Abstract
FasL, perforin, TNFα, IL-1 and NO have been considered as effector molecule(s) leading to β-cell death in autoimmune diabetes. However, the real culprit(s) of β-cell destruction have long been elusive despite intense investigation. Previously we have suggested IFNγ/TNFα synergism as the final effector molecules in autoimmune diabetes of NOD mice. A combination of IFNγ and TNFα but neither cytokine alone, induced classical caspase-dependent apoptosis in murine insulinoma and pancreatic islet cells. IFNγ treatment conferred susceptibility to TNFα-induced apoptosis on otherwise resistant murine insulinoma cells by STAT1 activation followed by IRF-1 induction. Here we report that IFNγ/TNFα synergism induces apoptosis of human pancreatic islet cells. We also observed STAT1 activation followed by IRF-1 induction by IFNγ treatment in human islet cells. Taken together, we suggest that IFNγ/TNFα synergism could be involved in human islet cell death in type 1 diabetes, similar to murine type 1 diabetes.
Keywords: Diabetes, Apoptosis, Autoimmunity, Cytokines, Inflammatory mediators
INTRODUCTION
Recently, a great advance was achieved in the understanding of pathogenesis of autoimmune diabetes, particularly regarding the molecular mechanism of pancreatic β-cell apoptosis and immunological significance of β-cell apoptosis. Hence, it has been shown that pancreatic β-cell death is important not only in the final effector phase of autoimmune type 1 diabetes (1,2) but also in the initiation of β-cell autoimmunity (3,4). Such progress was mostly achieved by employing murine models of autoimmune diabetes because application of most essential genetic manipulation or immunological intervention is possible only in animal models. Murine autoimmune diabetes models and human type 1 diabetes have many common characteristics such as the presence of insulitis, requirement for specific MHC haplotypes and autoimmune responses to autoantigens including glutamic acid decarboxylase (GAD). Thus, many recent progress obtained employing murine autoimmune diabetes models would be applicable to human type 1 diabetes, implying important therapeutic potential. Autoreactive T lymphocytes are the most important effector cells in murine autoimmune diabetes (5-7), and probably in human type 1 diabetes. They will ultimately induce apoptosis of β-islet cells in murine autoimmune diabetes (8,9), while apoptosis of pancreatic β-cells in human type 1 diabetes has not been demonstrated because of the difficulty in procuring human pancreatic tissue and inability to synchronize disease process in human type 1 diabetes.
We have shown that IFNγ/TNFα synergism is responsible for murine pancreatic β-cell apoptosis both in vitro and in vivo (2), which was critically dependent on STAT1 activation by IFNγ (1). In vivo role of STAT1 activation by IFNγ was also demonstrated using STAT1-knockout NOD mouse model (1). Other death effector molecules such as Fas ligand (FasL) might be an effector molecule for a small number of autoreactive lymphocytes (10) but not for the majority of final death effector cells (9,11). IFNγ appears to sensitize otherwise resistant pancreatic islet cells or insulinoma cells to TNF α-mediated apoptosis by activating STAT1/IRF-1 signal pathway (2). Most such pancreatic β-cell death and signal pathways were studied in murine autoimmune diabetes models. Such findings might also be relevant in human type 1 diabetes also. In an effort to elucidate the possible mechanism of human pancreatic islet cell apoptosis leading to human type 1 diabetes, we investigated if similar cytokine synergism could induce apoptosis of human pancreatic islet cells and similar signal molecules are induced in them.
MATERIALS AND METHODS
Isolation of human islet cells
Human pancreatic islets were obtained from brain-dead patients between 1998 and 2002 at Samsung Medical Center, using a modification of the automated method for human islet isolation (12). Briefly, 350 ml of Hank's buffered salt solution (HBSS) containing 9.1µmol/l collagenase solution (Liberase, Boehringer-Mannheim, Mannheim, Germany) was injected through the pancreatic duct after cannulation. The pancreas was loaded into a stainless steel digestion chamber, and islets were separated during a continuous digestion process for 30~45 min. During the recirculation phase (flow rate, 85 ml/min), intrachamber temperature was increased at a rate of 2℃/min by the passage of the solution through a stainless steel coil immersed in a water bath (50℃). The chamber containing the distended pancreas was gently agitated and samples were taken every 2 min to monitor digestion. After 20~30 min of recirculation, digestion was stopped by dilution in 400 ml/min ice-cold HBSS. In this phase, the digested tissue was rapidly collected in sterile bottles containing 200 ml FCS. Purification of the islets was carried out over discontinuous Euro-Ficoll gradients (1.108, 1.096, 1.037 and HBSS). Purified islets were then washed twice, evaluated for purity, and counted after dithizone (Sigma, St. Louis, MO) staining. To make single islet cells, islets were spinned at 1,000 rpm for 2 min. The pellet was resuspended in 3 ml warm 53.7µmol/l trypsin-3 mmol/l EDTA. After incubation at 37℃ for 5 min and pipetting for 2~3 min, islets became invisible. Then, cells were washed with warm RPMI-10% FCS at 1,500 rpm for 5 min. Single islet cells were frozen in liquid nitrogen until use. At the time of assays, single islet cells were thawed and 1.4×105 cells were plated per single well of 96-well plates. Viability of the islet cells after thawing was above 90%, as judged by trypan blue staining and acridine orange/propidium iodide staining. Informed consent was obtained from the family members of the patients. Isolated human pancreatic islet cells were grown in DMEM containing 15% FBS, 2 mM glutamine, and penicillin-streptomycin (Gibco-BRL, Gaithersburg, MD). Recombinant human IFNγ was from Green Cross (Yongin, Korea). Recombinant human TNFα and recombinant human IL-1β were purchased from R&D Systems (Minneapolis, MN). All other chemicals were from Sigma Chemical Co., St. Louis, MO) unless stated otherwise.
Assessment of cytotoxicity by MTT assays
Cells (2×104/well for human single islet cells) were seeded in 96-well microtiter plates and treated with various combinations of cytokines for the indicated time periods. The optimal concentrations of cytokines for the cytotoxic action were 1,000 U/ml for IFNγ, 10 ng/ml for TNFα, and 17.5 ng/ml for IL-1β. After cytokine treatment, the medium was removed and 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT, 0.5 mg/ml) was added followed by incubation at 37℃ for 2 hr in CO2 incubator. After a brief centrifugation, supernatant was carefully removed and DMSO was added. After insoluble crystals were completely dissolved, absorbance at 540 nm was measured using Thermomax microplate reader (Molecular Devices, Sunny Vale, CA).
Morphological analysis of apoptotic cells
Morphological changes in the nuclear chromatin of cells undergoing apoptosis were detected by staining with 2.5µg/ml of DNA-binding bisbenzimide Hoe33342 fluorochrome (Calbiochem, San Diego, CA), followed by an examination on a fluorescence microscope. For transmission electron microscopy, cells were fixed in 4% glutaraldehyde/1% paraformaldehyde/0.2 M phosphate (pH 7.2) at 4℃ for 2 hr. After two washes in 0.2 M phosphate, the cell pellets were postfixed with 2% OsO4 in the same buffer for 30 min. The pellets were dehydrated in ethanol and then in 100% propylene oxide, followed by embedding at 37℃ overnight and 60℃ for another 3 days. Ultrafine sections were cut and examined on an electron microscope (Hitachi H7100, 75 kV).
Western blot analyses
Cells were lysed in triple-detergent lysis buffer (50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 0.02% sodium azide, 0.1% SDS, 1% NP-40, 0.5% sodium deoxycholate, 1 mM PMSF). Protein concentration in cell lysates was determined using Bio-Rad protein assay kit. An equal amount of protein for each sample was separated by 10% or 12% SDS-PAGE and transferred to Hybond ECL nitrocellulose membrane (GE Healthcare Life Sciences, Buckinghamshire, UK). After blocking with 5% skim milk, the membranes were sequentially incubated with one of the primary antibodies (rabbit anti-mouse IRF-1, Santa Cruz; rabbit anti-phospho-STAT1, New England Biolabs, Ipswich, MA) and then HRP-conjugated secondary antibodies (anti-rabbit IgG, GE Healthcare Life Sciences, Buckinghamshire, UK), followed by ECL detection (GE Healthcare Life Sciences, Buckinghamshire, UK).
RESULTS AND DISCUSSION
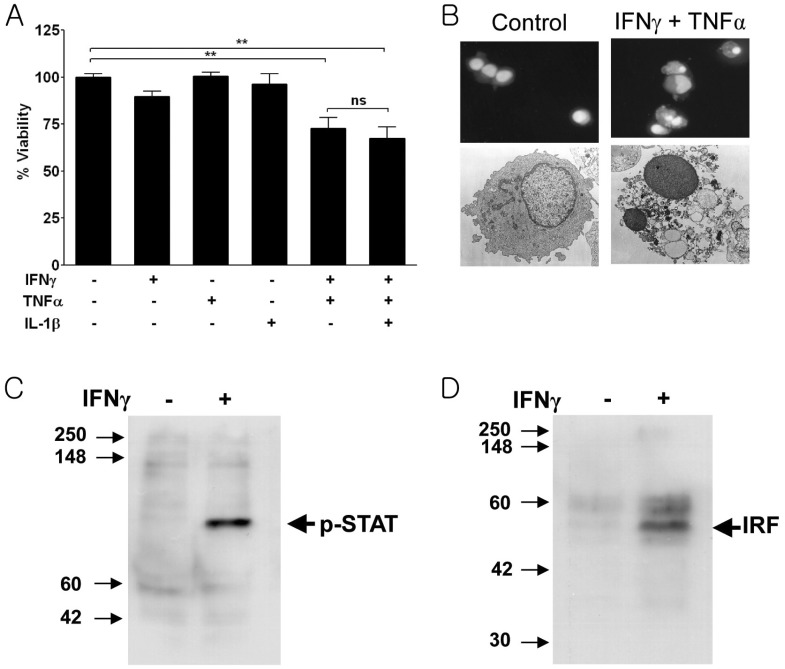
Treatment of single human islet cells with cytokines disclosed that combination of IFNγ, TNFα and IL-1β induced significant death after 5 days of incubation. When the effect of individual cytokine was studied, a combination of IFNγ and TNFα induced death of human islet cells while single cytokine did not exert significant cytotoxicity on human islet cells (Fig. 1A). The addition of IL-1β to the IFNγ/TNFα combination had only a minor effect on islet cell viability. Our results showing death of human islet cells by the triple combination of IFNγ, TNFα and IL-1β is similar to previous reports by others employing human islet cells (13-16). Percent death of human islet cells by the cytokine combination in this investigation was similar to a previous report (13), and was smaller than that of murine islet cells by the similar cytokine combination even after prolonged incubation for 5 days (2). Most of the death of human islet cells by the triple combination could be explained by IFNγ/TNFα combination because the addition of IL-1β to the IFNγ/TNFα combination increased human islet cell death only to a small degree, consistent with a previous paper (13).
Figure 1.
Human islet cell death by IFNγ/TNFα synergism. Single human islet cells were incubated with the cytokine combination for 5 days before assay. A combination of IFNγ (1,000 U/ml) and TNFα (10 ng/ml), but neither cytokine alone, induced human islet cell death as measured by MTT assays. IL-1β (17.5 ng/ml) had negligible effects. Graphs from one representative experiment among three independent experiments are shown. Values represent the means±SD from triplicate experiments. The means were compared using Student's unpaired t test. **p<0.01; ns, not significant (A). Apoptosis of human islet cells induced by IFNγ/TNFα. Nuclear condensation demonstrated by Hoechst staining (upper) or electron microscopy (lower) revealed that human islet cell death by IFNγ/TNFα was a classical apoptosis (B). IFNγ activates STAT1 and induces IRF-1 expression in human islet cells. Western blot analyses demonstrated that treatment of human islet cells with IFNγ (1,000 U/ml) for 30 min induced STAT1 phosphorylation (C). IFNγ also induced IRF-1 expression after 48 h of treatment (D).
Hoechst 33342 staining and electron microscopic examination showed that the death of human islet cells by IFNγ/TNFα combination was a typical apoptosis characterized by nuclear condensation and fragmentation with preserved plasma membrane integrity (Fig. 1B). These results are similar to previous reports showing nuclear condensation or TUNEL+ nuclei after cytokine treatment of human islet cells (15,16).
Next we asked if IFNγ activates STAT1 and induces IRF-1 which has been reported to be important for induction of TNFα susceptibility in otherwise resistant murine islet cells or cancer cells (2,17). Immunoblot analysis using antibody to phosphorylated STAT1 showed that STAT1 became activated 30 min after IFNγ treatment, similar to murine insulinoma cells (2) (Fig. 1C). IRF-1 was also induced by IFNγ treatment of human islet cells for 48 hrs again similar to murine insulinoma cells suggesting that STAT1 activation and IRF-1 induction by IFNγ may sensitize human islet cells to TNFα-induced apoptosis, similar to the case of murine islet cell death by IFNγ/TNFα synergism (2) (Fig. 1D). These results were similar to our previous study using murine islet/insulinoma cells and suggests the possibility that intracellular signal activated by IFNγ may sensitize otherwise resistant human pancreatic islet cells to TNFα-induced apoptosis, which could not be proved because of shortage of human islet cells, the absence of immortalized human islet cells and unavailability of genetic manipulation system. A recent paper has shown that Bim, a BH3-only proapoptotic Bcl-2 family member, is induced in human islets or rat islet cells after treatment with IFNγ+TNFα which plays an important role in β-cell death by IFNγ+TNFα synergism (18). In that model, Bim induction was attributed to IFNγ-induced activation of STAT1 which was bound to Bim promoter (18). While detailed biochemical and cellular mechanism of human islet cell death by cytokines could not be investigated further because of limited availability of human islets for diabetes research, our results suggest that the death of human islet cells is basically similar to that of murine islet cells.
ACKNOWLEDGMENTS
This study was supported by the Bio Research and Development Program (2008-04090), the Korea Healthcare Technology Research and Development Project, Ministry for Health, Welfare & Family Affairs, Korea (A080967) and National Cancer Center Grants (1110032). Lee M-S is the recipient of a Global Research Laboratory Grant (K21004000003-10A0500-00310) and Bio&Medical Technology Development Program (20110019335) of the National Research Foundation of Korea.
Footnotes
The authors have no financial conflict of interest.
References
- 1.Kim S, Kim HS, Chung KW, Oh SH, Yun JW, Im SH, Lee MK, Kim KW, Lee MS. Essential role for signal transducer and activator of transcription-1 in pancreatic beta-cell death and autoimmune type 1 diabetes of nonobese diabetic mice. Diabetes. 2007;56:2561–2568. doi: 10.2337/db06-1372. [DOI] [PubMed] [Google Scholar]
- 2.Suk K, Kim S, Kim YH, Kim KA, Chang I, Yagita H, Shong M, Lee MS. IFN-gamma/TNF-alpha synergism as the final effector in autoimmune diabetes: a key role for STAT1/IFN regulatory factor-1 pathway in pancreatic beta cell death. J Immunol. 2001;166:4481–4489. doi: 10.4049/jimmunol.166.7.4481. [DOI] [PubMed] [Google Scholar]
- 3.Kim HS, Han MS, Chung KW, Kim S, Kim E, Kim MJ, Jang E, Lee HA, Youn J, Akira S, Lee MS. TLR2 as a link between β-cell injury and initiation of autoimmune diabetes. Immunity. 2007;27:321–333. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med. 2003;198:1527–1537. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wicker LS, Miller BJ, Mullen Y. Transfer of autoimmune diabetes mellitus with splenocytes from nonobese diabetic (NOD) mice. Diabetes. 1986;35:855–860. doi: 10.2337/diab.35.8.855. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Pontesilli O, Gill RG, La Rosa FG, Lafferty KJ. The role of CD4+ and CD8+ T cells in the destruction of islet grafts by spontaneously diabetic mice. Proc Natl Acad Sci U S A. 1991;88:527–531. doi: 10.1073/pnas.88.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 8.Kurrer MO, Pakala SV, Hanson HL, Katz JD. Beta cell apoptosis in T cell-mediated autoimmune diabetes. Proc Natl Acad Sci U S A. 1997;94:213–218. doi: 10.1073/pnas.94.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YH, Kim S, Kim KA, Yagita H, Kayagaki N, Kim KW, Lee MS. Apoptosis of pancreatic beta-cells detected in accelerated diabetes of NOD mice: no role of Fas-Fas ligand interaction in autoimmune diabetes. Eur J Immunol. 1999;29:455–465. doi: 10.1002/(SICI)1521-4141(199902)29:02<455::AID-IMMU455>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 10.Amrani A, Verdaguer J, Anderson B, Utsugi T, Bou S, Santamaria P. Perforin-independent beta-cell destruction by diabetogenic CD8(+) T lymphocytes in transgenic nonobese diabetic mice. J Clin Invest. 1999;103:1201–1209. doi: 10.1172/JCI6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S, Kim KA, Hwang DY, Lee TH, Kayagaki N, Yagita H, Lee MS. Inhibition of autoimmune diabetes by Fas ligand: the paradox is solved. J Immunol. 2000;164:2931–2936. doi: 10.4049/jimmunol.164.6.2931. [DOI] [PubMed] [Google Scholar]
- 12.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 13.Rabinovitch A, Sumoski W, Rajotte RV, Warnock GL. Cytotoxic effects of cytokines on human pancreatic islet cells in monolayer culture. J Clin Endocrinol Metab. 1990;71:152–156. doi: 10.1210/jcem-71-1-152. [DOI] [PubMed] [Google Scholar]
- 14.Rabinovitch A, Suarez-Pinzon WL, Strynadka K, Schulz R, Lakey JR, Warnock GL, Rajotte RV. Human pancreatic islet beta-cell destruction by cytokines is independent of nitric oxide production. J Clin Endocrinol Metab. 1994;79:1058–1062. doi: 10.1210/jcem.79.4.7962274. [DOI] [PubMed] [Google Scholar]
- 15.Delaney CA, Pavlovic D, Hoorens A, Pipeleers DG, Eizirik DL. Cytokines induce deoxyribonucleic acid strand breaks and apoptosis in human pancreatic islet cells. Endocrinology. 1997;138:2610–2614. doi: 10.1210/endo.138.6.5204. [DOI] [PubMed] [Google Scholar]
- 16.Marselli L, Dotta F, Piro S, Santangelo C, Masini M, Lupi R, Realacci M, del Guerra S, Mosca F, Boggi U, Purrello F, Navalesi R, Marchetti P. Th2 cytokines have a partial, direct protective effect on the function and survival of isolated human islets exposed to combined proinflammatory and Th1 cytokines. J Clin Endocrinol Metab. 2001;86:4974–4978. doi: 10.1210/jcem.86.10.7938. [DOI] [PubMed] [Google Scholar]
- 17.Suk K, Chang I, Kim YH, Kim S, Kim JY, Kim H, Lee MS. Interferon gamma (IFNgamma) and tumor necrosis factor alpha synergism in ME-180 cervical cancer cell apoptosis and necrosis. IFNgamma inhibits cytoprotective NF-kappa B through STAT1/IRF-1 pathways. J Biol Chem. 2001;276:13153–13159. doi: 10.1074/jbc.M007646200. [DOI] [PubMed] [Google Scholar]
- 18.Barthson J, Germano CM, Moore F, Maida A, Drucker DJ, Marchetti P, Gysemans C, Mathieu C, Nuñez G, Jurisicova A, Eizirik DL, Gurzov EN. Cytokines tumor necrosis factor-α and interferon-γ induce pancreatic β-cell apoptosis through STAT1-mediated Bim protein activation. J Biol Chem. 2011;286:39632–39643. doi: 10.1074/jbc.M111.253591. [DOI] [PMC free article] [PubMed] [Google Scholar]