Abstract
The Sec61 protein translocon is a multimeric complex that transports proteins across lipid bilayers. We discovered that the Sec61β subunit modulates cellular sensitivity to chemotherapeutic agents, particularly the platinum drugs. To investigate the mechanism, expression of Sec61β was constitutively knocked down in 2008 ovarian cancer cells. Sec61β knockdown (KD) resulted in 8-, 16.8-, and 9-fold resistance to cisplatin (cDDP), carboplatin, and oxaliplatin, respectively. Sec61β KD reduced the cellular accumulation of cDDP to 67% of that in parental cells. Baseline copper levels, copper uptake, and copper cytotoxicity were also reduced. Because copper transporters and chaperones regulate platinum drug accumulation and efflux, their expression in 2008 Sec61β-KD cells was analyzed; ATP7A was found to be 2- to 3-fold overexpressed, whereas there was no change in ATP7B, ATOX1, CTR1, or CTR2 levels. Cells lacking ATP7A did not exhibit increased cDDP resistance upon knockdown of Sec61β. Sec61β-KD cells also exhibited altered ATP7A cellular distribution. We conclude that Sec61β modulates the cytotoxicity of many chemotherapeutic agents, with the largest effect being on the platinum drugs. This modulation occurs through effects of Sec61β on the expression and distribution of ATP7A, which was shown previously to control platinum drug sequestration and cytotoxicity.
Introduction
Although the ability of copper to undergo reversible oxidation is essential for the function of copper-requiring enzymes, this process produces reactive oxygen species that can cause severe cellular damage (Linder and Hazegh-Azam, 1996). Cells have evolved a complex system of copper transporters and chaperones that protect Cu(I) during its influx and distribution throughout the cytoplasm (Camakaris et al., 1999; O'Halloran and Culotta, 2000; Huffman and O'Halloran, 2001). ATP7A and ATP7B are P-type ATPases that sequester copper into the trans-Golgi network, where it is loaded onto ceruloplasmin and other copper-dependent enzymes (Dierick et al., 1997; Suzuki and Gitlin, 1999). Maintenance of the trans-Golgi compartments in which ATP7A and ATP7B reside may involve ADP-ribosylation factors (ARFs) and guanine nucleotide exchange factors (GEFs) (Holloway et al., 2007). Excess copper causes ATP7A and ATP7B to relocate to either the plasma membrane or vesicular compartments and is thought to be necessary for the efflux or exocytosis of copper (Camakaris et al., 1995; Petris et al., 1996; Petris and Mercer, 1999; Roelofsen et al., 2000; Setty et al., 2008).
Resistance to platinum-containing drugs may be the result of decreased drug uptake, changes in repair of DNA adducts, and/or alterations in apoptotic signaling pathways. We and others showed that proteins involved in copper homeostasis control both the influx and the efflux of platinum-containing drugs (Safaei and Howell, 2005). CTR1 and CTR2 regulate uptake, whereas ATP7A and ATP7B are involved in intracellular sequestration and drug export. Chaperones such as ATOX1 may transport the drugs between intracellular sites (Holzer et al., 2003; Samimi et al., 2004a; Safaei et al., 2007; Blair et al., 2009).
Cancer cell lines expressing high levels of ATP7A exhibit increased resistance to cDDP, carboplatin, and oxaliplatin (Samimi et al., 2004b). Clinical data suggested that increased expression of ATP7A in tumors is associated with worse outcomes among patients with ovarian or lung cancer who are treated with platinum-containing chemotherapeutic agents (Samimi et al., 2003; Li et al., 2012). ATP7A contains several metal-binding domains that can bind cDDP, and one hypothesis is that ATP7A exports the platinum-containing drugs in a similar manner as copper. In addition to being associated with resistance to platinum-containing drugs, ATP7A has been shown to confer resistance to other chemotherapeutic agents. It is more difficult to explain how ATP7A might mediate resistance to less structurally related compounds, and some investigators hypothesized that, in addition to serving as a copper export pump, ATP7A regulates an overall vesicle secretory process that is involved in the efflux of many compounds (Owatari et al., 2007; Furukawa et al., 2008).
The Sec61 translocon is an ER-resident multimeric protein complex that allows the transport of proteins from one side of a membrane to the other or laterally into a lipid bilayer (in the case of transmembrane proteins). It is composed of three subunits, α, β, and γ. Structural data on the highly conserved bacterial secYEG suggest that the α subunit forms the pore of the channel through which a polypeptide chain passes, whereas the γ subunit stabilizes the structure (Van den Berg et al., 2004). The β subunit makes only peripheral contact with the complex and seems largely dispensable for the forward-translocating function.
Less is known about retrotranslocation, but it was reported that this involves the ER-associated degradation pathway; the β subunit was shown to be important for this process in some cases (Liao and Carpenter, 2007; Scott and Schekman, 2008). Sec61β has also been associated with components of the exocyst complex and the reticulon family of proteins (Toikkanen et al., 2003; Zhao and Jäntti, 2009), whose main functions are thought to be tethering of transport vesicles trafficked from the ER to the plasma membrane and shaping of ER structure. Increased expression of Sec61β was reported for several types of cancer, including ovary, kidney, prostate, and brain, on the basis of microarray data (Wu et al., 2009), although no investigators have examined how its expression changes during platinum drug therapy.
The importance of trafficking for the function of copper export proteins led us to investigate the role of Sec61β. We made the novel observation that Sec61β modulates sensitivity to the cytotoxic effects of platinum-containing chemotherapeutic agents. Knockdown of Sec61β in ovarian cancer cells results in large increases in resistance to cDDP, carboplatin, and oxaliplatin. Knockdown of Sec61β also confers lesser degrees of resistance to several other classes of clinical chemotherapeutic agents. Because resistance to the platinum-containing drugs is linked to copper homeostasis, we analyzed the effects of Sec61β knockdown on the expression of copper transporters and chaperones, and we found a significant increase in the expression of ATP7A. Knockdown of Sec61β in cells lacking ATP7A failed to alter drug sensitivity, which demonstrates a specific role for ATP7A. Furthermore, the distribution of ATP7A is altered in Sec61β-KD cells. We conclude that Sec61β modulates the cytotoxicity of many chemotherapeutic agents, with the greatest effect being on the sensitivity to the platinum drugs, and this is mediated through effects on the expression and subcellular localization of ATP7A.
Materials and Methods
Drugs and Reagents.
Commercial formulations of cDDP, carboplatin, and oxaliplatin were obtained from the Moores Cancer Center pharmacy. Doxorubicin, paclitaxel, etoposide, and vincristine were gifts from the San Diego Veterans Affairs Infusion Center pharmacy (San Diego, CA). The drugs were diluted to the desired concentrations in RPMI 1640 medium or α-minimal essential medium (Thermo Fisher Scientific, Waltham, MA). A detergent-compatible protein kit was purchased from Bio-Rad Laboratories (Hercules, CA), and sulforhodamine B was obtained from MP Biomedicals (Solon, OH); sulforhodamine B was dissolved at 0.4% (w/v) in 1% (v/v) acetic acid solution.
Cell Types, Culture, and Molecular Engineering.
Human ovarian carcinoma 2008 (DiSaia et al., 1972), IGROV-1, and A2780 cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum. The Me32a and Me32a/MNK cell lines were grown in α-minimal essential medium supplemented with 10% fetal calf serum. To suppress constitutively the expression of Sec61β, human ovarian carcinoma 2008, IGROV-1, and A2780 cells and human fibroblast Me32a cells (Samimi et al., 2004a) were infected with a lentivirus expressing a shRNA targeting Sec61β mRNA (Sigma-Aldrich, St. Louis, MO). The sequence of the shRNA used for most knockdown procedures was ccggcagtattggttatgagtcttcctcgaggaagactcataaccaatactgttttttg. The shRNA sequences used for knockdown procedures targeted to other regions of the Sec61β gene were ccgggattctacacagaagattcacctcgaggtgaatcttctgtgtagaatcttttttg and ccggcccaacatttcttggaccaaactcgagtttggtccaagaaatgttgggttttttg, to create the 2008 Sec61β-KD 279 and 927 sublines, respectively. Infected cells were selected in appropriate medium containing 5 μg/ml puromycin. Cell survival after exposure to increasing concentrations of drugs was assayed by using the sulforhodamine B assay system (Monks et al., 1991). Four thousand cells were seeded into each well of a 96-well tissue culture plate. Cells were incubated overnight at 37°C in 5% CO2 and then were exposed to varying drug concentrations in 200 μl of complete medium. Cells were allowed to grow for 4 days after the addition of drug, after which the medium was removed and the protein was precipitated with 50% trichloroacetic acid and stained for 15 min at room temperature with 100 μl of 0.4% sulforhodamine B in 1% acetic acid. After washing, the absorbance of each well at 515 nm was recorded with a Versamax tunable microplate reader (Molecular Devices, Sunnyvale, CA). Results are plotted as drug concentration versus log10 cell survival. All experiments were repeated at least three times, by using three cultures for each drug concentration.
Western Blotting.
Whole-cell lysates were dissolved in lysis buffer (150 mM NaCl, 5 mM EDTA, 1% Triton X-100, and 10 mM Tris, pH 7.4) with protease inhibitors (Roche Diagnostics, Mannheim, Germany) and were subjected to electrophoresis on 4 to 15% gels in Tris-glycine (30–50 μg of protein per lane). Protein levels were determined with the DC protein assay (Bio-Rad Laboratories). A Bio-Rad Trans-Blot system was used to transfer the proteins to Immobilon-P or Immobilon-FL membranes (Millipore Corp., Billerica, MA). Blots were incubated overnight at 4°C with 5% dry nonfat milk in Tris-buffered saline/Tween 20 (150 mM NaCl, 300 mM KCl, 10 mM Tris, pH 7.4, 0.01% Tween 20) or Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE). Blots were incubated for 2 h at room temperature with anti-Sec61β antibody (1:1000 dilution; EMD Millipore, Billerica, MA), anti-ATP7A antibody (1:500; Abcam Inc., Cambridge, MA, or Neuromab, Davis, CA), anti-CTR2 antibody (1:500; custom antibody against peptide SQQTIAETDGDSAGSD, prepared by Biomatik, Wilmington, DE), anti-ATP7B antibody (1:1000; Novus Biologicals, Inc., Littleton, CO), anti-caveolin antibody (1:1000; BD Biosciences, San Jose, CA), or antibody to β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). Horseradish peroxidase-conjugated secondary antibody (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) or fluorescently labeled secondary antibody (LI-COR Biosciences) was dissolved in 5% milk in Tris-buffered saline/Tween 20 or Odyssey blocking buffer and was incubated with the blot for 1 to 2 h at room temperature. After three 10-min washes, blots were exposed to enhanced chemiluminescence reagent (Thermo Fisher Scientific) and were detected with X-ray films (HyBlot CL; Denville Scientific, Metuchen, NJ). Blots probed with fluorescently labeled antibody were imaged by using an Odyssey infrared imager (LI-COR Biosciences).
Assessment of ATP7A Protein Stability.
2008 cells or 2008 Sec61β-KD cells were incubated with 30 μg/ml cycloheximide for 0, 12, 24, or 48 h. Cells were then washed three times with PBS, and lysates were harvested for Western blotting as described above. Bands were quantitated by using the Odyssey infrared imager (LI-COR Biosciences), and results were normalized to actin levels.
Lipid Raft Isolation.
Fractions enriched in lipid rafts were prepared from the 2008 cells by using a sodium carbonate-based, detergent-free method, as described previously (Ostrom and Liu, 2007). 2008 cells were grown to 80 to 90% confluence and were scraped into 2 ml of 500 mM sodium carbonate with protease inhibitors. Homogenization was performed by using 10 strokes of a Dounce homogenizer, followed by four 20-s bursts from a probe sonicator. Homogenates were adjusted to 45% sucrose with 25 mM 4-morpholineethanesulfonic acid (MES), 0.15 M NaCl. A 5 to 35% discontinuous sucrose gradient was layered over this solution in an ultracentrifuge tube, which was centrifuged at 39,000 rpm for 20 h at 4°C in a SW-40Ti rotor (Beckman Coulter, Inc., Fullerton, CA). Eleven fractions were collected from the top and analyzed through Western blotting, as was a total lysate aliquot.
Assessment of ATP7A Cell Surface Expression.
2008 cells or 2008 Sec61β-KD cells were grown to ∼80 to 90% confluence and then were incubated with 200 μM CuSO4. Cell surface protein was biotinylated with a cell surface protein isolation kit (Thermo Fisher Scientific), according to the manufacturer's instructions. Samples were then analyzed through Western blotting, as described above. Antibodies to actin (Santa Cruz Biotechnology) and Na+/K+-ATPase (Cell Signaling Technology, Danvers, MA) were used as controls for assessment of the quality of preparations.
qRT-PCR.
Sec61β mRNA levels were measured by using a qRT-PCR method for detection of relative amounts of first-strand cDNA. cDNA was generated from mRNA isolated by using TRIzol (Invitrogen, Carlsbad, CA). Purified mRNA was converted to cDNA by using oligo(dT)20 priming and a SuperScript III first-strand kit (Invitrogen). qRT-PCR was performed with an MyiQ system (Bio-Rad Laboratories). Reactions were prepared by using iQ SYBR Green Supermix (Bio-Rad Laboratories), according to the manufacturer's recommendations. Samples were prepared in quadruplicate, and three independent sample sets were analyzed. Analyses were performed with Bio-Rad iQ5 system software.
Measurement of Platinum and Copper Accumulation.
Whole-cell platinum and copper contents were measured as reported previously (Larson et al., 2009). Cells were incubated in regular medium or 30 μM cDDP- or 100 μM CuSO4-containing, reduced-serum Dulbecco's modified Eagle's medium (Invitrogen) at 37°C for 60 min, after which the drug-containing medium was removed and the cultures were washed three times with ice-cold, high-performance liquid chromatography-grade PBS. To control for nonspecific binding of drug to the wells and cell surfaces, time 0 samples, for which drug-containing medium was aspirated within 15 s after the start of drug exposure, were obtained; these values were subtracted from all measurements, as described previously (Larson et al., 2009). Time 0 values were less than 2 to 3% of the levels measured after 1 h of drug exposure. Concentrated (70%) nitric acid was added to each well, and the plates were incubated overnight at room temperature, to dissolve all cellular debris thoroughly. After addition of an indium internal standard, platinum or copper concentrations were measured by using an Element 2 ICP-MS system (PerkinElmer Life and Analytical Sciences, Waltham, MA) located in the analytical facility at Scripps Institute of Oceanography (University of California, San Diego, CA). For normalization, total sulfur levels were measured by using an inductively coupled plasma-optical emission spectroscopy system (PerkinElmer Life and Analytical Sciences) located at Scripps Institute of Oceanography. All data presented are the means of at least three independent experiments, each performed with six cultures per concentration tested.
For measurement of platinum levels in DNA, cells were lysed and DNA was harvested by using DNAzol (Invitrogen), according to the manufacturer's protocol. For normalization, DNA levels were measured before the addition of nitric acid, by using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific). The DNA samples were then digested with nitric acid and prepared before ICP-MS measurement of platinum levels, as described above.
Deconvolution Microscopy.
Cells were grown on eight-well microscope chamber slides (Millipore). When cells reached ∼60 to 80% confluence, the medium was removed from each chamber. The chambers were then treated for 1 h with 300 μl of growth medium containing either 200 μM CuSO4 or 100 μM BCS. After 1 h of drug exposure, the medium was removed and the slides were treated and washed three times with PBS. Cells were fixed with 4% paraformaldehyde in PBS for 20 min, which was followed by three 10-min washes with PBS. Cells were then permeabilized with 0.3% Triton X-100 in PBS for 15 minutes, which was followed by a 10-min wash with 50 mM NH4Cl in PBS and two 10-min washes with PBS. The slides were blocked for 1 h with 5% bovine serum albumin in PBS, were treated with anti-ATP7A antibody (1:250 dilution; Neuromab), anti-syntaxin-6 antibody (1:500; Cell Signaling Technology), and Hoechst 33342 (1:20,000), and were washed with PBS (three 10-min PBS washes). The slides were viewed with a Deltavision deconvolution microscope (Applied Precision, Issaquah, WA).
Statistical Analysis.
All two-group comparisons used Student's t test, with the assumption of unequal variance. Data are presented as mean ± S.E.M.
Results
A 2008 Ovarian Cancer Sec61β-Knockdown Cell Line Was Created.
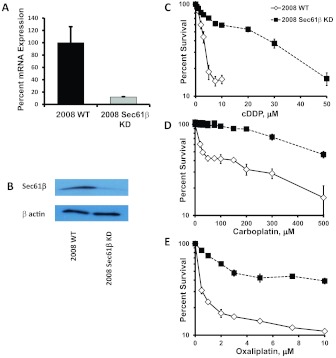
Sec61β expression was constitutively knocked down in the human ovarian cancer 2008 cell line through infection with a lentivirus expressing a shRNA targeted to human Sec61β. After selection of successfully infected cells with puromycin, cultures grown from small infected populations of approximately 50 to 100 cells were screened through qRT-PCR and Western blot analyses. As shown in Fig. 1, there was a 96% reduction in mRNA expression (Fig. 1A) and an 87% reduction in Sec61β protein levels (Fig. 1B) in the culture selected for further analysis.
Fig. 1.
Characterization of 2008 Sec61β-KD cells. A, relative Sec61β mRNA levels in parental 2008 wild-type (WT) cells and 2008 Sec61β-KD cells, as measured in qRT-PCR assays. B, Western blot analysis of Sec61β protein levels. Vertical bars, S.D. C–E, concentration-survival curves for parental 2008 wild-type and 2008 Sec61β-KD cells with cDDP (C), carboplatin (D), and oxaliplatin (E), as measured with the sulforhodamine B assay. Survival data were plotted on a logarithmic scale. Vertical bars, S.E.M.
Sec61β Controls Sensitivity to cDDP, Carboplatin, and Oxaliplatin.
The parental 2008 ovarian cancer cells and the 2008 Sec61β-KD cells were tested for sensitivity to the cytotoxic effects of platinum-containing drugs by using a sulforhodamine B assay, in which the cells were exposed continuously to increasing concentrations of cDDP, carboplatin, or oxaliplatin for 96 h (Monks et al., 1991). Figure 1, C–E, shows the concentration-survival curves for each of the cell lines with each of the platinum drugs. Knockdown of Sec61β resulted in an 8-fold increase in resistance to cDDP, compared with the parental 2008 cells. IC50 values (mean ± S.E.M.) were 2.6 ± 0.6 μM for the parental 2008 cells and 21.6 ± 4.9 μM for the 2008 Sec61β-KD cells (p < 0.001). Likewise, knockdown of Sec61β increased resistance to carboplatin and oxaliplatin. The IC50 for carboplatin increased 16.8- fold, from 28.8 ± 0.6 μM in the parental 2008 cells to 462.1 ± 11.4 μM in the knockdown cells (p < 0.001), and that for oxaliplatin increased 9.6-fold, from 0.33 ± 0.02 μM to 2.49 ± 0.30 μM (p < 0.01). These results indicated that Sec61β modulated one or more defense mechanisms that offset the toxicity of the platinum drugs.
To confirm that this effect was not cell line- or shRNA-specific, Sec61β was constitutively knocked down in two other human ovarian cancer cell lines, IGROV-1 and A2780. Sec61β mRNA levels were reduced 98% and 82% and protein expression was reduced to 53% and 24% of levels in the parental lines in IGROV-1 Sec61β-KD and A2780 Sec61β-KD cells, respectively (Supplemental Table 1; Supplemental Fig. 1). Both lines demonstrated increased cDDP resistance. IC50 values (mean ± S.E.M.) were 0.48 ± 0.06 μM for the parental IGROV-1 cells and 2.54 ± 0.01 μM for the IGROV-1 Sec61β-KD cells, which reflected a 5-fold increase in resistance (p < 0.001). IC50 values (mean ± S.E.M.) were 3.82 ± 0.27 μM for the parental A2780 cells and 6.42 ± 0.27 μM for the A2780 Sec61β-KD cells, which reflected a significant 1.7-fold increase in resistance (p < 0.05). Lentiviral shRNA vectors targeted to other segments of the Sec61β gene were used to generate two additional 2008 Sec61β-KD lines, namely, 2008 Sec61β-KD 279 and 2008 Sec61β-KD 927. Knockdown in these lines was confirmed with qRT-PCR analyses. The lines were found to be 9.3- and 14.0-fold resistant to cDDP, respectively (Supplemental Table 1). Knockdown of Sec61β mRNA consistently produced resistance to cDDP in multiple human ovarian cancer cell lines, and targeting of different parts of the mRNA reproduced the phenotype in the 2008 cells.
Sec61β Knockdown Impairs cDDP Accumulation.
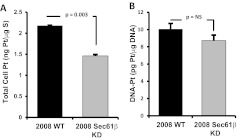
To define in more detail the mechanism through which Sec61β influenced the sensitivity to cDDP, the whole-cell contents of platinum in parental 2008 cells and 2008 Sec61β-KD cells were measured with ICP-MS after 1 h of exposure to 30 μM cDDP. Whereas the parental 2008 cells accumulated 2.18 ± 0.01 ng of platinum/μg of sulfur, the knockdown cells accumulated 1.46 ± 0.03 ng of platinum/μg of sulfur (i.e., 33% reduction in the total accumulation of platinum) (Fig. 2A). At least part of the observed reduction in drug sensitivity might be accounted for by decreased drug accumulation.
Fig. 2.
Effect of Sec61β knockdown on platinum accumulation. A, whole-cell platinum accumulation in 2008 wild-type (WT) and 2008 Sec61β-KD cells after 1-h exposure to 30 μM cDDP, as measured with ICP-MS. B, extent of DNA-platinum adduct formation after the same treatment as in A. Vertical bars, S.E.M.
Formation of DNA-platinum adducts is thought to be the primary mechanism through which platinum-containing drugs cause cell cytotoxicity. Platinum levels were measured in DNA isolated from cells that had been exposed to 30 μM cDDP for 1 h (Fig. 2B). The 2008 Sec61β-KD cells contained 14% less platinum in their DNA (8.65 ± 0.64 pg of platinum/μg of DNA) than did the parental cells (10.05 ± 0.65 pg of platinum/μg of DNA), although this difference was not statistically significant (p = 0.22).
Sec61β Knockdown Increases ATP7A Levels.
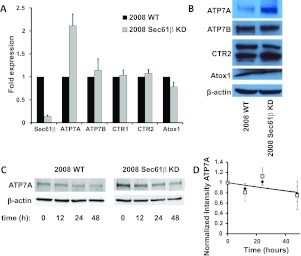
Given the observations that knockdown of Sec61β rendered cells resistant to cDDP and that sensitivity to cDDP was mediated in part by copper transporters and chaperones, the relative expression of copper transporters and chaperones shown previously to be capable of modulating the cellular pharmacokinetics of cDDP was quantified with qRT-PCR and Western blot analyses (Fig. 3). The ATP7A mRNA level was 2.1 ± 0.4-fold higher in the 2008 Sec61β-KD cells, but there were no significant changes in the expression of mRNA for ATOX1, ATP7B, CTR1, or CTR2 (Fig. 3A). Consistent with the change in the mRNA level, the ATP7A protein level was 2.5 ± 0.3-fold higher in the Sec61β-KD cells, as assessed through Western blotting (Fig. 3B). No significant differences in the levels of ATOX1, ATP7B, or CTR2 were found in Western blot analyses; CTR1 was not tested because of the lack of a reliable antibody capable of detecting the endogenous protein in these cells. ATP7A protein levels were also increased in the other cell lines in which Sec61β expression was knocked down; levels were increased 3.8 ± 1.8-fold in the IGROV-1 Sec61β-KD cells and 2.9 ± 1.7-fold in the A2780 Sec61β-KD cells, compared with their respective parental cells (Supplemental Fig. 1).
Fig. 3.
Analysis of expression of proteins involved in copper homeostasis. A, qRT-PCR analysis of the effect of Sec61β knockdown on mRNA expression of proteins involved in copper homeostasis in 2008 wild-type (WT) and 2008 Sec61β-KD cells. Vertical bars, S.E.M. B, Western blot analysis of the effect of Sec61β knockdown. Blots are representative of at least three independent experiments. C, Western blot analysis of the effect of cycloheximide on ATP7A stability. The blot is representative of three independent experiments. D, quantification of bands and normalization of results with respect to actin intensity, for 2008 wild-type cells (♦) and 2008 Sec61β-KD cells (□). Vertical bars, S.E.M.
To determine whether the ability of Sec61β knockdown to increase ATP7A protein expression was attributable only to a change in ATP7A mRNA levels, the stability of ATP7A protein was assessed by blocking new protein synthesis in 2008 and 2008 Sec61β-KD cells with cycloheximide and monitoring the disappearance of ATP7A through serial Western blot analyses. ATP7A was found to be very stable, with a half-life of >48 h, similar to values reported by others (Pase et al., 2004; Holloway et al., 2007). In a comparison of the rates of ATP7A degradation between the parental and 2008 Sec61β-KD cells, no significant difference was detected (Fig. 3, C and D). With the caution that long exposure to cycloheximide can affect a variety of cellular processes, this result suggests that the increases in ATP7A protein levels that resulted from Sec61β knockdown were attributable to increases in mRNA levels and not changes in protein stability.
Sec61β Knockdown Perturbs Copper Homeostasis.
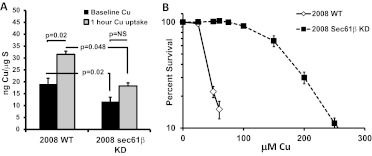
ATP7A is responsible for pumping intracellular copper into the secretory compartment and thence out of the cell, and ATP7A overexpression is associated with reduced accumulation of copper and resistance to its cytotoxic effects. To determine whether the change in ATP7A protein expression was sufficient to perturb copper homeostasis, whole-cell copper contents were measured in parental and 2008 Sec61β-KD cells with ICP-MS. As shown in Fig. 4A, the basal copper level in the 2008 Sec61β-KD cells was 40% lower than that in the parental cells. The 2008 Sec61β-KD cells contained 11.8 ± 1.3 ng of copper/μg of sulfur, whereas the parental cells contained 19.1 ± 2.4 ng of copper/μg of sulfur, which is a statistically significant difference (p < 0.05). To measure the effect on copper accumulation, cells were exposed to 100 μM CuSO4 for 1 h and washed, and whole-cell copper contents were measured. Net uptake values after copper exposure were 31.6 ± 1.8 ng of copper/μg of sulfur and 18.3 ± 1.3 ng of copper/μg of sulfur for the 2008 and 2008 Sec61β-KD cells, respectively (p < 0.01). Knockdown of Sec61β reduced both basal copper contents and rates of copper accumulation, consistent with enhanced export of copper mediated by the elevated levels of ATP7A.
Fig. 4.
Effects of Sec61β knockdown on copper accumulation and sensitivity. A, basal copper contents and copper accumulation levels in parental 2008 wild-type (WT) and 2008 Sec61β-KD cells after exposure to 100 μM CuSO4. B, copper concentration-survival curves for parental 2008 wild-type and 2008 Sec61β-KD cells. Survival data were plotted on a logarithmic scale. Vertical bars, S.E.M.
The parental and Sec61β-knockdown cells were tested for their sensitivity to copper. The concentration-survival curves shown in Fig. 4B indicate that the knockdown cells were 4.6-fold resistant to CuSO4, with the parental cells exhibiting an IC50 of 36.1 ± 0.1 μM and the knockdown cells an IC50 of 166.0 ± 0.1 μM (p < 0.01). The 2.5-fold increase in ATP7A protein levels produced with Sec61β knockdown was sufficient to reduce initial copper accumulation significantly and to render cells quite resistant to the growth-inhibitory effect of copper, which indicated that the excess ATP7A was fully functional with respect to copper homeostasis.
Sec61β Controls Sensitivity to Other Chemotherapeutic Agents.
Overexpression of ATP7A was reported previously to render cells resistant to several classes of chemotherapeutic drugs in addition to the platinum-containing agents (Owatari et al., 2007; Furukawa et al., 2008). To determine whether loss of Sec61β modulated sensitivity to other classes of drugs in human ovarian cancer, parental cells and 2008 Sec61β-KD cells were exposed to increasing concentrations of etoposide, vincristine, doxorubicin, and paclitaxel, each of which belongs to a different mechanistic class. As shown in Table 1, loss of Sec61β function reduced sensitivity to all of these drugs but the magnitude of resistance was substantially less than for the platinum drugs. Because these drugs injure cells through different mechanisms, this result indicates that Sec61β participates in some mechanism of cellular resistance that is common to all of these agents.
TABLE 1.
IC50 values (mean ± S.E.M.) for various chemotherapeutic agents and copper
| Drug | IC50 |
Increase | p | |
|---|---|---|---|---|
| 2008 Wild-Type Cells | 2008 Sec61β-KD Cells | |||
| μM | fold | |||
| Cisplatin | 2.60 ± 0.62 | 21.63 ± 4.88 | 8 | <0.001 |
| Carboplatin | 28.84 ± 0.63 | 462.13 ± 11.36 | 16.8 | <0.001 |
| Oxaliplatin | 0.33 ± 0.02 | 2.49 ± 0.30 | 9.6 | <0.01 |
| Copper | 36.05 ± 0.05 | 166.02 ± 0.08 | 5 | <0.01 |
| Etoposide | 1.05 ± 0.16 | 7.50 ± 2.29 | 7 | <0.05 |
| Vincristine | 0.0067 ± 0.0004 | 0.0129 ± 0.002 | 2.1 | <0.01 |
| Doxorubicin | 0.074 ± 0.036 | 0.221 ± 0.093 | 3.1 | <0.01 |
| Paclitaxel | 0.0020 ± 0.0002 | 0.0034 ± 0.0002 | 1.7 | <0.001 |
Copper and cDDP Do Not Alter ATP7A Expression Levels.
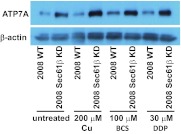
We and others showed that the expression of some copper transporters, such as CTR1, varied in response to changes in the availability of copper or platinum drug exposure. This presumably serves to buffer cells from high or low environmental copper levels (Guo et al., 2004; Larson et al., 2009; Blair et al., 2010). To determine whether copper or cDDP acutely influenced the level of expression of ATP7A, parental 2008 cells and 2008 Sec61β-KD cells were exposed to 200 μM CuSO4, 100 μM BCS (a copper chelator), or 30 μM cDDP for 1 h and ATP7A levels were assessed through Western blotting. As shown in Fig. 5, the increase in ATP7A protein expression was maintained in all three comparisons of parental 2008 cells and 2008 Sec61β-KD cells. Under all conditions tested, the 2008 Sec61β-KD cells maintained higher levels of expression of ATP7A, compared with similarly treated parental 2008 cells. Although quantification of protein band densities suggested possibly higher levels of expression of ATP7A in Sec61β-KD cells after CuSO4 treatment (5.4 ± 3-fold greater, compared with parental 2008 cells), this was not statistically greater than the 2- to 2.4-fold increases observed for the other three conditions. It seems that the effect on ATP7A expression mediated by Sec61β KD is not sensitive to acute changes in extracellular copper levels, although changes with extended periods of exposure cannot be ruled out. This is in contrast to known effects on other copper transporters such as CTR1 and CTR2, whose expression is rapidly altered with exposure to excess copper or copper chelators; changes were reported to occur within minutes (Holzer et al., 2004; Blair et al., 2010).
Fig. 5.
Western blot analysis of the effects of copper, BCS, and cDDP on ATP7A expression in parental 2008 cells and 2008 Sec61β-KD cells. Cell lysates were prepared from 2008 wild-type (WT) and 2008 Sec61β-KD cells after a 1-h incubation with 200 μM CuSO4, 100 μM BCS, or 30 μM cDDP, and Western blots were probed with antibodies against the proteins indicated.
Resistance to Platinum Drugs Mediated by Sec61β KD Requires ATP7A Expression.
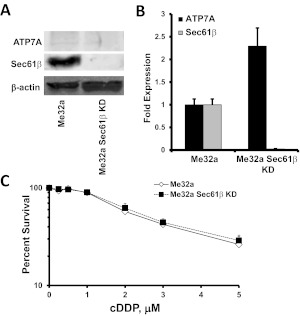
If the change in sensitivity mediated by Sec61β KD requires ATP7A expression, then cells lacking ATP7A should have similar sensitivities to cDDP regardless of Sec61β expression. The Me32a cell line was derived from fibroblasts from a patient with Menkes' disease, in which a mutation inactivates ATP7A through creation of a premature stop codon (La Fontaine et al., 1998). Sec61β was knocked down in the Me32a cell line through lentiviral expression of a shRNA directed against Sec61β, in a manner similar to that described above, which yielded Me32a Sec61β-KD cells. Knockdown of Sec61β in these cells was confirmed with qRT-PCR and Western blot analyses (Fig. 6, A and B). Although no ATP7A protein was detectable in Me32a or Me32a Sec61β-KD cells through Western blotting, as expected, a 2.3 ± 0.4-fold increase in ATP7A mRNA levels was detected with qRT-PCR assays, which suggests that the transcriptional effects of Sec61β KD were still operative (Fig. 6B). Knockdown of Sec61β in the Me32a cell line did not result in any appreciable changes in cDDP sensitivity (Fig. 6C). This result provides evidence that functional ATP7A is required for knockdown of Sec61β to produce cDDP resistance.
Fig. 6.
Effects of Sec61β knockdown in Me32a human fibroblasts lacking ATP7A. A, Western blots of ATP7A and Sec61β in Me32a and Me32a Sec61β-KD cells. B, qRT-PCR analysis of the effects of Sec61β knockdown on ATP7A and Sec61β mRNA expression in Me32a and Me32a Sec61β-KD cells. Vertical bars, S.E.M. C, cDDP concentration-survival curves for parental Me32a and Me32a Sec61β-KD cells. Survival data were plotted on a logarithmic scale. Vertical bars, S.E.M.
Knockdown of Sec61β Disrupts Normal Cellular Localization of ATP7A.
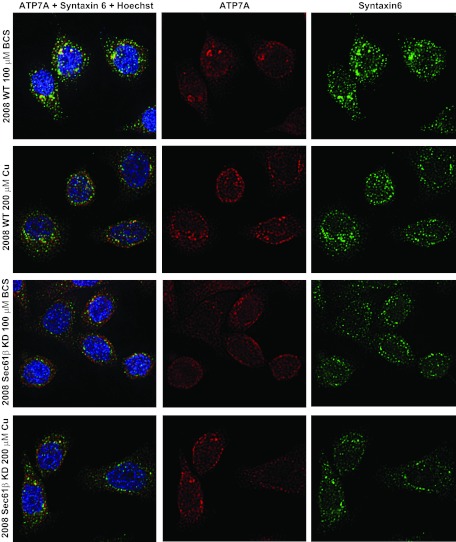
ATP7A resides in the trans-Golgi network in the absence of extracellular copper. We and others showed previously that ATP7A is redistributed from this perinuclear localization to other locations near or at the cell surface upon exposure to excess levels of copper (Petris et al., 1996; Samimi et al., 2004a), a process that may direct copper to export pathways. Maintenance of the compartment responsible for ATP7A localization is in part attributable to the activity of ARFs and GEFs (Holloway et al., 2007). In contrast, cDDP does not cause subcellular redistribution in response to copper, although higher ATP7A levels in cells may help to sequester the drug into cellular vesicles, thereby limiting its cytotoxicity. The distribution of ATP7A in parental and 2008 Sec61β-KD cells was examined through deconvolution microscopy. In the parental cells, ATP7A was concentrated in perinuclear structures and colocalized with the trans-Golgi network marker syntaxin-6 when cells were grown in medium without added copper (Fig. 7). Upon exposure to 200 μM CuSO4, ATP7A was redistributed throughout the cell, including areas close to the plasma membrane, similar to findings reported previously. ATP7A failed to colocalize significantly with the endosomal markers EEA1, rab11, and rab4 in either parental or 2008 Sec61β-KD cells (Supplemental Figs. 2–7). Knockdown of Sec61β altered the localization of ATP7A; it was no longer concentrated in perinuclear structures, and it failed to colocalize with syntaxin-6 to the same degree as in parental cells (Fig. 7). Syntaxin-6 staining was more diffuse in the 2008 Sec61β-KD cells, which raises the question of whether the altered distribution of ATP7A was attributable to a disturbance in the structure of the trans-Golgi network. Knockdown of Sec61β did not alter the subcellular distribution of EEA1, rab11, or rab4. Exposure of 2008 Sec61β-KD cells to 200 μM CuSO4 did not appreciably alter the distribution of ATP7A, which was already diffusely distributed throughout the cell, as detected microscopically. Immunoprecipitation of Sec61β failed to precipitate ATP7A and vice versa, which suggested that these two proteins were not stably binding to each other (data not shown).
Fig. 7.
Deconvolution microscopic analysis of 2008 wild-type (WT) and 2008 Sec61β-KD cells. Cells were fixed and stained for ATP7A, the trans-Golgi network marker syntaxin-6, and DNA (Hoechst 33342) after a 1-h treatment with 100 μM BCS or 200 μM CuSO4.
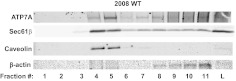
Because a proportion of ATP7A was shown to reside in membrane lipid rafts (Ashino et al., 2010), we sought to determine whether Sec61β was present within the same fractions. Parental 2008 cells were lysed, and fractions containing lipid rafts were isolated through detergent-free sucrose density centrifugation (Ostrom and Liu, 2007; Ashino et al., 2010). Similar to findings reported previously, ATP7A was found in fractions containing the lipid raft marker caveolin (Fig. 8). Sec61β was preferentially enriched in the same raft fractions, which is consistent with the possibility that Sec61β plays a role in the partitioning or trafficking of ATP7A in lipid rafts.
Fig. 8.
Lipid raft fractionation of ATP7A and Sec61β in 2008 cells. Sucrose gradient centrifugation of 2008 wild-type (WT) cells was performed. Eleven fractions from the top (fractions 1–11) and a total lysate sample (L) were isolated and assessed in Western blot analyses with the antibodies indicated.
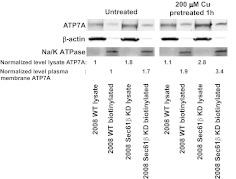
Although ATP7A was no longer localized to the trans-Golgi network in cells in which Sec61β was knocked down, this did not eliminate the possibility that ATP7A could still traffic to plasma membrane sites in response to copper. To determine whether Sec61β was required for the trafficking of ATP7A to the plasma membrane, the amounts of ATP7A on the cell surface that were susceptible to biotinylation were quantified before and after exposure to 200 μM CuSO4. As shown in Fig. 9, plasma membrane ATP7A levels were approximately 1.8-fold higher in untreated 2008 Sec61β-KD cells, compared with parental 2008 cells. Copper exposure increased plasma membrane ATP7A levels approximately 2-fold in parental cells, an observation consistent with a previous report (Nyasae et al., 2007). Copper exposure increased cell surface ATP7A levels in the 2008 Sec61β-KD cells by a similar proportion, which indicated that Sec61β was not required for the plasma membrane insertion step. These results demonstrate that Sec61β plays a pivotal role in controlling the subcellular distribution of ATP7A as well as the level of its expression and that both proteins are located in the membrane fraction containing lipid rafts. Loss of Sec61β does not eliminate the ability of ATP7A to sense copper and to traffic to the plasma membrane in response to copper.
Fig. 9.
Cell surface expression of ATP7A in response to copper through cell surface biotinylation in parental 2008 wild-type (WT) cells and 2008 Sec61β-KD cells. Cells were left untreated or were incubated with 200 μM CuSO4 for 1 h, after which cell surface proteins were biotinylated, precipitated with immobilized streptavidin, and assessed through Western blotting. β-Actin and Na+/K+-ATPase were probed as controls, to confirm the quality of sample preparation.
Discussion
We made the novel observation that Sec61β produces large changes in sensitivity to the cytotoxic effects of platinum-containing drugs. In investigations of the mechanism, it was discovered that knockdown of Sec61β resulted in increased expression of ATP7A at both the RNA and protein levels and altered subcellular distribution. The resistance to the platinum-containing drugs induced by Sec61β knockdown was mediated by ATP7A, as evidenced by the finding that knockdown of Sec61β in a cell line in which ATP7A was nonfunctional failed to induce resistance. Additional evidence consistent with a central role for ATP7A included reduced basal copper content, impaired copper accumulation, concomitant resistance to copper cytotoxicity, reduced cDDP uptake, and a trend toward reduced adduct formation.
ATP7A was shown previously to mediate resistance to copper, the platinum-containing drugs cisplatin, carboplatin, and oxaliplatin, and other chemotherapeutic agents (Samimi et al., 2004a,b; Furukawa et al., 2008). In the case of copper, ATP7A mediates resistance by sequestering the metal into the vesicles of the secretory system and thus enhancing export. Copper is known to trigger the relocalization of ATP7A-expressing vesicles from the Golgi complex to a region just under or at the plasma membrane, whence it is presumed that direct plasma membrane export or exocytosis of copper occurs (Petris et al., 1996; Nyasae et al., 2007). In the case of cDDP (which, unlike copper, does not trigger the relocalization of ATP7A-expressing vesicles), the mechanism through which ATP7A mediates resistance is less clear. In some cases, ATP7A seems to work primarily by detoxifying cDDP through intracellular sequestration into ATP7A-expressing vesicles, which may increase rates of drug exocytosis (Katano et al., 2002; Samimi et al., 2004a). Whether these vesicles are distinct from those associated with the trans-Golgi network or some other specific intracellular compartment is not known.
The number of identified functions of the Sec61 translocon, of which Sec61β is a part, is growing. It seems unlikely that forward translocation is the function of Sec61 that is related to the chemotherapeutic drug resistance observed in this study, because the Sec61β subunit was found to be largely unnecessary for forward translocation (Görlich and Rapoport, 1993; Toikkanen et al., 1996). The channel also functions in the reverse direction, however, for the retrotranslocation and degradation of misfolded ER-resident or transmembrane proteins, such as the cystic fibrosis transmembrane conductance regulator (Bebök et al., 1998). More-recent work showed that retrotranslocation is used not only for degradation but also for management of cellular signaling pathways (Liao and Carpenter, 2007). Compared with forward translocation, retrotranslocation is more dependent on the function of Sec61β, which suggests that this subunit plays a more important role during the reverse process. Sec61β was shown to interact physically with some retrotranslocated proteins, such as the epidermal growth factor receptor and Gurken (Liao and Carpenter, 2007; Kelkar and Dobberstein, 2009). In the case of ATP7A, we were unable to demonstrate such an interaction through coimmunoprecipitation experiments, and knockdown of Sec61β did not change the stability of ATP7A, which argues against a direct role for Sec61β in its degradation. Although we show here that the ability of Sec61β knockdown to mediate resistance to the platinum-containing drugs is dependent on ATP7A, it is possible that effects on other proteins that rely on the Sec61 translocon for retrotranslocation or degradation contribute to the resistant phenotype.
The Sec61β subunit also has functions that are not necessarily tied to protein translocation. Sec61β seems to be highly conserved, because human Sec61β can complement loss of the homologous yeast protein Sbh1p (Leroux and Rokeach, 2008). Sbh1p associates with components of the exocyst complex and the reticulon family of proteins (Toikkanen et al., 2003; Zhao and Jäntti, 2009), which are involved in directing vesicles from the Golgi complex to specific locations at the plasma membrane and tethering them there before vesicle fusion. Exocyst proteins have also been linked to control of cell polarity and the cytoskeleton (Lipschutz et al., 2003; Guo and Novick, 2004; He and Guo, 2009; Braun and Brumell, 2010). In yeast, the Sec61β homolog Sbh1p was shown to interact physically with exocyst complex proteins such as Sec15p and Sec8p, and overexpression of Sbh1p suppressed growth defects in various exocyst mutants, which suggests that it plays a central role in exocyst function (Toikkanen et al., 2003).
The findings that knockdown of Sec61β led to apparent dissolution of the trans-Golgi compartment associated with ATP7A and that ATP7A retained its ability to traffic to the plasma membrane in response to copper were very similar to the phenotype reported by Holloway et al. (2007). In their study of yeast, knockdown of tethering factor p115 led to dissolution of trans-Golgi structures associated with ATP7A, but this did not interfere with the ability of ATP7A to traffic in response to elevated copper levels. In the same study, those investigators showed that expression of a dominant-negative GEF of GBF1 (which can interact with ARF1) also led to dissolution of the trans-Golgi and ATP7A-containing compartments, although copper-responsive trafficking was lost. The Sec61β yeast homologs sbh1p and sbh2p were shown in a different study to function as GEFs, consistent with the observation that they contain homologous sec7 domains required for GEF function (Helmers et al., 2003). Our data suggest that Sec61β may function as a GEF and may modulate the ATP7A trans-Golgi compartment in a manner similar to that of the yeast GEF GBF1, possibly in concert with the ARF family of proteins. If this is the case, then it may be that compartments “loaded” with platinum-containing drug by ATP7A are more efficient at sequestration or export when more diffusely distributed in Sec61β-KD cells.
ATP7A function is largely regulated through post-translational control of trafficking. When copper is abundant, it is transported in vesicles to areas at or near the plasma membrane. ATP7A-containing vesicles can relocalize to the leading edge in vascular smooth muscle cells upon stimulation of migration with platelet-derived growth factor or cell culture wounding (Ashino et al., 2010). Elements of the exocyst complex are likely involved in the transport of ATP7A-containing vesicles, and knockdown of Sec61β probably interferes with this process, either by impairing the exocyst or by altering trans-Golgi network structure. Whether Sec61β controls the loading of ATP7A into vesicles bound for export or the trafficking of vesicles already loaded with platinum drugs by ATP7A is not known. It is notable that knockdown of Sec61β increases ATP7A expression, in addition to altering the cellular distribution of ATP7A. Although the increase in ATP7A expression might represent a direct effect of Sec61β knockdown and the resultant overwhelming of normal trafficking mechanisms, the higher expression levels also might reflect a cellular response to disrupted ATP7A localization, although our data from Me32a Sec61β-KD cells would not support this. Because no clear physical interaction between ATP7A and Sec61β could be demonstrated through either immunopreciptation or microscopic colocalization (Supplemental Fig. 8; data not shown), it seems likely that Sec61β controls ATP7A localization not through direct association but rather through effects on other aspects of the cellular trafficking machinery. The enrichment of both proteins in membrane fractions containing lipid rafts supports this hypothesis. It is noteworthy that Sec61β knockdown did not alter the expression of other copper homeostasis-related proteins and produced no large effects on cell growth or morphological features.
Knockdown of Sec61β resulted in greater resistance to the platinum-containing drugs than to copper. Previous studies repeatedly showed that relatively small increments of resistance to copper were accompanied by substantially larger increments of resistance to the platinum-containing drugs (Safaei and Howell, 2005). Unlike copper, cDDP does not trigger the relocalization of ATP7A from the Golgi apparatus (Samimi et al., 2004a). However, expression of wild-type ATP7A in fibroblasts that contain no endogenous ATP7A or overexpression in ovarian cancer cells results in resistance to cDDP (Komatsu et al., 2000; Samimi et al., 2004a; Owatari et al., 2007). The finding of cross-resistance between copper and cDDP led to recognition that the resistance was related to drug accumulation, as opposed to downstream effects on apoptosis. The resistance to cDDP produced by knockdown of Sec61β was associated with increased cell surface ATP7A levels, a ∼30% reduction in cDDP accumulation, and a trend toward reduced DNA adduct formation, all of which are consistent with enhanced ATP7A-mediated export. It was noted previously that very small changes in the extent of adduct formation were associated with relatively large changes in platinum drug sensitivity (Johnson et al., 1997).
How Sec61β affects resistance to the other chemotherapeutic agents tested is unclear. The results obtained with 2008 cells confirmed earlier observations that increased expression of ATP7A (including cells that overexpress ATP7A after transfection) was associated with resistance to various other classes of chemotherapeutic agents (Owatari et al., 2007; Furukawa et al., 2008). Although, like ATP7B (Safaei et al., 2008), ATP7A may transport cDDP directly, it seems unlikely that ATP7A would transport all of the drugs for which resistance has been demonstrated; it is more likely that Sec61β controls other types of transporters or alters vesicle pH or that Sec61β or ATP7A alters the activity of the vesicle secretory pathway. These hypotheses remain to be tested. It is notable that ATP7A is involved in cellular migration (Furukawa et al., 2008; Ashino et al., 2010), because migration also involves trafficking and cytoskeletal modifications, which probably depend on processes analogous to vesicle trafficking and exocytosis.
In conclusion, we have shown that Sec61β has large effects on cellular sensitivity to the platinum drugs and more-modest effects on other classes of chemotherapeutic agents. In the case of the platinum drugs, resistance is ATP7A-dependent and at least partially attributable to changes in platinum drug accumulation. Sec61β is required for the trans-Golgi localization of ATP7A but not for the ability of ATP7A to respond to copper. The mechanism through which Sec61β alters ATP7A levels is a subject for future study.
Supplementary Material
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by grants from the National Institutes of Health National Cancer Institute [Grants T32-CA121938, CA152185, CA095298]; and a grant from the Tower Cancer Research Foundation.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- ARF
- ADP-ribosylation factor
- GEF
- guanine nucleotide exchange factor
- BCS
- bathocuproine disulfonate
- cDDP
- cisplatin
- ICP
- inductively coupled plasma
- MS
- mass spectrometry
- KD
- knockdown
- PBS
- phosphate-buffered saline
- ER
- endoplasmic reticulum
- MES
- 4-morpholineethanesulfonic acid
- qRT-PCR
- quantitative reverse transcription-polymerase chain reaction
- shRNA
- short hairpin RNA.
Authorship Contributions
Participated in research design: Abada, Larson, and Howell.
Conducted experiments: Abada, Larson, Manorek, and Adams.
Contributed new reagents or analytic tools: Abada.
Performed data analysis: Abada and Howell.
Wrote or contributed to the writing of the manuscript: Abada and Howell.
References
- Ashino T, Sudhahar V, Urao N, Oshikawa J, Chen GF, Wang H, Huo Y, Finney L, Vogt S, McKinney RD, et al. (2010) Unexpected role of the copper transporter ATP7A in PDGF-induced vascular smooth muscle cell migration. Circ Res 107:787–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebök Z, Mazzochi C, King SA, Hong JS, Sorscher EJ. (1998) The mechanism underlying cystic fibrosis transmembrane conductance regulator transport from the endoplasmic reticulum to the proteasome includes Sec61β and a cytosolic, deglycosylated intermediary. J Biol Chem 273:29873–29878 [DOI] [PubMed] [Google Scholar]
- Blair BG, Larson CA, Adams PL, Abada PB, Safaei R, Howell SB. (2010) Regulation of copper transporter 2 expression by copper and cisplatin in human ovarian carcinoma cells. Mol Pharmacol 77:912–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair BG, Larson CA, Safaei R, Howell SB. (2009) Copper transporter 2 regulates the cellular accumulation and cytotoxicity of cisplatin and carboplatin. Clin Cancer Res 15:4312–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V, Brumell JH. (2010) Bacterial invasion: entry through the exocyst door. Curr Biol 20:R677–R679 [DOI] [PubMed] [Google Scholar]
- Camakaris J, Petris MJ, Bailey L, Shen P, Lockhart P, Glover TW, Barcroft C, Patton J, Mercer JF. (1995) Gene amplification of the Menkes (MNK; ATP7A) P-type ATPase gene of CHO cells is associated with copper resistance and enhanced copper efflux. Hum Mol Genet 4:2117–2123 [DOI] [PubMed] [Google Scholar]
- Camakaris J, Voskoboinik I, Mercer JF. (1999) Molecular mechanisms of copper homeostasis. Biochem Biophys Res Commun 261:225–232 [DOI] [PubMed] [Google Scholar]
- Dierick HA, Adam AN, Escara-Wilke JF, Glover TW. (1997) Immunocytochemical localization of the Menkes copper transport protein (ATP7A) to the trans-Golgi network. Hum Mol Genet 6:409–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiSaia PJ, Sinkovics JG, Rutledge FN, Smith JP. (1972) Cell-mediated immunity to human malignant cells: a brief review and further studies with two gynecologic tumors. Am J Obstet Gynecol 114:979–989 [DOI] [PubMed] [Google Scholar]
- Furukawa T, Komatsu M, Ikeda R, Tsujikawa K, Akiyama S. (2008) Copper transport systems are involved in multidrug resistance and drug transport. Curr Med Chem 15:3268–3278 [DOI] [PubMed] [Google Scholar]
- Görlich D, Rapoport TA. (1993) Protein translocation into proteoliposomes reconstituted from purified components of the endoplasmic reticulum membrane. Cell 75:615–630 [DOI] [PubMed] [Google Scholar]
- Guo W, Novick P. (2004) The exocyst meets the translocon: a regulatory circuit for secretion and protein synthesis? Trends Cell Biol 14:61–63 [DOI] [PubMed] [Google Scholar]
- Guo Y, Smith K, Lee J, Thiele DJ, Petris MJ. (2004) Identification of methionine-rich clusters that regulate copper-stimulated endocytosis of the human Ctr1 copper transporter. J Biol Chem 279:17428–17433 [DOI] [PubMed] [Google Scholar]
- He B, Guo W. (2009) The exocyst complex in polarized exocytosis. Curr Opin Cell Biol 21:537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmers J, Schmidt D, Glavy JS, Blobel G, Schwartz T. (2003) The beta-subunit of the protein-conducting channel of the endoplasmic reticulum functions as the guanine nucleotide exchange factor for the beta-subunit of the signal recognition particle receptor. J Biol Chem 278:23686–23690 [DOI] [PubMed] [Google Scholar]
- Holloway ZG, Grabski R, Szul T, Styers ML, Coventry JA, Monaco AP, Sztul E. (2007) Activation of ADP-ribosylation factor regulates biogenesis of the ATP7A-containing trans-Golgi network compartment and its Cu-induced trafficking. Am J Physiol Cell Physiol 293:C1753–C1767 [DOI] [PubMed] [Google Scholar]
- Holzer A, Samimi G, Katano K, Naedermann W, Howell SB. (2003) The role of human copper transporter hCTR1 in cisplatin uptake in human ovarian carcinoma cells (Abstract). Proc Am Assoc Cancer Res 44:923 [Google Scholar]
- Holzer AK, Katano K, Klomp LW, Howell SB. (2004) Cisplatin rapidly down-regulates its own influx transporter hCTR1 in cultured human ovarian carcinoma cells. Clin Cancer Res 10:6744–6749 [DOI] [PubMed] [Google Scholar]
- Huffman DL, O'Halloran TV. (2001) Function, structure, and mechanism of intracellular copper trafficking proteins. Annu Rev Biochem 70:677–701 [DOI] [PubMed] [Google Scholar]
- Johnson SW, Laub PB, Beesley JS, Ozols RF, Hamilton TC. (1997) Increased platinum-DNA damage tolerance is associated with cisplatin resistance and cross-resistance to various chemotherapeutic agents in unrelated human ovarian cancer cell lines. Cancer Res 57:850–856 [PubMed] [Google Scholar]
- Katano K, Kondo A, Safaei R, Holzer A, Samimi G, Mishima M, Kuo YM, Rochdi M, Howell SB. (2002) Acquisition of resistance to cisplatin is accompanied by changes in the cellular pharmacology of copper. Cancer Res 62:6559–6565 [PubMed] [Google Scholar]
- Kelkar A, Dobberstein B. (2009) Sec61beta, a subunit of the Sec61 protein translocation channel at the endoplasmic reticulum, is involved in the transport of Gurken to the plasma membrane. BMC Cell Biol 10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Sumizawa T, Mutoh M, Chen ZS, Terada K, Furukawa T, Yang XL, Gao H, Miura N, Sugiyama T, et al. (2000) Copper-transporting P-type adenosine triphosphatase (ATP7B) is associated with cisplatin resistance. Cancer Res 60:1312–1316 [PubMed] [Google Scholar]
- La Fontaine SL, Firth SD, Camakaris J, Englezou A, Theophilos MB, Petris MJ, Howie M, Lockhart PJ, Greenough M, Brooks H, et al. (1998) Correction of the copper transport defect of Menkes patient fibroblasts by expression of the Menkes and Wilson ATPases. J Biol Chem 273:31375–31380 [DOI] [PubMed] [Google Scholar]
- Larson CA, Blair BG, Safaei R, Howell SB. (2009) The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol Pharmacol 75:324–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux A, Rokeach LA. (2008) Inter-species complementation of the translocon beta subunit requires only its transmembrane domain. PLoS One 3:e3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZH, Qiu MZ, Zeng ZL, Luo HY, Wu WJ, Wang F, Wang ZQ, Zhang DS, Li YH, Xu RH. (2012) Copper-transporting P-type adenosine triphosphatase (ATP7A) is associated with platinum-resistance in non-small cell lung cancer (NSCLC). J Transl Med 10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HJ, Carpenter G. (2007) Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol Biol Cell 18:1064–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder MC, Hazegh-Azam M. (1996) Copper biochemistry and molecular biology. Am J Clin Nutr 63:797S–811S [DOI] [PubMed] [Google Scholar]
- Lipschutz JH, Lingappa VR, Mostov KE. (2003) The exocyst affects protein synthesis by acting on the translocation machinery of the endoplasmic reticulum. J Biol Chem 278:20954–20960 [DOI] [PubMed] [Google Scholar]
- Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A. (1991) Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst 83:757–766 [DOI] [PubMed] [Google Scholar]
- Nyasae L, Bustos R, Braiterman L, Eipper B, Hubbard A. (2007) Dynamics of endogenous ATP7A (Menkes protein) in intestinal epithelial cells: copper-dependent redistribution between two intracellular sites. Am J Physiol Gastrointest Liver Physiol 292:G1181–G1194 [DOI] [PubMed] [Google Scholar]
- O'Halloran TV, Culotta VC. (2000) Metallochaperones, an intracellular shuttle service for metal ions. J Biol Chem 275:25057–25060 [DOI] [PubMed] [Google Scholar]
- Ostrom RS, Liu X. (2007) Detergent and detergent-free methods to define lipid rafts and caveolae. Methods Mol Biol 400:459–468 [DOI] [PubMed] [Google Scholar]
- Owatari S, Akune S, Komatsu M, Ikeda R, Firth SD, Che XF, Yamamoto M, Tsujikawa K, Kitazono M, Ishizawa T, et al. (2007) Copper-transporting P-type ATPase, ATP7A, confers multidrug resistance and its expression is related to resistance to SN-38 in clinical colon cancer. Cancer Res 67:4860–4868 [DOI] [PubMed] [Google Scholar]
- Pase L, Voskoboinik I, Greenough M, Camakaris J. (2004) Copper stimulates trafficking of a distinct pool of the Menkes copper ATPase (ATP7A) to the plasma membrane and diverts it into a rapid recycling pool. Biochem J 378:1031–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petris MJ, Mercer JF. (1999) The Menkes protein (ATP7A; MNK) cycles via the plasma membrane both in basal and elevated extracellular copper using a C-terminal di-leucine endocytic signal. Hum Mol Genet 8:2107–2115 [DOI] [PubMed] [Google Scholar]
- Petris MJ, Mercer JF, Culvenor JG, Lockhart P, Gleeson PA, Camakaris J. (1996) Ligand-regulated transport of the Menkes copper P-type ATPase efflux pump from the Golgi apparatus to the plasma membrane: a novel mechanism of regulated trafficking. EMBO J 15:6084–6095 [PMC free article] [PubMed] [Google Scholar]
- Roelofsen H, Wolters H, Van Luyn MJ, Miura N, Kuipers F, Vonk RJ. (2000) Copper-induced apical trafficking of ATP7B in polarized hepatoma cells provides a mechanism for biliary copper excretion. Gastroenterology 119:782–793 [DOI] [PubMed] [Google Scholar]
- Safaei R, Howell SB. (2005) Copper transporters regulate the cellular pharmacology and sensitivity to Pt drugs. Crit Rev Oncol Hematol 53:13–23 [DOI] [PubMed] [Google Scholar]
- Safaei R, Otani S, Larson BJ, Rasmussen ML, Howell SB. (2008) Transport of cisplatin by the copper efflux transporter ATP7B. Mol Pharmacol 73:461–468 [DOI] [PubMed] [Google Scholar]
- Safaei R, Rasmussen ML, Francisco KS, Howell SB. (2007) The copper chaperone Atox1 is involved in the intracellular sequestration of cisplatin (Abstract). Proc Am Assoc Cancer Res 48:1330 [Google Scholar]
- Samimi G, Katano K, Holzer AK, Safaei R, Howell SB. (2004a) Modulation of the cellular pharmacology of cisplatin and its analogs by the copper exporters ATP7A and ATP7B. Mol Pharmacol 66:25–32 [DOI] [PubMed] [Google Scholar]
- Samimi G, Safaei R, Katano K, Holzer AK, Rochdi M, Tomioka M, Goodman M, Howell SB. (2004b) Increased expression of the copper efflux transporter ATP7A mediates resistance to cisplatin, carboplatin, and oxaliplatin in ovarian cancer cells. Clin Cancer Res 10:4661–4669 [DOI] [PubMed] [Google Scholar]
- Samimi G, Varki NM, Wilczynski S, Safaei R, Alberts DS, Howell SB. (2003) Increase in expression of the copper transporter ATP7A during platinum drug-based treatment is associated with poor survival in ovarian cancer patients. Clin Cancer Res 9:5853–5859 [PubMed] [Google Scholar]
- Scott DC, Schekman R. (2008) Role of Sec61p in the ER-associated degradation of short-lived transmembrane proteins. J Cell Biol 181:1095–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setty SR, Tenza D, Sviderskaya EV, Bennett DC, Raposo G, Marks MS. (2008) Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature 454:1142–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Gitlin JD. (1999) Intracellular localization of the Menkes and Wilson's disease proteins and their role in intracellular copper transport. Pediatr Int 41:436–442 [DOI] [PubMed] [Google Scholar]
- Toikkanen J, Gatti E, Takei K, Saloheimo M, Olkkonen VM, Söderlund H, De Camilli P, Keränen S. (1996) Yeast protein translocation complex: isolation of two genes SEB1 and SEB2 encoding proteins homologous to the Sec61 beta subunit. Yeast 12:425–438 [DOI] [PubMed] [Google Scholar]
- Toikkanen JH, Miller KJ, Söderlund H, Jäntti J, Keränen S. (2003) The beta subunit of the Sec61p endoplasmic reticulum translocon interacts with the exocyst complex in Saccharomyces cerevisiae. J Biol Chem 278:20946–20953 [DOI] [PubMed] [Google Scholar]
- Van den Berg B, Clemons WM, Jr, Collinson I, Modis Y, Hartmann E, Harrison SC, Rapoport TA. (2004) X-ray structure of a protein-conducting channel. Nature 427:36–44 [DOI] [PubMed] [Google Scholar]
- Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW, 3rd, et al. (2009) BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol 10:R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Jäntti J. (2009) Functional characterization of the trans-membrane domain interactions of the Sec61 protein translocation complex beta-subunit. BMC Cell Biol 10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.