Abstract
OBJECTIVE
Hydrogen-1 MR spectroscopy (1H-MRS) is gaining acceptance as a noninvasive technique for assessment of hepatic steatosis, and the findings have been found to correlate closely with histopathologic grade. The aims of this study were to validate 1H-MRS performed with a 3-T MRI system for quantifying hepatic steatosis and to determine threshold values of 1H-MRS proton density fat fraction corresponding to standard histopathologic grade in patients undergoing diagnostic liver biopsy.
SUBJECTS AND METHODS
We conducted a prospective cross-sectional liver MRS study with 52 subjects undergoing diagnostic liver biopsy. The diagnostic accuracy of 1HMRS was evaluated with receiver operating characteristic curves.
RESULTS
The diagnostic accuracy of 1H-MRS for hepatic steatosis was high with an area under the receiver operating characteristic curve of 0.94 (95% CI, 0.88–1.0). Results were similar for three 1H-MRS measurements obtained at different locations in the liver, for two independent pathologists, and whether fibrosis was present or absent. One third of participants had elevated transaminase concentrations of unknown cause, and 1H-MRS estimates of steatosis had perfect agreement with histopathologic grade in this group. Calculated 1H-MRS proton density fat fraction thresholds for histologic grades were less than 17% for grade 0 or trace steatosis, 17–38.6% for grade 1, and greater than 38.6% for grade 2 or higher.
CONCLUSION
Hydrogen-1 MR spectroscopy is an effective, noninvasive technique that can be used to diagnose and quantify hepatic steatosis. Hydrogen-1 MR spectroscopy thresholds corresponded with histopathologic grades and may be useful in the workup of patients with elevated transaminase concentrations.
Keywords: 3-T MR spectroscopy, hepatic steatosis, histopathologic grading
Hepatic steatosis is recognized as an important contributing factor to liver disease in the context of viral hepatitis [1] and with the rise of obesity, diabetes, and metabolic syndrome is seen increasingly in the general population [2]. Liver biopsy with histopathologic grading remains the reference standard for assessment of hepatic steatosis, but noninvasive hydrogen-1 MR spectroscopy (1H-MRS) is gaining acceptance as a technique for evaluation of hepatic steatosis. Szczepaniak et al. [3] performed hepatic 1H-MRS in the evaluation of a large cohort of participants in the Dallas Heart Study and found with good reproducibility that 33.6% of the participants had steatosis (defined as > 5% hepatic triglyceride content). The reliability of 1H-MRS for quantifying hepatic fat noninvasively may prove useful in the care of patients for whom liver biopsy is contraindicated.
A number of studies [4–9] in which 1H-MRS findings were directly compared with histopathologic grades of steatosis in patients with and without disease associated with fatty liver showed good agreement between the two methods. In addition, compared with ultrasound and CT, 1H-MRS was found to have superior sensitivity and specificity for the diagnosis of hepatic steatosis [7, 9]. Several earlier investigations also validated 1H-MRS with chemical analysis of liver biopsy tissue and detailed morphometric assessments of biopsy samples in a limited number of subjects [10, 11].
Although liver biopsy remains the reference standard, 1H-MRS is increasingly recognized as a noninvasive alternative for the evaluation of hepatic steatosis and in some instances may obviate liver biopsy. Although the utility of 1H-MRS continues to improve, the clinical correlation between 1H-MRS proton density fat fraction and standard histopathologic grade remains unclear. Furthermore, a uniform approach to 1H-MRS methods has yet to be established. In this prospective cross-sectional study we further validate 1H-MRS for quantitative analysis of hepatic steatosis, introduce 1H-MRS proton density fat fraction thresholds for correlation with standard histopathologic grading, and outline 1H-MRS methods for potential clinical use in a broad spectrum of patients undergoing diagnostic liver biopsy.
Subjects and Methods
Patients
Fifty-two patients undergoing percutaneous diagnostic liver biopsy at the National Institutes of Health Clinical Center were enrolled in this study. Participants were required to be 18 years old or older without known or recent pregnancy, have no contraindication to MRI, and be able to complete 1H-MRS of the liver within 30 days of the liver biopsy. The median time between liver biopsy and MRI was 15 days. No subject was known to be actively abusing alcohol. Subjects were not included if the liver biopsy was being performed to evaluate a specific lesion or if there had been relevant medication changes between biopsy and 1H-MRS.
Subjects were recruited through the National Institutes of Health Intramural HIV Program and the National Institute of Diabetes and Digestive and Kidney Diseases Intramural Hepatology Clinic. The study was approved by the National Institute of Allergy and Infectious Diseases institutional review board, and written informed consent was obtained from each patient. The study was registered at clinicaltrials.gov with the identification number NCT00594412.
Methods
Histopathologic assessment
A pathologist scored histopathologic grade of steatosis 0–3, a score of 1 or greater representing clinically relevant steatosis [12, 13]. Hepatic steatosis was graded on the steatosis scale of the Nonalcoholic Steatohepatitis Clinical Research Network [12] with the caveat that a distinction was made between biopsies that showed no steatosis at all (score of 0) and those that showed less than 5% steatosis (trace). Estimates of steatosis were based on the proportion of hepatocytes containing fat vacuoles visible at medium (×10) magnification. Biopsies reported to show trace amounts of steatosis were considered grade 0 for analyses. Forty-four of the 52 biopsies were scored for steatosis by a second independent pathologist. Percentage agreement between pathologists was 86% in the binary classification of no steatosis versus steatosis grade 1 or greater (κ = 0.69, p < 0.001). Biopsies were also scored for degree of fibrosis (scale, 0–4), and any biopsy scored grade 1 or higher was considered positive for fibrosis [14]. Results of 1H-MRS were not available to the pathologists at the time of scoring.
Hydrogen-1 MR spectroscopic data acquisition and phantom validation
A 3-T MRI system (Achieva, Philips Healthcare) was used. Patient studies were performed with a medium-size flex surface coil for both spectroscopy and imaging. Phantom studies were performed with a standard multichannel head coil. Phantom experiments entailed a single measurement of each serial dilution of 10%, 15%, 17.5%, and 20% IV fat emulsion (Intralipid, Kabi- Vitrum) in 50-mL sample tubes suspended in water at room temperature. Phantom data were collected by imaging-based relaxometry and single-voxel spectroscopy with protocols set to mimic subsequent clinical studies.
For accurate spectroscopic quantification, both T1 and T2 relaxation data were acquired to correct spectroscopic raw data. T1 maps were acquired with a fat-suppressed dual flip-angle spoiled gradient- recalled echo technique [15, 16]. Values were reviewed to ensure that T1 was less than TR. T2 relaxation measurements were performed with a fat-suppressed respiratory triggered multiecho Carr-Purcell-Meiboom-Gill technique at 30, 40, 50, and 70 ms TE at a TR of 3 seconds. Four echoes were used to achieve a good signalto- noise ratio without substantially increasing acquisition time. Data were corrected for T2 relaxation with a group average of the tissue water and a reference value for T2 of fat. Spectroscopy data were acquired with a breath-hold point-resolved spectroscopy (PRESS)-based single-voxel technique; TR/TE, 2000/50; imaging time, 12 seconds; number of phase cycles, 4; spectral resolution, 1.95 Hz; no water suppression.
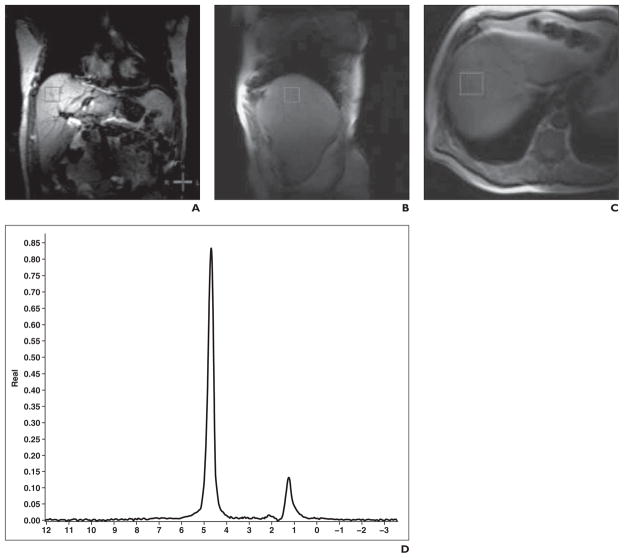
For patient studies, voxel localization was standardized to minimize signal contamination from adjacent structures and to avoid large intrahepatic vessels. Localization voxels (30 × 30 × 30 mm) were placed in three standardized locations—dome of right hepatic lobe, left lobe, and right inferior lobe— by trained radiology technicians and reviewed by a single radiologist for image quality and accuracy of voxel placement. The measurement of the dome of right hepatic lobe was selected as the primary outcome measure because this location most often allowed excellent intrahepatic voxel placement and yielded high-quality spectra (Fig. 1).
Fig. 1.
63-year-old man with hepatitis C. A–C, Hydrogen-1 MR spectroscopic image shows representative spectra of hepatic triacylglycerol. D, Representative spectra of hepatic triacylglycerol.
Hydrogen-1 MR spectroscopic analysis
Postprocessing steps were performed on each patient’s acquired spectroscopic data with a vendor postprocessing package requiring approximately 10 minutes per patient. Manual phase adjustment was used, as was spectral shift of the display before integration. Lipid and water peak integral areas were obtained at set frequency limits and then corrected for relaxation. Finally, each spectral acquisition was assessed by a single investigator for quality of voxel placement, ability to verify voxel location in all three anatomic planes, and quality of spectra (i.e., signal-to-noise ratio). Images of poor quality based on any of these three parameters were excluded. One subject was excluded from the study because of voxel placement, and one was excluded because MRI data were lost owing to a technical backup failure. These two subjects were not among the 52 whose data were analyzed. Proton density fat fraction was calculated from integral peak areas with a standardized formula from Longo et al. [17] for determining lipid volume fraction (Ff) in which FTSA is detectable fat-to-total signal peak area: Ff = FTSA/(1.138 – 0.339 FTSA).
Statistical Methods
For the primary analysis, the diagnosis of liver steatosis was defined as histopathologic grade 1 or greater. The histopathologic grades provided by the primary pathologist were used, although additional analyses were used to evaluate the performance of 1H-MRS with respect to a second pathologist. Further analyses were performed to evaluate accuracy relative to the indication for liver biopsy (e.g., hepatitis C infection, elevated transaminase concentration of unknown cause), effect of fibrosis, and region of interest (ROI) (voxel location in dome, inferior right lobe, versus left lobe).
Diagnostic accuracy was evaluated with receiver operating characteristic (ROC) curves. The area under the ROC curve (AUC) and corresponding 95% CI were estimated with a nonparametric estimator [18]. An AUC of 1.0 indicated perfect accuracy. In addition, the differences in 1H-MRS proton density fat fraction between subjects with steatosis (grade ≥1) and those with no steatosis were compared by Wilcoxon rank-sum test.
An additional analysis was performed to assess ability to distinguish steatosis according to histopathologic grade using 1H-MRS. Because of the limited sample size, grades were grouped as follows: grade 0 (grade 0 and trace steatosis noted at histologic examination), grade 1, and grade 2 or 3. Optimal 1H-MRS thresholds were selected to maximize the correct classification of grade 0 and high-grade steatosis. Analyses were conducted with Stata software (version 9.0, StataCorp).
Results
Phantom Validation Study
The measured proton density fat fraction values were 12.1%, 15.0%, 19.2%, and 21% (for the corresponding known concentrations of 10%, 15%, 17.5%, and 20%). These values were within 5% of the expected values and generated a regression coefficient (r2 = 0.95, p = 0.02). After T2 relaxation coefficients were applied, the measured values were 9.65%, 12.02%, 15.56%, and 17.08%. These values were within 5% of the expected value at the low end of the range and within 15% at the high end. Because of the long acquisition TR and the short T1 of tissues for the patient studies, T1 correction was not necessary for clinical data. The measured T2 of the patient data was within the expected normal range, so subsequent T2 correction was based on a group average value (mean, 63.34 ± 4.57 [SD] ms).
Clinical Hydrogen-1 MR Spectroscopic Studies
Fifty-two patients underwent liver biopsy and 1H-MRS within 30 days of biopsy (Table 1). One half of the subjects who participated in the study had HIV infection. The most frequent indications for liver biopsy were hepatitis C virus (HCV) infection (48%) and transaminase elevations of unclear cause not associated with viral hepatitis (33%). Other indications were hemochromatosis, hepatitis B, and autoimmune hepatitis; only one patient had a diagnosis of nonalcoholic steatohepatitis before biopsy. Thirty of the 52 patients (58%) had a biopsy finding of grade 0 steatosis; 29%, grade 1; 8%, grade 2; and 6%, grade 3 steatosis. Sixty-four percent of the biopsies showed grade 1 or higher fibrosis. Three participants had biopsy evidence of cirrhosis, and 12 patients had bridging fibrosis at biopsy.
TABLE 1.
Demographic and Clinical Characteristics
| Characteristic | Value |
|---|---|
|
| |
| No. of patients | 52 |
| Age (y) | 50.6 ± 11.1 |
| Body mass index | 27.4 ± 4.1 |
| Sex (%) | |
| Men | 73 |
| Women | 27 |
| Race (%) | |
| African American | 21 |
| American Indian, Alaskan native | 2 |
| Asian | 9 |
| White | 60 |
| Other | 8 |
| Ethnicity (%) | |
| Hispanic | 15 |
| Non-Hispanic | 81 |
| Unknown | 4 |
| Liver biopsy indication (no. of patients) | |
| Hepatitis Ca | 25 |
| Elevated transaminase concentrationsb | 17 |
| Hemochromatosis | 3 |
| Hepatitis B | 5 |
| Nonalcoholic steatohepatitis | 1 |
| Autoimmune hepatitis | 1 |
| Time between biopsy and MRI (d) | 15 ± 9 |
| Aspartate aminotransferase concentration (U/L) | 65.4 ± 51.0 |
| Alanine aminotransferase concentration (U/L) | 90.0 ± 75.0 |
| Alkaline phosphatase concentration (U/L) | 95.9 ± 59.1 |
| Bilirubin concentration (mg/dL) | |
| Total | 0.8 ± 0.4 |
| Direct | 0.17 ± 0.09 |
Note—Values are expressed as number of patients or mean ± SD.
Twelve patients had hepatitis C and HIV coinfection.
Fourteen patients had elevated transaminase concentrations and HIV-positive status.
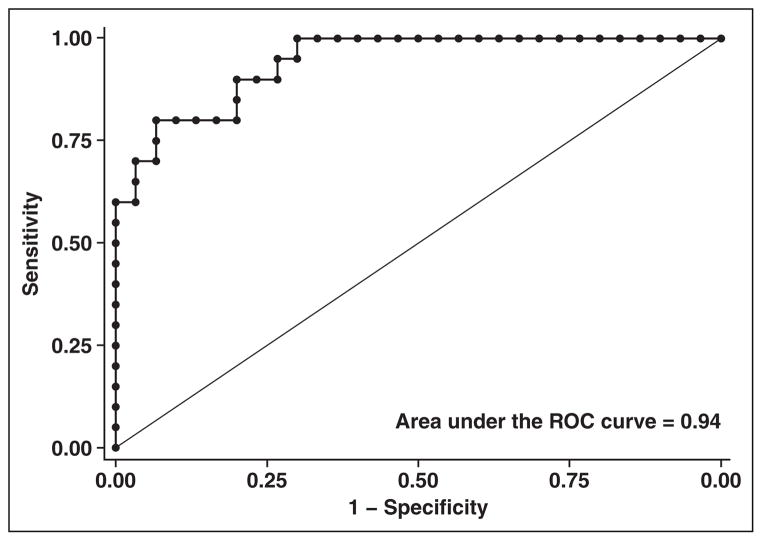
The diagnostic accuracy of 1H-MRS was very good with an AUC of 0.94 (95% CI, 0.88–1.0) (Fig. 2). The average 1H-MRS proton density fat fraction among patients with a steatosis grade less than 1 was 9.4%; for those with grade 1 or higher steatosis, the average was 31.8% (p < 0.00001). For evaluation of the performance of 1H-MRS with respect to findings by a second independent pathologist, the diagnostic accuracy was similarly high (AUC, 0.95; 95% CI, 0.89–1.0).
Fig. 2.
Receiver operating characteristic curve shows performance of hydrogen-1 MR spectroscopy in diagnosis of hepatic steatosis.
Indication for Liver Biopsy and Accuracy of Hydrogen-1 MR Spectroscopy
The diagnostic accuracy of 1H-MRS for steatosis was evaluated according to indication for liver biopsy, in particular for patients with HCV infection and those with transaminase elevations of unclear cause. Among the participants with HCV infection the AUC was 0.85 (95% CI, 0.69–1.0). Among participants with elevated transaminase levels of unclear cause, eight had normal and eight had abnormal pathologic findings, and 1H-MRS measurement of steatosis had perfect discrimination. The sample size was not sufficiently large to statistically compare accuracy between groups.
Fibrosis and Accuracy of Hydrogen-1 MR Spectroscopy
Although there was limited power to test differences in diagnostic accuracy according to fibrosis grade, AUCs were estimated separately for patients with no fibrosis and those with fibrosis grade 1 or greater at liver biopsy. The AUC was 0.96 (95% CI, 0.88–1.0) for subjects with no fibrosis and 0.92 (95% CI, 0.82–1.0) for those with fibrosis grades 1–4.
Voxel Location in the Liver
The ROI placed in the right dome of the liver was used for the primary outcome in this study. The ROIs placed in the inferior right lobe and the left lobe had AUC estimates similar to the estimate from the dome of the liver. Specifically, the inferior right lobe had an AUC of 0.84 (95% CI, 0.73–0.96), and the left lobe ROI an AUC of 0.92 (95% CI, 0.83 – 1.0). Neither of these AUCs was statistically significantly different from that obtained for ROIs in the dome of the liver.
Thresholds of Hydrogen-1 MR Spectroscopy for Classification of Steatosis Grade
The optimal thresholds for classifying subjects as having grade 0, 1, or 2 or higher steatosis with 1H-MRS were calculated by selection of thresholds that minimized the overall misclassification rate. These results corresponded to a 1H-MRS proton density fat fraction threshold of less than 17.0% for grade 0 steatosis, 17.0–38.6% for grade 1 steatosis, and greater than 38.6% for grade 2 or higher (Table 2). The overall cross-validated misclassification rate was 26% (13/50; 95% CI, 14.6–40.3%).
TABLE 2.
Hydrogen-1 MR Spectroscopic Proton Density Fat Fraction Thresholds and Standard Histopathologic Steatosis Grade
| No. of Patients | Histopathologic Grade | Percentage of Hepatocytes with Steatosisa | Percentage Triacylglycerol Detected With 1H-MRS |
|---|---|---|---|
|
| |||
| 30 | 0 or trace | < 5 | < 17 |
| 15 | 1 | 5–33 | 17–38.6 |
| 7 | 2 or 3 | > 33 | > 38.6 |
Note—1H-MRS = hydrogen-1 MR spectroscopy.
Based on percentage of hepatocytes with steatosis [12].
Discussion
Our study showed that 1H-MRS is an effective, noninvasive technique that can be used for the quantitative analysis of hepatic steatosis. In agreement with recent findings in the literature [4, 5, 7, 9], we found that 3-T 1H-MRS performed well in the diagnosis of hepatic steatosis relative to histopathologic grading in a group of patients undergoing liver biopsy for a broad range of indications. We also provide novel 1H-MRS proton density fat fraction thresholds for approximation of hepatic triglyceride content using single breath-hold sequences; these thresholds correspond to histopathologic grading and classification of steatosis.
Although we did not attempt to colocalize voxel placement with biopsy site, we obtained similar results from three separate locations in the liver: the right hepatic dome, the inferior right lobe, and the left lobe. Previous studies [6, 19] have shown similar consistency between 1H-MRS measurements in various segments of the liver. Although it is unclear whether histologic lesions associated with steatosis are unevenly distributed throughout the liver [20, 21], the use of multiple voxels allows noninvasive quantification of proton density fat fraction in any segment of the liver where a voxel can be accurately placed. Like liver biopsy findings, 1H-MRS results may be prone to sampling error. However, a single voxel represents 27,000 mm3 of liver tissue, which is considerably more than is sampled in a standard needle biopsy. Emerging quantitative MRI techniques that entail chemical shift–based approaches may surpass spectroscopy for assessment of hepatic lipid because these techniques sample the entire liver and have high spatial resolution [22–24].
After confirming strong agreement between 1H-MRS results and the readings of two independent pathologists, we calculated optimal 1H-MRS proton density fat fraction thresholds for correlation with standard histopathologic grading. Szczepaniak et al. [3] identified 5.56% as the upper limit of normal for percentage hepatic fat content based on the 95th percentile 1H-MRS triacylglycerol content of a subset of 345 individuals without known risk factors for steatosis. This value is similar to the often used histologic cutoff of less than 5% of hepatocytes with steatosis [12]. We identified a proton density fat fraction threshold less than 17% for cases of grade 0 and trace steatosis, which may be higher than 5%, in part owing to the inclusion of mild steatosis cases in the category and the inclusion of patients with medically indicated liver biopsy rather than healthy volunteers. Our threshold for grade 2 or higher steatosis of 38.6% is consistent with findings in a previous study [4] in which biochemical analysis, 1H-MRS, and histopathologic grading were used in the evaluation of subjects with chronic HCV infection. That study showed subjects with grades 2 and 3 steatosis had a mean triglyceride content of 33.7 mg/g at biopsy. These observed thresholds may have clinical utility for the diagnosis and tracking of steatosis. Studies with larger samples are needed to validate these findings.
The participants in our study underwent liver biopsy for a broad range of indications. One third of these participants had elevated transaminase concentrations of unknown cause. In the United States, the most likely diagnosis in association with chronically elevated transaminase concentrations without a known cause is steatosis [25]. In our study, 1H-MRS measurement of proton density fat fraction performed exceptionally well in discriminating clinically significant steatosis in participants with elevated transaminase levels. The effectiveness of 1H-MRS in this small sample suggests a role for this technology in the evaluation of the growing population of persons with hepatic steatosis.
With regard to our 1H-MRS technique, this study is unique in the use of breath-hold sequences. In many previous studies spectroscopy data were obtained under free breathing [5, 7, 8], which can introduce motion artifact related to respiratory movement and thereby affect the quality of spectral data. Technical improvements have allowed the use of breath-hold sequences and therefore shorter acquisition times for patients. In this study, participants were asked to hold their breath for approximately 20 seconds, which all 52 subjects were able to do without difficulty. In assessing appropriate voxel placement and spectral quality, we were able optimize the 1H-MRS data generated for comparison with biopsy results.
In the context of chronic HCV infection, as fibrosis advances, steatosis tends to recede [26]. McPherson and colleagues [27] also found this inverse relation and found that with 1H-MRS, the percentage of steatotic hepatocytes in patients with more advanced fibrosis tended to be underestimated. However, because 1H-MRS yields an estimate of proton density fat fraction and not a measure of the degree of hepatocellular involvement, this result would be expected. When ROCs were generated for the diagnosis of steatosis with 1H-MRS according to fibrosis stage, the values were only slightly lower in cases of more advanced fibrosis (AUC, 0.97 for stage 0–1 fibrosis versus 0.95 for stage 2–3 fibrosis) [27]. Although our sample was small, we also found only a small decrease in the ROC for 1H-MRS in subjects with fibrosis.
There were several limitations to this study. Because of the clinical referral base, one half of our subjects also had HIV infection, which might have limited the generalizability of our findings to other clinical populations. However, hepatic steatosis is an important contributor to liver injury in the context of HIV and has been associated with antiretroviral therapy, lipid disturbances, body fat distribution, insulin resistance, and HCV coinfection [28, 29]. The ability to evaluate hepatic steatosis with noninvasive methods in this population is increasingly important.
In addition to patients with HIV infection in our highly inclusive strategy, we incorporated patients with a broad range of indications for liver biopsy. We used 3-T MRI to obtain spectroscopic data, and although the use of 3-T machines is growing and previous studies have been conducted with 3-T 1H-MRS of the liver [4, 7, 9], most health centers still use 1.5-T machines for hepatic imaging. It is also important to note the intrinsic difference between histopathologic grade, which is determined by the relative physical appearance of hepatocellular fat particles in a histologic sample, and 1H-MRS results, which are determined by the number of fat protons versus water protons in a given region of liver sampled. As such, the two measures of steatosis are not necessarily linearly related. Furthermore, pathologist determination of histopathologic grade introduces the potential for interobserver variability (although minimal in our study). We used liver biopsy and histologic examination as the reference standard for the diagnosis of steatosis, but we did not use more detailed morphometric or direct quantification techniques to evaluate hepatic triglyceride content. It is also important to recognize that hepatic steatosis is not a disease but is a component of a pathologic process, and 1H-MRS cannot be used to make the distinction between steatosis and steatohepatitis.
Conclusion
Hydrogen-1 MR spectroscopy is an effective, noninvasive technique that can be used for diagnosis and quantitative analysis of hepatic steatosis. The introduction of 1H-MRS proton density fat fraction thresholds that correspond to clinical histopathologic grade is an important step forward in establishing the clinical utility of 1H-MRS. Furthermore, the accuracy of 1H-MRS among individuals with elevated transaminase concentrations of unknown cause suggests its usefulness in the growing population with hepatic steatosis.
Acknowledgments
Supported by the Intramural Research Program of the National Institutes of Health.
References
- 1.Ghany MG, Lok AS, Everhart JE, et al. Predicting clinical and histologic outcomes based on standard laboratory tests in advanced chronic hepatitis C. Gastroenterology. 2010;138:136–146. doi: 10.1053/j.gastro.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 3.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 4.Krssák M, Hofer H, Wrba F, et al. Non-invasive assessment of hepatic fat accumulation in chronic hepatitis C by 1H magnetic resonance spectroscopy. Eur J Radiol. 2010;74:e60–e66. doi: 10.1016/j.ejrad.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 5.Orlacchio A, Bolacchi F, Cadioli M, et al. Evaluation of the severity of chronic hepatitis C with 3-T 1H-MR spectroscopy. AJR. 2008;190:1331–1339. doi: 10.2214/AJR.07.2262. [DOI] [PubMed] [Google Scholar]
- 6.d’Assignies G, Ruel M, Khiat A, et al. Noninvasive quantitation of human liver steatosis using magnetic resonance and bioassay methods. Eur Radiol. 2009;19:2033–2040. doi: 10.1007/s00330-009-1351-4. [DOI] [PubMed] [Google Scholar]
- 7.Lee SS, Park SH, Kim HJ, et al. Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol. 2010;52:579–585. doi: 10.1016/j.jhep.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Ghotb A, Noworolski SM, Madden E, et al. Adipose tissue and metabolic factors associated with steatosis in HIV/HCV coinfection: histology versus magnetic resonance spectroscopy. J Acquir Immune Defic Syndr. 2010;55:228–231. doi: 10.1097/QAI.0b013e3181e1d963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Werven JR, Marsman HA, Nederveen AJ, et al. Assessment of hepatic steatosis in patients undergoing liver resection: comparison of US, CT, T1-weighted dual-echo MR imaging, and pointresolved 1H MR spectroscopy. Radiology. 2010;256:159–168. doi: 10.1148/radiol.10091790. [DOI] [PubMed] [Google Scholar]
- 10.Petersen KF, West AB, Reuben A, Rothman DL, Shulman GI. Noninvasive assessment of hepatic triglyceride content in humans with 13C nuclear magnetic resonance spectroscopy. Hepatology. 1996;24:114–117. doi: 10.1002/hep.510240119. [DOI] [PubMed] [Google Scholar]
- 11.Thomsen C, Becker U, Winkler K, Christoffersen P, Jensen M, Henriksen O. Quantification of liver fat using magnetic resonance spectroscopy. Magn Reson Imaging. 1994;12:487–495. doi: 10.1016/0730-725x(94)92543-7. [DOI] [PubMed] [Google Scholar]
- 12.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 13.Perumalswami P, Kleiner DE, Lutchman G, et al. Steatosis and progression of fibrosis in untreated patients with chronic hepatitis C infection. Hepatology. 2006;43:780–787. doi: 10.1002/hep.21078. [DOI] [PubMed] [Google Scholar]
- 14.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 15.Wang HZ, Riederer SJ, Lee JN. Optimizing the precision in T1 relaxation estimation using limited flip angles. Magn Reson Med. 1987;5:399–416. doi: 10.1002/mrm.1910050502. [DOI] [PubMed] [Google Scholar]
- 16.Deoni SC. High-resolution T1 mapping of the brain at 3T with driven equilibrium single pulse observation of T1 with high-speed incorporation of RF field inhomogeneities (DESPOT1-HIFI) J Magn Reson Imaging. 2007;26:1106–1111. doi: 10.1002/jmri.21130. [DOI] [PubMed] [Google Scholar]
- 17.Longo R, Pollesello P, Ricci C, et al. Proton MR spectroscopy in quantitative in vivo determination of fat content in human liver steatosis. J Magn Reson Imaging. 1995;5:281–285. doi: 10.1002/jmri.1880050311. [DOI] [PubMed] [Google Scholar]
- 18.Dodd LE, Pepe MS. Partial AUC estimation and regression. Biometrics. 2003;59:614–623. doi: 10.1111/1541-0420.00071. [DOI] [PubMed] [Google Scholar]
- 19.van Werven JR, Hoogduin JM, Nederveen AJ, et al. Reproducibility of 3. 0 Tesla magnetic resonance spectroscopy for measuring hepatic fat content. J Magn Reson Imaging. 2009;30:444–448. doi: 10.1002/jmri.21837. [DOI] [PubMed] [Google Scholar]
- 20.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 21.Larson SP, Bowers SP, Palekar NA, Ward JA, Pulcini JP, Harrison SA. Histopathologic variability between the right and left lobes of the liver in morbidly obese patients undergoing Roux-en-Y bypass. Clin Gastroenterol Hepatol. 2007;5:1329–1332. doi: 10.1016/j.cgh.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Qayyum A, Goh JS, Kakar S, Yeh BM, Merriman RB, Coakley FV. Accuracy of liver fat quantification at MR imaging: comparison of out-of-phase gradient-echo and fat-saturated fast spin-echo techniques–initial experience. Radiology. 2005;237:507–511. doi: 10.1148/radiol.2372040539. [DOI] [PubMed] [Google Scholar]
- 23.Meisamy S, Hines CD, Hamilton G, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767–775. doi: 10.1148/radiol.10100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reeder SB, Cruite I, Hamilton G, Sirlin CB. Quantitative assessment of liver fat with magnetic resonance imaging and spectroscopy. J Magn Reson Imaging. 2011;34:729–749. doi: 10.1002/jmri.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daniel S, Ben-Menachem T, Vasudevan G, Ma CK, Blumenkehl M. Prospective evaluation of unexplained chronic liver transaminase abnormalities in asymptomatic and symptomatic patients. Am J Gastroenterol. 1999;94:3010–3014. doi: 10.1111/j.1572-0241.1999.01451.x. [DOI] [PubMed] [Google Scholar]
- 26.Lok AS, Everhart JE, Chung RT, et al. Evolution of hepatic steatosis in patients with advanced hepatitis C: results from the hepatitis C antiviral long-term treatment against cirrhosis (HALT-C) trial. Hepatology. 2009;49:1828–1837. doi: 10.1002/hep.22865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McPherson S, Jonsson JR, Cowin GJ, et al. Magnetic resonance imaging and spectroscopy accurately estimate the severity of steatosis provided the stage of fibrosis is considered. J Hepatol. 2009;51:389–397. doi: 10.1016/j.jhep.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Hadigan C, Kottilil S. Hepatitis C virus infection and coinfection with human immunodeficiency virus: challenges and advancements in management. JAMA. 2011;306:294–301. doi: 10.1001/jama.2011.975. [DOI] [PubMed] [Google Scholar]
- 29.Guaraldi G, Squillace N, Stentarelli C, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47:250–257. doi: 10.1086/589294. [DOI] [PubMed] [Google Scholar]