (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV) or with the mGluR4 selective agonist l-(+)-2-amino-4-phosphonobutyric acid (l-AP4); however, full activation was apparent when both ligands were applied together, confirming that mGluR dimers require ligand binding in both subunits for full activation. Properties of allosteric modulators were also examined, including the findings that negative allosteric modulators (NAMs) have two binding sites per dimer and that positive allosteric modulators (PAMs) have only a single site per dimer. In SCG neurons, mGluR2/4 dimers were not inhibited by the mGluR2-selective NAM (Z)-1-[2-cycloheptyloxy-2-(2,6-dichlorophenyl)ethenyl]-1H-1,2,4-triazole (Ro 64-5229), supporting the two-site model. Furthermore, application of the mGluR4 selective PAMs N-(4-chloro-3-methoxyphenyl)-2-pyridinecarboxamide (VU0361737) or N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide (PHCCC) and combined application of mGluR4 PAMs with the mGluR2 selective PAM biphenyl indanone-A failed to potentiate glutamate responses through mGluR2/4, suggesting that mGluR2/4 heterodimers are not modulatable by PAMs that are currently available.
Introduction
Metabotropic glutamate receptors (mGluRs) are class C G protein-coupled receptors with widespread nervous system expression that are involved in an array of important physiological and pathological processes (Schoepp 2001; Grueter et al., 2007; Moussawi and Kalivas 2010; Vinson and Conn, 2012). There are eight mammalian mGluR genes (GRM1–8), subdivided into three groups, I through III. On the basis of an array of structural (Kunishima et al., 2000; Tsuchiya et al., 2002; Jingami et al., 2003; Sato et al., 2003), biochemical (Romano et al., 1996; Robbins et al., 1999; Selkirk et al., 2002), and physiological (Tateyama et al., 2004; Hlavackova et al., 2005; Beqollari and Kammermeier 2010; Huang et al., 2011; Tateyama and Kubo 2011) evidence, mGluRs are thought to express and function primarily as stable homodimers. However, evidence is beginning to emerge to suggest that at least under some circumstances, mGluRs may form heteromeric receptors, including heterodimers. Indeed, evidence from a recent study suggests that several mGluRs may physically interact with each other, including nearly all of the group II and III mGluRs (Doumazane et al., 2011). This finding is potentially very important in part because it suggests that the number of unique mGluRs in the nervous system that can serve as potential therapeutic targets is much more vast than previously thought. Still, for this potential to be realized, a great deal more information is required, including the expression patterns and dimerization tendencies of each mGluR pair. To date, however, neither the basic pharmacological profiles of mGluR heterodimers nor their basic signaling properties have been characterized.
Here, the pharmacology and signaling properties of the mGluR2/4 receptor, a combination described as forming heterodimers (Doumazane et al., 2011), were examined. The strategy was to express each receptor alone, or together, in isolated sympathetic neurons from the rat superior cervical ganglion (SCG) and to examine mGluR-mediated inhibition of the native calcium currents as an assay for receptor function. Currents were monitored using the patch-clamp technique in a whole-cell mode. Modulation of these channels has been well characterized in this system and, for Gi/o-coupled receptors, is known to proceed via a Gβγ-mediated pathway identifiable by its voltage dependence (Herlitze et al., 1996; Ikeda 1996). Thus, the effectors (CaV2.2 and CaV2.3) (Zhu and Ikeda 1993) provide a readout that is relatively proximal to receptor activation, so signaling can be easily monitored in real time. It is noteworthy that Doumazane et al. (2011) recently showed by using FRET studies and some elegant competition experiments that mGluR2 and -4 combine to form heterodimers, rather than tetramers, or higher-order multimeric receptors. This finding provided the opportunity to test some assumptions about the function of mGluR dimers that could not previously be addressed using wild-type mGluR subunits in a functional assay. First, mGluR dimers require ligand binding in both subunits for (full) activation (Kniazeff et al., 2004; Kammermeier and Yun 2005). Second, each subunit in a dimer has a binding site for negative allosteric modulators (NAMs), each of which must be occupied for inhibition of receptor signaling (Hlavackova et al., 2005; Lundström et al., 2011). Third, each dimer has only one binding site for positive allosteric modulators (PAMs) (Lundström et al., 2011), and/or mGluR dimers require PAM binding to only one dimer subunit (Goudet et al., 2005).
Materials and Methods
SCG Neuron Isolation, cDNA Injection, and Molecular Methods.
Detailed descriptions of the isolation and injection procedures have been described previously(Ikeda 1997). In brief, the SCGs were dissected from adult Wistar rats and incubated in Earle's balanced salt solution (Invitrogen, Carlsbad, CA) containing 0.5 mg/ml trypsin (Worthington Biochemicals, Freehold, NJ) and 1 mg/ml collagenase D (Roche Diagnostics, Indianapolis, IN) for 1 h at 35°C. Cells were centrifuged twice, transferred to minimum essential medium (Thermo Fisher Scientific, Waltham, MA), plated, and placed in an incubator at 37°C until cDNA injection. Injection of cDNA was performed with an Eppendorf 5247 microinjector and Injectman NI2 micromanipulator (Eppendorf, Madison, WI) 4 to 6 h after cell isolation. Plasmids were stored at −20°C as a 1 μg/μl stock solution in TE buffer (10 mM Tris, 1 mM EDTA, pH 8). The mGluR2 insert was subcloned into pCI, and mGluR4 was in pCDNA3.1 (Invitrogen). Concentrations of cDNAs injected were as indicated in the text. All neurons were coinjected with green fluorescent protein cDNA (0.02 μg/μl; pEGFPC1; Clontech Laboratories, Mountain View, CA) or other fluorescent marker if necessary for identification of injected cells. After injection, cells were incubated overnight at 37°C, and experiments were performed the next day.
Electrophysiology and Data Analysis.
Patch-clamp recordings were made using 8250 glass (King Precision Glass, Claremont, CA). Pipette resistances were 0.8 to 3 MΩ, yielding uncompensated series resistances of 1 to 5 MΩ. Series resistance compensation of 80% was used in all recordings. Data were recorded using an EPC-7 patch-clamp amplifier from HEKA Elektronik (Lambrecht, Germany). Voltage protocol generation and data acquisition were performed using custom data acquisition software (donated by Stephen R. Ikeda, National Institutes of Health National Institute on Alcohol and Alcoholism) on a Macintosh G3 computer with an Instrutech ITC16 data acquisition board (HEKA Elektronik). Currents were sampled at 0.5 to 5 kHz, low-pass filtered at 3 kHz, digitized, and stored on the computer for later analysis. All patch-clamp experiments were performed at 21–24°C (room temperature). Data analysis was performed using Igor Pro software (WaveMetrics, Lake Oswego, OR). The external (bath) calcium current recording solution contained 145 mM tetraethylammonium methanesulfonate, 10 mM HEPES, 15 mM glucose, 10 mM CaCl2, and 300 nM tetrodotoxin, pH 7.4; osmolality, 320 mOsmol/kg. The internal (pipette) solution contained 120 mM N-methyl-d-glucamine methanesulfonate, 20 mM tetraethylammonium, 11 mM EGTA, 10 mM HEPES, 10 mM sucrose, 1 mM CaCl2, 4 mM MgATP, 0.3 mM Na2GTP, and 14 mM Tris-creatine phosphate, pH 7.2; osmolality, 300 mOsmol/kg. The number of cells obtained for experiments summarized in each experiment is reported in figure legends or in text. For every experiment, data were obtained from at least two animals, with the exception of the mGluR2/7 experiment shown in Fig. 2, the combined BINA/VU036 experiment shown in Fig. 6, and the pertussis toxin (PTX) experiment described in the text.
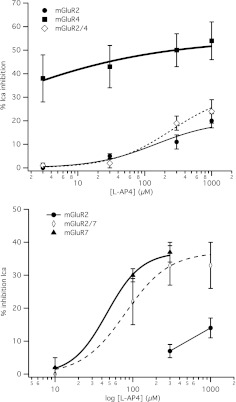
Fig. 2.
Responses to the selective group III mGluR agonist l-AP4 in SCG neurons expressing mGluR2 alone or coexpressed with a group III mGluR. Top, average (±S.E.M.) l-AP4 dose-response curves for cells expressing mGluR2 (black circles) and mGluR4 (gray squares) and coexpressing mGluR2 and -4 (open diamonds), generated as described for the glutamate responses in Fig. 1. Data at 3, 30, 300, and 1000 μM l-AP4 in mGluR2-expressing cells are averages of 3, 3, 9, and 6 cells, respectively. For mGluR4 cells, data are averages of four cells at each point. For mGluR2/4 cells, data are averages of 5, 6, 7, and 7 cells, respectively, in both the top and bottom graphs. Bottom, average (±S.E.M.) l-AP4 dose-response curves for cells expressing mGluR2 (black circles) and mGluR7 (gray triangles) and coexpressing mGluR2 and -7 (open diamonds), generated as described for the glutamate responses in Fig. 1. Data at 300 and 1000 μM l-AP4 in mGluR2-expressing cells are averages of six and three cells, respectively. For mGluR7 cells, data are averages of four cells at each point. For mGluR2/7 cells, data are averages of five cells at each concentration.
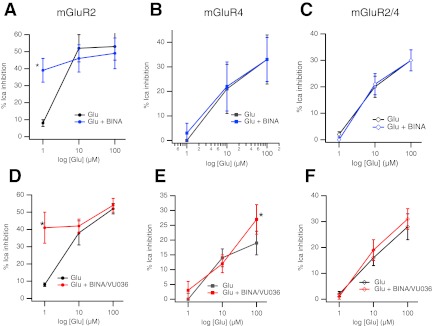
Fig. 6.
Simultaneous application of mGluR2 and mGluR4 selective PAMs fails to potentiate mGluR2/4 responses. A–C, effect of the mGluR2 PAM BINA on glutamate responses in SCG neurons expressing mGluR2 (A), mGluR4 (B), and mGluR2/4 (C). Control glutamate responses are shown in black, and responses in the presence of 100 nM BINA are shown in blue.*, significantly different from response to glutamate applied alone; n = 4 for each point. D–F, glutamate dose-response curves in the absence (black) and presence (red) of simultaneous application of 100 nM BINA and 1 μM VU036 in SCG neurons expressing mGluR2 (D), mGluR4 (E), or mGluR2/4 (F). Points represent average (±S.E.M.) calcium current inhibitions at each [glutamate]. Number of cells represented by points at 1, 10, and 100 μM glutamate are as follows: for mGluR2, -6, -3, and -4, respectively; for mGluR4, -6, -3, and -4; and for mGluR2/4, -7, -7, and -4. *, significantly different from response to glutamate applied alone, at the same concentration, paired t test comparing 100 μM Glu responses ± PAMs.
All allosteric compounds (PHCCC, VU0361737, BINA, and Ro64-5229) and selective ligands (l-AP4 and DCG-IV) were obtained from Tocris Bioscience (Bristol, UK). PTX treatment was done by expression of the PTX S1 subunit, as described previously (Ikeda et al., 1999).
Results
Expression of mGluR2 and mGluR4 in SCG Neurons.
Sympathetic neurons from the adult rat SCG provide a null-mGluR background (Kammermeier and Ikeda 1999; Kammermeier and Yun 2005) on which to examine mGluR signaling and the effects of various mGluR-targeted pharmacological compounds (Ishida et al., 1993; Maj 2003; Galici et al., 2006; Beqollari and Kammermeier 2008). Thus, to examine the signaling and pharmacological properties of mGluR2 and -4 and the putative mGluR2/4 heterodimer (Doumazane et al., 2011), each receptor was expressed alone or both were expressed together in isolated SCG neurons, and mGluR-mediated modulation of endogenous calcium currents was used as an assay for receptor signaling (Kammermeier and Ikeda 1999). Primary SCG cultures from adult rats natively express mainly CaV2.2 channels (N-type) and some CaV2.3 channels (R-type) (Zhu and Ikeda 1993), which are both strongly inhibited by free Gβγ, typically from the pertussis toxin-sensitive Gi/o family of heterotrimeric G proteins (Herlitze et al., 1996; Ikeda 1996). Because both group II (mGluR2 and -3) and group III mGluRs (mGluR4 and -6–8) couple exclusively to this G protein family, calcium channel inhibition provided a robust and relatively proximal effector to use as an assay for receptor function.
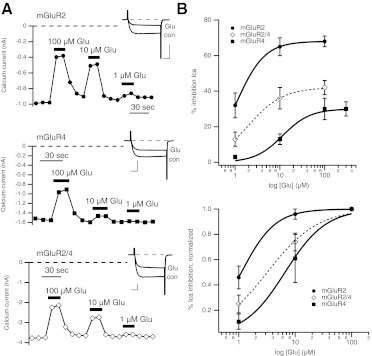
Figure 1A shows calcium current amplitude time courses and sample current traces (inset) for glutamate-mediated inhibition of currents elicited using 25-ms voltage steps to + 10 mV from a holding potential of −80 mV every 10 s in sample cells expressing mGluR2 (top), mGluR4 (middle), or both receptors (bottom), as indicated. Note that in each case the inhibition was relatively rapid, reversible, and showed the hallmarks of Gβγ-mediated inhibition, including slowing of activation kinetics (see inset currents) and reversibility upon strong depolarization (not shown), as expected. Also note that in uninjected SCG neurons, application of glutamate up to 10 mM has no detectable effect on the calcium currents (Kammermeier and Yun 2005).
Fig. 1.
Coexpression of mGluR2 and mGluR4 in SCG neurons results in glutamate responses that differ from either receptor alone. A, time course of native SCG calcium current amplitudes during application and wash of multiple glutamate concentrations (as indicated) in sample cells expressing mGluR2 (top) and mGluR4 (middle) and coexpressing both receptors (bottom). Insets show sample control and inhibited currents for each expression condition, from a test pulse to +10 mV from the holding potential of −80 mV. Scale bars indicate 0.5 nA (top), 1 nA (middle and bottom), and 5 ms. B, average (±S.E.M.) glutamate dose-response curves for cells expressing mGluR2 (black circles; n = 10) and mGluR4 (gray squares; n = 11) and coexpressing mGluR2 and -4 (open diamonds; n = 15). Bottom plot shows curves normalized to responses to 100 μM glutamate for each cell.
Experiments such as those shown in Fig. 1A were used to generate the average dose-response curves shown in Fig. 1B (top) for SCG neurons expressing mGluR2, mGluR4, or both receptors together (mGluR2/4), as indicated. In these experiments, as in all subsequent experiments in which dose-response curves were generated, the order of application of various drug doses was altered from cell to cell to avoid systematic errors in measuring efficacy at a specific concentration due to factors such as desensitization. However, it should be noted that, under the conditions used in these studies, significant desensitization of responses was not seen (see time courses of inhibition by drugs in Fig. 3). At the expression levels attained in these experiments, mGluR2 clearly showed a much greater efficacy than mGluR4, with maximal effects upon 100 μM glutamate application of 68 ± 3% (n = 10) and 30 ± 5% (n = 11), respectively. When both receptors were expressed together, the apparent efficacy was intermediate at 42 ± 4% (n = 15). When these responses were normalized to the effect of 100 μM glutamate, a slight difference in apparent potency between mGluR2 and mGluR4 was also evident (Fig. 1B, bottom). The half-maximal effects (EC50) for mGluR2 and mGluR4 from fits of these data to the Hill equation were estimated to be ∼1.1 and ∼6.6 μM, respectively. The observed potency of the response when both receptors were expressed was also intermediate, with an EC50 of ∼3.2 μM. Although these data provide some indication of the signaling properties of mGluR2 and mGluR4 in this assay, they cannot allow a determination of whether, under mGluR2/4 coexpression conditions and to what degree, the receptors act independently as mGluR2 or mGluR4 homodimers or as the putative mGluR2/4 heterodimer. To achieve this, responses to selective agonists must be examined. However, it should be noted that the intermediate responses observed when both receptors were expressed indicate that each receptor subunit is probably expressed to at least some degree or the responses would seem identical to those in cells expressing one or the other receptor alone.
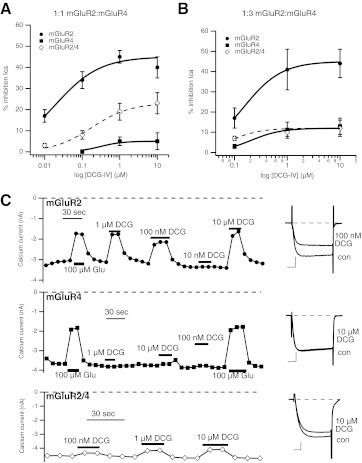
Fig. 3.
Responses to the selective group II mGluR agonist DCG-IV in SCG neurons expressing mGluR2, mGluR4, or both receptors together. A, average (±S.E.M.) DCG-IV dose-response curves for cells expressing a 1:1 injected [cDNA] ratio (150 ng/μl of each) of mGluR2 (black circles) and mGluR4 (gray squares) and coexpressing mGluR2 and -4 together (open diamonds), generated as described above. Data at 0.01, 0.1, 1, and 10 μM DCG-IV in mGluR2-expressing cells are averages of 12, 12, 12, and 3 cells, respectively. For mGluR4 cells, data are averages of 0, 7, 8, and 5 cells, respectively. For mGluR2/4 cells, data are averages of 6, 13, 13, and 6 cells, respectively. B, average (±S.E.M.) DCG-IV dose-response curves for cells expressing a 1:3 injected [cDNA] ratio (50 and 150 ng/μl, respectively) of mGluR2 (black circles) and mGluR4 (gray squares) and coexpressing mGluR2 and -4 together (open diamonds). Data at 0.1, 1, and 10 μM DCG-IV in mGluR2-expressing cells are averages of 9, 4, and 4 cells, respectively. For mGluR4 cells, data are averages of 8, 4, and 4 cells, respectively. For mGluR2/4 cells, data are averages of 12, 5, and 5 cells, respectively. C, time courses of glutamate and DCG-IV inhibition in example SCG neurons expressing mGluR2 (top) and mGluR4 (middle) and mGluR2 and -4 at a 1:3 ratio (bottom). Glutamate or DCG-IV was applied as indicated. Insets to the right show control and inhibited sample current traces (by the indicated concentration of DCG-IV) from the cells shown to the left. Scale bars in the insets indicate 1 nA and 5 ms.
Responses to the Group III Selective Agonist l-AP4.
To begin to examine the pharmacological properties and the molecular mechanism of activation of the putative mGluR2/4 heterodimer, responses to the group III mGluR-selective agonist l-AP4 were examined in SCG neurons expressing mGluR2, mGluR4, or both receptors (Fig. 2). As expected, l-AP4 produced strong responses in mGluR4-expressing cells at all concentrations examined (3–1000 μM) (Fig. 2A, gray squares). SCG neurons expressing only mGluR2 responded poorly to l-AP4, although some activation was observed at [l-AP4] above 30 μM (Fig. 2A, black circles). It is noteworthy that when mGluR2 and -4 were coexpressed, the l-AP4 dose-response curve was indistinguishable from that of mGluR2 expressed alone. These data suggest that either little or no mGluR4 is expressed or that both receptor subunits are expressed and that mGluR2/4 heterodimers form and have an l-AP4 dose-response relationship very similar to mGluR2. It should be noted that the expression conditions in these experiments were identical to those described in Fig. 1 in that the amount and ratio of cDNA for each receptor injected were the same (150 ng/μl for both receptors, a 1:1 ratio). Because those data demonstrate significant differences between the mGluR2 and mGluR2/4 conditions, they suggest that the latter interpretation is more likely. It is noteworthy that these data are consistent with recent work demonstrating that mGluR homodimers seem to require ligand binding in both subunits for signaling in SCG neurons (Kammermeier and Yun 2005) or for full activity in a heterologous expression system (Kniazeff et al., 2004). Those studies, however, relied on mutant or chimeric group I mGluRs to demonstrate those findings. Thus, these data suggest that this property is apparent in wild-type mGluRs.
Potential interactions between mGluR2 and mGluR7 were also tested by performing analogous experiments in SCG neurons. As shown in Fig. 2B, mGluR7 was activated by l-AP4 with an EC50 of 97 μM while activating mGluR2 similarly to the experiments shown in Fig. 2A. In contrast to the experiment in which mGluR2 and -4 were coexpressed, expression of mGluR2 and mGluR7 together resulted in an l-AP4 dose-response curve similar to that of mGluR7 alone. These results are consistent with a model in which mGluR2 and -7 function independently or at least do not show functional interdependence in the same manner as mGluR2 and -4. Thus, the apparent interaction between mGluR2 and -4 seem to have some specificity.
Responses to the Group II Selective Agonist DCG-IV.
Experiments were next conducted using the group II selective agonist DCG-IV (Ishida et al., 1993). Selectivity of this compound was confirmed in SCG neurons expressing mGluR2 or mGluR4 alone (Fig. 3, A and B). In this set of experiments, the ratio of injected mGluR2 and -4 [cDNA] was identical to that described in Figs. 1 and 2 in which [cDNA] was injected at a 1:1 ratio (150 ng/μl each plasmid). In mGluR2-expressing cells, DCG-IV produced a relatively strong maximal response (45 ± 3%, n = 12 at 1 μM DCG-IV; Fig. 3A, black circles), with an EC50 of ∼16 nM, comparable with mGluR2 responses reported in the literature (Ishida et al., 1993; Picconi et al., 2002). By contrast, DCG-IV had little effect in cells expressing mGluR4 alone (Fig. 3A, gray squares). In each cell, receptor expression was confirmed with a separate application of 100 to 300 μM glutamate (not shown). These data confirm the selectivity of DCG-IV.
It is unexpected that responses in cells expressing both receptors (Fig. 3A, open diamonds) had an intermediate response (19 ± 4%, n = 13 at 1 μM, EC50 ∼130 nM). This result was unexpected based on the prediction that agonist binding to only one subunit of an mGluR dimer should be insufficient to activate the receptor (Kniazeff et al., 2004; Kammermeier and Yun 2005) and on our l-AP4 data (Fig. 2). However, it is possible that an excess of mGluR2 expression results under these expression conditions (1:1 cDNA ratio). This could lead to expression of both mGluR2/4 heterodimers and some mGluR2 homodimers. Such a scenario would not be detected using l-AP4 as an agonist (Fig. 2) but would become apparent using DCG-IV. To test this theory, the DCG-IV experiment was repeated using a 1:3 mGluR2/mGluR4 [cDNA] ratio achieved by reducing the mGluR2 [cDNA] by two thirds (to 50 ng/μl injected [cDNA]) while leaving the mGluR4 [cDNA] constant (Fig. 3B). When the experiment was conducted under the 1:3 conditions, the maximal response of mGluR2-expressing cells was similar (41 ± 10%, n = 4, at 1 μM), but the 10-fold shift in DCG-IV potency (to an EC50 of 140 nM) is consistent with reduced receptor expression levels, leading to a lessened receptor reserve effect (Stephenson 1997). Again, responses in mGluR4-expressing cells were quite weak, even at 10 μM (8 ± 4%, n = 4). Now, when mGluR2 and -4 are coexpressed, the responses to DCG-IV are identical to those with mGluR4 alone, as expected for mGluR2/4 heterodimers. These data indicate that mGluR2/4 heterodimers cannot be activated by agonists selective for either subunit alone, as predicted from experiments using mutant group I mGluRs (Kniazeff et al., 2004; Kammermeier and Yun 2005), provided it can be shown that, under these expression conditions, the presumptive mGluR2/4 heterodimer is functional (can be activated by glutamate) and activated by combined application of both selective ligands.
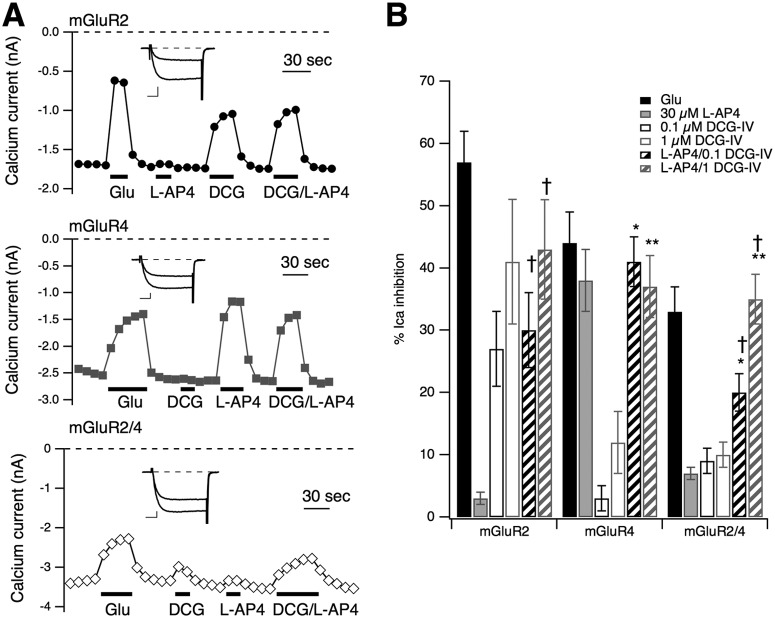
mGluR2/4 Responds to Combined DCG-IV/l-AP4 Ppplication but Not to Either Agonist Alone.
The data above provide some evidence that when mGluR2 and -4 are coexpressed in SCG neurons at a 1:3 [cDNA] ratio, much of the resulting signaling is attributed to putative mGluR2/4 heterodimers. However, more convincing evidence would come from an unambiguous demonstration that the active receptor under mGluR2/4 conditions is pharmacologically distinct from either receptor expressed alone. The model for mGluR dimer signaling suggests that both dimer subunits must bind ligand for efficient activation. Thus, the mGluR2/4-expressing SCG neurons should be largely unresponsive to both l-AP4 and DCG-IV applied alone while being strongly activated by both ligands applied together. By contrast, mGluR2 should be activated by DCG-IV only and mGluR4 by l-AP4 only, and neither receptor should yield enhanced activation when the two agonists are applied together. Indeed, when this experiment was conducted, this was precisely what was seen. Figure 4A illustrates sample time courses of calcium current amplitudes from each group, with control and glutamate-inhibited currents also shown (inset), as indicated. As a reference, 100 μM glutamate was also applied to each cell. l-AP4 (30 μM) and two concentrations of DCG-IV (0.1 and 1 μM) were used either alone or together. In the sample cells (Fig. 4A), 0.1 μM DCG-IV was applied. As these data illustrate, glutamate inhibition was relatively strong in each group and roughly consistent with the responses seen in Fig. 1. Cells expressing mGluR2 (Fig. 4A, top) were also strongly inhibited by DCG-IV but not by l-AP4, as expected. In addition, no additional inhibition was seen when l-AP4 was simultaneously applied with either concentration of DCG-IV (Fig. 4, A and B). Likewise, mGluR4-expressing cells exhibited strong responses to l-AP4 alone but only weak responses to DCG-IV and no additional effect when the two agonists were combined (Fig. 4, A, middle, and B). However, in mGluR2/4-expressing cells, neither agonist produced even 10% inhibition on average, but responses were significantly stronger when l-AP4 was combined with both concentrations of DCG-IV (Fig. 4, A, bottom, B). Note that in Fig. 4B, the results of statistical tests are only shown for the combined agonist applications versus each agonist alone. However, there were some other statistically significant differences that emerged. For example, the response of mGluR2/4 cells to l-AP4/1 μM DCG-IV was significantly greater than the response to l-AP4/0.1 μM DCG-IV. These data confirm that, under the expression conditions used, mGluR2/4 cells express primarily mGluR2/4 dimers and that these receptors require agonist binding in both subunits for activation.
Fig. 4.
Neurons coexpressing mGluR2/4 at a 1:3 [cDNA] ratio respond only to combined l-AP4/DCG-IV application. A, time course of native SCG calcium current amplitudes during application and wash of 100 μM glutamate, 30 μM l-AP4, 100 nM DCG-IV, and l-AP4 and DCG-IV together (as indicated) in sample cells expressing mGluR2 (top) and mGluR4 (middle) and coexpressing both receptors at a 1:3 ratio (bottom). Insets show control and Glu-inhibited currents (during a test pulse to +10 mV) for each group. Inset scale bars represent 0.5 nA and 5 ms. B, average (±S.E.M.) responses to glutamate (filled black), l-AP4 (filled gray), 100 nM DCG-IV (open/black), 1 μM DCG-IV (open/gray), l-AP4 + 100 nM DCG-IV (hatched/black), and l-AP4 + 1 μM DCG-IV (hatched/gray). Number of cells in each group was (left to right) as follows: for mGluR2, -5, -3, -5, -4, -5, and -4; for mGluR4, -4, -3, -4, -4, -4, and -4; and for mGluR2/4, -7, -12, -7, -5, -7, and -5. Analysis of variance was run comparing responses to all drug applications in each group, but for clarity, only significant (P ≤ 0.05) differences from l-AP4 and each [DCG-IV] applied alone are reported here.*, different from 100 nM DCG-IV alone. **, different from 1 μM DCG-IV alone. †, different from l-AP4 alone, using analysis of variance to evaluate responses within each expression condition.
With an experimental protocol that seemed to allow measurement of mGluR2/4 dimers in near isolation, the PTX sensitivity of the heterodimer was examined to determine whether the heterodimer, such as mGluR2 and mGluR4 homodimers, expressed alone (Gomeza et al., 1996) coupled to the Gi/o family of PTX-sensitive G proteins. As expected, under 1:3 [cDNA] expression conditions as described above, mGluR2/4-mediated modulation of SCG calcium currents was nearly completely abolished in PTX cells. The average inhibition of calcium currents by mGluR2/4 was reduced from 34 ± 4% (n = 6) in control cells to 5 ± 1% (n = 7) in PTX cells, confirming that no unexpected change in G protein coupling occurs with mGluR2/4 heterodimerization.
Requirements for NAM and PAM Action on mGluR2/4 Heterodimers.
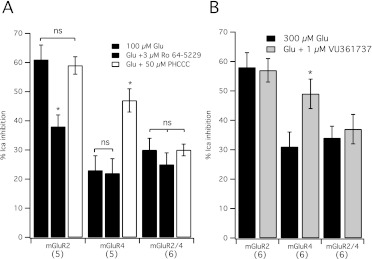
The current dogma regarding PAM and NAM association with mGluR dimers suggests that, although two NAMs are required for inhibition (Hlavackova et al., 2005; Lundström et al., 2011), only one PAM per dimer is required for potentiation (Goudet et al., 2005; Lundström et al., 2011). However, these conclusions rely largely on binding, or studies using chimeric mGluRs. Thus, these questions have not been addressed to date with wild-type mGluRs in functional studies using neural cells. These questions were addressed here by examining mGluR2/4 heterodimer function in SCG neurons, as described above, and examining the ability of Ro 64-5229, an allosteric antagonist (NAM) selective for mGluR2 (Kolczewski et al., 1999) to inhibit receptor activation by glutamate. The ability of PHCCC, an mGluR4 selective PAM (Maj 2003), to enhance glutamate responses was also tested. First, the selectivity of the compounds was examined in neurons expressing mGluR2 or mGluR4 alone. Figure 5A illustrates the results of these experiments. In mGluR2-expressing cells (Fig. 5A), 300 μM glutamate produced a 61 ± 5% (n = 5) inhibition of the calcium current. In the presence of 3 μM Ro 64-5229, the inhibition by glutamate was significantly reduced to 38 ± 4% (n = 5), whereas 50 μM PHCCC did not alter the glutamate response (59 ± 3%, n = 4). In mGluR4-expressing cells (Fig. 5A), the effect of glutamate on five cells was typically weaker and unaffected by Ro 64-5229 (22 ± 5%, n = 5) at 23 ± 5% but potently and significantly enhanced by PHCCC (47 ± 4%, n = 5). In cells expressing mGluR2/4 heterodimers, the control glutamate response was 30 ± 4% (n = 6). Responses to glutamate were not detectably altered by either Ro 64-5229 or PHCCC. When combined with these compounds, glutamate produced responses of 25 ± 4% (n = 6) and 30 ± 2% (n = 5), respectively (Fig. 5A). The lack of effect of Ro 64-5229 on the glutamate response on mGluR2/4 was predicted by binding studies on mGluR2 (Lundström et al., 2011) and functional studies using chimeric receptors based on mGluR1 and -5 (Hlavackova et al., 2005); thus, these results were expected and provide further support for the finding that mGluR dimers require NAM binding in both subunits for receptor inhibition. However, the result with the PAM PHCCC was unexpected. Previous work using radiolabeled compound binding has shown that mGluR2 seems to have half of the number of binding sites for an mGluR2 selective PAM than for an orthosteric, mGluR2 selective ligand (Lundström et al., 2011). Furthermore, studies using chimeric group I mGluRs show that PAMs can potentiate responses in receptors that contain only one subunit capable of interacting with the PAM (Goudet et al., 2005). For this reason, the PHCCC results (Fig. 5A) were unexpected. Therefore, this experiment was repeated using another mGluR4 selective PAM, VU0361737 [VU036 (Engers et al., 2009)]. As shown in Fig. 5B, 300 μM glutamate applied to six mGluR2-expressing cells produced a calcium current inhibition of 58 ± 5% in the absence of VU036 and 57 ± 4% in the presence of 1 μM VU036. Calcium current in cells expressing mGluR4 alone were inhibited 31 ± 5% by 300 μM glutamate, and 49 ± 5% in the presence of VU036 (n = 6), a significant increase (Fig. 5B). Consistent with the results using PHCCC, VU036 failed to enhance glutamate responses in cells expressing mGluR2/4. In these cells, 300 μM glutamate inhibited the calcium current 34 ± 4% in the absence and 37 ± 5% in the presence of VU036 (n = 6).
Fig. 5.
Selective NAMs and PAMs fail to alter signaling through mGluR2/4. A, average (±S.E.M.) calcium current inhibition in response to 300 μM glutamate alone (filled black) or in the presence of the mGluR2 selective NAM 3 μM Ro 64-5229 (filled gray) or the mGluR4 selective PAM 50 μM PHCCC (open) in SCG neurons expressing mGluR2, mGluR4, or mGluR2/4, as indicated. Number of cells in each group is shown in parentheses.*, different from (P ≤ 0.05) the response to glutamate alone, analysis of variance. B, average (±S.E.M.) calcium current inhibition in response to 300 μM glutamate alone (filled black) or in the presence of the mGluR4 selective PAM 1 μM VU0361737 (filled gray) in SCG neurons expressing mGluR2, mGluR4, or mGluR2/4, as indicated. Number of cells in each group shown in parentheses.*, different from (P ≤ 0.05) the response to glutamate alone, t test.
Finally, responses of the mGluR2/4 heterodimer to an mGluR2 selective PAM BINA (Galici et al., 2006) were examined. Because mGluR2 responses were generally of very high efficacy when the receptor was expressed alone, the effect of 100 nM BINA was examined against a low glutamate concentration (1 μM). BINA strongly enhanced glutamate responses at this glutamate concentration in mGluR2-expressing cells from 8 ± 2 to 39 ± 7% (n = 4) when BINA was absent or present, respectively. By contrast, BINA did not enhance responses to mGluR4- or mGluR2/4-expressing neurons. In mGluR4-expressing cells, responses were 0 ± 2 and 3 ± 3% (n = 4), respectively. In mGluR2/4 cells, the responses were 2 ± 1 and 0 ± 1% (n = 4), respectively. Together, these data confirm that mGluR dimers require NAM association with both subunits to produce inhibition of receptor activity. However, the data indicate that, at least for the mGluR2/4 heterodimer, a single PAM binding site in the dimer seems insufficient to result in potentiation of the glutamate response.
mGluR2/4 Heterodimers Are Not Susceptible to PAM Effects.
The data above indicate that signaling of the mGluR2/4 heterodimer is not potentiated in the presence of PAMs that can interact with either the mGluR4 or the mGluR2 subunits. One interpretation of these data is that the dogma regarding PAM interactions with mGluR dimers is flawed in the prediction that only a single PAM is required per mGluR dimer for potentiation of signaling (Goudet et al., 2005; Lundström et al., 2011). An alternate interpretation, however, is that the mGluR2/4 heterodimer is not, unlike mGluR2 or mGluR4 homodimers, susceptible to positive allosteric modulation, at least by the compounds used here. To distinguish between these possibilities, the activity of mGluR2, mGluR4, and mGluR2/4 was examined in the absence and presence of simultaneous application of the mGluR2 PAM BINA and the mGluR4 PAM VU036 at three glutamate concentrations (Fig. 6). If mGluR2/4 requires PAM association with both dimer subunits, simultaneous application of both PAMs should potentiate responses.
At higher glutamate concentrations, potentiation of mGluR2 responses by combined BINA/VU036 application (at 100 nM BINA and 1 μM VU036) was not apparent, probably because of the high efficacy of the control responses (Fig. 6, left). However, responses to 1 μM glutamate were strongly potentiated, similar to those seen with BINA alone (see above). In cells expressing mGluR4, significant potentiation of the 100 μM glutamate response was observed with BINA/VU036 (Fig. 6, middle). The magnitude of this potentiation was similar to that observed with VU036 alone (Fig. 5). By contrast, in cells expressing mGluR2/4, no potentiation of responses to 1, 10, or 100 μM glutamate was apparent with combined application of BINA/VU036 (Fig. 6, right).
Finally, combining BINA with 50 μM PHCCC was likewise ineffective at potentiating responses to mGluR2/4 at 3 and 300 μM glutamate, which produced 13 ± 2 and 25 ± 4% inhibition of the calcium currents, respectively, in the absence of BINA and PHCCC and 11 ± 5 and 24 ± 4% (n = 6) inhibition, respectively, in the presence of 100 nM BINA and 50 μM PHCCC. These data demonstrate the novel finding that, unlike mGluR2 or mGluR4 homodimeric receptors, mGluR2/4 heterodimers do not seem to be modulated with conventional PAMs.
Discussion
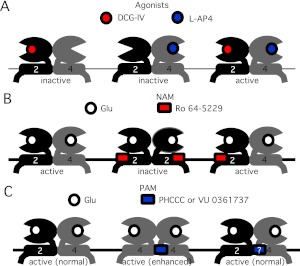
In the present study, signaling of mGluR2, mGluR4, and a putative mGluR2/4 heterodimer was examined by expressing each receptor(s) in sympathetic neurons from the rat SCG and using Gβγ-mediated modulation of the native calcium currents as an assay for receptor signaling. By use of this system and several available pharmacological tools, including selective orthosteric ligands, NAMs, and PAMs, several dogmatic assumptions about the pharmacological properties of mGluR dimers were tested, including the notions that 1) mGluR dimers require ligand binding in both subunits for activity (Fig. 7A), 2) each mGluR dimer has two NAM binding sites that must be occupied for receptor inhibition (Fig. 7B), and 3) each mGluR dimer has only one PAM binding site (Fig. 7C). Note that, coupled with the finding that NAM and PAM binding sites at least partially overlap, the notion that each type of compound has a different number of binding sites per mGluR dimer gives rise to an apparent contradiction. The recent finding that mGluR2 and -4 can associate as heterodimers (Doumazane et al., 2011) provided the opportunity to test these assumptions in a series of experiments using wild-type mGluR dimers functioning in intact neural cells.
Fig. 7.
Schematic model describing action of agonists, NAMs, and PAMs on the mGluR2/4 heterodimer. A, selective agonists fail to activate mGluR2/4 but combined application of both activates the receptors as well as glutamate. B, application of an mGluR2 selective NAM is insufficient to inhibit mGluR2/4 signaling. C, selective PAMs fail to potentiate mGluR2/4 responses, possibly because of the lack of a binding site, which may contact both dimer subunits in homodimers.
To observe activity of apparent mGluR2/4 heterodimers in SCG neurons, it was necessary to carefully titrate the ratio of cDNA intranuclearly injected into the neurons such that the resulting receptors showed no evidence of expression of either mGluR2 or mGluR4 homodimers (no meaningful responses to selective ligands; Figs. 3 and 4) but responded as well to the combined mGluR2 and -4 selective agonists to a saturating concentration of glutamate. These data were rather convincing to the notion that most of the expressed receptors were mGluR2/4 heteromers. It should be noted that from these data it cannot be determined what propensity each subunit has for forming homodimers versus heterodimers. However, other than adjusting the relative amounts of injected cDNAs, no special precautions were taken to ensure that mGluR2/4 heteromers would form. The fact that the resultant receptors had a unique pharmacological profile suggested that mGluR2/4 heterodimers (Doumazane et al., 2011) were the predominant species. This finding was entirely fortuitous and may suggest that these receptors “prefer” to form heterodimers to homodimers when both receptors are present in the same cell. Otherwise, some evidence for homodimer formation should have been apparent, especially in the experiment described in Fig. 4.
The data indicate that, at least for the mGluR2/4 heterodimer, potentiation of receptor activity using PAMs does not seem to be possible, despite the reports showing that only one PAM per mGluR dimer is necessary (Goudet et al., 2005) (mGluR1 and -5) or even able to bind (Lundström et al., 2011) (mGluR2) for potentiation of responses. The data presented here were surprising but not necessarily contradictory. An alternate interpretation that is consistent with those data and with the present study is that the PAM binding site may involve contact with both dimer subunits but also partially overlap the NAM site rather than a single site located entirely within a single subunit. This interpretation could explain the single PAM site per homodimer model and is also consistent with the data presented here showing that, for mGluR2/4 heterodimers, neither mGluR2-selective PAMs, mGluR4-selective PAMs, or pairing of the two was effective. This idea may also be consistent with current models suggesting overlapping PAM and NAM binding sites in mGluR2 because at least some NAMs seem to contact helix 5 in mGluR2 (Lundström et al., 2011), and this helix is thought to lie in close proximity to its counterpart in mGluR dimers (Yanagawa et al., 2011). Thus, it seems plausible that a small molecule at this site could span dimer subunits and that a PAM binding site for mGluR2 or mGluR4 selective compounds may be absent in mGluR2/4 heterodimers. However, it should be noted that it is not strictly necessary to invoke overlapping NAM and PAM binding sites, because the data (Lundström et al., 2011) are also consistent with a model in which NAM binding induces a conformational change that occludes PAM binding indirectly. Furthermore, the precise locations of PAM and NAM binding sites in the helical domains of mGluRs are not precisely known, so much of the interpretation remains subject to further study. At present, it cannot be determined whether PAMs are unable to interact with mGluR2/4 heterodimers or that they bind but do not potentiate responses.
The assumption of a dimer-spanning PAM binding site may seem contradictory to the findings of Goudet et al. (2005), which show that chimeric group I mGluRs engineered to assemble as mGluR1/5 heterodimers could be potentiated with mGluR1- or mGluR5-selective PAMs. However, this potential for PAM interaction was assessed by effects of PAMs on each receptor expressed as homodimers, with the assumption that each subunit possessed a distinct PAM binding site. An alternate model that is consistent with those data as well as those of Lundström et al. (2011) (data with mGluR2) and the present study is that PAMs bind to a single site in each dimer that may span subunits. This interpretation would necessitate the argument that selectivity arises from interaction of the PAM with its target subunit [e.g., mGluR5 (Goudet et al., 2005)] but still requires interaction with the “other” dimer subunit [e.g., mGluR1 (Goudet et al., 2005)] such that this portion of the PAM site is less selective. The loss of selective PAM effects on mGluR2/4 dimers, but not on chimeric mGluR1/5 dimers (Goudet et al., 2005), can be plausibly explained by the fact that mGluR1 and -5 share much greater homology than mGluR2 and -4.
The results in this study demonstrate for the first time that mGluR2 and -4 can combine to form functional heterodimers in neurons and that these novel receptors exhibit a unique pharmacological profile observable in the presence of selective orthosteric agonists, NAMs, and PAMs. Further studies will be necessary to determine whether the mGluR2/4 heterodimer exists in native tissues where mGluR2 and -4 subunits are natively expressed, but some overlap of mGluR2 and -4 expression in the brain is apparent (e.g., in cerebellar granule neurons) (Prézeau et al., 1994). Nevertheless, the findings of this study may provide a pharmacological road map to address this question more definitively.
This work was supported in part by the National Institutes of Health National Institute of General Medical Sciences [Grant GM101023].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- mGluRs
- metabotropic glutamate receptors
- SCG
- superior cervical ganglion
- NAM
- negative allosteric modulator
- PAM
- positive allosteric modulator
- VU0361737 (VU036)
- N-(4-chloro-3-methoxyphenyl)-2-pyridinecarboxamide
- BINA
- biphenyl indanone-A
- PHCCC
- N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide
- l-AP4
- l-(+)-2-amino-4-phosphonobutyric acid
- DCG-IV
- (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine
- Ro 64-5229
- (Z)-1-[2-cycloheptyloxy-2-(2,6-dichlorophenyl)ethenyl]-1H-1,2,4-triazole
- PTX
- pertussis toxin.
Authorship Contributions
Participated in research design: Kammermeier.
Conducted experiments: Kammermeier.
Performed data analysis: Kammermeier.
Wrote or contributed to the writing of the manuscript: Kammermeier.
References
- Beqollari D, Kammermeier PJ. (2008) The mGlu(4) receptor allosteric modulator N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide acts as a direct agonist at mGlu(6) receptors. Eur J Pharmacol 589:49–52 [DOI] [PubMed] [Google Scholar]
- Beqollari D, Kammermeier PJ. (2010) Venus fly trap domain of mGluR1 functions as a dominant negative against group I mGluR signaling. J Neurophysiol 104:439–448 [DOI] [PubMed] [Google Scholar]
- Engers DW, Niswender CM, Weaver CD, Jadhav S, Menon UN, Zamorano R, Conn PJ, Lindsley CW, Hopkins CR. (2009) Synthesis and evaluation of a series of heterobiarylamides that are centrally penetrant metabotropic glutamate receptor 4 (mGluR4) positive allosteric modulators (PAMs).J Med Chem 52:4115–4118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doumazane E, Scholler P, Zwier JM, Eric T, Rondard P, Pin JP. (2011) A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors.FASEB J 25:66–77 [DOI] [PubMed] [Google Scholar]
- Galici R, Jones CK, Hemstapat K, Nong Y, Echemendia NG, Williams LC, de Paulis T, Conn PJ. (2006) Biphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic- and anxiolytic-like effects in mice. J Pharmacol Exp Ther 318:173–185 [DOI] [PubMed] [Google Scholar]
- Gomeza J, Mary S, Brabet I, Parmentier ML, Restituito S, Bockaert J, Pin JP. (1996) Coupling of metabotropic glutamate receptors 2 and 4 to G alpha 15, G alpha 16, and chimeric G alpha q/i proteins: characterization of new antagonists. Mol Pharmacol 50:923–930 [PubMed] [Google Scholar]
- Goudet C, Kniazeff J, Hlavackova V, Malhaire F, Maurel D, Acher F, Blahos J, Prézeau L, Pin JP. (2005) Asymmetric functioning of dimeric metabotropic glutamate receptors disclosed by positive allosteric modulators. J Biol Chem 280:24380–24385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, McElligott ZA, Winder DG. (2007) Group I mGluRs and long-term depression: potential roles in addiction? Mol Neurobiol 36:232–244 [DOI] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. (1996) Modulation of Ca2+ channels by G-protein beta gamma subunits. Nature 380:258–262 [DOI] [PubMed] [Google Scholar]
- Hlavackova V, Goudet C, Kniazeff J, Zikova A, Maurel D, Vol C, Trojanova J, Prézeau L, Pin JP, Blahos J. (2005) Evidence for a single heptahelical domain being turned on upon activation of a dimeric GPCR. EMBO J 24:499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Cao J, Jiang M, Labesse G, Liu J, Pin JP, Rondard P. (2011) Interdomain movements in metabotropic glutamate receptor activation. Proc Nat Acad Sci USA 108:15480–15485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda SR. (1997) Heterologous expression of receptors and signaling proteins in adult mammalian sympathetic neurons by microinjection. Methods Mol Biol 83:191–202 [DOI] [PubMed] [Google Scholar]
- Ikeda SR. (1996) Voltage-dependent modulation of N-type calcium channels by G-protein beta gamma subunits. Nature 380:255–258 [DOI] [PubMed] [Google Scholar]
- Ikeda SR, Jeong SW, Kammermeier PJ, Ruiz-Velasco V, King MM. (1999) Heterologous expression of a green fluorescent protein-pertussis toxin S1 subunit fusion construct disrupts calcium channel modulation in rat superior cervical ganglion neurons. Neurosci Lett 271:163–166 [DOI] [PubMed] [Google Scholar]
- Ishida M, Saitoh T, Shimamoto K, Ohfune Y, Shinozaki H. (1993) A novel metabotropic glutamate receptor agonist: marked depression of monosynaptic excitation in the newborn rat isolated spinal cord. Br J Pharmacol 109:1169–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jingami H, Nakanishi S, Morikawa K. (2003) Structure of the metabotropic glutamate receptor. Curr Opin Neurobiol 13:271–278 [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Ikeda SR. (1999) Expression of RGS2 alters the coupling of metabotropic glutamate receptor 1a to M-type K+ and N-type Ca2+ channels. Neuron 22:819–829 [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Yun J. (2005) Activation of metabotropic glutamate receptor 1 dimers requires glutamate binding in both subunits. J Pharmacol Exp Ther 312:502–508 [DOI] [PubMed] [Google Scholar]
- Kniazeff J, Bessis AS, Maurel D, Ansanay H, Prézeau L, Pin JP. (2004) Closed state of both binding domains of homodimeric mGlu receptors is required for full activity. Nat Struct Mol Biol 11:706–713 [DOI] [PubMed] [Google Scholar]
- Kolczewski S, Adam G, Stadler H, Mutel V, Wichmann J, Woltering T. (1999) Synthesis of heterocyclic enol ethers and their use as group 2 metabotropic glutamate receptor antagonists. Bioorg Med Chem Lett 9:2173–2176 [DOI] [PubMed] [Google Scholar]
- Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, Nakanishi S, Jingami H, Morikawa K. (2000) Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature 407:971–977 [DOI] [PubMed] [Google Scholar]
- Lundström L, Bissantz C, Beck J, Wettstein JG, Woltering TJ, Wichmann J, Gatti S. (2011) Structural determinants of allosteric antagonism at metabotropic glutamate receptor 2: mechanistic studies with new potent negative allosteric modulators. Br J Pharmacol 164:521–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj M, Bruno V, Dragic Z, Yamamoto R, Battaglia G, Inderbitzin W, Stoehr N, Stein T, Gasparini F, Vranesic I, et al. (2003) (−)-PHCCC, a positive allosteric modulator of mGluR4: characterization, mechanism of action, and neuroprotection. Neuropharmacology 45:895–906 [DOI] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. (2010) Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. Eur J Pharmacol 639:115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picconi B, Pisani A, Centonze D, Battaglia G, Storto M, Nicoletti F, Bernardi G, Calabresi P. (2002) Striatal metabotropic glutamate receptor function following experimental parkinsonism and chronic levodopa treatment. Brain 125:2635–2645 [DOI] [PubMed] [Google Scholar]
- Prézeau L, Carrette J, Helpap B, Curry K, Pin JP, Bockaert J. (1994) Pharmacological characterization of metabotropic glutamate receptors in several types of brain cells in primary cultures. Mol Pharmacol 45:570–577 [PubMed] [Google Scholar]
- Robbins MJ, Ciruela F, Rhodes A, McIlhinney RA. (1999) Characterization of the dimerization of metabotropic glutamate receptors using an N-terminal truncation of mGluR1alpha. J Neurochem 72:2539–2547 [DOI] [PubMed] [Google Scholar]
- Romano C, Yang WL, O'Malley KL. (1996) Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J Biol Chem 271:28612–28616 [DOI] [PubMed] [Google Scholar]
- Sato T, Shimada Y, Nagasawa N, Nakanishi S, Jingami H. (2003) Amino acid mutagenesis of the ligand binding site and the dimer interface of the metabotropic glutamate receptor 1. Identification of crucial residues for setting the activated state. J Biol Chem 278:4314–4321 [DOI] [PubMed] [Google Scholar]
- Schoepp DD. (2001) Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299:12–20 [PubMed] [Google Scholar]
- Selkirk JV, Challiss RA, Rhodes A, McIlhinney RA. (2002) Characterization of an N-terminal secreted domain of the type-1 human metabotropic glutamate receptor produced by a mammalian cell line. J Neurochem 80:346–353 [DOI] [PubMed] [Google Scholar]
- Stephenson RP. (1997) A modification of receptor theory. 1956. Br J Pharmacol 120 (Suppl 1):106–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateyama M, Abe H, Nakata H, Saito O, Kubo Y. (2004) Ligand-induced rearrangement of the dimeric metabotropic glutamate receptor 1alpha. Nat Struct Mol Biol 11:637–642 [DOI] [PubMed] [Google Scholar]
- Tateyama M, Kubo Y. (2011) The intra-molecular activation mechanisms of the dimeric metabotropic glutamate receptor 1 differ depending on the type of G proteins. Neuropharmacology 61:832–841 [DOI] [PubMed] [Google Scholar]
- Tsuchiya D, Kunishima N, Kamiya N, Jingami H, Morikawa K. (2002) Structural views of the ligand-binding cores of a metabotropic glutamate receptor complexed with an antagonist and both glutamate and Gd3+. Proc Natl Acad Sci USA 99:2660–2665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson PN, Conn PJ. (2012) Metabotropic glutamate receptors as therapeutic targets for schizophrenia. Neuropharmacology 62:1461–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa M, Yamashita T, Shichida Y. (2011) Comparative fluorescence resonance energy transfer analysis of metabotropic glutamate receptors: implications about the dimeric arrangement and rearrangement upon ligand bindings. J Biol Chem 286:22971–22981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Ikeda SR. (1993) Adenosine modulates voltage-gated Ca2+ channels in adult rat sympathetic neurons. J Neurophysiol 70:610–620 [DOI] [PubMed] [Google Scholar]