Abstract
Rationale and objective
Repeated and/or heightened elevations in glucocorticoids (e.g., repeated stress) can promote escalated drug-taking behaviors and induce compromised HPA axis function. Given that interoceptive/subjective drug cues are a fundamental factor in drug-taking behavior, we sought to determine the effects of exposure to repeated elevations in the glucocorticoid corticosterone (CORT) on the interoceptive effects of alcohol in rats using drug discrimination techniques.
Methods
Male Long Evans rats trained to discriminate alcohol (1 g/kg, IG) vs. water were exposed to CORT (300 μg/ml) in the home cage drinking water for 7 days. The interoceptive effects of experimenter- and self-administered alcohol were assessed and HPA axis function was determined.
Results
The interoceptive effects of experimenter- and self-administered alcohol were blunted following CORT. Control experiments determined that this decreased sensitivity was unrelated to discrimination performance impairments or decreased CORT levels at the time of testing and was dependent on repeated CORT exposure. Susceptibility to compromised HPA axis function following CORT exposure was suggested by an altered pattern of CORT secretion and blunted CORT response following injection of the synthetic glucocorticoid dexamethasone.
Conclusions
These findings present a possible behavioral mechanism for escalated alcohol drinking during episodes of heightened elevations in glucocorticoids (e.g., stress). That is, during these episodes, individuals may consume more alcohol to achieve the desired interoceptive effects. Understanding these behavioral mechanisms may lead to a better understanding of factors that promote alcoholism and alcohol abuse in at risk populations.
Keywords: Drug discrimination, HPA axis dysregulation, Alcoholism, Discriminative stimulus, Ethanol, Stress, Glucocorticoids, Drinking, Interoceptive, Subjective, Alcohol
Introduction
Glucocorticoids (cortisol in the human; corticosterone in the rodent) are secreted upon hypothalamic–pituitary–adrenal (HPA axis) activation and play a major role in normal negative feedback control of the HPA axis, the major neuroendocrine system involved in regulating response to stressors (see de Kloet et al. 2005; McEwen 2007). However, repeated and/or persistent elevations in glucocorticoids can have detrimental consequences, such as impaired HPA axis function (McEwen 2007) and increased risk for developing some affective disorders (Brown et al. 2004; Gold and Chrousos 2002; Sapolsky 2000); e.g., depression). Compromised HPA axis function, including dysregulation of glucocorticoid feedback and blunted response to HPA axis activation, is a characteristic feature of alcoholism (Adinoff et al. 1990; Berman et al. 1990; King et al. 2006; Lovallo et al. 2000; Majumdar et al. 1988; Wand and Dobs 1991) and can persist into abstinence (Adinoff et al. 1990; Costa et al. 1996; Errico et al. 1993), highlighting the enduring nature of these changes.
There is a substantial literature showing that repeated and/or heightened elevations in glucocorticoids (e.g., resulting from chronic or repeated stress exposure) escalate drug-taking and -seeking behaviors (see Goeders 2002; Hoffmann and Su 1998; Koob 1999; Kreek and Koob 1998; Lu et al. 2003; Piazza and Le Moal 1998; Sillaber and Henniger 2004). A fundamental factor in drug-taking behavior is the interoceptive (subjective) drug cues (Stolerman 1992), such as the feeling of “drunkenness” or lightheadedness that can accompany alcohol drinking. Therefore, it is plausible that increased drug taking may be related to changes in the interoceptive effects of the drug. For example, if an individual is sensitive to the subjective/ interoceptive cues produced by alcohol drinking, and those cues are blunted, then the likelihood of escalated alcohol drinking may be increased. Indeed, individuals with decreased subjective sensitivity to alcohol may be more likely to drink more alcohol than individuals with greater sensitivity (see Pollock 1992). Interestingly, these less sensitive individuals, such as individuals with a family history of an alcohol use disorder (AUD), are at higher risk for developing AUDs (Schuckit 2009; Schuckit and Smith 2000; Trim et al. 2009) and show dysregulated response to HPA axis challenges (Dai et al. 2002; Hernandez-Avila et al. 2002; Uhart et al. 2006; Waltman et al. 1994).
The primary goal of the present work was to examine the effects of repeated elevations in CORT on the interoceptive effects of alcohol in rats. Elevations in glucocorticoids can be induced by exposure to stressors or directly, by exogenous administration of CORT. In the present work, we utilized CORT in drinking water as the method to induce repeated heightened elevations in glucocorticoids (Gourley and Taylor 2009; Karatsoreos et al. 2010). Therefore, to address the primary hypothesis that repeated elevations in CORT result in decreased sensitivity to the interoceptive effects of alcohol, we assessed whether CORT exposure altered the discriminative stimulus effects of 1) experimenter-administered, and 2) self-administered alcohol in rats trained to discriminate a moderate dose of alcohol (1 g/kg, IG) from water (IG) using well-characterized drug discrimination methods. Control experiments were also conducted to eliminate memory/discrimination performance impairment explanations, to determine the necessity of repeated CORT exposure, and to assess the role of reduced CORT levels. Further, experiments were conducted to characterize the CORT exposure model and to assess HPA axis function in response to a challenge. Understanding the behavioral mechanisms by which repeated elevations in glucocorticoids can alter the interoceptive effects of alcohol is an important approach and may lead to a better understanding of factors that promote alcohol abuse and alcoholism.
Methods
Animals
Male Long Evans rats (Harlan Sprague Dawley, Indianapolis, IN), maintained at approximately 325–340 g, were fed at approximately 3 pm each day. Food restriction is common in drug discrimination procedures and was carried out in Experiments 1a, 1b and 3 to maintain consistency with the discrimination experiments. The colony room was maintained on a 12-h light/dark cycle (lights on at 7 am) and experiments were conducted during the light portion of the cycle (approximately between 10 am and noon unless otherwise noted). Animals were under continuous care and monitoring by veterinary staff from the Division of Laboratory Animal Medicine (DLAM) at UNC-Chapel Hill. All procedures were also carried out in accordance with the NIH Guide to Care and Use of Laboratory Animals and institutional guidelines.
Experiment 1: characterization of the corticosterone exposure model
Experiment 1a: pattern of CORT levels during CORT exposure
In naïve rats, tail blood was collected to determine baseline plasma CORT levels (at approximately 10 am). Immediately after the blood collection, rats were given one bottle (1-ml graduates) fitted with a ball bearing stopper (to limit spillage) containing corticosterone (CORT; 300 μg/ml) or water (n=8/group) as the sole available fluid for 7 days (24 h daily access). The CORT concentration was chosen based on literature (Conrad et al. 2007; Karatsoreos et al. 2010; Pung et al. 2003) and on pilot experiments showing significant changes in plasma CORT levels and no severe effects on general health using similar protocols. Rats were weighed daily and fluid was measured and changed daily. This CORT exposure protocol and concentration was used in all the experiments, except where noted. To determine the pattern of plasma CORT levels, during the sevent day of CORT/water exposure (n=8/group), tail blood was collected at 10 pm and again at 10 am (at the completion of Day 7).
Experiment 1b: HPA axis challenge (dexamethasone test)
Dysregulation of the HPA system is commonly characterized by impaired negative feedback mechanisms as evidenced by resistance/decreased sensitivity to dexamethasone-induced CORT secretion (reviewed in Ising et al. (2005)). Rats underwent CORT exposure, as described in Experiment 1a, and on the seventh day of CORT/water exposure (7 am; n=17/group), rats were injected with the synthetic glucocorticoid dexamethasone (0 or 0.1 mg/kg, IP), and tail blood was collected at 11 am (i.e., at the completion of 7 days of CORT exposure). Dexamethasone doses in this range are commonly used in rodent studies to assess HPA axis function (Buwalda et al. 1999; Griesbach et al. 2011; Hatzinger et al. 1996; Mantsch et al. 2007; Raone et al. 2007).
Discriminative stimulus effects of experimenter-administered alcohol
Experiment 2a: effects of repeated CORT exposure on the discriminative stimulus effects of alcohol
Seven-day CORT exposure (cumulative alcohol test)
Rats were trained on a two-lever alcohol discrimination task. The same training procedures and conditioning chambers described in Besheer et al. (2009), Besheer and Hodge (2005), Besheer et al. (2006), and Cannady et al. (2011) were used. Briefly, following alcohol administration (1 g/kg by intragastric (IG) gavage; 10 min before the 15-min session), completion of ten responses on the alcohol-appropriate lever (e.g., right lever) throughout the session resulted in the presentation (4 s) of the sucrose (10% w/v) reinforcer. Similarly, following water administration (IG), completion of ten responses on the water-appropriate lever (e.g., left lever) throughout the session resulted in sucrose reinforcer delivery. Daily training continued until the following accuracy criteria were met: the percentage of appropriate lever responses before the first reinforcer and during the entire session was >80% for at least eight out of ten consecutive days.
After the training criteria were met, a cumulative alcohol substitution curve (0.1, 0.5, 1.0, and 1.7 g/kg, IG) was determined. Cumulative dosing procedures were used as previously described (Besheer et al. 2009; Cannady et al. 2011) and these cumulative dosing test sessions were identical to training sessions except that they were 2 min in duration (after the initial 10-min delay) and ten responses on either lever resulted in sucrose delivery. Thirty minutes after the final alcohol administration, tail blood was collected for determination of baseline plasma CORT levels. Immediately thereafter, homecage CORT exposure began where CORT or water (n=8/group) was the sole available fluid for 7 days (as described in Experiment 1a). Discrimination training was withheld during this time and rats remained in the homecage for the 7-day duration. Immediately upon completion of Day 7 (at approximately 10 am), a cumulative alcohol curve was determined and tail blood was collected 30 min after the final alcohol dose administration for determination of plasma CORT levels.
One-day CORT exposure (cumulative alcohol test)
To determine if changes in the discriminative stimulus effects of alcohol were due to “repeated” CORT exposure, another group of discrimination-trained rats followed the same procedure; however, they were exposed to CORT in drinking water or water only for 1 day. After 24 h of CORT exposure, a cumulative alcohol curve was determined and tail blood was collected. For this group (n=8), a within-subjects design was used (treatment order was counterbalanced) given that no changes in behavior were observed following the first half of CORT exposure and as an effort to reduce the number of rats needed. For these reasons, within-subjects designs were used whenever possible as noted in the remaining experiments. Rats were given 2 weeks of discrimination training following the exposure cycles.
Experiment 2b: testing memory/discrimination performance impairment: 7-day CORT exposure (cumulative water test)
To determine whether the reductions in alcohol-appropriate responding after 7 days of CORT were due to discrimination performance impairment, another group of discrimination-trained rats was tested. For this group, after determination of baseline measures, rats began CORT (n=7) or water (n=8) exposure in drinking water for 7 days. Immediately upon completion of Day 7, a cumulative water curve was determined and tail blood was collected. This cumulative test was identical to the alcohol cumulative curve test, except that water was administered in place of alcohol. Tail blood was collected 30 min after the final water administration for determination of plasma CORT levels.
Experiment 3: blood alcohol levels following CORT exposure
To determine whether 7 days of CORT exposure reduces blood alcohol levels, naïve rats were exposed to CORT (n=9) or Water for 7 days (n=10). At the completion of Day 7 (10 am), rats were administered alcohol (1 g/kg, IG) and tail blood was collected 30, 60, 120 and 240 min later.
Experiment 4: CORT synthesis inhibition on the discriminative stimulus effects of alcohol
To assess whether reduced CORT level was related to inhibition of the discriminative stimulus effects of alcohol, discrimination-trained rats were administered the CORT synthesis inhibitor metyrapone (0, 25, and 50 mg/kg, SC; n=8) 90 min before a cumulative alcohol test. Tail blood was collected 30 min after the final alcohol administration. A within-subjects counterbalanced design was used with at least 1 week between tests.
Discriminative stimulus effects of self-administered alcohol
Experiment 5a: effects of repeated CORT exposure on the discriminative stimulus effects of self-administered alcohol (in discrimination-trained rats)
Sweetened alcohol reinforcer (10S/10A)
To determine whether CORT exposure would alter the discriminative stimulus effects of self-administered alcohol, in addition to the effects of experimenter-administered alcohol (Experiment 2a), discrimination performance during discrimination/self-administration (Discrim/SA) test sessions was assessed as detailed in Besheer et al. (2006). Specifically, discrimination-trained rats underwent a baseline Discrim/SA test session. For these Discrim/SA test sessions, rats were administered water (IG) and placed in the chambers for a 30-min session (following the initial 10-min delay). However, alcohol (A; 10% v/v) was added to the standard sucrose (S; 10% w/v) reinforcer (10S/10A) to assess the interoceptive effects of the self-administered alcohol. As described in Besheer et al. (2006), following water (IG), rats begin responding on the water-appropriate lever; as the session continues and rats have consumed significant amounts of the sweetened alcohol reinforcer, responding shifts to the alcohol-appropriate lever, indicating that the interoceptive effects of the consumed alcohol are detected by the animal. During these sessions, behavior is free to vary between the two levers since completion of an FR10 on either lever produces access to the sweetened alcohol solution. Fifteen minutes after the completion of this baseline Discrim/SA session, tail blood was collected and rats began CORT (n=8) or water (n=6) exposure for 7 days. Upon completion of Day 7, rats experienced another Discrim/SA session (10S/10A reinforcer) and tail blood was collected 15 min after the completion of the session.
Experiment 5b: testing memory/discrimination performance impairment and specificity to alcohol interoceptive effects: sucrose reinforcer (10S)
To confirm that any CORT-induced changes in discrimination performance were not due to discrimination performance impairments, another group of rats (n=7) was tested using the same Discrim/SA procedure with the exception that a sucrose (10% w/v) reinforcer was used (i.e., no alcohol was added). For this group, a within-subjects design was used (treatment order counterbalanced) and rats were given 2 weeks of discrimination training between CORT/water exposure cycles.
Drugs and dosing
Alcohol (95%) was diluted in distilled water to a concentration of 20% (v/v) and administered IG, with volumes varied to obtain the desired dose. Corticosterone hemisuccinate (4-pregnen-11β, 21-DIOL-3, 20-DIONE 21-hemisuccinate; Steraloids, Inc., Newport, RI) was dissolved in tap water (for drinking studies; prepared daily) by addition of NaOH and neutralized with HCl, to a final pH of 7.0–7.4 (Gourley and Taylor 2009). Metyrapone (2-Methyl-1,2-di-3-pyridyl-1-propanone; Sigma-Aldrich) was suspended in a 0.5% carboxymethocellulose solution (vehicle) and injected subcutaneously (SC) at a volume of 2 ml/kg. Dexamethasone (Sigma-Aldrich) was dissolved in saline and injected at a volume of 1 mg/kg (SC).
Data analysis
Response accuracy was expressed as the percentage of alcohol-appropriate lever presses upon delivery of the first reinforcer. Response rate (responses/min) was analyzed for the entire session and provided an index of locomotor ability. Complete expression of the discriminative stimulus effects of alcohol (i.e., full substitution) was defined as >80% choice of the alcohol lever upon completion of the first FR10 during test sessions. One- or two-way repeated measures analysis of variance (RM ANOVA) was used to analyze response accuracy and response rate data. Paired or unpaired t-tests were used for two group comparisons. Tukey post hoc analyses were used to explore significant main effects and interactions. Statistical significance was declared at P≤0.05.
Results
For all experiments, baseline discrimination performance (where appropriate), baseline plasma CORT levels and average daily fluid and CORT dose consumed are shown in Tables 1 and 2. No group differences in any of these measures were observed.
Table 1.
Mean (±S.E.M.) baseline alcohol discrimination performance
| Alcohol-appropriate responses (%) Cumulative alcohol dose (g/kg, IG) |
Response rate (response/min) Cumulative alcohol dose (g/kg, IG) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.3 | 1.0 | 1.7 | 0.1 | 0.3 | 1.0 | 1.7 | |
| Experiment 2a: effects of repeated CORT exposure on the discriminative stimulus effects of alcohol | ||||||||
| 7-day CORT | ||||||||
| Water | 14.6±9.5 | 4.1±4.1 | 47.9±15.9 | 94.5±2.3 | 72.9±2.9 | 70.4±4.3 | 62.1±2.2 | 59.1±4.7 |
| CORT | 11.6±4.0 | 8.4±5.8 | 53.8±16.2 | 86.5±7.9 | 72.6±3.4 | 70.6±2.8 | 67.1±3.9 | 63.5±2.1 |
| 1-day CORT | ||||||||
| Water | 23.2±12.5 | 15.0±10.8 | 44.4±17.6 | 83.1±10.7 | 63.9±6.4 | 60.3±5.4 | 56.1±4.5 | 49.7±3.2 |
| CORT | 25.6±10.5 | 13.4±5.3 | 46.9±16.2 | 87.5±11.3 | 54.4±6.9 | 56.4±3.8 | 55.7±2.9 | 52.7±3.9 |
| Experiment 2b: testing memory/performance impairment: 7-day CORT exposure (cumulative water test) | ||||||||
| Water | 14.0±3.7 | 13.1±8.7 | 73.5±13.9 | 81.8±11.0 | 61.7±5.1 | 61.1±4.7 | 57.3±4.0 | 50.6±5.2 |
| CORT | 15.6±6.5 | 13.7±5.1 | 79.3±9.5 | 95.0±2.6 | 67.6±5.4 | 61.2±4.4 | 57.7±3.9 | 53.4±4.0 |
| Alcohol-appropriate responses (%) Time (min) | Cumulative alcohol intake (g/kg) Time (min) | |||||||
| 10 | 20 | 30 | 10 | 20 | 30 | |||
| Experiment 5a: effects of repeated CORT exposure on the discriminative stimulus effects of self-administered alcohol (in discrimination-trained rats) | ||||||||
| Water | 9.3±6.1 | 39.8±16.4 | 73.8±14.9 | 1.2±0.1 | 2.2±0.3 | 2.9±0.3 | ||
| CORT | 11.3±6.8 | 77.1±13.1 | 92.0±4.0 | 1.2±0.1 | 2.1±0.1 | 2.9±0.2 | ||
| Alcohol-appropriate responses (%) Time (min) | ||||||||
| 10 | 20 | 30 | Response rate (response/min) | |||||
| Experiment 5b: testing memory/performance impairment and specificity to alcohol interoceptive effects | ||||||||
| Water | 14.8±14.2 | 15.1±13.5 | 28.1±17.3 | 49.6±4.6 | ||||
| CORT | 0.48±0.4 | 12.9±11.1 | 19.4±13.7 | 50.3±2.6 | ||||
Table 2.
Mean (±S.E.M.) baseline corticosterone and daily consumption measures
| Baseline plasma corticosterone (ng/ml) |
Daily fluid consumption (ml) |
Daily CORT dose consumed (mg/kg) CORT | |||
|---|---|---|---|---|---|
| Water | CORT | Water | CORT | ||
| Experiment 1a | 80.6±24.6 | 87.1±33.0 | 23.7±1.5 | 25.7±1.5 | 24.5±1.5 |
| Experiment 1b | |||||
| Vehicle | 101.0±10.5 | 87.0±18.5 | 24.3±1.4 | 22.4±1.3 | 20.8±1.2 |
| Dexamethasone | 119.0±25.4 | 133.0±33.3 | 22.8±0.7 | 25.0±1.6 | 23.6±1.5 |
| Experiment 2a | |||||
| 7-Day CORT | 152.8±25.8 | 161.3±34.1 | 21.7±1.9 | 23.3±1.4 | 21.5±1.3 |
| 1-Day CORT | 131.1±29.2 | 137.4±40.0 | 18.3±0.5 | 21.9±1.8 | 19.8±1.7 |
| Experiment 2b | 109.1±29.5 | 92.3±22.2 | 24.1±1.7 | 25.6±2.2 | 23.8±2.1 |
| Experiment 3 | 103.5±17.1 | 99.8±11.3 | 24.6±0.9 | 25.8±1.1 | 25.6±1.4 |
| Experiment 5a | 129.1±25.8 | 120.2±36.1 | 21.8±3.5 | 22.7±2.0 | 21.1±1.9 |
| Experiment 5b | 124.0±11.6 | 108.8±17.5 | 21.7±0.9 | 23.9±1.9 | 22.1±1.8 |
Experiment 1: characterization of the corticosterone exposure model
Experiment 1a: pattern of CORT elevations during CORT exposure
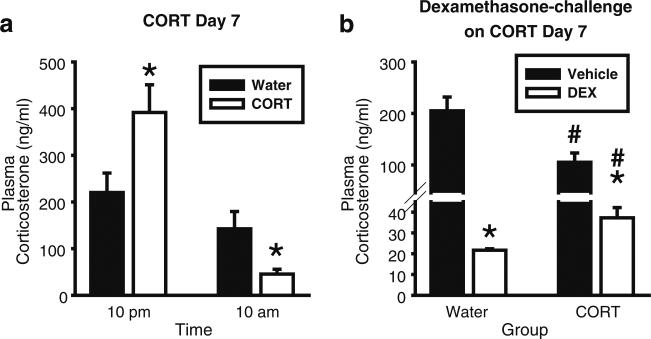
Figure 1a shows the pattern of CORT levels on Day 7. There was a significant main effect of time [F(1,14)=27.11, p<0.001], and a significant interaction [F(1,14)=10.86, p=0.005; Fig. 1a]. CORT levels were significantly elevated as compared to the Water group at 10 pm (p<0.05), corresponding to CORT intake of 15.8±1.2 mg/kg (10 am on the start of Day 7 to 10 pm). At 10 am, CORT levels were significantly lower in the CORT and Water groups (corresponding CORT intake 4.1±0.6 mg/kg— 10 pm to 10 am). Given that the remaining experiments utilize the same CORT exposure procedure, a decrease in CORT levels at the 10 am time point (i.e., approximate time at which blood is collected in all the behavioral experiments) will serve as a physiological index of efficacy of the CORT-drinking procedures.
Fig. 1.
Corticosterone exposure (7 days) produces elevations in CORT and blunts response to dexamethasone. a On the seventh day of CORT exposure (300 μg/ml), plasma CORT levels were significantly higher than the Water group at 10 pm, and significantly lower than the Water group at 10 am (n=8/group). Asterisk signifies significant difference from the Water group (p<0.05). b CORT exposure (300 μg/ml; 7 days) resulted in significantly less suppression of plasma CORT levels in the CORT group after dexamethasone (0.1 mg/kg, SC) administration (blood collected 4 h after dexamethasone administration at 11 am; n=9/group). Asterisk signifies significant difference from the respective vehicle group (p<0.05); number sign signifies significant difference from the respective Water groups. Values on graphs represent mean ± S.E.M.
Experiment 1b: HPA axis challenge (dexamethasone test)
Dexamethasone test
Dexamethasone produced a significant reduction in plasma CORT levels in both the Water and CORT groups (Fig. 1b). A two-way ANOVA showed a significant main effect of group [F(1,30)=7.5, p=0.01], a significant main effect of injection (dexamethasone or saline; F(1,30)=66.8, p<0.001), and a significant interaction [F(1,30)=14.1, p<0.001]. Consistent with the Experiment 1a (10 am), plasma CORT levels were significantly reduced in the vehicle-CORT group relative to the vehicle-Water group (p<0.05), confirming efficacy of the CORT procedure. Dexamethasone induced significantly less CORT suppression in the CORT group relative to the Water group (p<0.05). Given the group difference in CORT levels after vehicle injection, a difference from control measure was used to assess the relative change in plasma CORT levels after dexamethasone injection. Significantly less suppression was evident in the CORT group [Water: –183.4±14.5 ng/ml; CORT: –69.4±4.9 ng/ml; t=23.0, p<0.001]. Together, these results show less sensitivity to dexamethasone challenge following CORT exposure, suggesting a possible impairment in negative feedback mechanisms.
Discriminative stimulus effects of experimenter-administered alcohol
Experiment 2a: effects of repeated CORT exposure on the discriminative stimulus effects of alcohol
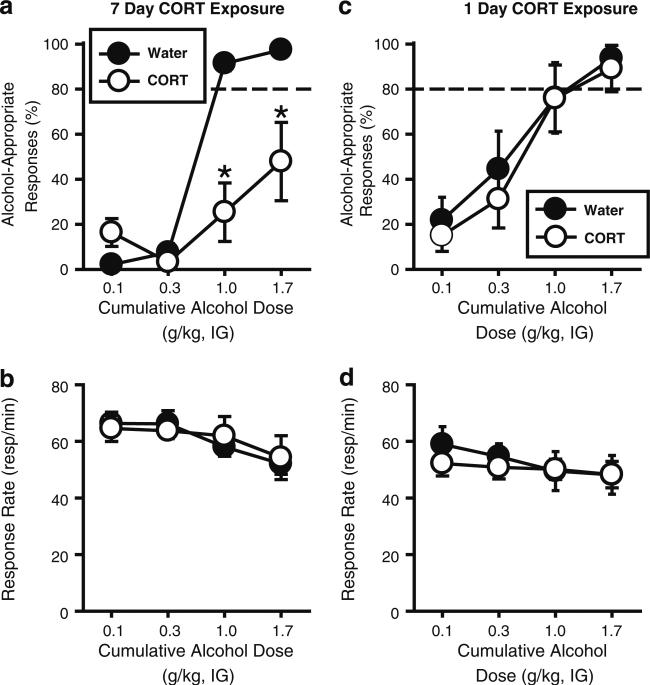
Seven-day CORT exposure (cumulative alcohol test) CORT exposure blunted the interoceptive effects of alcohol as indexed by a significant reduction in alcohol-appropriate responding, and prevented full substitution at 1.0 and 1.7 g/kg (Fig. 2a), indicating decreased sensitivity to the discriminative stimulus (interoceptive) effects of alcohol. This was confirmed by analysis of alcohol-appropriate responses in which a significant main effect of exposure (CORT or water; F(1,14)=55.82, p<0.001), alcohol dose [F(3,40)=24.60, p<0.001], and a significant interaction [F(3,40)=7.43, p<0.001] were observed. Response rate was not altered following CORT exposure (Fig. 2b), indicating the absence of nonspecific motor effects. Plasma CORT levels (ng/ml) following 7 days of CORT exposure (blood collected 30 min after the final alcohol administration at approximately 10 am) were significantly reduced relative to the Water group [t=4.11, p=0.001; Water: 192.8±78.0; CORT: 78.0±21.8], confirming efficacy of the CORT exposure procedure.
Fig. 2.
Corticosterone exposure (7 days in drinking water) blunts the interoceptive effects of experimenter-administered alcohol. a Corticosterone exposure (CORT; 300 μg/ml; 7 days; n=8) significantly reduced the percentage of alcohol-appropriate responding relative to Water controls (n=8), and prevented full expression of the discriminative stimulus effects of alcohol (1 and 1.7 g/kg, IG), without altering b response rate. c One day of CORT exposure (300 μg/ml) did not alter the discriminative stimulus effects of alcohol or d response rate. Horizontal dashed line (>80%) represents full expression of the discriminative stimulus effects of alcohol. Asterisk signifies significant difference from Water group (p<0.05). Values on graphs represent mean ± S.E.M.
One-day CORT exposure (cumulative alcohol test)
Acute CORT exposure (1 day) did not alter alcohol-appropriate responding (Fig. 2c; i.e., sensitivity to the interoceptive effects of alcohol were not changed). There was a significant main effect of alcohol dose [F(3,21)=14.64, p<0.001], and no main effect of treatment or interaction. Response rate was also not altered by CORT exposure (Fig. 2d). Further, 1 day of CORT exposure did not alter plasma CORT levels [Water: 226.4±78.0; CORT: 176.3±31.1; blood collected 30 min after final alcohol administration at approximately 10 am]. These results confirm that “repeated” elevations in CORT (7 days) are necessary to induce reduced sensitivity to the interoceptive effects of alcohol.
Experiment 2b: testing memory/performance impairment: 7-day CORT exposure (cumulative water test)
As shown in Table 3, CORT exposure (7 days) did not alter response accuracy (i.e., alcohol-appropriate responses) when water was administered at each test during the cumulative testing procedure (i.e., responding predominantly on water-appropriate lever; low responding on the alcohol-appropriate lever). This accurate discrimination performance argues against a memory/discrimination performance impairment contributing to the reduction in the discriminative stimulus effects of alcohol after CORT exposure (7 days; Experiment 2a). Plasma CORT levels were significantly reduced in the CORT group following 7 days of CORT exposure [t=2.99, p=0.03; Water: 150.8±40.4; CORT: 22±3.6; blood collected 30 min after final water administration at approximately 10 am], confirming efficacy of the CORT exposure procedure.
Table 3.
Experiment 2b: mean (±S.E.M.) discrimination performance after corticosterone exposure (7 days) with water administered at each test
| Alcohol-appropriate responses (%) Cumulative water (IG) test |
Response rate (response/min) Cumulative water (IG) test |
Plasma CORT (ng/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||
| Water | 23.0±10.9 | 15.0±6.4 | 18.5±5.6 | 8.3±3.4 | 56.2±6.3 | 58.4±6.9 | 55.3±5.5 | 54.0±6.4 | 150.8±40.4 |
| CORT | 8.4±5.7 | 6.7±4.0 | 13.4±5.5 | 5.7±3.7 | 57.9±5.8 | 55.5±4.5 | 52.5±6.7 | 49.2±4.2 | 22.5±3.6* |
p<0.05 (water vs. CORT)
Experiment 3: blood alcohol levels following CORT exposure
Blood alcohol levels were examined after CORT exposure (7 days) and were found to be unaltered by CORT exposure (Table 4). A mixed two-way ANOVA (time as a repeated measure) showed that blood alcohol decreased across time [F(3,51)=564.9, p<0.001] as would be expected, and this pattern was unaffected by CORT exposure (7 days). Therefore, reduced sensitivity to the discriminative stimulus effects of alcohol following CORT exposure is likely not due to altered pharmacokinetics of alcohol.
Table 4.
Experiment 3: mean (±S.E.M.) blood alcohol levels (mg/dl) after alcohol (1 g/kg IG)
| Time (min) after alcohol administration | |||||||
|---|---|---|---|---|---|---|---|
| 30 | 60 | 120 | 240 | ||||
| Water | CORT | Water | CORT | Water | CORT | Water | CORT |
| 82.7±5.7 | 83.3±4.6 | 79.2±6.3 | 78.1±3.9 | 52.6±6.5 | 48.8±4.7 | 12.1±2.5 | 10.5±1.9 |
Experiment 4: CORT synthesis inhibition on the discriminative stimulus effects of alcohol
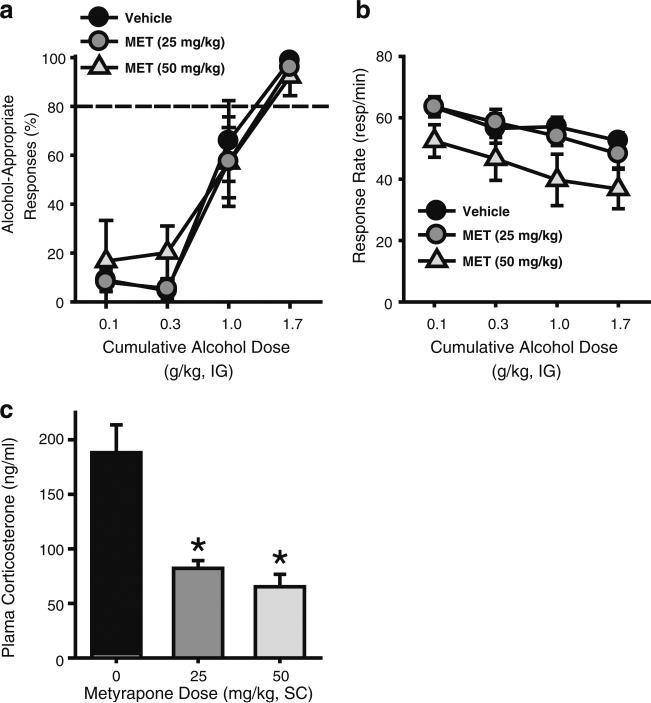
In addition to confirming efficacy of the CORT-exposure procedure, decreased CORT levels at the time of testing, could contribute to the decreased sensitivity to the discriminative stimulus effects of alcohol (i.e., Experiment 2a). To address this possibility, the CORT synthesis inhibitor metyrapone was tested in discrimination-trained rats. Metyrapone did not alter the discriminative stimulus effects of alcohol (Fig. 3a), as appropriate alcohol discriminative stimulus control was observed. This was confirmed by a two-way RM ANOVA that showed a significant main effect of alcohol dose [F(3,15)=33.83, p<0.001], and no main effect of metyrapone dose or interaction. The highest metyrapone dose (50 mg/kg) induced a significant reduction in response rate as indicated by a significant main effect of metyrapone dose [two-way RM ANOVA F(2,10)=11.96, p=0.002; no main effect of alcohol dose or interaction; Fig. 3b]. Consistent with its known mechanism of action, metyrapone significantly reduced plasma CORT levels [RM ANOVA: F(2,12)=23.64, p<0.001; Fig. 3c]. One rat did not show reduced CORT level following metyrapone (25 mg/kg) injection, indicating a possible missed injection and therefore was not included in the discrimination performance or CORT level analyses. Metyrapone pretreatment resulted in a greater than two-fold reduction in plasma CORT levels, similar to the change between Water- and CORT-exposed rats in Experiment 2a. These results suggest that reduced sensitivity to the interoceptive effects of alcohol following CORT exposure (Experiment 2a) is likely not a direct function of reduced CORT levels.
Fig. 3.
Inhibition of CORT synthesis by metyrapone does not alter the interoceptive effects of alcohol. a Metyrapone pretreatment (25 or 50 mg/kg, SC) did not alter the percentage of alcohol-appropriate responding, but induced b an overall reduction in response rate at the highest (50 mg/kg) dose (n=7). Horizontal dashed line (>80%) represents full expression of the discriminative stimulus effects of alcohol. c Plasma CORT levels were reduced by metyrapone pretreatment. Asterisk signifies significant difference from vehicle group (p<0.05). Values on graphs represent mean ± S.E.M.
Discriminative stimulus effects of self-administered alcohol
Experiment 5a: effects of repeated CORT exposure on the discriminative stimulus effects of self-administered alcohol (in discrimination-trained rats)
Sweetened alcohol reinforcer (10S/10A)
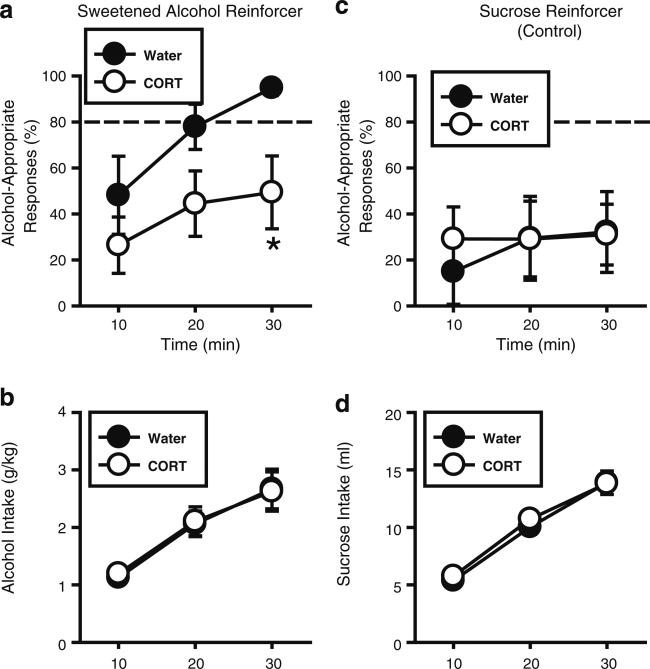
Overall, alcohol-appropriate responses increased across time as more alcohol was consumed during the Discrim/SA session (Fig. 4a), indicating that behavior was under discriminative stimulus control of the consumed alcohol. This was confirmed by a significant main effect of time [F(2,23)=4.73, p=0.02]. However, CORT exposure significantly reduced alcohol-appropriate responding as indicated by a significant main effect of treatment [F(1,12)=4.95, p=0.046], indicating decreased sensitivity to the discriminative stimulus effects of the consumed alcohol. Planned comparisons showed a trend for a reduction at 20 min into the session (p=0.09), and a significant reduction at 30 min (p=0.04) in the CORT relative to the Water group. As shown in Fig. 4b, alcohol intake (g/kg) increased across time [F(2,24)=58.69, p<0.001] and importantly, did not differ between groups. Thus, reductions in discrimination performance (Fig. 4a) were directly related to decreased sensitivity to the discriminative stimulus effects of the consumed alcohol and not to differences in the alcohol dose consumed. Analysis of plasma CORT (ng/ml) showed significant decreases in the CORT group [t=2.31, p=0.04; Water: 98.2±26.7; CORT: 43.9±16.3; blood collected 15 min after the session at approximately 10 am], confirming efficacy of the CORT exposure procedure. Together, these findings further support and extend the initial findings in Experiment 2a to show that the interoceptive effects of both experimenter- and self-administered alcohol are blunted following repeated elevations in circulating CORT levels.
Fig. 4.
Corticosterone exposure (7 days) blunts the interoceptive effects of self-administered alcohol. a In the Water controls (n=6), alcohol-appropriate responding increased across time as greater alcohol was consumed, indicating sensitivity to the discriminative stimulus effects of the consumed sweetened alcohol reinforcer (10% w/v sucrose/10% v/v alcohol). CORT exposure (300 μg/ml; 7 days; n=8) blunted the full expression of the discriminative stimulus effects of the consumed alcohol, and b did not alter alcohol intake (g/kg). c In the Water controls, alcohol-appropriate responding remained low throughout the session when sucrose (10% w/v) was the reinforcer, indicating accurate discrimination performance. CORT exposure (300 μg/ml; 7 days) did not alter this pattern of responding, or d sucrose intake (ml; n=7/group). Horizontal dashed line (>80%) represents full expression of the discriminative stimulus effects of alcohol. Asterisk signifies significant difference from Water group (p<0.05). Values on graphs represent mean ± S.E.M.
Experiment 5b: testing memory/performance impairment and specificity to alcohol interoceptive effects: Sucrose Reinforcer (10S)
CORT exposure did not alter discrimination performance when sucrose (10% w/v) was the reinforcer (Fig. 4c; no significant main effects of CORT exposure or time and no interaction). As expected, alcohol-appropriate responding remained low throughout the session, since no alcohol was present. As shown in Fig. 4d, sucrose intake (ml) increased across time [F(2,12)=255.11, p<0.001] and was unaffected by CORT exposure. Further, significant reductions in plasma CORT levels (ng/ml) were observed in the CORT group [t=3.44, p=0.01; Water: 71.5±17.6; CORT: 8.82±2.1]. These results show that reduced sensitivity to the interoceptive effects of self-administered alcohol (Experiment 5a) was not related to memory/discrimination performance impairments.
Discussion
The current study shows for the first time that repeated CORT exposure results in decreased sensitivity to the interoceptive effects of both experimenter- and self-administered alcohol. Using drug discrimination techniques, we show decreased alcohol-appropriate responding and a failure of any experimenter-administered alcohol dose tested to produce full substitution for the 1 g/kg training dose. A possible explanation for the reduction in alcohol-appropriate responding is that CORT exposure disrupted memory processes. Indeed, glucocorticoid receptors are highly expressed within the hippocampus (Chao et al. 1989; Van Eekelen et al. 1988), a brain region known to regulate aspects of learning and memory processes and to show adaptive changes after alcohol discrimination training (Besheer et al. 2008). Further, chronic glucocorticoid administration or repeated stress has been shown to produce morphological changes in the hippocampus, such as dendritic atrophy (Conrad et al. 1999; Magarinos et al. 1998; McLaughlin et al. 2007). In the discrimination task, memory impairment would result in the inability to distinguish between the alcohol- and water-appropriate levers (i.e., 50% responding on a two-lever task). However, this pattern of responding did not occur during the cumulative alcohol substitution test as responding after low alcohol doses remained on the water-appropriate lever as would be expected. Further, a direct test for memory/ discrimination performance impairment was the CORT group that received water-only during the test (Experiment 2b), and given that responding remained predominantly on the water-appropriate lever throughout the test a memory impairment explanation is less tenable. Another possible explanation for the reduction in alcohol-appropriate responding is that CORT exposure reduced blood alcohol levels. However, this is not a likely explanation as demonstrated by similar blood alcohol levels in the CORT and Water groups in Experiment 3. Lastly, reduced plasma CORT levels as evidenced in the CORT group (Experiment 2a), may have contributed to blunted interoceptive effects in that group and this potential mechanism was evaluated directly by inhibition of CORT synthesis (Experiment 4). That is, while metyrapone pretreatment significantly reduced plasma CORT levels, the interoceptive effects of alcohol were unaltered. This pattern of results argues against a direct relation between reduced CORT level and blunted interoceptive effects of alcohol.
To date preclinical studies have shown mixed results of acute manipulation of the HPA axis on the interoceptive effects of drugs (Filip et al. 2000; Lu et al. 2003; Mantsch and Goeders 1998; Miczek et al. 1999). In relation to alcohol, the discriminative stimulus effects of alcohol are not altered by acute restraint stress (Koros et al. 1999) or acute foot shock stress (Bowen et al. 1999). In contrast, social drinkers exposed to an acute social stressor prior to alcohol consumption report blunted subjective response to alcohol on ratings of “cheerful”, “focused”, and “outgoing” (de Wit et al. 2003) and a subpopulation show blunted subjective stimulant effects of intravenously administered alcohol (Childs et al. 2011). The present findings, showing that acute CORT exposure (1 day) did not alter the interoceptive effects of alcohol, are consistent with the previous acute stress/alcohol discrimination findings. Interestingly, CORT plasma levels were not elevated after the test and did not differ from the Water group. However, if blood was collected closer in time to fluid consumption, increases would most likely have been observed.
Consistent with the inhibition of the interoceptive effects of experimenter-administered alcohol, the interoceptive effects of self-administered alcohol were also blunted after CORT exposure (Experiment 5a). This was evidenced by decreased alcohol-appropriate responding when alcohol (10% v/v) was added to the standard sucrose reinforcer, and extends the findings of Experiment 2a to show that the interoceptive effects of both experimenter-and self-administered alcohol are blunted following repeated elevations in CORT. Importantly, alcohol intake, as directly estimated from response rate, did not differ between the Water and CORT groups. Therefore, the reduction in alcohol-appropriate responding was not due to a direct reduction in the consumed dose of alcohol, which would result in weaker or less detectable interoceptive effects, but rather to decreased sensitivity to the interoceptive effects of the consumed alcohol. An important issue to address is that the rats in these Discrim/SA experiments are trained on the drug discrimination procedure. Accordingly, they respond at high rates for sucrose (~60 responses/min) and are not trained to respond for alcohol (i.e., not alcohol self-administration). Therefore, this procedure assesses the interoceptive effects of consumed alcohol, not alcohol self-administration or alcohol reinforcement processes per se. It will be critical for future work to directly evaluate whether alcohol self-administration is escalated following this CORT exposure procedure using self-administration techniques.
A potential neurobiological mechanism(s) underlying the blunting of the interoceptive effects of alcohol may be related to maladaptive changes in the function of limbic systems known to modulate the discriminative stimulus effects of alcohol. Indeed, repeated elevations in glucocorticoids profoundly impact numerous limbic systems that control emotion and addiction processes (Herman et al. 2005; Piazza and Le Moal 1998). Previous work has shown that the nucleus accumbens and amygdala play prominent roles in modulation of the interoceptive effects of alcohol (Besheer et al. 2003, 2009; Hodge and Aiken 1996; Hodge and Cox 1998). Thus, decreased sensitivity to the interoceptive effects of alcohol following repeated CORT exposure may be related, in part, to maladaptive changes (i.e., neurochemical, transcription, and signaling) within these limbic brain regions (Buffalari and Grace 2009; Makino et al. 1994; Morales-Medina et al. 2009; Otero Losada 1988; Perrotti et al. 2004; Wang et al. 2010). Therefore, it will be of interest for future work to determine the functional neurobiological mechanism underlying the related decreased sensitivity to the interoceptive effects of alcohol.
The altered pattern of circulating CORT levels and the blunted suppression of plasma CORT levels after dexamethasone administration following repeated CORT exposure are suggestive of maladaptive changes in the HPA axis (Harris et al. 2004; Mantsch et al. 2007; Marti and Armario 1997; Mizoguchi et al. 2008; Pung et al. 2003; Romeo et al. 2006). Repeated CORT exposure resulted in significantly decreased daytime basal CORT levels (10 am) as compared to Water controls (confirmed in all experiments). This suggests that repeated CORT exposure produced a compensatory downregulation in circulating CORT levels via negative feedback mechanisms, a likely consequence of the heightened nighttime elevations in CORT levels (Fig. 1a; 10 pm). Accordingly, under these conditions the HPA axis may be differentially responsive to challenge of negative feedback systems. Indeed, repeated CORT exposure was associated with blunted response to dexamethasone challenge. Together, altered basal CORT levels and blunted response to dexamethasone suggest adaptive change in the HPA axis system, and the possibility of compromised or dysregulated function. It will be important for future work to further evaluate HPA axis function under these CORT exposure conditions.
The present findings are highly relevant because they present a possible behavioral mechanism for escalated alcohol drinking during episodes of heightened elevations in glucocorticoid levels as is common in drug and alcohol addiction, as well as mental health disorders, such as depression and posttraumatic stress disorder (PTSD). That is, during these episodes, individuals may consume more alcohol to achieve the desired interoceptive effects. Consideration of the present findings within the context of altered HPA axis function could also have significant implications given that compromised HPA axis function is a feature common to populations at risk for increased alcohol drinking (Hasin et al. 2005; Jacobsen et al. 2001; Schneier et al. 2010; Schuckit 2009; Schuckit and Smith 2000; Trim et al. 2009), such as individuals with alcohol use disorders or a family history of an alcohol use disorder, some forms of depression, PTSD, and other mood and anxiety disorders (Brown et al. 2004; Crum and Pratt 2001; Dai et al. 2002; Davidson et al. 1990; Gold and Chrousos 2002; Hernandez-Avila et al. 2002; Kodl et al. 2008; Sapolsky 2000; Uhart et al. 2006; Waltman et al. 1994). The findings of this work and future mechanistic work examining the interaction between heightened elevations in glucocorticoids and decreased subjective effects of alcohol can lead to better understanding of factors that promote alcohol drinking during periods of stress and in at risk populations.
Acknowledgments
The authors would like to thank Dr. Cynthia Kuhn for discussions on corticosterone data interpretation, and Drs. Sarah Holstein and Sara Faccidomo, and Kelly Psilos for help with blood collection and/or blood analyses. This work was supported in part by funds from the National Institutes of Health AA016009 (JB), AA019682 (JB), AA014983 (CWH), ABMRF/The Foundation for Alcohol Research (JB), and by the Bowles Center for Alcohol Studies.
Contributor Information
Joyce Besheer, Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, Thurston-Bowles Building, CB#7178, Chapel Hill, NC 27599, USA; Curriculum in Neurobiology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
Kristen R. Fisher, Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, Thurston-Bowles Building, CB#7178, Chapel Hill, NC 27599, USA
Julie J. M. Grondin, Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, Thurston-Bowles Building, CB#7178, Chapel Hill, NC 27599, USA
Reginald Cannady, Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, Thurston-Bowles Building, CB#7178, Chapel Hill, NC 27599, USA; Curriculum in Neurobiology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
Clyde W. Hodge, Bowles Center for Alcohol Studies, University of North Carolina at Chapel Hill, Thurston-Bowles Building, CB#7178, Chapel Hill, NC 27599, USA Curriculum in Neurobiology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; Department of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA; Department of Pharmacology, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599, USA.
References
- Adinoff B, Martin PR, Bone GH, Eckardt MJ, Roehrich L, George DT, Moss HB, Eskay R, Linnoila M, Gold PW. Hypothalamic–pituitary–adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Arch Gen Psychiatry. 1990;47:325–330. doi: 10.1001/archpsyc.1990.01810160025004. [DOI] [PubMed] [Google Scholar]
- Berman JD, Cook DM, Buchman M, Keith LD. Diminished adrenocorticotropin response to insulin-induced hypoglycemia in nondepressed, actively drinking male alcoholics. J Clin Endocrinol Metab. 1990;71:712–717. doi: 10.1210/jcem-71-3-712. [DOI] [PubMed] [Google Scholar]
- Besheer J, Hodge CW. Pharmacological and anatomical evidence for an interaction between mGluR5- and GABA(A) alpha1-containing receptors in the discriminative stimulus effects of ethanol. Neuropsychopharmacology. 2005;30:747–757. doi: 10.1038/sj.npp.1300616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Cox AA, Hodge CW. Coregulation of ethanol discrimination by the nucleus accumbens and amygdala. Alcohol Clin Exp Res. 2003;27:450–456. doi: 10.1097/01.ALC.0000057036.64169.C1. [DOI] [PubMed] [Google Scholar]
- Besheer J, Stevenson RA, Hodge CW. mGlu5 receptors are involved in the discriminative stimulus effects of self-administered ethanol in rats. Eur J Pharmacol. 2006;551:71–75. doi: 10.1016/j.ejphar.2006.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Schroeder JP, Stevenson RA, Hodge CW. Ethanol-induced alterations of c-Fos immunoreactivity in specific limbic brain regions following ethanol discrimination training. Brain Res. 2008;1232:124–131. doi: 10.1016/j.brainres.2008.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Grondin JJ, Salling MC, Spanos M, Stevenson RA, Hodge CW. Interoceptive effects of alcohol require mGlu5 receptor activity in the nucleus accumbens. J Neurosci. 2009;29:9582–9591. doi: 10.1523/JNEUROSCI.2366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen CA, Purdy RH, Grant KA. An investigation of endogenous neuroactive steroid-induced modulation of ethanol's discriminative stimulus effects. Behav Pharmacol. 1999;10:297–311. doi: 10.1097/00008877-199905000-00006. [DOI] [PubMed] [Google Scholar]
- Brown ES, Varghese FP, McEwen BS. Association of depression with medical illness: does cortisol play a role? Biol Psychiatry. 2004;55:1–9. doi: 10.1016/s0006-3223(03)00473-6. [DOI] [PubMed] [Google Scholar]
- Buffalari DM, Grace AA. Chronic cold stress increases excitatory effects of norepinephrine on spontaneous and evoked activity of basolateral amygdala neurons. Int J Neuropsychopharmacol. 2009;12:95–107. doi: 10.1017/S1461145708009140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buwalda B, de Boer SF, Schmidt ED, Felszeghy K, Nyakas C, Sgoifo A, Van der Vegt BJ, Tilders FJ, Bohus B, Koolhaas JM. Long-lasting deficient dexamethasone suppression of hypothalamic–pituitary–adrenocortical activation following peripheral CRF challenge in socially defeated rats. J Neuroendocrinol. 1999;11:513–520. doi: 10.1046/j.1365-2826.1999.00350.x. [DOI] [PubMed] [Google Scholar]
- Cannady R, Grondin JJ, Fisher KR, Hodge CW, Besheer J. Activation of group I metabotropic glutamate receptors inhibits the discriminative stimulus effects of alcohol via selective activity within the amygdala. Neuropsychopharmacology. 2011 doi: 10.1038/npp.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HM, Choo PH, McEwen BS. Glucocorticoid and mineralocorticoid receptor mRNA expression in rat brain. Neuroendocrinology. 1989;50:365–371. doi: 10.1159/000125250. [DOI] [PubMed] [Google Scholar]
- Childs E, O'Connor S, de Wit H. Bidirectional interactions between acute psychosocial stress and acute intravenous alcohol in healthy men. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–913. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- Conrad CD, McLaughlin KJ, Harman JS, Foltz C, Wieczorek L, Lightner E, Wright RL. Chronic glucocorticoids increase hippocampal vulnerability to neurotoxicity under conditions that produce CA3 dendritic retraction but fail to impair spatial recognition memory. J Neurosci. 2007;27:8278–8285. doi: 10.1523/JNEUROSCI.2121-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Bono G, Martignoni E, Merlo P, Sances G, Nappi G. An assessment of hypothalamo–pituitary–adrenal axis functioning in non-depressed, early abstinent alcoholics. Psychoneuroendocrinology. 1996;21:263–275. doi: 10.1016/0306-4530(96)00001-7. [DOI] [PubMed] [Google Scholar]
- Crum RM, Pratt LA. Risk of heavy drinking and alcohol use disorders in social phobia: a prospective analysis. Am J Psychiatry. 2001;158:1693–1700. doi: 10.1176/appi.ajp.158.10.1693. [DOI] [PubMed] [Google Scholar]
- Dai X, Thavundayil J, Gianoulakis C. Response of the hypothalamic–pituitary–adrenal axis to stress in the absence and presence of ethanol in subjects at high and low risk of alcoholism. Neuropsychopharmacology. 2002;27:442–452. doi: 10.1016/S0893-133X(02)00308-1. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Kudler HS, Saunders WB, Smith RD. Symptom and comorbidity patterns in World War II and Vietnam veterans with posttraumatic stress disorder. Compr Psychiatry. 1990;31:162–170. doi: 10.1016/0010-440x(90)90020-s. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- de Wit H, Soderpalm AH, Nikolayev L, Young E. Effects of acute social stress on alcohol consumption in healthy subjects. Alcohol Clin Exp Res. 2003;27:1270–1277. doi: 10.1097/01.ALC.0000081617.37539.D6. [DOI] [PubMed] [Google Scholar]
- Errico AL, Parsons OA, King AC, Lovallo WR. Attenuated cortisol response to biobehavioral stressors in sober alcoholics. J Stud Alcohol. 1993;54:393–398. doi: 10.15288/jsa.1993.54.393. [DOI] [PubMed] [Google Scholar]
- Filip M, Nowak E, Siwanowicz J, Przegalinski E. Effects of corticosterone and its synthesis blockade on the cocaine-induced discriminative stimulus effects in rats. Pol J Pharmacol. 2000;52:411–421. [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs. low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Taylor JR. Recapitulation and reversal of a persistent depression-like syndrome in rodents. Curr Protoc Neurosci. 2009 doi: 10.1002/0471142301.ns0932s49. Chapter 9: Unit 9 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesbach GS, Hovda DA, Tio DL, Taylor AN. Heightening of the stress response during the first weeks after a mild traumatic brain injury. Neuroscience. 2011;178:147–158. doi: 10.1016/j.neuroscience.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RB, Gu H, Mitchell TD, Endale L, Russo M, Ryan DH. Increased glucocorticoid response to a novel stress in rats that have been restrained. Physiol Behav. 2004;81:557–568. doi: 10.1016/j.physbeh.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Goodwin RD, Stinson FS, Grant BF. Epidemiology of major depressive disorder: results from the National Epidemiologic Survey on Alcoholism and Related Conditions. Arch Gen Psychiatry. 2005;62:1097–1106. doi: 10.1001/archpsyc.62.10.1097. [DOI] [PubMed] [Google Scholar]
- Hatzinger M, Reul JM, Landgraf R, Holsboer F, Neumann I. Combined dexamethasone/CRH test in rats: hypothalamo–pituitary–adrenocortical system alterations in aging. Neuroendocrinology. 1996;64:349–356. doi: 10.1159/000127138. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo–pituitary–adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Oncken C, Van Kirk J, Wand G, Kranzler HR. Adrenocorticotropin and cortisol responses to a naloxone challenge and risk of alcoholism. Biol Psychiatry. 2002;51:652–658. doi: 10.1016/s0006-3223(01)01334-8. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Aiken AS. Discriminative stimulus function of ethanol: role of GABAA receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1996;20:1221–1228. doi: 10.1111/j.1530-0277.1996.tb01116.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Cox AA. The discriminative stimulus effects of ethanol are mediated by NMDA and GABA(A) receptors in specific limbic brain regions. Psychopharmacology (Berl) 1998;139:95–107. doi: 10.1007/s002130050694. [DOI] [PubMed] [Google Scholar]
- Hoffmann JP, Su SS. Stressful life events and adolescent substance use and depression: conditional and gender differentiated effects. Subst Use Misuse. 1998;33:2219–2262. doi: 10.3109/10826089809056256. [DOI] [PubMed] [Google Scholar]
- Ising M, Kunzel HE, Binder EB, Nickel T, Modell S, Holsboer F. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1085–1093. doi: 10.1016/j.pnpbp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. Am J Psychiatry. 2001;158:1184–1190. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat SM, Bowles NP, Weil ZM, Pfaff DW, McEwen BS. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in, ouse. Endocrinology. 2010;151:2117–2127. doi: 10.1210/en.2009-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Munisamy G, de Wit H, Lin S. Attenuated cortisol response to alcohol in heavy social drinkers. Int J Psychophysiol. 2006;59:203–209. doi: 10.1016/j.ijpsycho.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Kodl MM, Fu SS, Willenbring ML, Gravely A, Nelson DB, Joseph AM. The impact of depressive symptoms on alcohol and cigarette consumption following treatment for alcohol and nicotine dependence. Alcohol Clin Exp Res. 2008;32:92–99. doi: 10.1111/j.1530-0277.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Stress, corticotropin-releasing factor, and drug addiction. Ann N Y Acad Sci. 1999;897:27–45. doi: 10.1111/j.1749-6632.1999.tb07876.x. [DOI] [PubMed] [Google Scholar]
- Koros E, Kostowski W, Danysz W, Bienkowski P. Ethanol discrimination in the rat: lack of modulation by restraint stress and memantine. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:117–122. doi: 10.1007/pl00005330. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ. Blunted stress cortisol response in abstinent alcoholic and polysubstance-abusing men. Alcohol Clin Exp Res. 2000;24:651–658. [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci Biobehav Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Orchinik M, McEwen BS. Morphological changes in the hippocampal CA3 region induced by noninvasive glucocorticoid administration: a paradox. Brain Res. 1998;809:314–318. doi: 10.1016/s0006-8993(98)00882-8. [DOI] [PubMed] [Google Scholar]
- Majumdar SK, Shaw GK, Bridges PK. The dexamethasone suppression test in chronic alcoholics with and without depression and its relationship to their hepatic status. Drug Alcohol Depend. 1988;21:231–235. doi: 10.1016/0376-8716(88)90074-9. [DOI] [PubMed] [Google Scholar]
- Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Goeders NE. Generalization of a restraint-induced discriminative stimulus to cocaine in rats. Psychopharmacology (Berl) 1998;135:423–426. doi: 10.1007/s002130050531. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Cullinan WE, Tang LC, Baker DA, Katz ES, Hoks MA, Ziegler DR. Daily cocaine self-administration under long-access conditions augments restraint-induced increases in plasma corticosterone and impairs glucocorticoid receptor-mediated negative feedback in rats. Brain Res. 2007;1167:101–111. doi: 10.1016/j.brainres.2007.05.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti O, Armario A. Influence of regularity of exposure to chronic stress on the pattern of habituation of pituitary–adrenal hormones, prolactin and glucose. Stress. 1997;1:179–189. doi: 10.3109/10253899709001107. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Mutschler NH, van Erp AM, Blank AD, McInerney SC. d-amphetamine “cue” generalizes to social defeat stress: behavioral sensitization and attenuated accumbens dopamine. Psychopharmacology (Berl) 1999;147:190–199. doi: 10.1007/s002130051160. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Shoji H, Ikeda R, Tanaka Y, Tabira T. Persistent depressive state after chronic stress in rats is accompanied by HPA axis dysregulation and reduced prefrontal dopaminergic neurotransmission. Pharmacol Biochem Behav. 2008;91:170–175. doi: 10.1016/j.pbb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Morales-Medina JC, Sanchez F, Flores G, Dumont Y, Quirion R. Morphological reorganization after repeated corticosterone administration in the hippocampus, nucleus accumbens and amygdala in the rat. J Chem Neuroanat. 2009 doi: 10.1016/j.jchemneu.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Otero Losada ME. Changes in central GABAergic function following acute and repeated stress. Br J Pharmacol. 1988;93:483–490. doi: 10.1111/j.1476-5381.1988.tb10302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–10602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Pollock VE. Meta-analysis of subjective sensitivity to alcohol in sons of alcoholics. Am J Psychiatry. 1992;149:1534–1538. doi: 10.1176/ajp.149.11.1534. [DOI] [PubMed] [Google Scholar]
- Pung T, Zimmerman K, Klein B, Ehrich M. Corticosterone in drinking water: altered kinetics of a single oral dose of corticosterone and concentrations of plasma sodium, albumin, globulin, and total protein. Toxicol Ind Health. 2003;19:171–182. doi: 10.1191/0748233703th182oa. [DOI] [PubMed] [Google Scholar]
- Raone A, Cassanelli A, Scheggi S, Rauggi R, Danielli B, De Montis MG. Hypothalamus–pituitary–adrenal modifications consequent to chronic stress exposure in an experimental model of depression in rats. Neuroscience. 2007;146:1734–1742. doi: 10.1016/j.neuroscience.2007.03.027. [DOI] [PubMed] [Google Scholar]
- Romeo RD, Bellani R, Karatsoreos IN, Chhua N, Vernov M, Conrad CD, McEwen BS. Stress history and pubertal development interact to shape hypothalamic–pituitary–adrenal axis plasticity. Endocrinology. 2006;147:1664–1674. doi: 10.1210/en.2005-1432. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000;57:925–935. doi: 10.1001/archpsyc.57.10.925. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Foose TE, Hasin DS, Heimberg RG, Liu SM, Grant BF, Blanco C. Social anxiety disorder and alcohol use disorder co-morbidity in the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med. 2010;40:977–988. doi: 10.1017/S0033291709991231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. An overview of genetic influences in alcoholism. J Subst Abuse Treat. 2009;36:S5–S14. [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. J Stud Alcohol. 2000;61:827–835. doi: 10.15288/jsa.2000.61.827. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Henniger MS. Stress and alcohol drinking. Ann Med. 2004;36:596–605. doi: 10.1080/07853890410018862. [DOI] [PubMed] [Google Scholar]
- Stolerman I. Drugs of abuse: behavioural principles, methods and terms. Trends Pharmacol Sci. 1992;13:170–176. doi: 10.1016/0165-6147(92)90059-f. [DOI] [PubMed] [Google Scholar]
- Trim RS, Schuckit MA, Smith TL. The relationships of the level of response to alcohol and additional characteristics to alcohol use disorders across adulthood: a discrete-time survival analysis. Alcohol Clin Exp Res. 2009;33:1562–1570. doi: 10.1111/j.1530-0277.2009.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhart M, Oswald L, McCaul ME, Chong R, Wand GS. Hormonal responses to psychological stress and family history of alcoholism. Neuropsychopharmacology. 2006;31:2255–2263. doi: 10.1038/sj.npp.1301063. [DOI] [PubMed] [Google Scholar]
- Van Eekelen JA, Jiang W, De Kloet ER, Bohn MC. Distribution of the mineralocorticoid and the glucocorticoid receptor mRNAs in the rat hippocampus. J Neurosci Res. 1988;21:88–94. doi: 10.1002/jnr.490210113. [DOI] [PubMed] [Google Scholar]
- Waltman C, McCaul ME, Wand GS. Adrenocorticotropin responses following administration of ethanol and ovine corticotropin-releasing hormone in the sons of alcoholics and control subjects. Alcohol Clin Exp Res. 1994;18:826–830. doi: 10.1111/j.1530-0277.1994.tb00046.x. [DOI] [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the hypothalamic–pituitary–adrenal axis in actively drinking alcoholics. J Clin Endocrinol Metab. 1991;72:1290–1295. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Wang W, Sun D, Pan B, Roberts CJ, Sun X, Hillard CJ, Liu QS. Deficiency in endocannabinoid signaling in the nucleus accumbens induced by chronic unpredictable stress. Neuropsychopharmacology. 2010 doi: 10.1038/npp.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]