Abstract
Background
Inflammation plays a critical role in adverse cardiac remodeling and heart failure. Therefore, approaches geared towards inhibiting inflammation may provide therapeutic benefits. We tested the hypothesis that genetic deletion of interleukin-10 (IL10), a potent anti-inflammatory cytokine, exacerbates pressure-overload induced adverse cardiac remodeling and hypertrophy and that IL10 therapy inhibits this pathology.
Methods and Results
Cardiac hypertrophy was induced in Wild-type (WT) and IL10-knockout (KO) mice by isoproterenol (ISO) infusion. ISO-induced left ventricular (LV) dysfunction and hypertrophic remodeling, including fibrosis and fetal gene expression, were further exaggerated in KO mice compared to WT. Systemic recombinant mouse IL10 administration markedly improved LV function and not only inhibited but also reversed ISO-induced cardiac remodeling. Intriguingly, very similar cardio-protective response of IL10 was found in transverse aortic constriction (TAC)-induced hypertrophy and heart failure model. In neonatal rat ventricular myocytes (NRCM) and H9c2 myoblasts, ISO activated NFκB while it inhibited STAT3 phosphorylation. Interestingly, IL10 suppressed ISO-induced NFκB activation and attenuated STAT3 inhibition. Moreover, pharmacological and genetic inhibition of STAT3 reversed the protective effects of IL10 while ectopic expression of constitutively active STAT3 mimicked the IL10 responses on the ISO effects, confirming that IL10 mediated inhibition of NFκB is STAT3 dependent.
Conclusions
Taken together our studies suggest IL10 treatment as a potential therapeutic approach to limit the progression of pressure overload-induced adverse cardiac remodeling.
Keywords: heart failure, hypertrophy, interleukins, myocardium, signal transduction
Introduction
Hypertrophic cardiac remodeling has been demonstrated to contribute significantly to ventricular dysfunction in various heart diseases including cardiomyopathy, hypertension, and ischemia-reperfusion 1. Although Gq-protein coupled receptor (GqPCR) signaling is well accepted in the pathophysiology of chronic heart diseases, its mechanisms are poorly understood 2–4. Mechanical load on the heart is accompanied by various molecular and cellular changes, including myocyte apoptosis, inflammatory/fibrogenic responses and hypertrophy, which lead to myocardial remodeling and subsequent ventricular dysfunction and heart failure 1, 5, 6. The factors that regulate these cardiac events during pressure overload, however, are not fully elucidated.
The adult heart’s response to excessive hemodynamic overload results in the secretion of many cytokines and growth factors due to hypertrophic growth of cardiac myocytes (CM), which ultimately may lead to heart failure. Experimental evidence suggests that the inflammatory response plays a key role in the development of pressure overload-induced cardiac hypertrophy and heart failure 7–9. In addition, the balance between pro-inflammatory and anti-inflammatory cytokines tends to shift towards the pro-inflammatory cytokines during prolonged/chronic stress on the heart. These notions are supported by the observation that heart failure patients demonstrate elevated plasma levels of pro-inflammatory cytokines, which correlate with severity of disease10, 11. The deleterious effects of these cytokines include myocardial cell death, blunted β-adrenergic signaling, fetal gene re-activation, endothelial dysfunction, and collagen deposition6–8. These processes lead to cellular breakdown, impaired cardiac contractility and enhancement of the remodeling process. In addition, pro-inflammatory cytokines have been implicated in the development of cardiac hypertrophy, most likely via downstream activation of many signaling mediators (such as p38 and ERK) and transcription factors such as nuclear factor-κB (NFκB) 3,12. Recent studies from others and our laboratory have suggested that activation of inflammatory mediators and subsequent left ventricular (LV) dysfunction and remodeling (dilation and fibrosis) are major determinants in the pathogenesis of cardiac disorders 12–15. Mice with increased expression of cardiac-specific TNF-α develop dilated cardiomyopathy 16 and increased fibrosis in the heart 7, 17. It has also been shown that IL-1β promotes myocyte hypertrophy and mice with cardiac-targeted IL-1β over-expression exhibit concentric LV hypertrophy with preserved LV systolic function 6. In addition, the increased cytokine gene expression during the acute phase of inflammation evokes secondary, self-sustaining autocrine and paracrine growth factor and cytokine expression. Both hypertrophic and inflammatory signaling cascades have common downstream transcriptional targets (NFκB, NAFT and CREB), and thus the modulation of immune response towards anti-inflammatory signaling cascades may provide a potential therapeutic avenue for the treatment of hypertrophy-related remodeling and heart failure 18.
Interleukin-10 (IL10), a potent anti-inflammatory cytokine, inhibits infiltration of monocytes/macrophages into the injured site and is a strong repressor of pro-inflammatory cytokines and chemokines 19. Previously, we have shown that IL10 inhibited inflammation-mediated intimal hyperplasia following carotid artery injury in mice via post-transcriptional mRNA destabilization of TNFα 15. We also demonstrated that IL10 suppresses the inflammatory response and contributes to improved LV function and remodeling in acute myocardial infarction (AMI) models. These beneficial effects of IL10 were mediated via suppression of HuR/MMP-9 and by enhancing capillary density through activation of STAT3 14.
In spite of these observations, very little is known about the role of IL10 anti-inflammatory therapy for the treatment of cardiomyopathy especially cardiac hypertrophy and remodeling. Therefore, we hypothesize that recombinant IL10 protein alters the pressure overload-induced myocardial remodeling process, and prevents the development of dilated cardiomyopathy and ultimately heart failure. Here for the first time we provide evidence that IL10 knockout mice (KO) exhibit severe cardiac hypertrophy, fibrosis and inflammatory cytokine gene expression in response to isoproterenol (ISO) induced pressure overload and that IL10 therapy significantly improves left ventricular function, attenuates cardiac fibrosis and cell death induced by (ISO). Furthermore, we demonstrate the cardio-protective effect of IL10 in the transverse aortic constriction (TAC) model, where IL10 improved cardiac function and reduced mortality. Mechanistically, we show that IL10, via activation of STAT3 signaling, inhibited NFκB mediated hypertrophic and inflammatory gene expression.
Materials and Methods
The detailed and expanded methodology is provided in the online-only Data Supplement.
Animals and treatments
All animal experiments performed in this study adhered to the protocols approved by the Institutional Animal Care and Use Committee of the Northwestern University. Six to ten-week-old wild-type (WT) and IL10-KO (IL10tm1Cgn) mice of C57BL/6J background were procured from Jackson Research Laboratory (Bar Harbor, ME). Before treatments all animals were screened for baseline echocardiography. The cardiac hypertrophy and heart failure were induced by isoproterenol osmotic pump and transverse aortic constriction surgery (TAC) as described in the online-only Data Supplement.
Cardiac Imaging
Trans-thoracic two-dimensional M-mode echocardiography was performed using Vevo 770 (VisualSonics, Toronto, Canada) equipped with a 30 MHz transducer. Echocardiographic studies were performed before (baseline) and at 7, 14 and 28 days after TAC surgery or pump implantation. Percent fractional shortening (% LVFS) and ejection fraction (% LVEF) were calculated as described previously 14.
Quantitative Real-Time PCR
Gene expression levels of ANP, BNP, CD 68, IL-1β, TGF-β and TNF-α were quantified in the left ventricular tissue, NRCM and H9c2 myoblasts as described previously 13, 14.
Isolation of neonatal rat ventricular myocytes and treatments
NRCM were prepared by enzymatic digestion of hearts obtained from newborn (0–2 day old) Sprague–Dawley rat pups as described, previously 20.
Electrophoretic Mobility Shift Assay
The following oligonucleotide sequence containing binding sites for NFκB/c-Rel homodimeric and heterodimeric complexes were purchased from Santa Cruz Biotech. (sc 2505) and used for EMSA: 5'-AGT TGA GGG GAC TTT CCC AGG C-3'. Nuclear protein isolation and EMSAs were carried out as described, elsewhere 21.
Immunofluorescent Staining
Immunofluorescent staining for p65/RelB (NFkB), Cd68 and α-SA was performed in tissue sections or NRCM plated onto chamber slides (100 viable cells/mm2) coated with 1 µg/cm2 collagen IV as described in the online-only Data Supplement.
Statistical Analysis
Differences between data groups were calculated for significance by using unpaired t test or 1-way analysis of variance (ANOVA), as appropriate and Tukey`s multiple comparison post hoc test (Graph Pad prism Software Inc., San Diego, CA). The post hoc testing was performed if overall comparison across groups was statistically significant. Two-way repeated-measures ANOVA was used to evaluate the statistical significance of data acquired from the same animal over multiple time points. Survival analysis was performed by the Kaplan-Meier method, and between-group differences in survival were tested by the Log-rank (Mantel-Cox) test using Graph Pad prism Software. Data are expressed as mean ± SEM. For all tests, a probability value of > 0.05 was considered to denote statistical significance.
Results
IL10-KO Mice Are More Susceptible to Pressure Overload-Induced Left Ventricular Dysfunction
M-mode echocardiography revealed no baseline differences in LV structure or function between WT and KO mice. Left ventricular hypertrophy and heart failure was induced in these mice with isoproterenol infusion using mini-osmotic pumps as described in methods section. There was a trend toward increased mortality among KO mice, however the difference was not statistically significant (Supplementary Figure S1c). Significant impairment in LV function was observed from day 7 until 28 after ISO treatment, as evidenced by reduced ejection fraction, fractional shortening and increased LV mass (Figure 1a–d and Supplementary Table S1). It is noteworthy that ISO-induced impairment in heart function was further aggravated in KO mice compared to WT mice at all the time points (Figure 1a–d and Supplementary Table S1). Interestingly, marked increase in LV volume and mass (especially in KO mice) at 28 days clearly suggests that IL10 KO mice are more susceptible to chronic ISO treatment and inclined towards heart failure after ISO treatment (Figure 1d and Supplementary Table S1). Therefore, we hypothesized that systemic administration of mouse recombinant IL10 (IL10) might prevent ISO-induced hypertrophic remodeling.
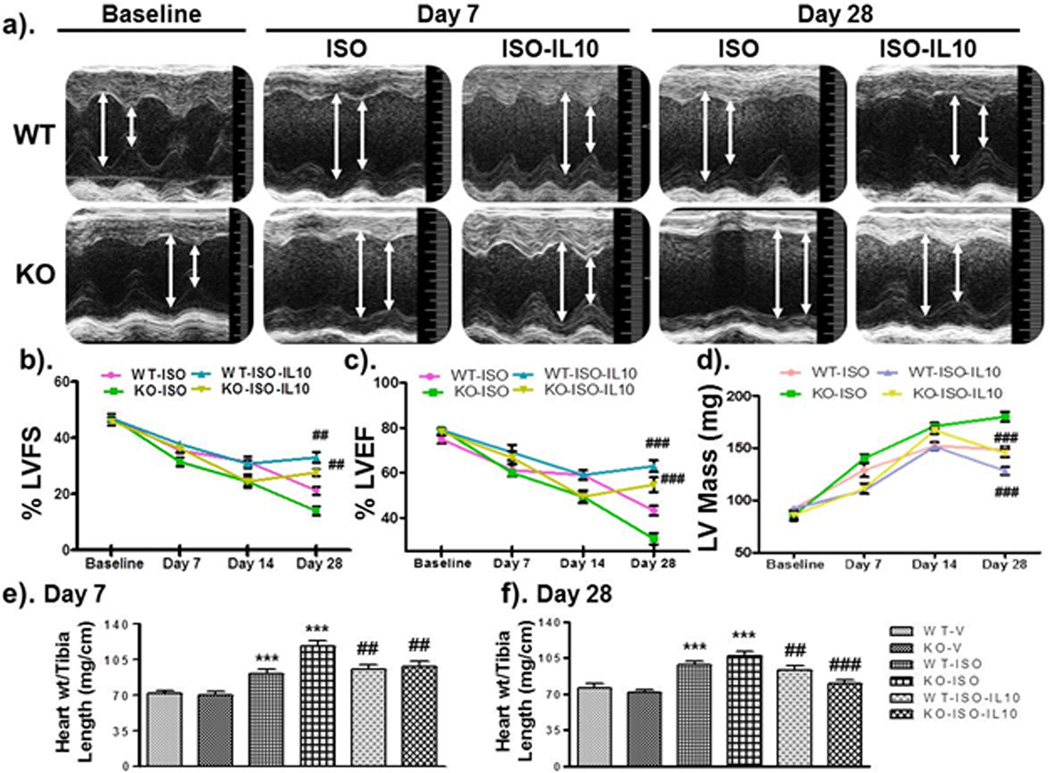
Figure 1.
IL10 improves left ventricular (LV) functions & attenuates ISO-induced hypertrophic remodeling. WT and KO mice were treated with ISO and IL10 as described. After serial Echocardiographic analysis mice were euthanized and heart tissue was extracted for biochemical analysis. a). M-mode echocardiography indicated that chronic ISO treatments exaggerated chamber size and reduced wall thicknesses in KO compared to WT mice & IL10 significantly reversed this effect. b & c). ISO-induced decreased FS and EF were significantly improved by IL10. d). Chronic ISO treatment markedly increased left ventricular mass, which was significantly recovered by IL10. e & f). Heart weight and tibia length ratio were calculated at 7 and 28 days respectively. ISO treatment significantly increased this ratio; however IL10 markedly abolished ISO effect. *** p<0.001 vs. respective vehicle, ###p<0.001 vs. respective ISO treated, ##p<0.01 vs. respective ISO treated. N=6–8.
Recombinant IL10 Administration Improves LV function in Pressure Overload Hypertrophy and Heart Failure Models
Administration of IL10 (50µg/kg) significantly improved heart function as evidenced by the increased ejection fraction (Figure 1b; p<0.001) and fractional shortening (Figure 1c; p<0.01) both in WT and KO mice (Supplementary Table S1). These functional data indicate the direct role of IL10 in the attenuation of pressure-overload induced LV dysfunction. To further assess the hypertrophic remodeling, heart weight vs. tibia length ratio was calculated. ISO-treatment significantly increased heart weight/tibia length ratio from day 7 (p<0.001) to 28 (p<0.001) both in WT and KO mice (Figure 1e & f). This ratio was larger in KO mice (p<0.001) which was significantly corrected by IL10 administration both at day 7 (WT, p<0.01 and IL10KO, p<0.01) and 28 (WT, p<0.01 and KO, p< 0.001) (Figure 1e & f). Similar results were observed on heart weight and body weight ratio in these mice (Supplementary Figure S1a & b).
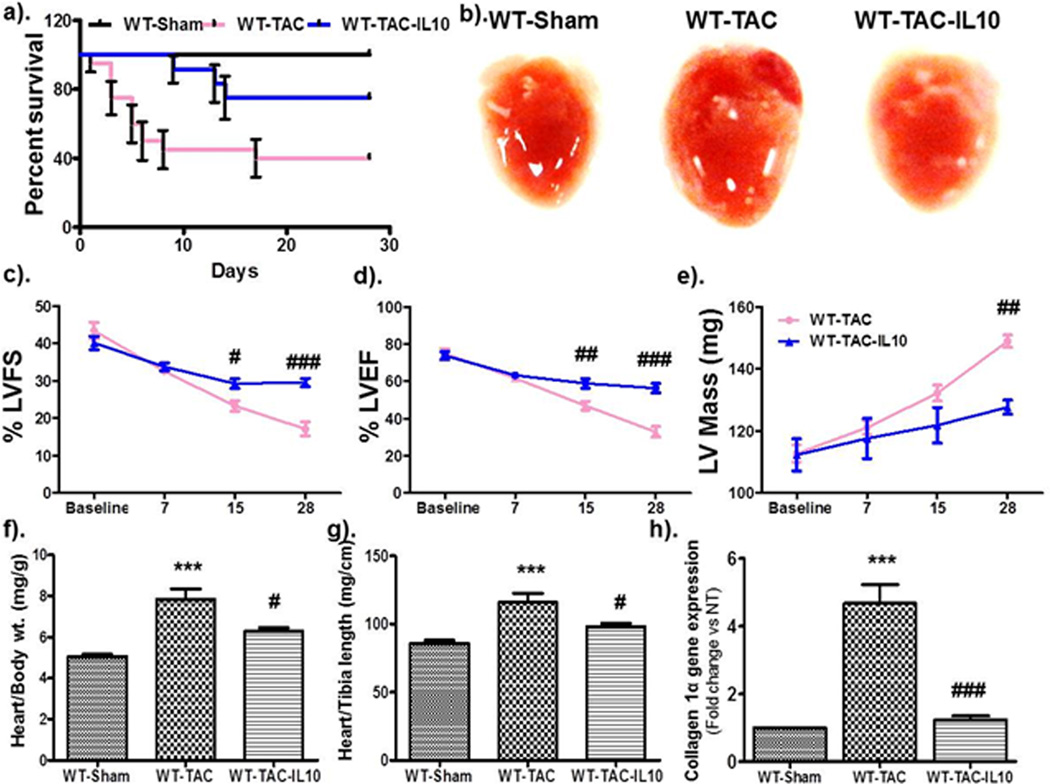
To further verify the cardio-protective effect of IL10 in pressure overload, we conducted an additional series of experiments utilizing the transverse aortic constriction (TAC) model in WT mice. Importantly, IL10 treatment significantly reduced TAC-induced mortality in WT animals (Figure 2a). Furthermore, IL10 treatment prevented TAC induced hypertrophy and prevented TAC induced heart failure (Figure 2 c–f). Therefore, we have demonstrated the protective role of IL10 in two complementary in vivo models of pressure overload induce hypertrophy and heart failure.
Figure 2.
IL10 therapy improves left ventricular (LV) functions & reduces transverse aortic constriction (TAC)-induced hypertrophic remodeling. Hypertrophic remodeling was induced by constricting the transverse aorta in WT mice followed by IL10 treatments, as described. After serial Echocardiographic analysis mice were euthanized and heart tissue was extracted for biochemical analysis. a). Kaplan-Meier plot indicating that IL10 treatment significantly improved the survival of mice after TAC surgery. b). Representative images of whole hearts. c & d). Hypertrophic remodeling-induced decreased in %FS and %EF were significantly improved by IL10. e). Mice subjected to TAC surgery showed marked increase in left ventricular mass, which was significantly reduced by IL10 treatment. f & g). Heart weight and body weight ratio and heart weight and tibia length ratio were calculated at 28 days. IL10 treatment markedly abolished the TAC-induced heart weight. *** p<0.001 vs. sham, ###p<0.001 vs. TAC, ##p<0.01 vs. TAC, #p<0.05 vs. TAC. N=5–6. h). IL10 therapy markedly reduced the expression of collagen 1-α gene expression in hearts of mice with TAC surgery.
IL10 Therapy Substantially Reduces ISO and TAC Induced Cardiac Fibrosis
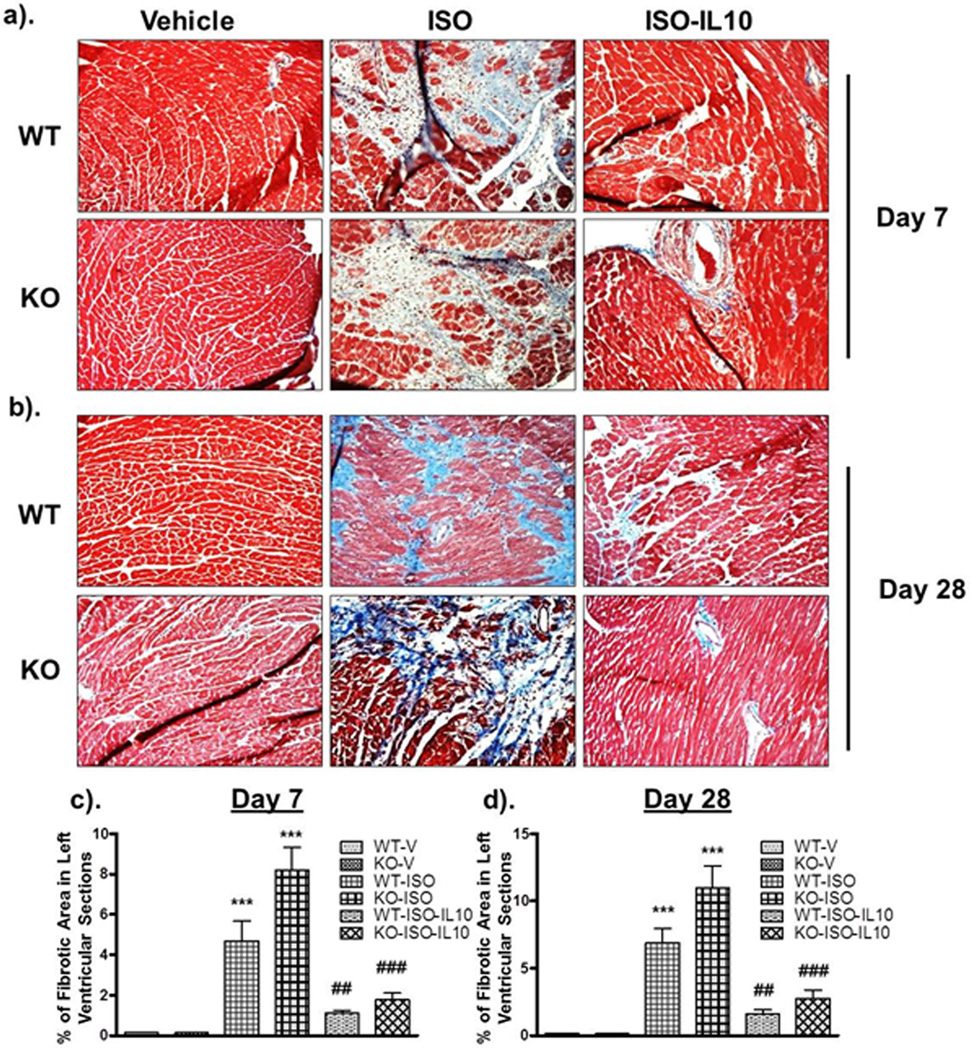
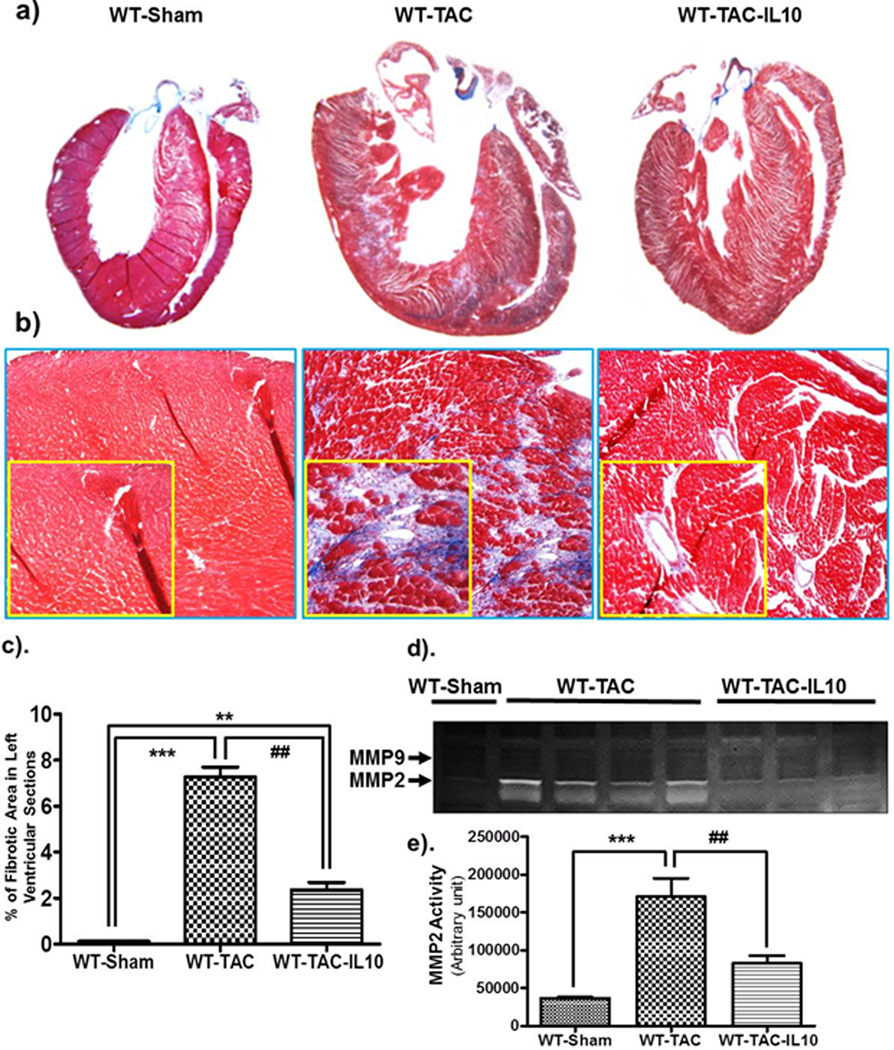
As cardiac fibrosis is a key process in the progression of pathological cardiac remodeling, we next assessed the level of fibrosis in mice after ISO and/or IL10 treatments. Masson`s Trichome staining was performed to show collagen deposition in left ventricular sections. ISO markedly increased the amount of fibrosis both in WT and KO mice (Figure 3). At both time points, KO mice demonstrated exaggerated fibrosis compared to WT (Figure 3c & d) indicating a significant involvement of inflammation in progression of cardiac fibrotic remodeling. Interestingly, IL10 treatment markedly inhibited ISO-induced fibrosis both in WT (day 7, p<0.01; day 28 p<0.01) and KO mice (day 7 p<0.001 & day 28 p<0.001) (Figure 3c & d). A significant increase in fibrosis was also observed 28 days after TAC surgery and IL10 treatment markedly reduced fibrosis (Figure 4a–c). Therefore, IL10 demonstrates anti-fibrotic properties in both the ISO and TAC models.
Figure 3.
IL10 treatment diminishes ISO-induced left ventricular fibrosis. Masson`s Trichome staining was performed to check the collagen deposition in LV tissue sections. Stained sections were analyzed with Image-Pro Plus 6.3 software. IL KO mice showed significant increased interstitial and perivascular fibrosis compared to WT ISO-treated mice both at day 7 (a & c) and at day 28 (b & d). ISO-induced increased fibrosis was attenuated by IL10 treatment. OM=20X. ***p<0.001 vs. respective vehicle, ###p<0.001 vs. respective ISO treated, ##p<0.01 vs. respective ISO treated. N=4–6.
Figure 4.
IL10 treatment diminishes TAC-induced left ventricular fibrosis. Masson`s Trichome staining was performed to check the collagen deposition in LV tissue sections. Stained sections were analyzed with Image-Pro Plus 6.3 software. a–c). TAC significant increased interstitial and perivascular fibrosis. TAC-induced increased fibrosis was attenuated by IL10 treatment. OM=20X. d & e). Matrix metalloproteinase activity was measured in whole tissue lysates by gelatin zymography. A significant increase in MMP2 activity was observed in constricted animals. IL10 treatment largely abolished the TAC induced MMP2 activity. ***p<0.001 vs. sham, **p<0.01 vs. sham, ##p<0.01 vs. WT-TAC (N=4–5).
To assess the recruitment of inflammatory cells in heart tissues, CD 68 gene expression was measured by Q-PCR. A significant increase in the expression of CD 68 was observed at day 7 suggesting the recruitment of inflammatory cells after ISO treatment (Supplementary Figure S2a). KO mice infused with ISO showed markedly enhanced CD 68 gene expression (>10 fold vs. vehicle, p< 0.001) as compared to WT (5.5 fold vs. vehicle, p<0.001) (Supplementary Figure S2a). At day 28, CD 68 expression was significantly increased in ISO treated groups, although to a lesser degree (Supplementary Figure S2c). Interestingly, increased CD 68 gene expression was markedly reduced by IL10 administration both in WT and KO mice at both time points (Supplementary Figure S2a & c). In addition, infiltration of inflammatory cells was also measured by immunohistochemical staining in left ventricular tissue sections after TAC and TAC-IL10 treatment in WT mice. Significant increase in CD 68 cells was observed after TAC and was almost completely inhibited with IL10 treatments (Supplementary Figure 3a & b).
Since TGF-β signaling plays a significant role in abnormal extracellular matrix deposition, we examined the expression of TGF-β after different treatments. ISO infusion significantly increased TGF-β mRNA expression (Supplementary Figure S2b & d). IL10 inhibited ISO-induced TGF-β gene expression in KO mice (p<0.001). To assess the involvement of matrix metalloproteinases on excessive fibrosis, gelatin zymography was performed in heart tissue lysates from TAC or TAC plus IL10 treated mice. Pressure-overload markedly increased the MMP2 activity. Interestingly, pressure-induced MMP2 activity was almost totally reduced in IL10 treated animals. In contrast, there was no change in MMP9 activity after aortic constriction (Figure 4d & e).
IL10 Treatment Inhibits ISO and TAC Induced Apoptosis
To investigate potential causes of cardiac dysfunction and excessive fibrosis after ISO and TAC, we analyzed cardiac myocyte apoptosis. Paraffin embedded sections of hearts isolated from mice receiving ISO for 7 days were co-stained for α-sarcomeric actinin (α-SA) and TUNEL to determine the rate of cardiac myocyte apoptosis. A noticeable increase in cardiac myocyte apoptosis was observed after ISO treatments both in WT and KO mice (Figure 5a–g; p<0.001). KO mice showed noticeably increased apoptosis compared to WT ISO-treated mice (Figure 5g; p< 0.001). IL10 administration substantially reduced ISO-induced cell death both in WT-ISO (p< 0.05) and KO-ISO (P<0.01) mice (Figure 5g). In addition, apoptotic cardiomyocytes were increased after TAC in WT mice and TAC induced apoptosis was inhibited with IL10 treatment (Figure 6a & b). Next, we investigated the molecular mediators of apoptosis in WT mice with TAC. TAC markedly increased active caspase-3 and IL10 treatment significantly reduced TAC-induced active caspase-3. Furthermore, Bcl2, an anti-apoptotic protein, was increased with IL10 treatment in the TAC model (Figure 6c). Therefore, IL10 treatment inhibits key pro-apoptotic mediators and augments anti-apoptotic factors.
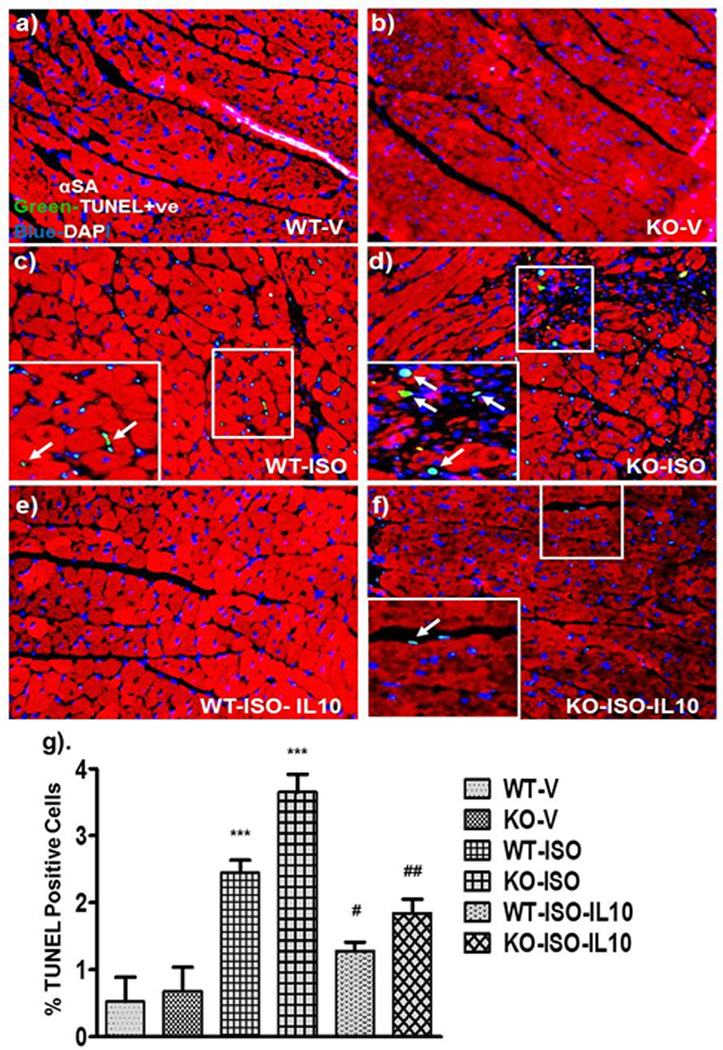
Figure 5.
IL10 administration reduces ISO-induced myocardial cell death. Paraffin embedded sections were stained with TUNEL reagents (green) and α-SA (Texas-red). Nuclei were counter stained with DAPI. TUNEL positive cells (bright green) were visualized by fluorescence microscopy at 20X magnification. a, b & g). No cell death in vehicle treated mice. c, d & g). ISO treatment increased cell death with a greater magnitude in KO mice. e, f & g). IL10 significantly reduced ISO-induced cell death in both strain. ***p<0.001 vs. respective vehicle. ##p<0.01 vs. respective ISO treated, #p<0.05 vs. respective ISO treated (N=4).
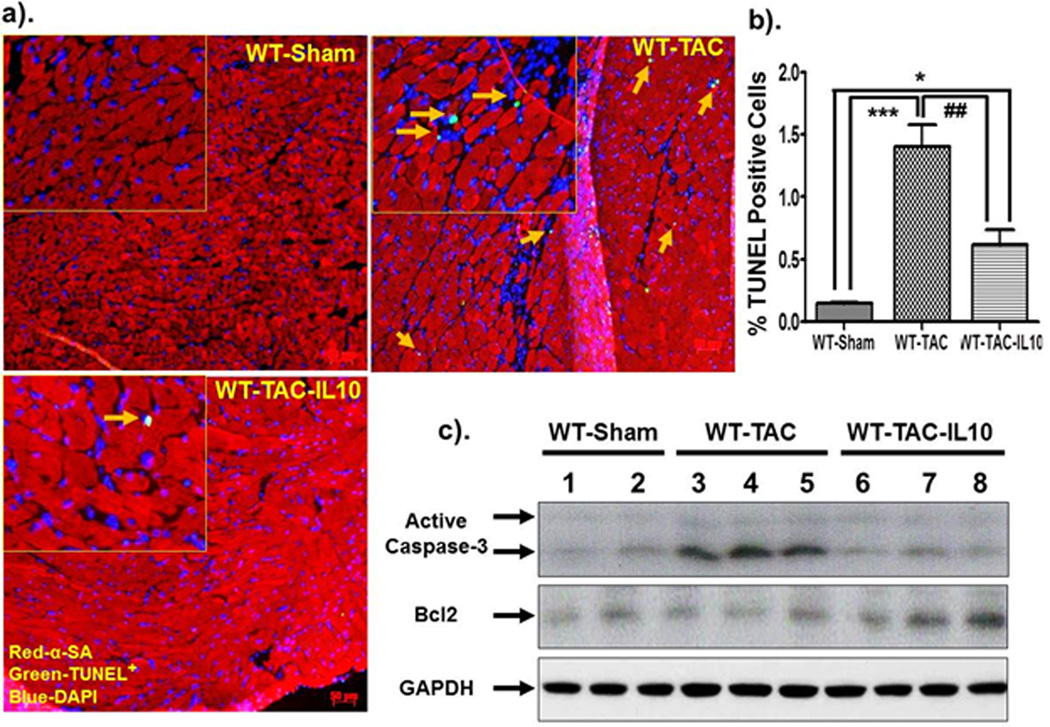
Figure 6.
IL10 treatment reduces TAC-induced apoptotic cell death and caspase3 activation in mice at day 28-post constriction. Formalin fixed and paraffin embedded sections were stained with TUNEL reagents (green) and α-SA (Texas-red). Nuclei were counter stained with DAPI. TUNEL positive cells (bright green) were visualized by fluorescence microscope at 20X magnification. a & b). No cell death were observed in WT-sham mice. Transverse aortic constriction significantly increased cell death in mice. IL10 significantly reduced TAC-induced cell death. c). Whole tissue lysates were prepared from left ventricle and cleaved caspase3 and Bcl2 levels were measured. Increase caspase3 cleavage were found in WT-TAC animals, however, IL10 treatment markedly reduced it. In contrast, we did not see any significant difference on Bcl2 level after TAC surgery or with IL10 treatment. ***p<0.001 vs. WT-sham, *p<0.05 vs. WT-sham, ##p<0.01 vs. WT-TAC (N=4).
IL10 Suppresses ISO and TAC Induced Activation of Hypertrophic and Pro-Inflammatory Genes
Next, we measured inflammatory and hypertrophic gene expression in left ventricular tissues by Q-PCR both at day 7 and day 28 post-ISO infusion and at day 28 after TAC. ISO-infusion in both WT and KO mice showed increased expression of pro-inflammatory cytokines and hypertrophic markers at Day 7 (Supplementary Figure S4a–d). Co-treatment of ISO and IL10 together from day 1–7, significantly inhibited the ISO-induced expression of these genes (Figure S4a–d). Chronic treatment of ISO for 28 days further increased the expression of ANP (p<0.001) and BNP (p<0.001) (Supplementary Figure S4e–h). Furthermore, ISO treated KO mice showed a greater increase in hypertrophic markers than WT mice. Interestingly, systemic IL10 injection remarkably suppressed ISO-induced expression of hypertrophic and inflammatory markers both in WT and KO mice (Supplementary Figure S4a–h). Importantly, IL10 inhibited hypertrophic and inflammatory gene expression in WT mice 28 days after TAC (Supplementary Figure S5a–d). Therefore, IL10 treatment inhibits hypertrophic and inflammatory gene expression in the setting of pressure overload.
IL10 Treatment Markedly Reduces TAC-Induced Hypertrophic and Inflammatory Signaling
Similar to ISO-induced cardiac hypertrophy model, TAC-induced pressure overload significantly decreased in STAT3 phosphorylation in the hearts of WT untreated animals which was remarkably restored by IL10 treatment. Interestingly, p38 phosphorylation was markedly increased after TAC, however IL10 significantly inhibited pressure overload induced p38 activation (Supplementary Figure S6a–c). Therefore, IL10 rescues STAT3 activity and inhibits p38 activity in the TAC model.
IL10 Inhibits ISO-Induced Hypertrophic and Inflammatory Responses in Neonatal Rat Cardiomyocytes
In order to understand the molecular mechanisms of IL10 mediated inhibition of cardiac hypertrophy, we performed a series of in vitro experiments in neonatal rat ventricular myocytes (NRCM) and the H9c2 rat cardiomyoblast cell line. Cells were treated with ISO (1 µM) to induced hypertrophy. ISO treatment for 24 hours significantly increased NRCM surface area (Supplementary Figure S7a; p< 0.01). In addition, ISO treatment significantly increased ANP (p<0.01), BNP (p<0.001), TNF-α (p<0.001) and IL-1β (p<0.01) gene expression (Supplementary Figure S7b–e). Further, to check the effect of IL10 on ISO-induced gene expression, NRCM were pre-treated with IL10 for 1 hours followed by ISO for 24 hours. IL10 markedly reduced ISO-induced expression of inflammatory and hypertrophic genes (Supplementary Figure S7 b–e). Moreover, to study the intermediate signaling components, Western blot analysis was performed for p38 MAP kinase, a known signaling pathway linked to both hypertrophy and inflammation, in the total cell lysates from NRCM after ISO with and without IL10 treatments. ISO-induced p38 phosphorylation was markedly reduced by IL10 treatment (Supplementary Figure S7f). To test the possibility for activation of survival signaling by IL10, STAT3 activation was also measured in NRCM. ISO alone inhibited STAT3 activation, while IL10 treatment significantly increased STAT3 activity and thus activated survival signaling (supplementary Figure S7f).
STAT3 Inhibition Attenuates IL10 Effects on ISO-Induced Hypertrophic Responses in NRCM
Next, we hypothesized that IL10 mediated STAT3 activation leads to inhibition of ISO-induced hypertrophic responses. To test this hypothesis, we took the advantage of both genetic and pharmacological tools to regulate STAT3 function. ISO treatment increased p38 phosphorylation and IL10 treatment attenuated this response. Pharmacological inhibition of STAT3 with curcurbitacin I (CB I) resulted in robust activation of both basal and ISO-mediated p38 phosphorylation, suggesting that the anti-hypertrophic responses of IL10 are likely mediated by STAT3. Furthermore, CB I attenuated the IL10 mediated decrease in ISO induced p38 phosphorylation (Supplementary Figure S7f).
In vivo tissue sections demonstrated a marked increase in apoptotic cell death in ISO treated animals. To understand the anti-apoptotic mechanism of IL10, pro-apoptotic, cleaved caspase-3 and anti-apoptotic, Bcl2 levels were measured in the total cell lysate prepared from NRCM treated with different combinations of ISO, IL10 and STAT3 inhibitor for 24 hours (Supplementary Figure S8). ISO treatment significantly increased active caspase-3. ISO-induced caspase-3 activation was attenuated by IL10 treatment. Interestingly, the anti-apoptotic effect of IL10 was markedly reduced by STAT3 inhibitor. Therefore, the anti-apoptotic action of IL10 is mediated by STAT3. In contrast, there were no significant effects of ISO or IL10 on Bcl2 levels.
IL10 Inhibits ISO-Induced NFκB Activation in a STAT3 Dependent Manner
Previous reports have documented the involvement of NFκB signaling in both inflammation and hypertrophic processes 10, 18. Therefore, we next investigated the interaction of IL10 and STAT3 signaling in regulation of NFκB activation by tracking the translocation of p65, the active form of NFkB, from the cytosol to the nucleus in NRCM. ISO and/or STAT3 inhibitor (CB I) treatment significantly induced the translocation of p65 to the nucleus (magenta nuclei in merged image; Supplementary Figure S9c & e). ISO-induced p65 nuclear translocation was markedly attenuated by IL10 treatment (Supplementary Figure S9d). Intriguingly, the effect of IL10 on the attenuation of ISO-induced p65 translocation was almost completely abolished by STAT3 inhibitor (Supplementary Figure S9f). These data suggest that IL10 prevents ISO-induced NFkB activation in a STAT3 dependent manner.
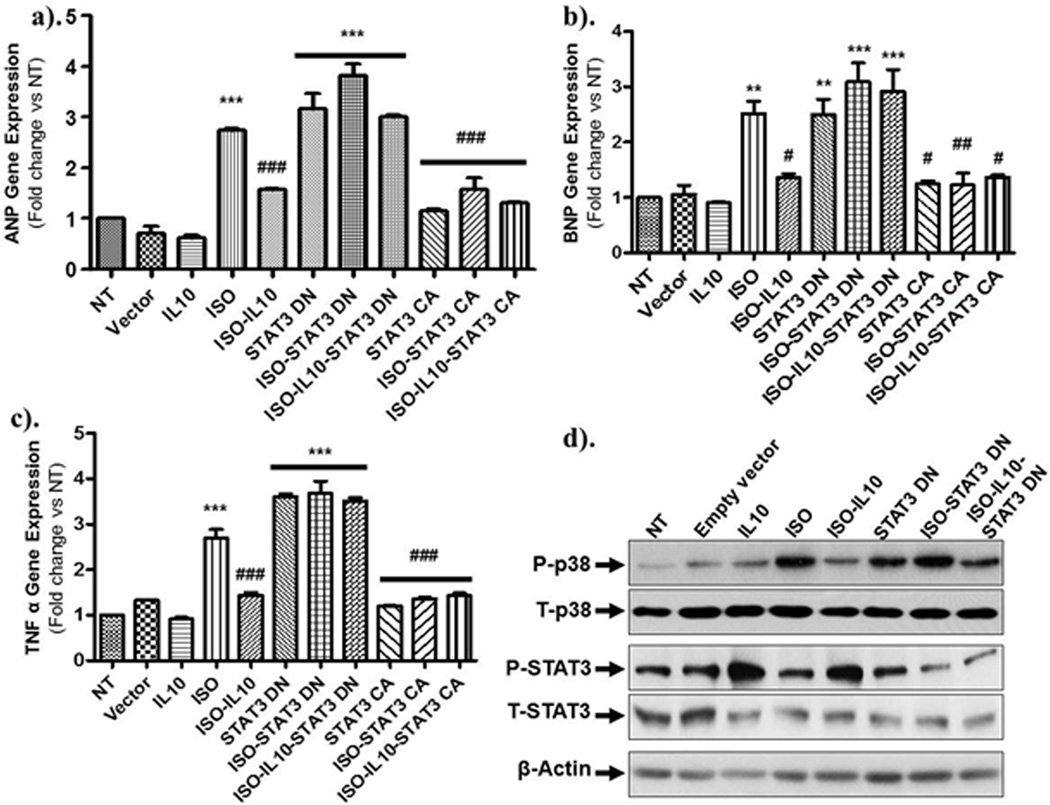
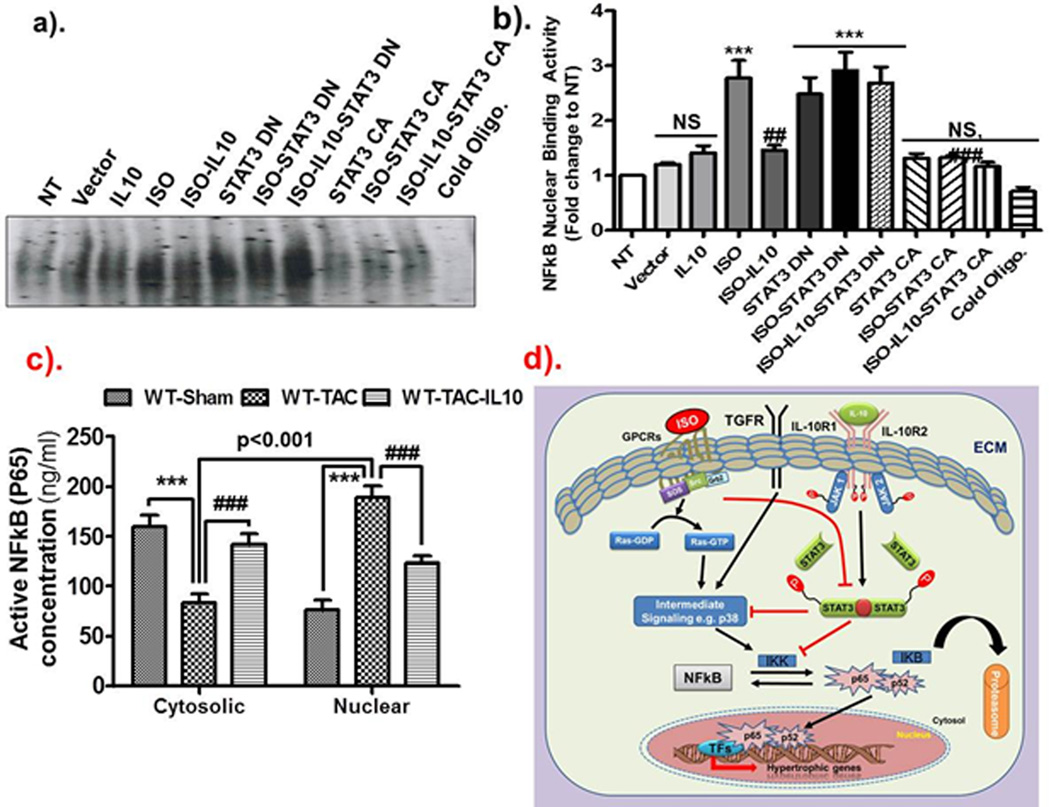
To further corroborate our pharmacological inhibitor findings, we next utilized genetic approach for STAT3 inhibition by over-expressing the dominant negative (DN, Y705F) and constitutively active (CA) STAT3 plasmid vectors in H9c2 myoblasts. Hypertrophic and inflammatory mRNA expression was assessed by Q-PCR. Similar to NRCM, ISO markedly induced ANP (p<0.001), BNP (p<0.01) and TNF-α (p<0.001) gene expression in H9c2 cells. ISO-induced ANP, BNP and TNF-α gene expression was significantly reduced by IL10 (Figure 7a p<0.001; b p<0.05 & c p<0.001 respectively). Ectopic expression of dominant negative STAT3 alone or in combination with ISO markedly induced the ANP (p<0.001 for other), BNP (p<0.01 for STAT3DN and p<0.001 for others) and TNF-α (p<0.001 for others) expression. Intriguingly, the protective effect of IL10 was almost totally attenuated in the presence of DN-STAT3 (Figure 7a–c). In contrast, gain of function experiments using over-expression of the constitutively active form of STAT3 (CA-STAT3) alone or with IL10 significantly reduced ANP, BNP and TNF-α gene expression (Figure 7a–c). ISO-induced p38MAPK activation was inhibited by IL10 treatments and DN-STAT3 reversed IL10 effects (Figure 7d). Furthermore, electrophoretic mobility shift assays (EMSA) were also performed in the nuclear fraction of H9c2 myoblasts to further confirm that NFκB is the downstream signaling molecule for IL10-STAT3 signal transduction. As demonstrated in Figure 8, ISO drastically increased NFκB DNA binding activity, which was substantially reduced by IL10 (Figure 8a–b). Interestingly, DN-STAT3 alone or in combination with ISO increased NFκB activity while CA-STAT3 over-expression alone or with IL10 almost completely abolished ISO-induced NFκB binding activity (Figure 8a–b).
Figure 7.
STAT3 activation is required for IL10 function in H9c2 cells. Dominant negative and constitutively active STAT3 were expressed for 36 hours followed by IL10 and ISO. Expression of ANP, BNP & TNF-α in H9c2 myocytes was quantified by Q-PCR. Relative mRNA expression of target genes was normalized to the endogenous 18S and represented as fold change vs. no treatment group. ISO-induced increased expression of (a) ANP, (b) BNP, and (c) TNF-α was significantly attenuated by IL10. Furthermore, IL10 mediated inhibition of gene expression is dependent on STAT3. d). Western blots were performed in total cell lysates for p38 and STAT3 activation after different treatments. Respective total proteins were used as normalizing control. IL10 treatment markedly reduced the ISO-induced p38 activation and activated STAT3 phosphorylation. Interestingly, IL10 mediated inhibition of P38 is reasonably dependent on STAT3. β actin was used as loading control. ***p<0.001 vs. NT, **p<0.01 vs. NT, *p<0.05 vs. NT (N=4–6).
Figure 8.
IL10-mediated inhibition of NFkB activity is dependent on STAT3. Dominant negative and constitutively active STAT3 were expressed in H9c2 myocytes for 36 hours followed by IL10 and ISO treatments. a–b). EMSA was performed to check the binding of p65 subunit of NFkB to its promoter. Densitometric analysis was done by Image Quant-5.0 computer software. ISO induced NFkB activity was markedly reduced by IL10 and/or STAT3 CA. Expression of STAT3 DN reduced IL10-mediated inhibition of NFkB activity. ***p<0.001 vs. NT, ###p<0.001 vs. ISO treated group, ##p<0.01 vs. ISO treated group, NS not signifiant. (N=3). c). TAC-induced increase in nuclear expression of NFkB is markedly reduced by IL10. d). Hypothetical model: ISO-after binding with GPCRs induces many intermediate signaling cascades and induced hypertrophic remodeling. IL10 by interacting with IL10 receptor activates the JAK/STAT3 pathway. IL10 activated STAT3 can either directly inhibit the intermediate signaling components (p38 MAP kinase in our studies) or inhibit the activation of NFkB transcription factor and thus inhibiting hypertrophic remodeling. Our results strongly suggest that IL10 has potential to alleviate pathological hypertrophic remodeling by inhibiting either hypertrophic signaling components or transcription factor NFkB.
To confirm the in vitro findings in our in vivo model, we next assessed NFkB activity using NF-κB/p65 ELISA. By this method, we measured the active NFkB (i.e. p65) levels both in the cytosolic and nuclear fractions of heart lysates from WT mice after TAC. Our results indicated that IL10 treatment markedly inhibited the TAC-induced translocation of p65 from the cytosol to the nucleus, consistent with our observations in the cell culture system (Figure 8c). Therefore, these in vitro and in vivo findings demonstrate that the cardio-protective effect of IL10 is mediated via STAT3 dependent inhibition of NFkB signaling. A signaling model of IL-10 effects is shown in Figure 8d.
Discussion
Physiological cardiac hypertrophy is an adaptive response to preserve left ventricular (LV) function in response to stress, but sustained hypertrophic growth of the myocardium leads to an increased risk of cardiovascular events, heart failure and death. Inflammation plays a significant role in this transition 22. Therefore, a deeper understanding of anti-inflammatory cytokines on the regulation of pressure overload-induced cardiac hypertrophy and remodeling is a largely open area of study. In the present study we focused on the role of anti-inflammatory cytokine therapy for pressure overload-induced cardiac hypertrophy and remodeling. The essential and clinically relevant finding of this study is that IL10 therapy prevents hypertrophy, reduces fibrosis, and preserves cardiac function in the face of pressure overload stress. Most importantly, IL10 administration significantly inhibited the deleterious effects of ISO and inhibited the transition of hypertrophy to heart failure in both the ISO and TAC models. To the best of our knowledge, this is the first report showing the importance of IL10 therapy to inhibit the progression of heart failure. The functional significance of IL10 on ISO-induced cardiac hypertrophy and heart failure is further evident from our findings that KO mice exhibit an exaggerated response to pressure load compared to the WT mice and the exogenous supplementation of IL10 significantly mitigates adverse cardiac remodeling in these mice. In addition, the therapeutic benefit of IL10 treatment was verified in the TAC model where IL10 prevented TAC-induced mortality and preserved cardiac function. At the molecular level we demonstrate that the beneficial effects of IL10 are mediated by a novel STAT3-NFκB signaling pathway.
Chronic inflammation is a hallmark of heart failure and is a predictor of overall prognosis 7, 23. Failing myocardium exhibits augmentation of both pro-inflammatory cytokine expression, re-expression of fetal genes and uncoordinated contractile functions 24. In our study increased expression of CD 68 (monocytes/macrophage marker) in ISO treated and TAC mouse hearts suggests a significant recruitment of inflammatory cells that was associated with an increase in mRNA expression of various pro-inflammatory cytokines (IL-1β and TNF-α). In the myocardium, production of cytokines is mainly mediated by inflammatory cells during acute stress, however, during chronic stress activated cardiac cells can also start secretion of these cytokines thereby augmenting the chronic inflammatory responses. Previous studies have strongly suggested that persistent expression of pro-inflammatory cytokines cause pathological remodeling of the heart 7, 13, 14. Pathological remodeling of the heart is accompanied by increased apoptosis, fibrosis, and alteration in cardiac gene expression and myocyte contractile dysfunction7. An adverse accumulation of extracellular matrix structural protein leads to abnormal tissue stiffness and adversely affects myocardial viscoelasticity 25. Extracellular fibrillar collagen functions as scaffolding tissue to maintain cardiac structure and stiffness. Accumulation of fibrillar collagen leads to diastolic and systolic ventricular dysfunction 5. Our data also show that ISO treatment severely increases interstitial and perivascular fibrosis with greater extend in IL10 KO mice, signifying the involvement of unregulated inflammation in the progression of the cardiac fibrosis and heart failure.
Furthermore, KO mice showed a significant increase in apoptosis after ISO treatments compared to WT. This increase in apoptosis is mostly of cardiac myocytes as evident from the reduced staining of α-sarcomeric actinin in KO tissue sections compared to WT tissue sections. Previous studies have advocated that cell death is one of the hallmarks of heart failure and the faster transition to heart failure observed in KO mice may reflect this phenomenon. The greater extent of fibrosis and cell death in the KO mice further suggests that inflammatory cytokines and chemokines play major roles in the progression of heart failure. Our in vitro findings demonstrate that IL10 inhibits ISO-induced caspase-3 activation. Caspase-3 activation is a well-known process in apoptotic cell death. Inflammatory cytokines play a significant role in progression of apoptosis. Previous work has demonstrated that TNF-α induced cardiac myocyte apoptosis via activation of caspase-3 26. In addition, the therapeutic benefit of exogenous IL10 in prevention of pressure overload induced heart failure signifies the importance of inflammation as a driving force in pathological remodeling. Interruption of inflammatory signaling with IL10 reduced cardiomyocyte apoptosis leading to a reduction in fibrosis and preservation of cardiac function in the face of sustained pressure overload.
IL10 is a pleotropic cytokine and is known to act through a wide range of signaling cascades, largely in a cell and gene dependent manner. To tease out the mechanisms of IL10 action on myocyte hypertrophy in response to ISO, we induced hypertrophy in primary neonatal rat ventricular myocytes and H9c2 myoblasts. ISO treatment significantly induced hypertrophic and inflammatory gene expression both in NRCMs and H9c2 myoblasts. In corroboration with our in vivo data, IL10 co-treatment significantly inhibited the expression of these genes. We have reported that IL10 mediated improvement in heart function is dependent on STAT3 signaling in the myocardial infarction model14. In accordance to our previous finding, IL10 markedly activated STAT3 in both myocyte cell types and reduced myocyte cell death 14, 27. The role of p38 dependent and independent NFκB activation in the progression of inflammation and hypertrophic heart failure is well established 28. Recent findings have shown that STAT3 can directly influence the NFκB transcriptional activities 29. We found that in our system inhibition of STAT3 activation blocked IL10 mediated inhibition of NFκB activation. Previous studies have suggested significant activation of NFκB both in vivo and in vitro in hypertrophic heart failure models 18, 28. Furthermore, inhibition of NFκB by various inhibitors protects the heart from cardiac hypertrophy 30. It still remains to be established how NFκB induces cardiac hypertrophy and fetal gene re-expression because NFκB binding sites have not been identified in the promoter regions of adult or fetal cardiac genes associated with cardiac hypertrophy. Therefore, we speculate that NFκB induces cardiac remodeling either through a physical interaction with another hypertrophic transcription factor such as myocarditin and MEF2 or through an indirect effect, perhaps by activating the expression of another regulator of cardiac remodeling, such as Histone modifying enzymes, PKC δ, BMP-2, or FGF8, which have been shown to be NFκB target genes in other cell systems and have been demonstrated to regulate cardiac growth 31. In conclusion, this is the first study describing the protective role of IL10 on pressure overload induced adverse remodeling. A model depicting the possible mechanisms of IL-10 mediated inhibition of pressure-overload induced cardiac remodeling is shown in Figure 8. Taken together, our studies suggest that IL10 treatment not only inhibits the progression but also reverses the pressure overload-induced adverse cardiac remodeling and IL10 therapy may in the future serve as a therapeutic modality to treat heart failure.
Supplementary Material
Clinical Perspective.
Chronic stress-induced hypertrophic growth of the myocardium leads to an increased risk of cardiovascular events, heart failure and death. Experimental evidence suggests a potential role of inflammation in the progression of heart failure; however the mechanisms of this process are not fully understood. The present study elucidates the novel therapeutic efficacy of anti-inflammatory cytokine, IL-10, in both the prevention and attenuation of pathologic hypertrophy and heart failure in two models of pressure over-load. Additionally, our study provides the mechanism through which IL-10 imparts its therapeutic benefits for cardio-protection. The significance of protective effect of IL-10 is further supported by an exaggerated adverse remodeling after pressure overload in IL-10 knock-out mice. Importantly, we show that pretreatment of mice with exogenous recombinant IL-10 before either ISO or TAC-induced hypertrophic stimulus, prevents ventricular remodeling, excessive fibrosis and cardiomyocyte death and preserves left ventricular functions. Moreover, IL-10 treatment, once the hypertrophy has been established, also attenuates adverse remodeling and fibrosis in animals with pressure over-load. These findings, showing that IL10 therapy prevents cardiac remodeling and preserves cardiac function in the face of pressure overload stress, have significant bearing on not only our understanding of the mechanisms involved in IL-10 action but also on the potential future clinical and therapeutic use of IL-10 and/or its downstream signaling components for the treatment of heart failure. Thus, IL10 treatment may be a novel therapeutic approach to prevent and cure the hypertrophic cardiac remodeling in patients.
Acknowledgments
Source of Funding: Work described in this manuscript was in part supported by National Institute of Health grants HL091983, HL105597, HL095874, HL053354 and HL108795 (R.K.) and American Heart Association National-The Davee Foundation SDG grant 0930219N (P.K.), NRSA F32 postdoctoral award HL107093 (M.T.) and AHA pre-doctoral fellowship 11PRE7360065 (E.H.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
References
- 1.Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 2.Sussman MA, McCulloch A, Borg TK. Dance band on the titanic: Biomechanical signaling in cardiac hypertrophy. Circ Res. 2002;91:888–898. doi: 10.1161/01.res.0000041680.43270.f8. [DOI] [PubMed] [Google Scholar]
- 3.Esposito G, Prasad SV, Rapacciuolo A, Mao L, Koch WJ, Rockman HA. Cardiac overexpression of a g(q) inhibitor blocks induction of extracellular signal-regulated kinase and c-jun nh(2)-terminal kinase activity in in vivo pressure overload. Circulation. 2001;103:1453–1458. doi: 10.1161/01.cir.103.10.1453. [DOI] [PubMed] [Google Scholar]
- 4.Malhotra R, D'Souza KM, Staron ML, Birukov KG, Bodi I, Akhter SA. G alpha(q)-mediated activation of grk2 by mechanical stretch in cardiac myocytes: The role of protein kinase c. J Biol Chem. 2010;285:13748–13760. doi: 10.1074/jbc.M110.109272. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, Zhang D, Nakamura T, Takimoto E, Kass DA. Pivotal role of cardiomyocyte tgf-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121:2301–2312. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honsho S, Nishikawa S, Amano K, Zen K, Adachi Y, Kishita E, Matsui A, Katsume A, Yamaguchi S, Nishikawa K, Isoda K, Riches DW, Matoba S, Okigaki M, Matsubara H. Pressure-mediated hypertrophy and mechanical stretch induces il-1 release and subsequent igf-1 generation to maintain compensative hypertrophy by affecting akt and jnk pathways. Circ Res. 2009;105:1149–1158. doi: 10.1161/CIRCRESAHA.109.208199. [DOI] [PubMed] [Google Scholar]
- 7.Sun M, Chen M, Dawood F, Zurawska U, Li JY, Parker T, Kassiri Z, Kirshenbaum LA, Arnold M, Khokha R, Liu PP. Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation. 2007;115:1398–1407. doi: 10.1161/CIRCULATIONAHA.106.643585. [DOI] [PubMed] [Google Scholar]
- 8.Smeets PJ, Teunissen BE, Planavila A, de Vogel-van den Bosch H, Willemsen PH, van der Vusse GJ, van Bilsen M. Inflammatory pathways are activated during cardiomyocyte hypertrophy and attenuated by peroxisome proliferator-activated receptors pparalpha and ppardelta. J Biol Chem. 2008;283:29109–29118. doi: 10.1074/jbc.M802143200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann S, Krause T, van Geel PP, Willenbrock R, Pagel I, Pinto YM, Buikema H, van Gilst WH, Lindschau C, Paul M, Inagami T, Ganten D, Urata H. Overexpression of the human angiotensin ii type 1 receptor in the rat heart augments load induced cardiac hypertrophy. J Mol Med. 2001;79:601–608. doi: 10.1007/s001090100246. [DOI] [PubMed] [Google Scholar]
- 10.Haugen E, Chen J, Wikstrom J, Gronros J, Gan LM, Fu LX. Parallel gene expressions of il-6 and bnp during cardiac hypertrophy complicated with diastolic dysfunction in spontaneously hypertensive rats. Int J Cardiol. 2007;115:24–28. doi: 10.1016/j.ijcard.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 11.McKinsey TA. Targeting inflammation in heart failure with histone deacetylase inhibitors. Mol Med. 2011;17:434–441. doi: 10.2119/molmed.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem. 2006;13:1877–1893. doi: 10.2174/092986706777585086. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamurthy P, Lambers E, Verma S, Thorne T, Qin G, Losordo DW, Kishore R. Myocardial knockdown of mrna-stabilizing protein hur attenuates post-mi inflammatory response and left ventricular dysfunction in il-10-null mice. FASEB J. 2010;24:2484–2494. doi: 10.1096/fj.09-149815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krishnamurthy P, Rajasingh J, Lambers E, Qin G, Losordo DW, Kishore R. Il-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of stat3 and suppression of hur. Circ Res. 2009;104:e9–e18. doi: 10.1161/CIRCRESAHA.108.188243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajasingh J, Bord E, Luedemann C, Asai J, Hamada H, Thorne T, Qin G, Goukassian D, Zhu Y, Losordo DW, Kishore R. Il-10-induced tnf-alpha mrna destabilization is mediated via il-10 suppression of p38 map kinase activation and inhibition of hur expression. FASEB J. 2006;20:2112–2114. doi: 10.1096/fj.06-6084fje. [DOI] [PubMed] [Google Scholar]
- 16.Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, Liu PP. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation. 2004;110:3221–3228. doi: 10.1161/01.CIR.0000147233.10318.23. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Chancey AL, Tzeng HP, Zhou Z, Lavine KJ, Gao F, Sivasubramanian N, Barger PM, Mann DL. The development of myocardial fibrosis in transgenic mice with targeted overexpression of tumor necrosis factor requires mast cell-fibroblast interactions. Circulation. 2011;124:2106–2116. doi: 10.1161/CIRCULATIONAHA.111.052399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freund C, Schmidt-Ullrich R, Baurand A, Dunger S, Schneider W, Loser P, El-Jamali A, Dietz R, Scheidereit C, Bergmann MW. Requirement of nuclear factor-kappab in angiotensin ii-and isoproterenol-induced cardiac hypertrophy in vivo. Circulation. 2005;111:2319–2325. doi: 10.1161/01.CIR.0000164237.58200.5A. [DOI] [PubMed] [Google Scholar]
- 19.Frangogiannis NG, Mendoza LH, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, Entman ML. Il-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol. 2000;165:2798–2808. doi: 10.4049/jimmunol.165.5.2798. [DOI] [PubMed] [Google Scholar]
- 20.Lal H, Verma SK, Golden HB, Foster DM, Smith M, Dostal DE. Stretch-induced regulation of angiotensinogen gene expression in cardiac myocytes and fibroblasts: Opposing roles of jnk1/2 and p38alpha map kinases. J Mol Cell Cardiol. 2008;45:770–778. doi: 10.1016/j.yjmcc.2008.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kishore R, Qin G, Luedemann C, Bord E, Hanley A, Silver M, Gavin M, Yoon YS, Goukassian D, Losordo DW. The cytoskeletal protein ezrin regulates ec proliferation and angiogenesis via tnf-alpha-induced transcriptional repression of cyclin a. J Clin Invest. 2005;115:1785–1796. doi: 10.1172/JCI22849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kania G, Blyszczuk P, Eriksson U. Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends Cardiovasc Med. 2009;19:247–252. doi: 10.1016/j.tcm.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Mann DL. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 24.Kassiri Z, Oudit GY, Sanchez O, Dawood F, Mohammed FF, Nuttall RK, Edwards DR, Liu PP, Backx PH, Khokha R. Combination of tumor necrosis factor-alpha ablation and matrix metalloproteinase inhibition prevents heart failure after pressure overload in tissue inhibitor of metalloproteinase-3 knock-out mice. Circ Res. 2005;97:380–390. doi: 10.1161/01.RES.0000178789.16929.cf. [DOI] [PubMed] [Google Scholar]
- 25.Burlew BS, Weber KT. Connective tissue and the heart. Functional significance and regulatory mechanisms. Cardiol Clin. 2000;18:435–442. doi: 10.1016/s0733-8651(05)70154-5. [DOI] [PubMed] [Google Scholar]
- 26.Dhingra S, Sharma AK, Arora RC, Slezak J, Singal PK. Il-10 attenuates tnf-alpha-induced nf kappab pathway activation and cardiomyocyte apoptosis. Cardiovasc Res. 2009;82:59–66. doi: 10.1093/cvr/cvp040. [DOI] [PubMed] [Google Scholar]
- 27.Wei L. Immunological aspect of cardiac remodeling: T lymphocyte subsets in inflammation-mediated cardiac fibrosis. Exp Mol Pathol. 2011;90:74–78. doi: 10.1016/j.yexmp.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Purcell NH, Molkentin JD. Is nuclear factor kappab an attractive therapeutic target for treating cardiac hypertrophy? Circulation. 2003;108:638–640. doi: 10.1161/01.CIR.0000085362.40608.DD. [DOI] [PubMed] [Google Scholar]
- 29.Yu Z, Zhang W, Kone BC. Signal transducers and activators of transcription 3 (stat3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor kappab. Biochem J. 2002;367:97–105. doi: 10.1042/BJ20020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang C, Li L, Zhang ZG, Fan D, Zhu Y, Wu LL. Globular adiponectin inhibits angiotensin ii-induced nuclear factor kappab activation through amp-activated protein kinase in cardiac hypertrophy. J Cell Physiol. 2010;222:149–155. doi: 10.1002/jcp.21931. [DOI] [PubMed] [Google Scholar]
- 31.Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of nf-{kappa}b in the heart: To be or not to nf-{kappa}b. Circ Res. 2011;108:1122–1132. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.