Abstract
The molecular complexity of the simple blowfly heart makes it an attractive preparation to delineate cardiovascular mechanisms. Blowfly cardiac activity consists of a fast, high frequency signal phase alternating with a slow, low frequency signal phase triggered by pacemakers located in the posterior abdominal heart and anterior thoracocephalic aorta, respectively. Mechanisms underlying FMRFamide-related peptides (FaRPs) effects on heart contractions are not well understood. Here, we report antisera generated to a FaRP, dromyosuppressin (DMS, TDVDHVFLRFamide), recognized neuronal processes that innervated the blowfly Protophormia terraenovae heart and aorta. Dromyosuppressin caused a reversible cardiac arrest. High and low frequency signals were abolished after which they resumed; however, the concentration dependent resumption of the fast phase differed from the slow phase. Dromyosuppressin decreased the frequency of cardiac activity in a dose dependent manner with threshold values between 5 fM and 0.5 fM (fast phase) and 0.5 fM and 0.1 fM (slow phase). Dromyosuppressin structure-activity relationship (SAR) for the decrease of the fast phase frequency was not the same as the SAR for the decrease of the slow phase frequency. The alanyl-substituted analog TDVDHVFLAFamide ([Ala9] DMS) was inactive on the fast phase, but active on the slow phase, a novel finding. FaRPs including myosuppressins are reported to require the C-terminal RFamide for activity. Our data are consistent with the conclusions DMS acts on posterior and anterior cardiac tissue to play a role in regulating the fast and slow phases of cardiac activity, respectively, and ligand-receptor binding requirements of the abdominal and thoracocephalic pacemakers are different.
Keywords: Aorta, DMS, FMRFamide, FaRP, heart, neurosecretion
1. Introduction
Contractions of heart muscle are critical for animal development and health; thus, it is crucial to understand the molecular mechanisms involved in regulating cardiac activity. The cardiac pump of the adult blowfly is a contractile tubular vessel composed of a single layer of myocytes that lies along the dorsal body midline. The cardiac pump or dorsal vessel consists of a posterior abdominal chambered heart and an anterior thoracocephalic unchambered aorta [5]. Cardiac activity of the blowfly contractile vessel is a cycle composed of a high frequency signal period, the fast phase, alternating with a low frequency signal period, the slow phase [4, 6, 7, 18, 35, 41]. Neural influence on the two alternating cardiac phases is established in the blowfly [2, 3, 40]. The fast phase and the slow phase may be generated from separate pacemakers located in the posterior abdominal heart and in the anterior thoracocephalic aorta, respectively [6]. However, no data are reported to describe a peptidergic innervation of posterior and anterior pacemakers or a molecular mechanism involved in blowfly cardiac activity.
Myosuppressin was first identified from cockroach head extracts based on the ability of the peptide to decrease the frequency of spontaneous hindgut contractions [15]. Myosuppressins also decrease the frequency of heart contractions [38]. Biochemical, immunohistochemical, and genomic data provide evidence that dromyosuppressin (DMS, TDVDHVFLRFamide) or a DMS-like material is present in the fruitfly Drosophila melanogaster [23], fleshfly Neobellieria bullata [13], horn fly and stable fly Haematobia irritans and Stomoxys calcitrans [17, 18], and the blowfly Phormia regina [36]. The myosuppressin consensus structure is XDVXHXFLRFamide. Myosuppressins are members of a superfamily related by a common RFamide C terminus to the tetrapeptide FMRFamide first discovered based on its effect on heart contractions [32]. Members of this superfamily known as FMRFamide-related peptides (FaRPs) including myosuppressins are reported to date to require RFamide for activity [14, 21, 22].
The effects of FaRPs on the frequency and amplitude of cardiac activity are reported in numerous invertebrates and vertebrates [14, 33, 34]. However, relatively little is known about the underlying molecular mechanisms involved in these effects. The molecular complexity of the simple blowfly heart makes it an attractive preparation in which to delineate fundamental mechanisms related to regulation of cardiovascular parameters. However, the effect of a myosuppressin on blowfly heart is not reported. We stained blowfly P. terraenovae cardiac tissue with DMS antisera. We measured the effect of DMS on the fast phase and the slow phase of the cardiac cycle over a range of concentrations. We also determined the activity of a series of DMS alanyl-substituted analogs, a series of DMS N-terminal truncated analogs, and the DMS free acid analog on the fast phase and the slow phase of the cardiac cycle in order to gain insight into the structure-activity relationships involved in the effects of the peptide.
2. Materials and methods
2.1 Flies
Three- to 5-day adult male blowflies obtained from a colony of P. terraenovae reared under standard conditions were used [1]. The flies were maintained at constant temperature (24°C), relative humidity (70/80%) and photoperiod (16:8 L:D cycle) conditions, and deprived of food and supplied with water ad libitum for 24 hours before experiments.
2.2 Immunolocalization
Indirect immunolocalization was performed with polyclonal antisera raised in rabbits according to the protocol previously described [16]. The antigen, TDVDHV-MAP, where MAP represents multiple antigenic peptide [27, 31], was designed to the N terminus of DMS, thus avoiding the common C-terminal RFamide contained in several structurally-related peptides [23 – 26]. Tissue was dissected from 3–5 day adult P. terraenovae. The heart or posterior region of the dorsal vessel remained attached to the body wall; however the aorta or anterior dorsal vessel and the central nervous system were dissected from the remaining tissue. Tissue was fixed in 4% paraformaldehyde for 4–6 hours at 4 °C, rinsed in PTN (0.1 M phosphate buffer, pH 7.2, containing 0.3% Triton X-100, 0.1% sodium azide, and 0.1% bovine serum albumin) for 30 minutes at room temperature, and incubated in primary antisera overnight at 4 °C. The tissue was rinsed in PTN for 75–90 min and incubated in Cy3-labelled goat anti-rabbit antibody (Jackson ImmunoResearch Labs; West Grove, PA, USA) and FITC-labelled phalloidin (Molecular Probes - Invitrogen; Carlsbad, CA, USA) for 4–6 hours at 4 °C. Phalloidin, a toxin that binds actin, was included in order to stain tissue for enhance visualization of the preparation. The tissue was then rinsed in PTN for 3–4 hours and placed in 4 mM sodium carbonate for 5–10 minutes prior to placing it in 80% glycerol with 5% n-propyl gallate. The preparation was mounted under a glass coverslip on a glass microscope slide for analysis. Fluorescence signals were imaged using a BioRad MRC 600 scanning confocal microscope (Hercules, CA, USA) equipped with a Kr-Ar laser and attached to a Nikon inverted microscope (Melville, NY, USA). Data z-series were collected with Comos software and processed with Adobe Photoshop version 6.0 (San Jose, CA, USA). No fewer than six preparations were analyzed for antisera and second antibody, antisera (pre-absorbed with antigen) and second antibody, antisera alone, secondary antibody alone, and no antisera and no second antibody. The results of the controls support the conclusion that staining is specific to the antisera and not due to cross-reactivity to a related antigen or to background. However, as with any immunohistochemical analysis, to unequivocally confirm the nature of the material recognized it would be necessary to isolate the antisera complex and identify the in vivo antigen.
2.3 Chemicals
Dromyosuppressin (DMS, TDVDHVFLRFamide), alanyl-substituted, truncated and free acid DMS analogs were synthesized by standard Fmoc protocol with a Protein Technologies Symphony synthesizer and purified by reversed phase HPLC. The structures of the peptides were confirmed by amino acid analysis and mass spectrometry. The nomenclature used for the alanyl-substituted analogs was: ADVDHVFLRFamide, [Ala1] DMS; TAVDHVFLRFamide, [Ala2] DMS; TDADHVFLRFamide, [Ala3] DMS; TDVAHVFLRFamide, [Ala4] DMS; TDVDAVFLRFamide, [Ala5] DMS; TDVDHAFLRFamide, [Ala6] DMS; TDVDHVALRFamide, [Ala7] DMS; TDVDHVFARFamide, [Ala8] DMS; TDVDHVFLAFamide, [Ala9] DMS, and TDVDHVFLRAamide, [Ala10] DMS. The nomenclature for N-terminal truncated analogs was: DVDHVFLRFamide, DMS (2–10); VDHVFLRFamide, DMS (3–10); DHVFLRFamide, DMS (4–10); HVFLRFamide, DMS (5–10); VFLRFamide, DMS (6–10), and FLRFamide, DMS (7–10). The structure of the DMS free acid was TDVDHVFLRF-OH. Peptide and analogs were diluted in series with a blowfly physiological solution [8] to obtain 10 μM, 1 μM, 0.1 ηM, 1 ρM, 5 fM, 0.5 fM, and 0.1 fM DMS solutions.
2.4 Bioassays
Each fly was fixed dorsal side down on a strip of low-melting-point dental wax (Tenatex; Imadent, Turin, Italy) and its legs were singly restrained in the wax. These manipulations were done viewing through a dissection microscope (Wild M5 A; Wild Leitz, Heerbrugg, Switzerland) provided with an optical fiber lighting system (KL 1500 electronic; Schott, Wiesbaden, Germany). Cardiac activity was monitored in the intact fly by means of surface electrocardiographic recording (ECG) as previously described [4], while maintaining climatic conditions as for rearing the colony. Prior to the recording, a small aperture (about 1 mm in diameter) was made in the ventrolateral surface of the third abdominal sclerite through which the solution of the chemical being tested was applied.
Monopolar ECGs were measured using metal electrodes (Ag-AgCl wires, 150 μm diameter), which were positioned on the fly cuticle with the aid of a pair of micromanipulators (M3301; Word Precision Instruments (WPI), Sarasota, FL, USA). A small amount of conductive ECG gel (Ultrasound; Meditec, Parma, Italy) was positioned at the electrode/cuticle interface to ensure a good electrical contact. The active electrode was positioned on the ventral cuticle of the second abdominal segment. The grounded reference electrode was positioned on the thoracic cuticle at the base of a foreleg. The positions of the electrodes were based on previous investigations [2 – 4], they are standard and accepted positions for ECG recordings. The electrode positions allowed the animals to breathe during the experiment; cardiac activity requires oxygen. Signals were displayed on the screen of a storage oscilloscope (5111; Tektronix, Beaverton, OR, USA) via a negative capacity amplifier (FD 223 Dual Differential Electrometer; WPI) and stored on magnetic tape (200T PCM Data Recorder; A.R. Vetter, Rebersburg, PA, USA). Recordings were later processed through an integrated system of hardware and software designed to record, display, and analyze ECG signals (PowerLab/4S; AD Instruments, Castle Hill NSW, Australia).
The ECG was monitored in each fly starting 20 minutes after electrode positioning. If spontaneous contractions of the body musculature affecting cardiac activity [30, 38] still occurred, additional resting time was allowed until regular fast phase and slow phase were observed. Thereafter, the ECG was recorded uninterruptedly starting 10 minutes before, during, and after solution administration. Using a microsyringe with a glass capillary (Digital Adjust Micro/Pettor; Scientific Manufacturing Industries, Berkeley, CA, USA), 0.5 μl of each of the solutions were applied into the abdominal hemocoel. Due to known mechanosensory-induced influence on fly heart activity [40], any contact with the abdominal cuticle was avoided by correctly positioning the injection system with a micromanipulator (MD4; WPI). Each solution was injected at a volume of 0.5 μl/fly (n = 10). The injection of this volume of blowfly physiological solution, previously tested in 2 groups of 10 flies each, had no effect on cardiac activity (control groups).
2.5 Data analysis
The signal frequencies of the fast phase and the slow phase in the five cardiac cycles were measured in each fly before injection of an experimental or control material. On the basis of these frequencies measured before injection frequency percentage variations following the injection were determined for each phase in each fly corresponding to the cardiac cycle immediately following the injection (time 0 in the figures) and, at 1 minute intervals, to the cycles in the following 10 minutes (times 1 to 10 in the figures). Mean values ± standard errors (SE) of frequency percentage variation/minute following the injection were then established for each experimental group and their statistical significance was analysed with Wilcoxon Test.
3. Results
3.1 Myosuppressin immunoreactivity in the dorsal vessel
Dromyosuppressin-like immunoreactivity was observed in cells and processes in the adult brain, and in processes in the thoracoabdominal ganglia. Processes from the medial superior protocerebrum cells were observed to send fibers posteriorly along the midline to the region of the esophageal opening. Several of these fibers extended further posteriorly through the subesophageal ganglion and innervated the anterior portion of the aorta (Fig. 1). DMS-like immunoreactivity was also present in two cells located in the posterior abdominal segments. These cells were positioned on either side of the midline. DMS antisera-stained immunoreactive fibers extended anteriorly from these cells to innervate the posterior of the heart (Fig. 1).
Fig. 1.
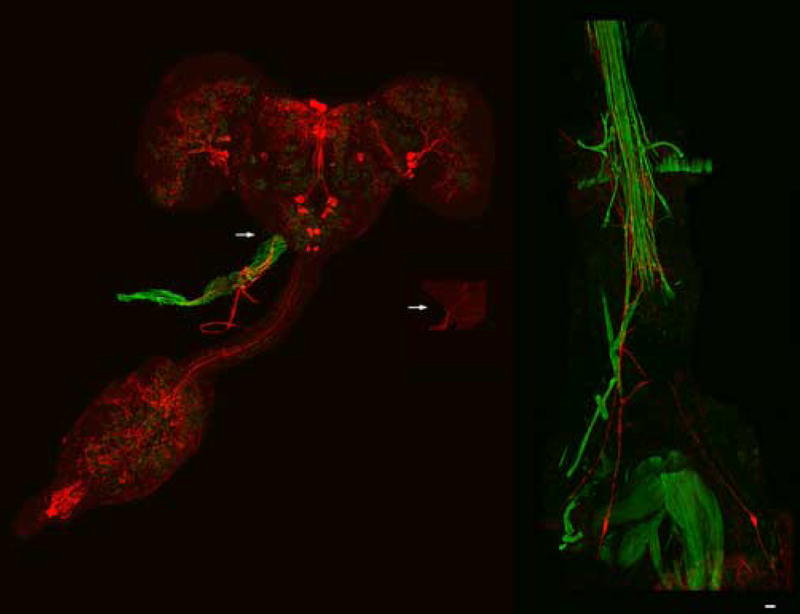
Dromyosuppressin (DMS) immunoreactive fibers innervate the aorta and the heart of the blowfly. Neurons (far left; enlargement, shown in center) were stained by fluorescently-labelled DMS antisera in the adult brain (cyanine; red) from which immunoreactive fibers projected to innervate the anterior of the dorsal vessel (arrows). Fluorescently-labelled phalloidin (fluorescein; green) was used to enhance the background in order to view the dorsal vessel. Bilaterally symmetric cells (far right) were stained by DMS antisera from which immunoreactive fibers projected to innervate the posterior of the dorsal vessel. Scale bar = 50 μM.
3.2 ECG changes following DMS injection
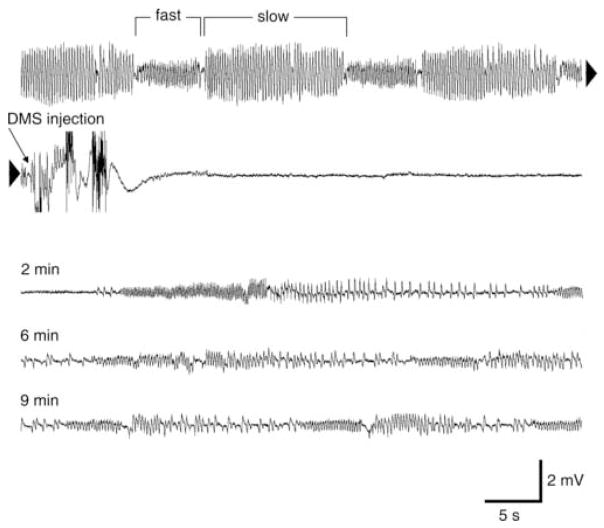
Injection of 0.5 μl of a 0.1 ηM solution of DMS into an intact fly was followed by a reversible arrest of ECG activity. In Fig. 2, the electrical signals (hereafter referred to as signals, y-axis, see figures) recorded from the electrodes during cardiac cycles prior to injection occurred in alternating phases of higher (fast phase: 5 signals/s) and lower signal frequencies (slow phase: 4 signals/s). Just a few seconds after the solution was injected, cardiac arrest occurred, no signals appeared in the recording for a duration of 2 minutes. This was followed by reversal of fast and slow ECG activity of considerably less amplitude compared to before the injection. However, after several minutes the activity was still reduced in amplitude and frequency compared to prior to injection. In the case of fast activity, signal amplitude was 50% lower both at 6 minutes and at 10 minutes after injection, while signal frequency was reduced by 20% and 10%, respectively. In the case of slow activity, signal amplitude was 57% lower both at 6 minutes and at 10 minutes after injection, while signal frequency was reduced by 40% and 26%, respectively.
Fig. 2.
Dromyosuppressin (DMS)-induced changes on the cardiac activity of the blowfly. Electrocardiogram (ECG) was continuously recorded before, during, and after injection of 0.5 μl of a 0.1 ηM DMS solution. Before injection, the fast phase and the slow phase alternated regularly in a cardiac cycle (first line). A few seconds after injection (arrow), there was no signal (second line; continuous recording from first line, see arrowheads). A recovery of alternating fast phase and slow phase occurred after 2 minutes (third line). However, signal frequency was reduced compared to before injection even 10 minutes later (end of the fifth line).
3.3 DMS-induced arrest of cardiac activity
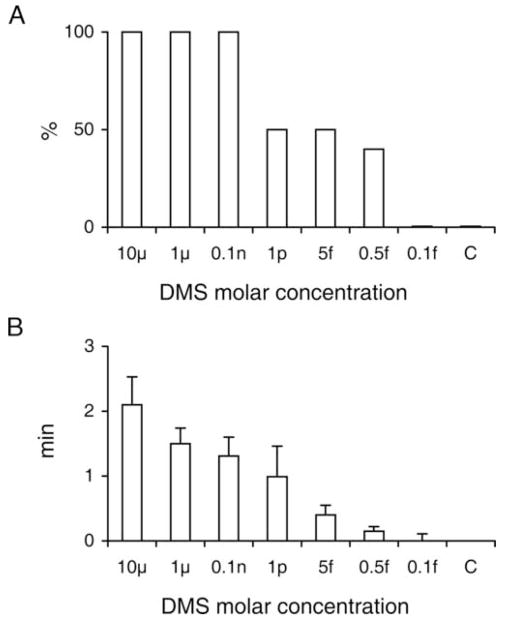
In order to determine the effect of DMS on cardiac arrest, various concentrations of the peptide were injected. At the three highest concentrations - 10 μM, 1 μM and 0.1 ηM - cardiac arrest was seen in 100% of the animals (n = 10; Fig. 3, A). At the three lower concentrations − 1 ρM, 5 fM, and 0.5 fM – cardiac arrest was still observed in about 50% of the animals. However, at 0.1 fM, cardiac arrest was not observed in any animal. Saline (control, C) injection did not result in cardiac arrest in any animal.
Fig. 3.
Cardiac arrest after injection of different concentrations of dromyosuppressin (DMS) in the blowfly. Percentage of flies with arrest (A) and mean value ± SE of duration of cardiac arrest (B). Each DMS concentration was tested in 10 flies. Saline was applied as a control (C) in 10 flies. The values were: 2.10 ± 0.43 minutes, 1.50 ± 0.24 minutes, 1.31 ± 0.29 minutes, 0.99 ± 0.47 minutes, 0.40 ± 0.15 minutes and 0.15 ± 0.07 minutes for the 10 μM, 1 μM, 0.1 ηM, 1 ρM, 5 fM and 0.5 fM solutions, respectively.
Signal resumed in specimens injected with the 10 μM DMS solution after a period of cardiac arrest lasting an average of 2.1 ± 0.43 minutes (Fig. 3, B). This period of time shortened progressively at lower DMS concentrations. The times were 2.1 ± 0.43 minutes, 1.5 ± 0.24 minutes, 1.31 ± 0.29 minutes, 0.99 ± 0.47 minutes, 0.40 ± 0.15 minutes, and 0.15 ± 0.07 minutes for 10 μM, 1 μM, 0.1 ηM, 1 ρM, 5 fM, and 0.5 fM, respectively.
3.4 Specificity of DMS on the signal frequency of the fast phase and the slow phase
In order to investigate the effect of DMS on the fast phase and the slow phase of the ECG, the peptide was injected at various concentrations. Cardiac arrest was reversible. In all cases cardiac arrest was followed by the resumption of signal. However, the time course of the reversal varied between the two phases. The time course was dependent on the concentration of DMS (Fig. 4). The signal frequencies were reduced compared to preinjection values, even several minutes after the end of the period of cardiac arrest. A marked inhibition of both cardiac activities was induced by the 10 μM DMS solution, the strong effect of which continued to appear even after 10 minutes from the injection (39.95 ± 19.03% and 41.60 ± 23.33% reduction for the fast and the slow activity, respectively). In the case of 1 μM, 0.1 ηM, 1 ρM and 5 fM DMS, there was also a marked inhibition of both cardiac activities. However, the effect on the fast phase ceased within 2 minutes from the injections of 1 μM and 0.1 ηM solutions, while the effect on the slow phase was observed throughout the entire recording period (10 minutes). Interestingly, a considerable and prolonged inhibition of the slow phase only was caused by the 0.5 fM DMS solution, while the fast phase showed no significant variation. Therefore, a concentration range of the DMS solution between 5 fM and 0.5 fM corresponded to the threshold value for the inhibitory effect on the fast phase. The injected solution no longer produced any variation even on slow activity signal frequency just by reducing the concentration to 0.1 fM. Therefore, a concentration range of the DMS solution between 0.5 fM and 0.1 fM corresponded to the threshold value for the inhibitory effect on slow activity. In the control group, signal frequency in both cardiac phases was not found to have changed immediately after injection (time 0; Fig. 4), and showed non-significant oscillations (empty squares and triangles) compared to the preinjection value in the following 10 minutes.
Fig. 4.
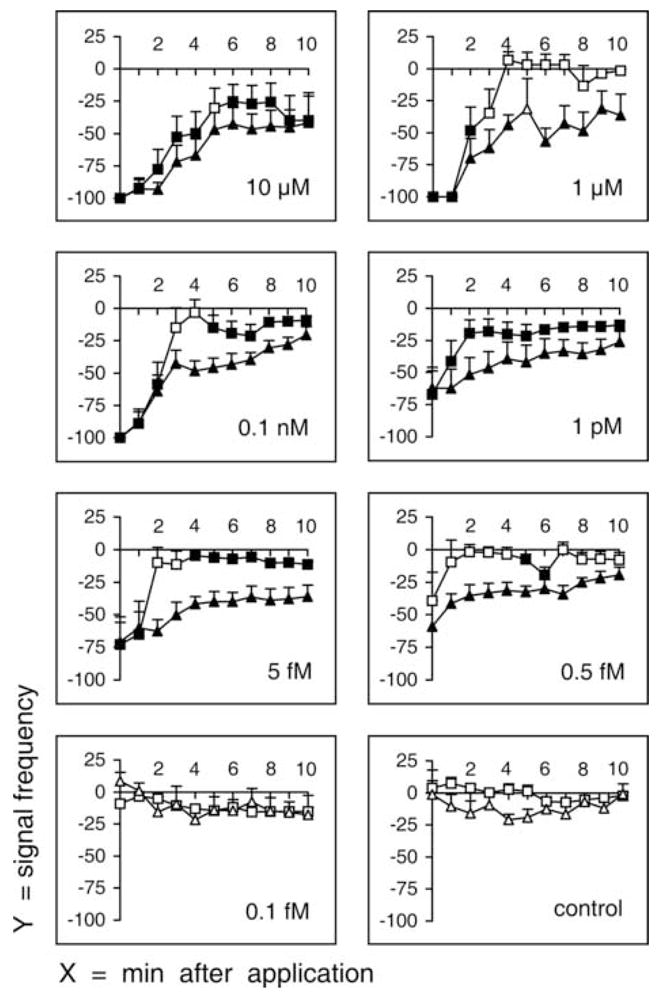
Signal frequency of fast phase and slow phase after injection of different concentrations of dromyosuppressin (DMS). Fast phases and slow phases were recorded immediately after injection (time 0) and continuously for 10 minutes (x-axis, minutes after injection). Signal frequency (y-axis) is reported as mean value ± SE (percentage variation following injection; squares, fast phase; triangles, slow phase). Each DMS concentration was tested in 10 flies. Saline was applied as a control in 10 flies. Filled symbols indicate significance (P < 0.05; Wilcoxon Test).
The effective concentrations for half-maximal effect (EC50s) at 2 minutes were determined to be 3 ρM for the fast phase and 65 ρM for the slow phase (Fig. 5). During the time interval from 0 to 2 minutes both the fast phase and the slow phase showed a full range of responses over the various DMS concentrations used. Thus, values at 2 minutes, a time point in this interval, were chosen for EC50 calculations.
Fig. 5.
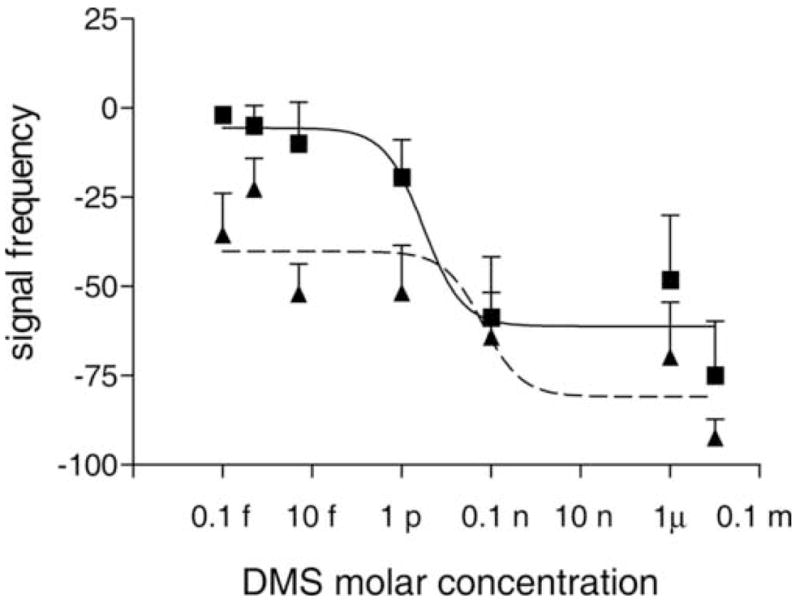
Dose dependence of the frequency of the fast phase and the slow phase after injection of dromyosuppressin (DMS). The effects of DMS on the signal frequencies of the fast and the slow phases were analyzed with Prism software version 3.0 (Prism Software Corporation, Irvine, CA, USA). The mean values and standard errors for the fast phase (squares; solid line) and slow phase (triangles; dashed line) are reported. The effective concentration for half-maximal effect (EC50) calculated with the software was 3 ρM for the fast phase and 65 ρM for the slow phase.
3.5 Effects of alanyl-substituted DMS and free acid analogs on the fast phase and the slow phase
The effects of alanyl-substituted DMS analogs and DMS free acid were determined at 1 μM. This concentration was used because 1 μM DMS caused noticeable but not maximal effects for the fast phase and the slow phase (Fig. 6). Thus, an analog more agonistic than the parent peptide might be identified.
Fig. 6.
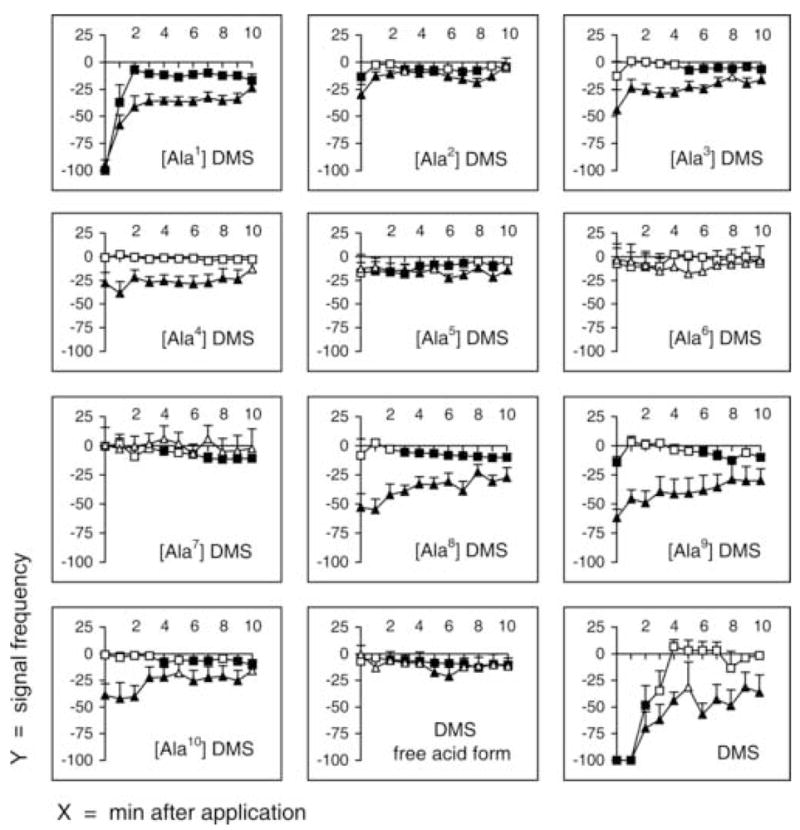
Signal frequency of fast phase and slow phase after injection of alanyl-substituted dromyosuppressin (DMS) analogs and DMS free acid. Fast phase and slow phase were recorded immediately after injection (time 0) and continuously for 10 minutes (x-axis, minutes after injection). Signal frequency (y-axis) is reported as mean values ± SE (percentage variation following injection; squares, fast phase; triangles, slow phase). DMS and each analog (1 μM) were tested individually, using an animal only once, in 10 flies. Filled symbols indicate significance (P < 0.05; Wilcoxon Test).
The [Ala1] DMS analog decreased the signal frequencies of both the fast phase and the slow phase similar to the parent peptide (Fig. 6). Cardiac arrest occurred at 0 minutes for both the fast phase and the slow phase. Similar to DMS, the effect on the frequency of the slow phase was prolonged throughout the 10-minutes recording period. However, at 2 minutes, the effect on the fast phase returned to −7.16 ± 3.78% which is approximately the value before injection. Thus, threonine (T1) was identified as not essential for the effects of DMS on the frequency of the fast and the slow phases.
None of the remaining nine alanyl-substituted DMS analogs, nor the DMS free acid, led to important variations in fast phase signal frequency, the maximum reductions of which amounted barely to 13.55 ± 3.51% and 14.29 ± 5.22% with the DMS analogs [Ala2] DMS and [Ala9] DMS, respectively (Fig. 6).
Contrary to signal frequency of the fast phase, that of the slow phase was consistently reduced by the effect of several of the remaining DMS analogs, specifically [Ala2] DMS, [Ala3] DMS, [Ala4] DMS, [Ala8] DMS, [Ala9] DMS, and [Ala10] DMS. Thus, replacement of the aspartic acid (position 2), valine (position 3), aspartic acid (position 4), leucine (position 8), arginine (position 9), and phenylalanine (position 10) with alanine led to a partial retention of slow activity inhibition. Overall, the effects of the analogs were much less in comparison to the effects of the un-substituted DMS parent peptide.
Interestingly, only three of all the alanyl-substituted DMS analogs, specifically [Ala5] DMS, [Ala6] DMS, [Ala7] DMS, and the DMS free acid form caused no variation in the slow phase signal frequency. Thus, replacement of histidine (position 5), valine (position 6), and phenylalanine (position 7) with alanine, as well as loss of the amide group led to inactive analogs.
3.6 Effects of truncated peptide DMS analogs on the fast phase and the slow phase
The effects of truncated analogs were determined at 1 μM (Fig. 7). N-terminal truncations were used because FaRPs are reported to require the C-terminal amide for activity [14, 21, 22]. The DMS (2–10) analog, DVDHVFLRFamide, in which the threonine or the N terminus is absent, decreased the frequencies of both the fast phase and the slow phase similar to the parent peptide (Fig. 7). At 0 minutes, the frequency of the fast phase was −84 ± 16.02% and the slow phase was −80.9 ± 9.75%. Similar to DMS, the effect on the frequency of the slow phase was prolonged throughout the 10-minutes recording period. Thus, the threonine (T1) was not essential for the effects of DMS on the frequency of the fast phase and the slow phase.
Fig. 7.
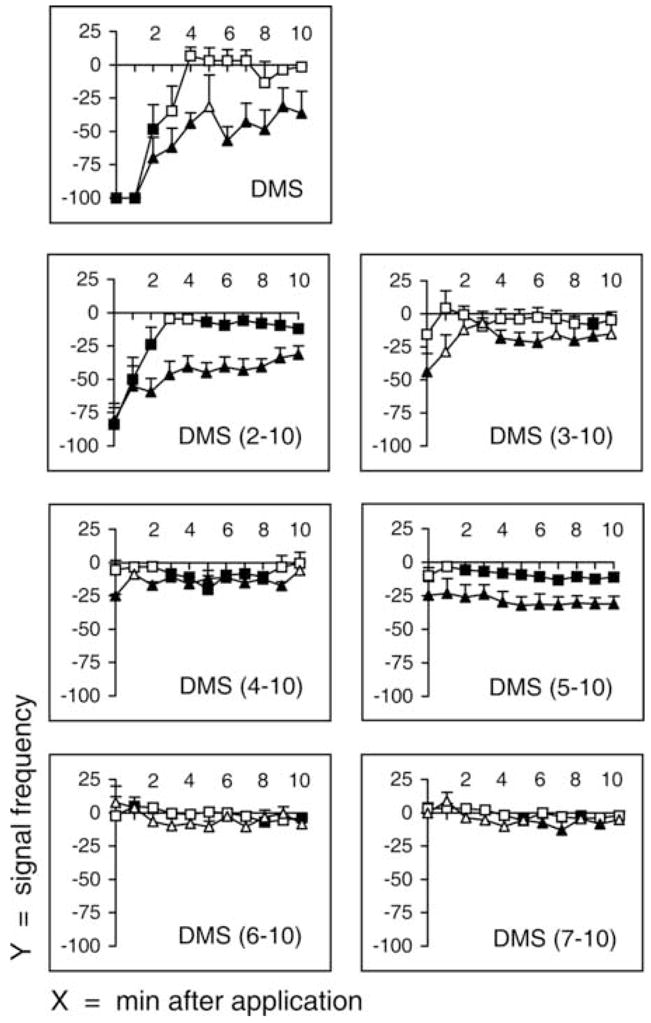
Signal frequency of fast phase and slow phase after injection of truncated dromyosuppressin (DMS) analogs. Fast and slow phases were recorded immediately after injection (time 0) and continuously for 10 minutes (x-axis, minutes after injection). Frequency (y-axis) is reported as mean values ± SE (percentage variation following injection; squares, fast phase; triangles, slow phase). DMS and each analog (1 μM) were tested individually, using an animal only once, in 10 flies. Filled symbols indicate significance (P < 0.05; Wilcoxon Test).
The N-terminally truncated analogs DMS (3–10), DMS (4–10), and DMS (5–10) did not arrest the fast phase; they were different from the parent peptide and DMS (2–10). Thus, aspartic acid (D2), valine (V3), and aspartic acid (D4) were essential for the effects of DMS on the frequency of the fast phase. In contrast to no effects of these analogs on the fast phase, the frequency of the slow phase was decreased by these analogs. Specifically, DMS (3–10), DMS (4–10), and DMS (5–10) decreased the frequency of the slow phase to −43.77 ± 13.74%, −25.07 ± 1.11%, and −24.76 ± 10.30% at 0 minutes, respectively. Thus, valine (V3), aspartic acid (D4), and histidine (H5) were not essential for the effects of DMS on the frequency of the slow phase. However, DMS (6–10) and DMS (7–10) did not decrease the frequency of both the fast phase and the slow phase. Thus, valine (V6) and phenylalanine (F7) were essential for the effects of DMS on the frequency of the fast phase and the slow phase.
4. Discussion
Cardiac activity is a critical biological process; thus, it is essential to delineate the molecular mechanisms involved in initiation and control of heartbeat. Peptides play important roles as regulators of cardiac muscle contractions. Therefore, it is crucial to elucidate peptidergic mechanisms involved in cardiac activity in order to understand how aberrant peptide synthesis, expression, or signalling may affect animal development and health.
The cardiac activity of the blowfly P. terraenovae is a good model for the study of heartbeat because it is composed of a fast phase and a slow phase. Here, we report the first data on blowfly cardiac peptidergic innervation and control of both the fast phase and the slow phase. Other publications describe the effects of biogenic amines and peptides on fly heartbeat [9 – 11, 15, 28]. However, to our knowledge, no literature reports on the sites and mechanisms by which a single peptide acts on fly cardiac tissue to regulate both the fast phase and the slow phase. Also, no report describes the structure-activity relationship of a molecular mechanism to produce cardiac arrest, a natural biological phenomenon.
Our immunological and molecular data showed DMS immunoreactive material is present in posterior and anterior cardiac tissue. Our data also demonstrate exogenously applied DMS acts at a physiological concentration on the fast phase and the slow phase, likely at two different pacemakers because of the different SARs, to cause cardiac arrest. Our immunological staining data show DMS antisera recognized neuronal processes that innervated the posterior and the anterior of the blowfly dorsal vessel where pacemakers are known to exist that generate the slow phase and the fast phase of cardiac activity. Thus, our immunological data are consistent, but not conclusive, with DMS being delivered from a posterior and an anterior pacemaker to act in the physiological effects we measured on cardiac activity.
Dromyosuppressin decreased the frequency of both the fast phase and the slow phase in a dose dependent manner. However, the dynamics of recovery for the fast phase and the slow phase differed. In addition, our SAR data demonstrate the amino acid residues required for the effects of DMS on the fast phase and the slow phase are different. These data are consistent with the conclusion that DMS acts through two different mechanisms to regulate the fast phase and the slow phase of the cardiac cycle. The D. melanogaster genome database contains two genes encoding predicted myosuppressin receptors; when the genes were expressed the proteins bound DMS [12]. The presence of two myosuppressin receptors in fruitfly is consistent with, but indirect support of the conclusion DMS acts through two different mechanisms in blowfly.
The [Ala9] DMS analog in which an alanyl residue is substituted for the arginyl residue was inactive on the fast phase, yet, active on the slow phase. These are the first data, to our knowledge, that demonstrate the arginyl residue in myosuppressin is not required which is in contrast to its requirement for hindgut muscle activity [21] or α-amylase release [22]. However, there are several experimental parameters that should be considered as a possible basis for the difference; the animals and tissues examined, blowfly heart versus cockroach gut, and types of assay, in vivo versus in vitro, and heart contractions versus stimulation of enzyme release. The in vitro ligand-receptor binding conducted on the expressed myosuppressin proteins did not specifically address the requirement for a C-terminal RFamide [12]. The molecular explanation for this difference in structure requirement awaits further investigations including delineating ligand-receptor binding studies and tissue specificity of the myosuppressin receptors
Our immunohistochemical data show DMS may be delivered to the heart and aorta by anterior and posterior dorsal vessel. Thus, our bioassay data and immunohistochemical data are consistent with the conclusion that DMS or a DMS-like material acts at the posterior and the anterior of the dorsal vessel, the heart and the aorta, respectively, to cause cardiac arrest by two different molecular mechanisms. The presence of FaRPs throughout the animal kingdom and the high degree of myosuppressin peptide structure conservation and activity suggest our findings are broadly applicable to advance the knowledge about the role of these conserved peptides in animal biology.
Acknowledgments
This research was supported by a MIUR-PRIN grant to AMA and by a NIMH grant 5R03MH062177 to RN
Abbreviations
- DMS
dromyosuppressin
- ECG
electrocardiogram
- EC50
effective concentration for half-maximal effect
- FaRP
FMRFamide-related peptide
- FMRFamide
phenylalanine-methionine-arginine-phenylalanine-NH2
- SAR
structure-activity relationship
Footnotes
DMS acts on fast and slow cardiac activity
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Angioy AM, Liscia A, Pietra P. Cyclic sensitivity variations in the labellar chemosensilla of Calliphora. Experientia. 1983;39:546–47. [Google Scholar]
- 2.Angioy AM, Tomassini Barbarossa I, Crnjar R, Liscia A, Pietra P. Reflex cardiac response to various olfactory stimuli in the blowfly, Protophormia terraenovae. Neurosci Lett. 1987;81:263–6. doi: 10.1016/0304-3940(87)90393-4. [DOI] [PubMed] [Google Scholar]
- 3.Angioy AM. Reflex cardiac response to a feeding stimulus in the blowfly Calliphora vomitoria L. J Insect Physiol. 1988;34:21–7. [Google Scholar]
- 4.Angioy AM, Pietra P. Mechanisms of beat reversal in semi-intact heart preparations of the blowfly Phormia regina (Meigen) J Comp Physiol B. 1995;165:165–170. [Google Scholar]
- 5.Angioy AM, Boassa D, Dulcis D. Functional morphology of the dorsal vessel in the adult fly Protophormia terraenovae (Diptera, Calliphoridae) J Morphol. 1999;240:15–31. doi: 10.1002/(SICI)1097-4687(199904)240:1<15::AID-JMOR2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 6.Brazeau L, Campan R. Sur l’automatisme cardiaque de Calliphora vomitoria. CR Acad Sci Paris. 1970;271:2354–6. [Google Scholar]
- 7.Campan R. Light-induced heart-beat disturbances: comparative studies in Calliphora vomitoria (Linnaeus 1758) (Diptera) and Nemobius sylvestris (Bosc 1792) (Orthoptera) Monit Zool Ital. 1972;6:269–89. [Google Scholar]
- 8.Chen AC, Friedman S. An isotonic saline for the adult blowfly, Phormia regina, and its application to perfusion experiments. J Insect Physiol. 1975;21:529–36. doi: 10.1016/0022-1910(75)90158-4. [DOI] [PubMed] [Google Scholar]
- 9.Dulcis D, Levine RB. Innervation of the heart of the adult fruit fly, Drosophila melanogaster. J Comp Neurol. 2003;465:560–78. doi: 10.1002/cne.10869. [DOI] [PubMed] [Google Scholar]
- 10.Dulcis, Levine RB. Glutamatergic innervation of heart initiates retrograde contractions in adult Drosophila melanogaster. J Neurosci. 2005;25:271–80. doi: 10.1523/JNEUROSCI.2906-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dulcis D, Levine RB, Ewer J. Role of the neuropeptide CCAP in Drosophila cardiac function. J Neurobiol. 2005;64:259–74. doi: 10.1002/neu.20136. [DOI] [PubMed] [Google Scholar]
- 12.Egerod K, Reynisson E, Hauser F, Cazzamali G, Williamson M, Grimmelikhuijzen CJ. Molecular cloning and functional expression of the first two specific insect myosuppressin receptors. Proc Natl Acad Sci USA. 2003;100:9808–13. doi: 10.1073/pnas.1632197100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fónagy A, Schoofs L, Proost P, Van Damme J, Bueds H, De Loof A. Isolation, primary structure, and synthesis of neomyosuppressin, a myoinhibiting neuropeptide from the grey fleshfly, Neobellieria bullata. Comp Biochem Physiol. 1992;102C:239–45. doi: 10.1016/0742-8413(92)90107-i. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg MJ, Price DA. Relationships among the FMRFamide-like peptides. Prog Brain Res. 1992;92:25–37. doi: 10.1016/s0079-6123(08)61162-0. [DOI] [PubMed] [Google Scholar]
- 15.Holman GM, Cook BJ, Nachman RJ. Isolation primary structure and synthesis of leucomyosuppressin, an insect neuropeptide that inhibits spontaneous contractions of the cockroach hindgut. Comp Biochem Physiol [C] 1986;85:329–33. doi: 10.1016/0742-8413(86)90077-0. [DOI] [PubMed] [Google Scholar]
- 16.Johnson E, Ringo J, Dowse H. Native and heterologous neuropeptides are cardioactive in Drosophila melanogaster. J Insect Physiol. 2000;46:1229–36. doi: 10.1016/s0022-1910(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 17.McCormick J, Nichols R. Spatial and temporal expression identify dromyosuppressin as a brain-gut peptide in Drosophila melanogaster. J Comp Neurol. 1993;338:272–88. doi: 10.1002/cne.903380210. [DOI] [PubMed] [Google Scholar]
- 18.Medioni J, Campan R. Etude préliminaire de l’effet de la stimulation lumineuse sur le rythme cardiaque de la mouche Calliphora. J Physiol Paris. 1967;59:264–5. [PubMed] [Google Scholar]
- 19.Meola SM, Wright MS, Holman GM, Thompson JM. Localization of leucomyosuppressin-like peptides in the central nervous system of the stable fly with immunocytochemistry. J Med Entomol. 1991;28:712–8. doi: 10.1093/jmedent/28.5.712. [DOI] [PubMed] [Google Scholar]
- 20.Meola SM, Wright MS, Nichols R, Pendleton MW. Localization of myosuppressin-like peptides in the hypocerebral ganglion of two blood-feeding flies: horn fly and stable fly (Diptera: Muscidae) J Med Entomol. 1996;33:473–81. doi: 10.1093/jmedent/33.3.473. [DOI] [PubMed] [Google Scholar]
- 21.Merte J, Nichols R. Drosophila melanogaster myotropins have unique functions and signaling pathways. Peptides. 2002;23:787–94. doi: 10.1016/s0196-9781(01)00670-2. [DOI] [PubMed] [Google Scholar]
- 22.Nachman RJ, Giard W, Lange A, Favrel P. Stimulation of alpha-amylase release in the scallop Pecten maximus by the myosuppressins. Structure-activity relationships. Ann N Y Acad Sci. 1999;897:273–81. doi: 10.1111/j.1749-6632.1999.tb07898.x. [DOI] [PubMed] [Google Scholar]
- 23.Nachman RJ, Holman GM, Hayes TK, Beier RC. Structure-activity relationships for inhibitory insect myosuppressins: contrast with the stimulatory sulfakinins. Peptides. 1993;14:665–70. doi: 10.1016/0196-9781(93)90095-x. [DOI] [PubMed] [Google Scholar]
- 24.Nichols R. Isolation and structural characterization of Drosophila TDVDHVFLRFamide and FMRFamide-containing neural peptides. J Mol Neurosci. 1992a;3:213–8. doi: 10.1007/BF03380141. [DOI] [PubMed] [Google Scholar]
- 25.Nichols R. Isolation and expression of the Drosophila drosulfakinin neural peptide gene product, DSK-I. Mol Cell Neurosci. 1992b;3:342–7. doi: 10.1016/1044-7431(92)90031-v. [DOI] [PubMed] [Google Scholar]
- 26.Nichols R, McCormick J, Lim I, Starkman J. Spatial and temporal analysis of the Drosophila FMRFamide neuropeptide gene product SDNFMRFamide: evidence for a restricted cellular expression pattern. Neuropeptides. 1995a;29:205–13. doi: 10.1016/0143-4179(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 27.Nichols R, McCormick J, Lim I, Caserta L. Cellular expression of the Drosophila neuropeptide, DPKQDFMRFamide: evidence for differential processing of the FMRFamide polypeptide precursor. J Mol Neurosci. 1995b;6:1–10. doi: 10.1007/BF02736754. [DOI] [PubMed] [Google Scholar]
- 28.Nichols R, McCormick J, Lim I. Multiple antigenic peptides designed to structurally-related Drosophila peptides. Peptides. 1997;18:41–5. doi: 10.1016/s0196-9781(96)00279-3. [DOI] [PubMed] [Google Scholar]
- 29.Nichols R, Kaminski S, Walling E, Zornik E. Regulating the activity of a cardioacceleratory peptide. Peptides. 1999;20:1153–8. doi: 10.1016/s0196-9781(99)00118-7. [DOI] [PubMed] [Google Scholar]
- 30.Nichols R. Signaling pathways and physiological functions of Drosophila melanogaster FMRFamide-related peptides. Annu Rev Entomol. 2003;48:485–503. doi: 10.1146/annurev.ento.48.091801.112525. [DOI] [PubMed] [Google Scholar]
- 31.Normann TC. Heart activity and its control in the adult blowfly, Calliphora erytrocephala. J Insect Physiol. 1972;18:1793–810. [Google Scholar]
- 32.Posnett DN, Tam JP. Multiple antigenic peptide method for producing antipeptide site-specific antibodies. In: Langone JJ, editor. Methods in Enzymology. Vol. 178. San Diego: Academic Press; 1989. pp. 739–746. [DOI] [PubMed] [Google Scholar]
- 33.Price DA, Greenberg MJ. Structure of a molluscan neuropeptide. Science. 1977;197:670–1. doi: 10.1126/science.877582. [DOI] [PubMed] [Google Scholar]
- 34.Price DA, Greenberg MJ. The hunting of the FaRPs: the distribution of FMRFamide-related peptides. Biol Bull. 1989;177:198–205. [Google Scholar]
- 35.Queinnec Y, Campan M. Influence of sexual maturation on cardiac activity and reactivity of Calliphora vomitoria. I -- Cardiac activity J Physiol Paris. 1975;70:457–66. [PubMed] [Google Scholar]
- 36.Raffa RB. The action of FMRFamide (Phe-Met-Arg-Phe-NH2) and related peptides on mammals. Peptides. 1988;9:915–22. doi: 10.1016/0196-9781(88)90141-6. [DOI] [PubMed] [Google Scholar]
- 37.Richer S, Stoffolano JG, Yin CM, Nichols R. Innervation of dromyosuppressin (DMS) immunoreactive processes and effect of DMS and benzethonium chloride on the Phormia regina (Meigen) crop. J Comp Neurol. 2000;421:136–42. [PubMed] [Google Scholar]
- 38.Robb S, Packman LC, Evans PD. Isolation, primary structure and bioactivity of SchistoFLRF-amide, a FMRF-amide-like neuropeptide from the locust, Schistocerca gregaria. Biochem Biophys Res Comm. 1989;160:850–6. doi: 10.1016/0006-291x(89)92512-6. [DOI] [PubMed] [Google Scholar]
- 39.Thon B. Habituation of cardiac and motor responses to a moving visual stimulus in the blowfly Calliphora vomitoria. J Comp Physiol Psychol. 1980;94:886–93. doi: 10.1037/h0077821. [DOI] [PubMed] [Google Scholar]
- 40.Thon B. Influence of the cardiac phase on the latency of a motor response to a visual stimulus in the blowfly. J Insect Physiol. 1982;28:411–6. [Google Scholar]
- 41.Thon B. Préparation à l’action et processus d’acquisition: une approche expérimentale chez l’insecte. Thèse Doct Spéc Univ Paul Sabatier; Toulouse, France. 1987. [Google Scholar]