Abstract
Human pancreatic cancer remains a highly malignant disease with almost similar incidence and mortality despite extensive research. Many targeted therapies are under development. However, clinical investigation showed that single targeted therapies and most combined therapies were not able to improve the prognosis of this disease, even though some of these therapies had excellent anti-tumor effects in pre-clinical models. Cross-talk between cell proliferation signaling pathways may be an important phenomenon in pancreatic cancer, which may result in cancer cell survival even though some pathways are blocked by targeted therapy. Pancreatic cancer may possess different characteristics and targets in different stages of pathogenesis, maintenance and metastasis. Sensitivity to therapy may also vary for cancer cells at different stages. The unique pancreatic cancer structure with abundant stroma creates a tumor microenvironment with hypoxia and low blood perfusion rate, which prevents drug delivery to cancer cells. In this review, the most commonly investigated targeted therapies in pancreatic cancer treatment are discussed. However, how to combine these targeted therapies and/or combine them with chemotherapy to improve the survival rate of pancreatic cancer is still a challenge. Genomic and proteomic studies using pancreatic cancer samples obtained from either biopsy or surgery are recommended to individualize tumor characters and to perform drug sensitivity study in order to design a tailored therapy with minimal side effects. These studies may help to further investigate tumor pathogenesis, maintenance and metastasis to create cellular expression profiles at different stages. Integration of the information obtained needs to be performed from multiple levels and dimensions in order to develop a successful targeted therapy.
Keywords: Pancreatic ductal adenocarcinoma, molecular targeted therapy, pancreatic stroma, drug resistance, gene therapy, monoclonal antibody, kinase inhibitors
INTRODUCTION
For decades, scientists, both in clinical as well as basic research areas, have worked intensively to make a major breakthrough in human pancreatic cancer treatment and to improve its clinical prognosis. Unfortunately, little progress has been achieved. In a recent report from Ellison et al., the five-year relative survival for pancreatic cancer remains at 6% [1]. Human pancreatic cancer treatment remains a serious challenge in oncology.
Although complete surgical resection of the tumor mass is the most effective regimen in pancreatic cancer treatment, it can only be used in limited cases with localized tumors and there exists a significant rate of cancer relapse [2]. Chemotherapy, radiation therapy and adjuvant therapy together with palliative treatment are other approaches for pancreatic cancer treatment. Among these therapies, gemcitabine has been recognized by many oncologists as the first-line drug to treat pancreatic cancer for more than a decade [3]. With the progression of in-depth research on pancreatic cancer, several important pathologic processes related to pancreatic cancer pathobiology have been elucidated and many therapeutic strategies targeting key molecules in these processes have been developed. For example, cetuximab as well as erlotinib are used to target the EGFR pathway to inhibit tumor growth. Bevacizumab is used for targeting VEGF to inhibit angiogenesis. Imetelstat was produced to inhibit telomerase activity in both pancreatic cancer cells as well as cancer stem cells [4]. GDC-0449 is used to target hedgehog pathway inhibiting Smoothened activation [5]. In recent years, many of these targeted therapeutic approaches in pancreatic cancer treatment have been tested in clinical trials (http://www.cancer.gov). However, most of these trials, including combination treatment with gemcitabine, did not improve overall survival rate of pancreatic cancer. It is reasonable to conclude that single pathway targeted treatments and currently used combined therapies do not block the pathogenesis and metastasis of pancreatic cancer sufficiently. Multidimensional therapeutic strategies for pancreatic cancer should be considered and designed by using network and systems biology approaches [6, 7]. In this review, currently available targeted therapies are discussed, with an emphasis on those that have been developed pre-clinically and translated into clinical trials. However, much work needs to be done in terms of how to use these therapies effectively to achieve success in pancreatic cancer treatment.
The optimal treatment of pancreatic cancer may rely on an improved understanding of genetics and biology of pancreatic cancer [8], which may be closely associated with tumorigenesis and metastasis formation. Further research to identify critical genes and the role of their downstream signals in pancreatic cancer pathogenesis is still an urgent need. However, individual signal transduction pathways may have cross-talk with other signaling cascades. In normal cells, the specific biological effect of a pathway in response to a stimuli may be maintained by filtering out spurious cross-talk through mutual inhibition [9]. Specific targeted therapy can easily hit the target in these conditions. In cancer cells, abundant crosstalk between different pathways could make the target a “moving target” [10]. Multiple points of interaction of two pathways allow for the discrete pairing of cell cycle activation and regulation of protein translation [11]. Thus, the specific target inhibitor may be ineffective and downstream signaling to promote tumor growth may escape the treatment. Cross-talk between insulin/insulin-like growth factor 1 (IGF-1) and G protein-coupled receptor (GPCR) signaling pathways has been proposed as a mechanism of pancreatic cancer cell proliferation [12]. Metformin, a drug used to treat diabetes was reported to break this cross-talk [13]. Similarly, crosstalk may disturb normal feedback mechanisms. In cancer, taking the interaction between kinases and proteases as an example, enormous diversity and complexity of their bidirectional communication processes were found [14]. To break this interrelationship among pathways, combined molecular targeted therapies can be applied. However, a significant number of clinical trials with various combinations of chemotherapy and molecular targeted therapies have not led to a major breakthrough yet. Chemotherapeutic agents are still the main components in pancreatic cancer treatment. A recent phase III trial using FOLFIRINOX (5-FU, leucovorin, irinotecan and oxaliplatin) vs. gemcitabine showed a significant prolongation of overall survival in metastatic pancreatic cancer patients (11.1 vs. 6.8 months) [15]. Minimizing side effects from the treatment is also an important issue. Integration of targeted therapies with chemotherapies may still be an applicable strategy to reach that goal. Additional investigation to further explore these promising regimens and to search for critical genes or proteins may supply valuable information leading to important new targeting agents. Identifying and combining molecular targeted therapies against different targets in pancreatic cancer treatment requires extensive analysis and integration of crucial interacting pathways [6]. Network and systems biology approaches are necessary in understanding and evaluation of multiple drug combinations in cancer treatment [6].
Another challenge in pancreatic cancer treatment is to identify underlying pathological defects and to integrate them with molecular biomarkers or gene mutations [10]. Analysis of histological classification and anatomical extent of tumor combined with their molecular features may help to create tailored, personalized multidimensional therapies [7]. Identification and analysis of effective, combined targeted therapies may help to generate better strategies in treating pancreatic cancer.
Targeted Molecules on the Cell Surface
In targeting cell surface molecules, monoclonal antibodies are the main tools that have been used. Monoclonal antibodies may serve as a receptor pathway inhibitor or as a ligand to activate certain pathways. Humanized monoclonal antibodies are used clinically to decrease the immune response to murine products.
EGF Receptor
EGF receptors comprise four subtypes EGFR/ERBB1, HER2/ErbB2, HER3/ErbB3 and HER4/ErbB4. EGF, TGF-α, heparin-binding EGF-like growth factor and neuregulins serve as their ligands. The EGFR signal transduction pathway is essential for tissue and organ development and regulation of cell migration, adhesion and proliferation. Activation of EGFR in cancer is associated with oncogenesis, angiogenesis, apoptosis inhibition and tumor metastasis. Recently, Fujita et al. reported that pancreatic cancer patients with high EGFR mRNA expression in tumor tissue had shorter disease-free-survival and overall survival [16].
Cetuximab is a chimeric mouse-human antibody that binds to EGFR. Pre-clinical studies showed cetuximab decreased cell proliferation and phosphorylation of EGFR, and blocked the binding of the adaptor protein Grb2 to EGFR upon activation by EGF [17]. Morgan et al. reported cetuximab together with gemcitabine and radiation effectively prolonged pancreatic cancer xenograft volume doubling time, and produced a synergistic effect with gemcitabine and radiation [18]. However, an enhanced antitumor effect was not observed in pancreatic cancer clinical trials with cetuximab combined with gemcitabine, or gemcitabine/cisplatin [19–21]. In a phase III study of gemcitabine plus cetuximab vs. gemcitabine alone the median survival was 6.3 months with progression-free survival of 3.4 months in the combination treatment group, vs. 5.9 months and 3 months respectively in the gemcitabine group [20]. Recently, cetuximab was used in combination with capecitabine, radiation and ixabepilone in phase II clinical trials, although no obvious improvement was observed [22, 23]. There are several other ongoing clinical trials although using a combination of cetuximab, irinotecan and oxaliplatin, or cetuximab, everolimus and capecitabine [http://www.cancer.gov].
Other antibodies against EGFR include panitumumab, matuzumab (EMD 72000), and nimotuzumab. Several studies have been conducted in pre-clinical as well as clinical trials using these antibodies [24–26]. Another chimeric monoclonal antibody CH 806 is an antibody against mutant EGFR. There is no information available yet for this antibody in pancreatic cancer treatment.
HER2/ErbB2
HER2/ErbB2, a member of the EGFR family, was found to be overexpressed in pancreatic cancer. Tsiambas et al. reported 42% positive staining of HER2 with 16% gene amplification in pancreatic cancer tissue [27]. A recent report showed overexpression of HER2 in 61.2% of tissue samples from pancreatic cancer patients without metastasis and overexpression of HER2 positively correlated with shorter survival time of these patients [28]. Expression of HER2 was found to be related with the expression of MUC4 protein. Down-regulation of MUC4 led to decreased expression and phosphorylation of HER2, with subsequent decreased phosphorylation of its downstream signal proteins FAK and p44/42 [29]. Trastuzumab (Herceptin) is a humanized antibody against HER2. Pre-clinical studies using this antibody showed significant growth inhibition of pancreatic cancer cell lines and xenografts. Additive effects were found when trastuzumab was combined with fluoropyrimidine S-1 [30]. In addition, trastuzumab induced antibody-dependent cellular cytotoxicity (ADCC) through antibody Fc receptors expressed on immune effector cells. ADCC contributed to the cytotoxicity of trastuzumab against pancreatic cancer cell lines [30, 31]. Clinical trials of trastuzumab have not yet achieved an improved prognosis for pancreatic cancer. Combination of trastuzumab with capecitabine treatment yielded a progression free survival at 12 weeks of 23.5% and median overall survival of 7.0 months [32]. Vidalpla et al. recently reported trastuzumab combined with cetuximab and gemcitabine induced a 2.1 fold increase in pancreatic cancer cell death and 2.3 fold reduction of vascularization in xenografts compared with gemcitabine plus either antibody as a double treatment [33]. Whether this pre-clinical study can be translated into a clinical trial with similar outcomes needs to be determined. 111In-labeled trastuzumab was generated and is now in a phase I clinical trial [34].
Another recombinant humanized monoclonal antibody against HER2, pertuzumab, has been used to treat solid tumors, including pancreatic cancer. Two pancreatic cancer patients showed partial responses with stable disease for 15.3 months in one patient [35]. A phase II clinical trial using this antibody together with erlotinib is ongoing [http://www.cancer.gov].
Death Receptor 5
Death receptor (DR) 5 belongs to the tumor necrosis factor receptor (TNFR) superfamily. Its ligand is TNF-related apoptosis-inducing ligand (TRAIL). This transmembrane receptor forms a homotrimer after ligand binding and subsequently induces the formation of a death-inducing signaling complex (DISC) which activates caspase 8-triggered apoptosis. The intrinsic mitochondrial pathway is also activated and contributes to apoptosis [36–39]. Normal cells are in general resistant to TRAIL induced apoptosis despite the presence of DR5 on the cell surface. Tumor cell lines from diverse origins including pancreatic cancer are sensitive to this apoptotic process [40].
Tigatuzumab (CS-1008) is a humanized antibody against DR5 [41]. CS-1008 is derived from a mouse anti-human DR5 antibody TRA-8 [41]. TRA-8 activates the apoptosis pathways in a variety of tumor cell lines [42]. In an orthotopic pancreatic cancer mouse model, TRA-8 produced synergistic cytotoxicity with gemcitabine in a panel of pancreatic cancer cell lines [43]. TRA-8 combined with irinotecan prolonged mouse survival time longer than monotherapy with TRA-8 or irinotecan [44]. In another pancreatic cancer xenograft model, TRA-8 treatment combined with gemcitabine significantly prolonged tumor doubling time compared with gemcitabine monotherapy. Combination treatment reduced the pancreatic cancer stem cell population in the tumor tissues as compared to monotherapy with gemcitabine [45].
Conatumumab (AMG655) is another humanized antibody against DR5 developed by Amgen Inc. In pre-clinical studies, AMG 655 produced synergistic cytotoxicity when combined with gemcitabine and irinotecan [46]. Ongoing phase I clinical trials are testing this antibody in solid tumor treatment [47, 48]. Another human single chain antibody against DR5, Apomab, was developed by Genentech. In pre-clinical models of pancreatic cancer, complete remission of tumors was observed in 100% of mice after a single injection of Apomab (10 mg/kg). Combination treatment with this antibody and gemcitabine induced partial remission in seven and a complete remission in one of ten mice [49]. It was reported recently that the sensitivity to Apomab was correlated with the expression of O-glycosyltransferase GALNT14 in pancreatic cancer cells [50]. More information needs to be obtained from further evaluation of TRAIL death receptor antibodies in clinical trials.
Insulin-like Growth Factor-1 Receptor (IGF-1R)
IGF-1R is a transmembrane receptor tyrosine kinase and has been found to be overexpressed in pancreatic cancer. Activation of IGF-1R has been correlated with decreased apoptosis, cancer cell proliferation and angiogenesis [51, 52]. IGF-1R induced pancreatic cancer proliferation and invasion were related to the down-regulation of the tumor suppressor chromosome 10 (PTEN) phosphorylation and activation of the PI3K/Akt signaling pathway [53]. Blockage of IGF-1R activation also decreased the expression of the matrix metalloproteinase, MMP7, which was shown to be related with tumor invasion [54]. Pancreatic cancer cell proliferation and motility were significantly suppressed by a specific IFG-1R inhibitor picropodophyllin in vitro [55]. Insulin receptor and IGF-1R share high homology in amino acid sequences. In human multiple myeloma cells, insulin was found to be a myeloma growth factor and to induce insulin receptor (IR) and IGF-1R hybridization and phosphorylation [56]. In a pancreatic neuroendocrine cancer mouse model, high expression level of IR (or high IR to IGF-1R expression ratio) was related to anti-IGF-1R treatment resistance [57]. Investigation of the receptor expression profile in tumor tissues may help to determine if dual targeting against IR and IGF-1R would be beneficial. As mentioned above, there exists cross-talk between IR/IGF-1R and GPCR signaling pathways. This cross-talk may occur at multiple levels, from receptors to downstream cascades, causing a series of long term biological consequences [12]. In a recent report by Kisfalvi et al., the cross-talk between IR/IGF-1R and GPCR pathways was mediated by mTOR, which was inhibited by rapamycin. Metformin, a drug to treat type II diabetes, disrupted this cross-talk by inhibition of mTOR function and inhibited human pancreatic PANC1 and MIA PaCa-2 xenograft growth in nude mice [13].
Cixutumumab (IMC-A12) is a human IgG1 antibody that binds to IGF-1R developed by ImClone Systems Incorporated. In mice bearing BxPC-3 tumors, IMC-A12 used a single agent at 1 mg/kg every 3 days inhibited tumor growth by 80% [58]. Additive effects were observed when IMC-A12 was combined with cetuximab and gemcitabine in pancreatic cancer xenograft models [59]. Combination therapy using this antibody together with gemcitabine and erlotinib is in a phase II clinical trial of pancreatic cancer patients [21].
AMG479, another human anti-IGF-1R antibody has been tested in vitro and in pancreatic cancer mouse models. AMG 479 completely inhibited ligand (IGF-1, IGF-II and insulin) induced activation of IGF-1R homodimer and IGF-1R/IR heterodimer, and decreased cell viability. In a mouse model, it inhibited by 80% xenograft tumor growth and an additive effect with gemcitabine was observed [60]. This antibody is now in a phase I clinical trial in patients with solid tumors and non-Hodgkin’s lymphoma [61]. RAV12 is a chimeric antibody against an N-linked carbohydrate antigen (RAAG12). RAAG12 was found to coexist with many membrane proteins, including IGF-1R, ALCAM, IFN-γR1, TfR, IR, and EGFR. RAV12 inhibited pancreatic cancer cell growth in vitro. Additive or synergistic effects were observed when combined with MAPK inhibitors. Tumor cells with less expression of RAAG12 had no response to RAV12 treatment [62]. In a pre-clinical study of a colon cancer xenograft mouse model, RAV12 showed a dose dependent inhibition of tumor growth [63]. However, this antibody has shown some intolerable side effects at effective doses in a recent phase I clinical trial to treat gastrointestinal tumors, including pancreatic cancer patients. Reengineering of this antibody and humanized product have been suggested to eliminate these side effects [64]. Another monoclonal anti-IGF-1R antibody, figitumumab (CP-751,871) has been tested in pre-clinical studies and showed the ability to suppress tumor growth in a dose-dependent manner [65, 66]. MK-0646, another humanized anti-IGF-1R antibody, is now in a phase I/II clinical trial [67, 68].
Carboni et al. found the IGF-1R/IR inhibitor BMS-754807 inhibited growth of a broad range of human tumor cells in vitro, including a series of pancreatic cancer cell lines. BMS-754807 synergized with other anti-tumor treatment. Combination of cetuximab and BMS-754807 showed improved outcome vs. single treatment in a pre-clinical study [69].
Mesothelin
Mesothelin is a 40 kDa cell surface protein anchored by glycosyl phosphatidylinositol. Mesothelin is a cleaved product from a precursor protein encoded by the Mesothelin gene (MSLN). Expression of mesothelin is normally seen in mesothelial cells lining peritoneal, pleural and pericardial cavities. Overexpression of the MSLN gene as well as mesothelin was found in about one third of cancers, including pancreatic cancer [70, 71]. The physiological roles of this protein are not fully understood. However, due to its unique tissue distribution profile in cancer and normal tissues, mesothelin has become an important target in cancer diagnosis and treatment. A truncated mutant of Pseudomonas exotoxin A was fused genetically with a single-chain Fv against mesothelin isolated from a phage display library (SS1P). In a pre-clinical study, SS1P plus radiation treatment significantly prolonged tumor doubling time in a mesothelin-expressing tumor xenograft model [72]. Synergy was observed when gemcitabine was used in combination with SS1P and this treatment induced complete xenograft regressions [73]. SS1P was found to be well tolerated in a phase I clinical trial. Of 34 patients treated, 12% showed minor responses (tumor size decreased between 20–50% and lasted for more than 4 weeks), 56% showed stable disease and 29% of the patients had progressive disease [74]. Kreitman et al. reported a recent phase I clinical trial and suggested bolus dosing in combination with other therapies [75]. Another chimeric antibody derived from a phage-display library, MORAB-009, was used in a pre-clinical study with a mesothelin expressing xenograft model. Monotherapy with MORAB-009 moderately inhibited tumor growth, while combination therapy with gemcitabine significantly improved the inhibitory effect [76]. M912, another humanized monoclonal antibody obtained from a phage-display library was generated and the cytotoxicity to mesothelin expressing cancer cells was mediated by ADCC [77].
MUC1
MUC1 is a heavily glycosylated type 1 membrane protein with several extracellular tandem repeat domains. These domains undergo cleavage between amino acid 1097 and 1098 after translation. MUC1 is expressed in nearly all human glandular epithelial tissues and throughout all regions of the gastrointestinal tract on the apical side of cells [78]. MUC1 expression is upregulated in almost all human adenocarcinomas with an expression pattern over the entire cell surface [78–80]. The expression of MUC1 in cancer cells is regulated by DNA methylation and histone H2 lysine 9 modification of the MUC1 promoter. Inhibition of DNA methylation enhanced MUC1 gene expression [81]. MUC1 serves as a counter-receptor for myelin-associated glycoprotein in pancreatic cancer and their interaction is considered to be related to pancreatic cancer perineural invasion [82]. The intact tandem repeats and cytoplasmic tail of MUC1 may play a role in preventing tumor invasion and metastasis [83]. Overexpressed MUC1 upregulated expression of transcription factors slug and snail with inhibition of E-cadherin, thus enhancing epithelial to mesenchymal transition, which was associated with cancer invasion and metastasis [84]. In the TNF-R1 signaling pathway, MUC1 was recruited to the TNF-R1 complex upon stimulation by TNF-α and interacted with the IκB kinase complex resulting in phosphorylation and degradation of IκBα, which favored cell survival [85]. MUC1 was also found to block death receptor-mediated apoptosis by binding to caspase 8 and FADD [86]. Down-regulation of MUC1 by RNA interference upregulated β-catenin and E-cadherin expression, promoting E-catenin/catenin complex formation which decreased in vitro cell invasion [87]. In PANC-1 cells, knockdown of MUC1 expression significantly decreased cell proliferation and slowed the growth rate of xenografts in SCID mice [88].
PM4 is a murine antibody against MUC1. The binding of this antibody to MUC1 is conformation dependent and its epitope may contain carbohydrate. HuPAB4 is a humanized form. This antibody has been radiolabeled with 90Y and is now under investigation in phase I/II clinical trials. In a phase I study, 15 patients with advanced pancreatic cancer were treated. Three patients showed 32–51% tumor shrinkage and other three patients had stable disease at 4 weeks after treatment [89]. TF-10, a humanized bispecific monoclonal antibody, which is diavalent for PAM4 and monovalent for monoclonal antibody 679, was used in a CaPan1 human pancreatic cancer xenograft model. Antibody 679 is reactive against the histamine-succinyl-glycine hapten (HSG-hapten peptide). Pretargeting TF-10 followed by 111In-labeled peptide IMP-288 containing HSG groups showed high 111In-IMP-288 uptake in tumors [90]. Recently, the same group reported combination treatment of CaPan1 xenografts with gemcitabine and TF-10 followed by 90Y-HSG peptide enhanced the tumor growth inhibition by gemcitabine monotherapy [91]. Glazer et al. recently reported administration of gold nanoparticles (AuNP) conjugated to PAM4 antibody induced intracellular hyperthermia of tumors. Combination treatment using PAM4 conjugated with AuNP followed by exposure to radiofrequency field 36 hours after the antibody limited the tissue destruction inside tumors despite whole body radiofrequency exposure [92].
CC49 is a mouse monoclonal antibody against a tumor-associated antigen TAG-72, which is a cell surface glycoprotein and has characteristics of a mucin. This antibody has been conjugated to radioisotopes for diagnosis and treatment purposes. Humanized CC49 was engineered. Branowska-Kortylewicz et al. reported that 131I-CC49 significantly prolonged pancreatic cancer xenograft quadrupling time and this effect was enhanced by a tyrosine kinase inhibitor imatinib [93].
A humanized monoclonal antibody HuC242 against CanAg (a glycoform of MUC1) was conjugated with antimicrotuble agent DM1 (N2′-deacetyl-N2′-[3-mercapto-1-oxopropyl]-maytansine, cantuzumab mertansine) and tested in a phase I clinical trial of 37 patients with solid tumors. Two patients with chemotherapy resistance had minor regression and four patients had stable disease during treatment [94]. Conjugation of HuC242 to another maytasinoid derivative (HuC242-DN4) is now in a phase I clinical trial for pancreatic cancer treatment.
Carcinoembryonic Antigen (CEA)
CEA has been recognized as an overexpressed cell surface antigen in many cancers, mainly in gastrointestinal tract malignancies. A humanized anti-CEA antibody MN-14 (labetuzumab) is now under clinical trial for pancreatic cancer treatment together with filgrastim (G-CSF). Labetuzumab has recently been conjugated with SN-38, an active form of CPT-11, and tested in pancreatic cancer and other cancer xenograft models. The results showed conjugated antibody prolonged the median survival time of mice and decreased the systemic toxicity due to a lower amount of drug in the conjugate [95].
Small Molecule Kinase Inhibitors
Many kinases including receptor tyrosine kinases or non-receptor kinases, have been found to be activated in pancreatic cancer tissues. Inhibition of these kinase activities using small molecules is now an important approach in pancreatic cancer treatment. Many kinase inhibitors have been tested in combination with other treatments in a variety of cancer therapy studies. Among these inhibitors, erlotinib (EGFR tyrosine kinase inhibitor) has been the one tested most in pancreatic cancer treatment with other drugs (Table (1)).
Table 1.
List of Small Molecule Kinase Inhibitors in Ongoing Pancreatic Cancer Clinical Trials
| Targets | Inhibitors | Drugs Combined in Trials | References |
|---|---|---|---|
| EGFR, ErbB2 | Lapatinib (GW572016) | Gemcitabine | * |
| Gemcitabine/oxaliplatin | [96] | ||
| Oxaliplatin/folinic acid/5-fluorouracil | [97] | ||
| Oxaliplatin, cetuximab | * | ||
| Erlotinib | Capecitabine | * | |
| MK-0646 (IGF-1R antibody), gemcitabine | [68] | ||
| Isoflavones, gemcitabine | [98] | ||
| Gemcitabine, IMC-A12 | [21] | ||
| Apricoxib, gemcitabine | [99] | ||
| Gemcitabine, capecitabine | [100] | ||
| Bevacizumab | [101] | ||
| Bevacizumab, gemcitabine, capecitabine | [102] | ||
| Gemcitabine | [103–105] | ||
| Gemcitabine vs. gemcitabine/docetaxel | [106] | ||
| Capecitabine, radiation | [107] | ||
| Rapamycin | [108] | ||
| Oxaliplatin | * | ||
| GDC-0449±gemcitabine | * | ||
| Gemcitabine, cisplatin | * | ||
| Pertuzumab | * | ||
| Gemcitabine+trastuzumab | * | ||
| ASD6244 (selumetinib) | * | ||
| Capecitabine, bevacizumab | * | ||
| Gemcitabine, nab-paclitaxel | * | ||
| VEGFR | Pazopanib | [109] | |
| Paclitaxel | [110] | ||
| Axitinib (AG013736) | Gemcitabine | [111] | |
| PDGFR | Imatinib (glivec) | Gemcitabine, oxaliplatin | [112] |
| IGF-1R | BMS-754807 | [69] | |
| mTOR | Temsirolimus (CCI-779) | [108, 113, 114] | |
| Everolimus (RAD001) | Gemcitabine | * | |
| Irinotecan, cetuximab | * | ||
| Cetuximab, capecitabine | * | ||
| MEK 1/2 | AS70326 (MSC1936369B) | * | |
| Multiple kinases | Sorafenib (Bay43-9006) | Tipifarnib | [115] |
| Gemcitabine | * | ||
| Gemcitabine, radiation | * | ||
| Everolimus | * | ||
| Erlotinib | * | ||
| GSK 1363089 (foretinib) | Gemcitabine | [116] | |
| Sunitinib | * | ||
| Src family | Saracatinib (AZD 0530) | [117] | |
| Dasatinib | * |
In a phase I study, 18 pancreatic cancer patients and 7 biliary cancer patients were administrated lapatinib at 1,500 mg/day daily with weekly gemcitabine or gemcitabine/oxaliplatin. Dose-limiting side effects of nausea or anorexia were found in 2 of 5 patients that received treatment combined with gemcitabine/oxaliplatin. Median survival in all patients was 11 months and 1-year survival was 48% [96]. Results of a phase III trial using erlotinib plus gemcitabine vs. gemcitabine alone in 569 patients with unresectable, locally advanced or metastatic pancreatic cancer showed significant improvement of clinical outcome. Median survival in the combination group was 6.24 months vs. 5.91 months in the gemcitabine group. One-year survival and progression-free survival were 23% and 3.75 months respectively in the combination group vs. 17% and 3.55 months in the monotherapy group [118]. Erlotinib combined with gemcitabine improved overall survival significantly for patients younger than 65 years old compared with a group treated with gemcitabine only as reported in a phase III trial recently [105]. Patients with skin rashes during treatment with erlotinib had better overall survival than the patients with fewer or no rashes [103, 104]. Combination of erlotinib (100 mg/day) with gemcitabine (1,000 mg/m2) did not show any benefit in overall survival rate compared with gemcitabine (1,000 mg/m2) combined with docetaxel (35 mg/m2) in a phase II study from 69 metastatic pancreatic cancer patients [106]. In a phase III study, erlotinib (150 mg/day) was given to 141 patients combined either with gemcitabine (1,000 mg/m2/week for 7 weeks), or capecitabine (2,000 mg/m2/day, day 1–14 every three weeks) until disease progression or toxicity appeared (TIF1). It was found that TIF1 was longer in the group receiving erlotinib with gemcitabine than that treated with erlotinib with capecitabine (3.4 months vs. 2.4 months, p=0.0036). Overall survival was longer in patients with wild type KRAS (8.0 months) than mutant type (6.6 months, p=0.011) in response to the treatment [100]. Watkins et al. reported a phase II study using the combination of gemcitabine (1,000 mg/m2, on day 1, 8, and 15), capecitabine (1,400 mg/m2 on day 1–21), bevacizumab (5 mg/m2 on day 1 and day 15) and erlotinib (100 mg daily) for every 28 days. Median and one year overall survival was 11.1 months and 49% in patients with metastatic disease and 14.8 months and 89% in patients with local advanced disease [102]. Erlotinib (150 mg daily) plus bevacizumab (15 mg/kg every 21 days) did not show any treatment benefit in gemcitabine-resistant metastatic pancreatic cancer patients [101]. Erlotinib was proposed to down-regulate activated phosphorylation of Akt induced by the mTOR inhibitor rapamycin, and thus produce a synergistic anti-tumor effect. Whether it is effective clinically is still under investigation [108].
The VEGFR inhibitor pazopanib was tested in a phase I trial of solid tumors together with paclitaxel. Among twenty-six patients enrolled (17 patients received maximum tolerated regimen of pazopanib at 800 mg daily with weekly paclitaxel at 80 mg/m2), six patients (23%) had a partial response and 15 (58%) of patients had stable disease [110]. Another inhibitor axitinib was evaluated in a phase II trial from 103 unresectable, locally advanced or metastatic pancreatic cancer patients. The results showed a small, non-significant difference in median overall survival between the group treated with gemcitabine plus axitinib and gemcitabine only (6.9 months vs. 5.6 months) and the drug needs to be further evaluated in a phase III study [111].
Imatinib (400 mg daily), a PDGFR inhibitor, was combined with different doses of gemcitabine and oxaliplatin and tested in a phase I trial with gemcitabine resistant locally advanced or metastatic pancreatic cancer patients. Twenty-six patients were enrolled. Two out of 26 patients had a partial response and 11/26 had stable disease. Median progression free survival and overall survival were 4.6 and 5.7 months respectively [112].
mTOR is an important kinase in the signaling pathway of PI3k/Akt, which is a downstream component of many growth factor receptors. Deregulation of mTOR is associated with tumor pathogenesis. mTOR inhibitors have been used as anti-cancer agents and have been summarized in a recent review [119]. Everolimus (RAD001) was evaluated in 58 specialized models of human cancers, including three cell lines of pancreatic cancer. The drug was given at 10 mg/kg/day, five days a week for three weeks. The mean change of relative tumor volume in treatment group/control group in pancreatic cancer models was 21 ± 5% [120]. Although mTOR inhibitors inhibit tumor growth and down-regulate protein synthesis, longer exposure to these inhibitors induced phosphorylation of Akt, which favors tumor growth as mentioned previously. This negative feedback loop might be related to rapid disease progression observed by Javle et al. in a phase II trial of the mTOR inhibitor temsirolimus (CCL-779) [108]. Schmid et al. reported in 2009 in a pancreatic cancer xenograft model (CA20948) that combination of the mTOR inhibitor everolimus (RAD001) with a multi-receptor ligand somatostatin analogue pasireotide (SOM230) produced additional tumor growth inhibition compared with monotherapy. The mechanism is still unknown [121]. Combination treatment with a MEK inhibitor RDEA119 and rapamycin in three pancreatic cancer xenograft models (OCIP 19, 21 AND 23) produced somewhat better tumor growth inhibition than rapamycin only, although the difference was not statistically significant [122].
Another target of kinase inhibitors is the Raf/MEK/ERK cascade. The anti-tumor activity of the MEK inhibitor AS703026 was tested in mouse xenografts of the pancreatic cancer cell line MIA PaCa-2. Tumor regression was observed at a low dose of 10 mg/kg twice a day [123]. Tumors bearing Ras and Raf mutations were more sensitive to this inhibitor [124].
Sorafenib, GSK1363089 (foretinib) and sunitinib are a group of agents which inhibit multi-kinases. In vitro tests showed that sorafenib induced apoptosis in pancreatic cancer cell lines by different mechanisms in different cell lines, which included inhibition of MEK/MAPK, dephosphorylation of BAD ser 122, up-regulation of PUMA, caspase 3 and 9 cleavage, down-regulation of the anti-apoptotic protein Mcl-1 and decreased Akt expression and phosphorylation, accompanied by different degrees of apoptosis [125]. In another in vitro study, sorafenib was found to inhibit constitutive signal transducer and activator of transcription 3 (STAT3) phosphorylation and suppress expression of Mcl-1 and BclxL. Sorafenib was also found to enhance the effect of TRAIL to induce apoptosis in pancreatic cancer cells [126]. GSK1363089, when combined with the HER1/2 inhibitor lapatinib, was found to decrease phosphorylation of Akt and ERK significantly in tumors overexpressing HER1/2 [127]. Hong et al. tested a combination of sorafenib with a farnesyltransferase inhibitor tipifarnib in a phase I clinical trial with 50 cancer patients, including 4 pancreatic cancer patients. One pancreatic cancer patient showed prolonged stable disease for 6 months [115]. In another phase I study of 40 solid tumor patients (including one pancreatic cancer patient), three patients had a partial response and another 22 patients had stable disease with a duration from 1 to 10 months [116]. Negative data was reported by Kindler et al. from a phase II study in pancreatic cancer patients. Patients received sorafenib 400 mg twice daily and gemcitabine 1,000 mg/m2 on days 1, 8 and 15 of a 28 day cycle. Thirteen patients were evaluated for response. Eighteen percent of the patients had stable disease. Median overall survival was 4.0 months (95% CI: 3.4, 5.9), median progression-free survival was 3.2 months [128].
Src kinase has been recognized as a proto-oncogene and is regulated by a variety of signals including activation by receptor tyrosine kinases and cytoplasmic phosphatases [129]. Many downstream substrates of Src have been identified, which makes Src a critical kinase in cellular signal transduction [129]. Inhibition of Src activity has been a focused target in cancer treatment. Cellular location of Src expression was correlated with patient survival in pancreatic cancer. Increased membranous Src expression was associated with a significantly lower overall survival, while a higher expression in the cytoplasmic compartment was associated with improved survival [130]. An in vitro study showed that the Src inhibitor dasatinib inhibited pancreatic cancer cell proliferation, migration and invasion accompanied by stimulated apoptosis, decreased phosphorylation of Src, focal adhesion kinase, paxillin, Akt, STAT3, ERK and MAPK. In an in vivo study, tumor volume in untreated mice bearing BxPC3 xenografts increased 62% compared with 17% in sorafenib treated (10 days treatment) mice. Tumor volume in untreated mice bearing PANC 1 xenografts increased 174% compared with 50% in treated mice [130]. Recently, a triple combination of dasatinib, erlotinib and gemcitabine were used in a pre-clinical study. The treatment was able to inhibit multiple signaling pathways including FAK, Akt, ERK, JNK, MAPK and STAT3, while single agent treatment or double agent treatment was ineffective. Similar results were reported in another in vivo xenograft study. Tumor volume decreased 93.6% with triple treatment compared with dasatinib plus erlotinib 79.8%, dasatinib plus gemcitabine 77.3%, and gemcitabine plus erlotinib 81.1% [131]. In another pre-clinical study, Evans found administration of dasatinib decreased the metastasis rate of pancreatic cancer in mice [132]. It was proposed that dasatinib not only inhibited Src activity but also inhibited Eph receptor tyrosine kinase and its downstream effectors [133]. Another Src inhibitor saracatinib (AZD5030) was evaluated in a phase I/II clinical trial in combination with gemcitabine. The results did not show any advantage when compared with gemcitabine alone [134]. In another phase II trial using saracatinib single agent therapy in a group of unselected, previously treated advanced pancreatic cancer patients, this drug failed to improve 6-month survival [117].
Stroma-Targeted Therapy
The abundance of stroma in pancreatic cancer tissue has drawn investigator’s attention regarding its role in aggressive tumor growth and metastasis. In pancreatic cancer, the extensive desmoplastic reaction may expand the stromal volume up to 90% of the whole tumor [135]. Pancreatic cancer stroma contains extracellular matrix, fibroblasts, myofibroblasts, and blood vessels. One important type of cell, pancreatic stellate cells (PSC), was found to play an important role in mediating epithelial-stromal interaction. This interaction and stromal biology were discussed in a recent review [135]. In animal models, PSC were found to migrate with cancer cells, and PSC were able to migrate through transendothelial monolayers, which was stimulated by cancer cells [136]. Ikenaga et al. recently reported that PSC in tumor tissue showed higher frequency expression of CD10, which significantly increased tumor growth and invasiveness than CD10− PSC cells in co-culture with pancreatic cancer cells. The stimulation of tumor growth and metastasis may be mediated by matrix metalloproteinase 3 secreted by CD10+ PSC [137]. The growth, angiogenesis and invasiveness of pancreatic cancer were also found related to the level of hepatocyte growth factor (HGF) from stromal cells. Also, the HGF level was stimulated by cancer cell derived IL-1α [138]. The desmoplastic reaction is accompanied by a relatively avascular tumor microenvironment, subsequent hypoperfusion and hypoxia of cancer tissue, which dramatically influences drug delivery to the tumor tissue. This partially explains why excellent pre-clinical therapy in xenograft models has not been translated into clinical trials successfully. Xenografts tend to be better vascularized. It was proposed that using transgenic mouse models of pancreatic cancer in pre-clinical studies would yield more accurate information about the potential for cancer treatment [11], since abundant pancreatic cancer stroma forms in these models. To improve drug delivery into tumor parenchyma, targeting cancer stroma seems a step which cannot be neglected. A hypothesis for “stroma depletion” to enhance drug delivery to tumor tissue was proposed [139].
One of the stroma targeting strategies is to use an antagonist of the hedgehog (Hh) signaling pathway. Hedgehog ligand receptor, Patched (Ptc) is a trans-membrane protein with 12 membrane spanning regions. Normally, Ptc represses its downstream protein, Smoothened (another trans-membrane protein with 7 membrane spanning regions, Smo) in the absence of Hh ligand. When Hh ligand binds Ptc, it is inactivated, thus activating Smo. Activation of Smo subsequently activates Gli transcription factors and activates Hh targeted genes (Fig. (1)) [140]. Activated Hh pathway was found in pancreatic cancer and its precursor lesions. Blocking this pathway with cyclopamine induced apoptosis and inhibited cancer cell proliferation [141, 142]. Over-expressed Hh ligand was found in pancreatic cancer cells [142], while Smo was found overexpressed in pancreatic cancer stromal cells [143]. Activation of the Hh signaling pathway through paracrine Hh ligand from cancer cells induced Hh signal activation in pancreatic cancer stroma [144], which made the Hh signal blocking strategy more specific towards stroma. The Smo inhibitor GDC-0449 (vismodegib) has been tested in some tumors. It is now in ongoing clinical trials to treat pancreatic cancer (NCT01096732, NCT01088814 and NCT00878163).
Fig. 1. Hedgehog signaling pathway.
Hedgehog ligand receptor (Ptc) is a transmembrane protein. (A) When there is no binding of Hh ligand to Ptc, Ptc represses the downstream protein Smo. Smo represses the downstream Gli family. (B)When Hh ligand binds to Ptc, Ptc activates Smo, which subsequently activates Gli family and induces transcription of Hh targeted genes.
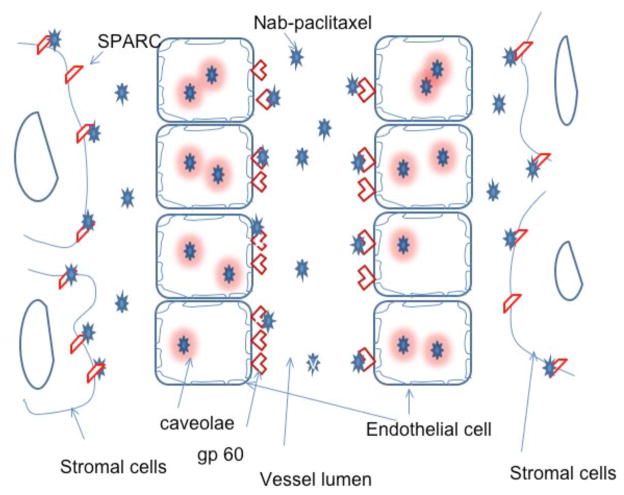
Another strategy of stromal targeting is administration of nab-paclitaxel. Nab-paclitaxel is an albumin-bound nanoparticle formulation of paclitaxel. The attached albumin molecules bind albumin receptor, glycoprotein 60, on endothelial cells and nab-paclitaxel molecules are transported into endothelial cells. Nab-paclitaxel is then transported across cells into the extracellular space through formation of caveolae, where it selectively binds to secreted protein, acidic and rich in cysteine (SPARC). Paclitaxel is then released from the complex (Fig. (2)) [145, 146]. SPARC is a glycoprotein with high affinity to albumin. The biological characterization of SPARC, its expression profile as well as its role in tumorigenesis was described in a review [147]. SPARC expression was found to be low in several pancreatic cancer cell lines while high in pancreatic stellate cells. The expression was mainly in peritumoral and distal stroma [147, 148]. Pancreatic cancer patients who expressed SAPRC in stromal cells, but not pancreatic cancer cells, had worse prognosis than those did not express SPARC [149]. SPARC expression in distal stroma correlated inversely with overall survival of the pancreatic cancer patients [148]. High SPARC mRNA expression was also found associated with a poor prognosis and was proposed as a prognostic marker for pancreatic cancer patients [150]. Recently, analysis of SPARC gene transcriptional regulation region showed two hypermethylation wave peak regions, and one of them was highly associated with the tumor size. Methylation of the SPARC gene may serve as a tumorigenesis marker for early detection of pancreatic cancer [151]. In a phase II clinical trial using nab-paclitaxel to treat 19 advanced pancreatic cancer patients who were previously treated with gemcitabine, nab-paclitaxel was given at 100 mg/m2 on days 1, 8, and 15 of a 28-day cycle. Six-month overall survival was 58%. Median overall survival was 7.3 months. One patient showed a partial response. Six patients showed stable disease. CA19-9 levels decreased by 52% in the patients who had stable disease or partial response compared with an 18% decrease in the patient who had progressive disease [152]. In a recent report by Thapaliya et al., nab-paclitaxel was combined with gemcitabine in 13 patients. Nine of 13 patients showed a partial response and three of 13 patients showed stable disease. One patient had progressive disease [153]. Nab-paclitaxel was combined with low dose 5-FU, leucovorin, oxaliplatin and bevacizumab to treat 28 pancreatic cancer patients. One patient showed a complete response and 13 patients showed a partial response. Overall survival was greater than 8 months [154]. Further studies using nab-paclitaxel in pancreatic cancer treatment is ongoing and is expanding to a larger cohort and is being tested in multiple institutions.
Fig. 2. Delivery of nab-paclitaxel to cancer cells from blood stream.
Injected nab-paclitaxel drug binds to albumin receptor, gp 60, through its albumin molecules which are transported into endothelial cells. The drugs are then transported across cells through formation of caveolae and released into extracellular space. The drugs selectively bind to SPARC, which are expressed on the stromal cells in pancreatic cancer tissue.
Anti-VEGF targeting is another important approach in controlling tumor growth and metastasis. The VEGF family consists of VEGF-A, -B, -C, -D and placenta growth factor (PLGF). The receptors for the VEGF family are VEGFR-1 (Flt-1), VEGFR-2 (KDR), and VEGFR-3. VEGF (usually referring to VEGF-A) was found to be expressed in 93% of pancreatic cancer samples and expression of VEGF was correlated with liver metastasis and prognosis. VEGF was also detected in bone marrow derived cells or mesenchymal stem cells [155, 156]. Expression of VEGF-C and -D in pancreatic cancer was found to be correlated with lymph node metastasis, lymphatic invasion and venous invasion [157]. Bevacizumab is a humanized murine anti-human VEGF-A monoclonal antibody and has been used in the treatment of pancreatic cancer together with other chemotherapy agents.
The preliminary clinical trial results of GDC-0449, nab-paclitaxel and bevacizumab, either monotherapy or combined with other drugs are listed in Table (2). It is still too early to identify the best combinations of these agents. According to most of the results, side effects or toxicities from these drugs were tolerable and manageable. However, a recent report showed that bevacizumab increased the treatment-related mortality in cancer patients. The most common side effects seen were hemorrhages, neutropenia and gastrointestinal tract perforation. These risks were seen more often when bevacizumab was combined with taxanes or platinum agents [158].
Table 2.
Clinical Trial Results of GDC-0449, Nab-paclitaxel and Bevacizumab
| Investigator | Phase | Patient numbers | Drug combined | Results | References |
|---|---|---|---|---|---|
| GDC-0449 | |||||
| Lorusso et al. | I/II | 68 solid tumor (8 PC) | Monotherapy | 20 response, 14 SD (no PC), 8 PD | [159] |
| Nab-paclitaxel | |||||
| Chien et al. | I | 25 solid tumors(2 PC) | Lapatinib | 65% PR or SD (no pancreatic cancer pa- tients) | [160] |
| Hosein et al. | II | 19 PC (progressed on gemcit- abine) | Monotherapy | 6 mo OS 58%, median OS 7.3 mo, median PFS 1.6 mo, 1 pt PR, 6 pts SD, 12 PD | [152] |
| Isacoff et al. | Pilot | 28 PC (advanced) | 5-FU (low dose), leucovorin, oxaliplatin, bevacizumab | 1 CR, 13 PR, OS>8 months | [154] |
| Thappliya | I/II | 13 PC (unresectable/borderline resectable) | Gemcitabine | 9 PD, 3 SD, 1 PD, EORTC PET response: 7/10 PR, 3/10 SD | [153] |
| Bevacizumab | |||||
| Berlin et al. | II | 25 PC (resected tumor) | Gemcitabine, capecitabine, radiation | At 2 years: DFS 22%, OS 37% | [22] |
| Watkins et al. | I/II | 39 PC (advanced) | Gemcitabine, capecitabine, erlotinib | Radiological response 23%, median PFS 8.5 mo, median OS 12.8 mo Median & 1 year survival 11.1 mo & 49% (pts with metastasis), 14.8 mo & 89% (pts with local advanced) |
[102] |
| Martin et al. | II | 39 PC (advanced) | Gemcitabine, 5-FU | Freedom from progression in 53% pts (24 weeks), 24% PR, 47% SD, mean time to progression 8.1 mo, median survival 10.7 mo | [161] |
Abbreviations: PC, pancreatic cancer, PR, partial response, SD, stable disease, OS, overall survival, DFS, disease-free survival, PD, progressive disease, PFS, progression free survival.
Heat Shock Proteins
Heat shock proteins (HSPs) are a set of highly conserved proteins whose expression is induced in response to a variety of physiological and environmental insults [162]. Once up-regulated, HSPs have been shown to protect against programmed cell death or apoptosis. By virtue of this pro-survival role, HSPs are believed to contribute to the pathogenesis of cancer. Heat shock protein 70 (HSP70) is one of the most extensively studied HSPs and multiple lines of evidence have established its role in the extreme resistance to cell death demonstrated by pancreatic cancer cells. Aghdassi et al. [163] showed that the HSP70 levels are markedly high in various pancreatic cancer cell lines at both protein and mRNA levels, thus suggesting increased transcription, as compared to normal pancreatic ductal cells, the cells of origin of pancreatic cancer. This finding is clinically relevant since HSP70 overexpression was also observed in human pancreatic cancer specimens when the levels were compared with normal pancreas margins [163]. This increased expression of HSP70 has been linked to increased level and activity of Heat Shock Factor-1 (HSF-1), the transcription factor that regulates the heat shock response [164]. Similar overexpression of HSP70 has been observed in other cancers like hepatocellular cancer [165] and colorectal cancer [166] and this increased expression has been correlated with advanced stage, poor differentiation and poor prognosis.
Further support for the role of HSP70 in the pathogenesis of pancreatic cancer comes from the studies which have shown that decreasing HSP70 leads to cell death in pancreatic cancer cells. As a proof of principle, silencing HSP70 expression by specific siRNA, leads to caspase dependent apoptotic cell death suggesting that HSP70 down-regulation could emerge as novel therapeutic strategy for pancreatic cancer [163]. This has led to a search for pharmacologic inhibitors of HSP70 which could be used clinically. Two such inhibitors include quercetin [163] and triptolide [167]. Though quercetin is effective in inhibiting HSP70 expression [163] as well as inducing cell death in pancreatic cancer cells [163], its toxicity profile, low potency and insolubility in water have been barriers to its application in clinical practice. Triptolide, a diterpene triepoxide from the Chinese plant Tripterygium wilfordii, was identified as an inhibitor of human heat shock gene transcription through a small molecule screen [168]. Inhibition of HSP70 expression in pancreatic cancer cells by triptolide [167] has been shown to induce apoptotic cell death in pancreatic cancer cells. Since cancer in itself is composed of a heterogeneous group of cells with variable aggressiveness, the effect of triptolide has been evaluated in both less aggressive pancreatic cancer cell lines like MIA PaCa-2 and Panc-1 developed from primary tumors, as well as in highly aggressive cell lines like S2013 and S2VP10 (developed from metastases) and ASPC-1 (developed from malignant ascites) and has been shown to be uniformly effective [167, 169]. Furthermore, in line with the hypothesis that HSP70 induces resistance to apoptosis, triptolide sensitizes pancreatic cancer cells to TRAIL induced apoptosis. Pancreatic cancer cells are known to be highly resistant to TRAIL induced apoptosis, and the fact that inhibition of HSP70 by triptolide could sensitize to TRAIL induce apoptosis opens up novel avenues for combination therapy for pancreatic cancer for which therapies have largely been ineffective.
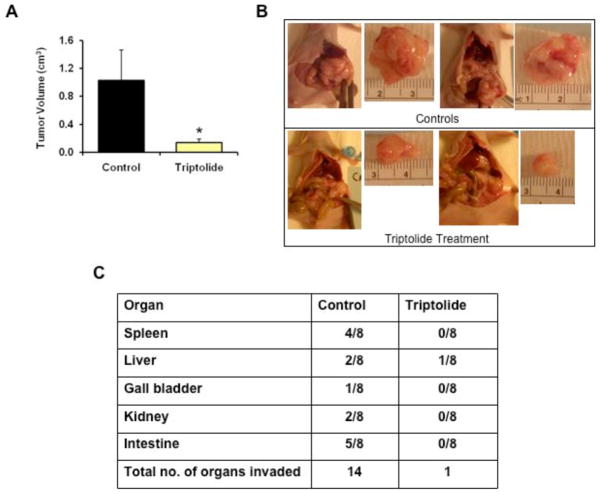
Triptolide has been evaluated in multiple animal models of pancreatic cancer and has been shown to be very effective in reducing the growth (Fig. (3A) and (3B)) as well as loco-regional spread of pancreatic tumors (Fig. (3C)) [163, 167]. Remarkably, inhibition of HSP70 expression by quercetin or triptolide does not induce apoptosis in normal pancreatic ductal cells [163, 167]. Though the reason for this specificity of anti-HSP70 therapy to the cancer cells is unclear, the data points toward certain mechanisms. The level of HSP70 in the normal ductal cells is very low as compared to pancreatic cancer cells. Thus, it seems that HSP70 expression is important for the survival of pancreatic cancer cells but may not be required for the survival of normal non-neoplastic cells under unstressed conditions [163]. Similar observations have been reported by others [170] where in contrast to cancer cells, non-transformed cells do not undergo cell death on depletion of HSP70 suggesting that tumor cells but not non-transformed cells cannot survive endogenously activated cell death if they lack HSP70 [171]. This along with the fact that triptolide is non-toxic to experimental animals clearly suggests that inhibition of HSP70 expression can emerge as a tumor selective therapy.
Fig. 3. Inhibition of HSP70 expression is highly effective as a therapeutic strategy in an animal model of pancreatic cancer.
(A) Inhibition of HSP70 expression by triptolide markedly reduced the growth of MIA PaCa-2 tumors in an orthotopic model of pancreatic cancer in nude mice. Triptolide was administered at a dose of 0.2 mg/kg/day for 60 days. Animals were sacrificed at the 60th day and the tumor volumes in the triptolide group were compared with the control group (treated with vehicle alone). n=8 in each group, *=p<0.05. (B) Representative pictures of tumor tissue in the control and triptolide treatment group. (C) Table demonstrating reduced loco-regional spread in the triptolide treatment group. (p < 0.001, n = 8, χ2 analysis) [167].
Similar efficacy of anti-HSP70 therapy has been demonstrated in other gastrointestinal and non-gastrointestinal tumors. Phillips et al. reported that besides inducing apoptosis in pancreatic cancer cells, triptolide induces cell death in colon cancer cell lines as well suggesting that anti-HSP70 therapy will be of broader value rather than for pancreatic cancer only [167]. Triptolide also induces apoptotic cell death in neuroblastoma cells and markedly reduces tumor growth in animal models [172]. Similar to its effects in pancreatic cancer cells, triptolide induces apoptotic cell death in cholangiocarcinoma cells and also sensitizes them to TRAIL induced cell death [173]. Thus, development of effective and bioavailable inhibitors of HSP70 expression will be useful in a wide variety of cancers as monotherapy or in combination with other chemotherapeutic agents. In this regard, triptolide [167] appears to be a very promising molecule which at very low doses (0.2 mg/kg) is able to inhibit HSP70 expression in mouse models and also produce anti-cancer effects with inhibition of both local tumor growth and loco-regional spread. One of the limitations in taking triptolide to clinical trial is that it is not soluble in water. This has been circumvented at the University of Minnesota by synthesis of its water-soluble derivative named minnelide. In pre-clinical trials (unpublished work), minnelide has been shown to be as effective as triptolide and clinical trials are in design phase. Once in clinical trials, minnelide will be one of the first HSP inhibitors to undergo basic and translational research against pancreatic cancer.
Targeting Drug Resistance
One of the reasons for poor prognosis of pancreatic cancer is the resistance to chemotherapy. Diagnosis of drug resistance in individual patients may improve treatment efficacy by avoiding inefficient treatment [174]. Targeting drug resistance of pancreatic cancer has drawn investigators’ attention. Drug resistance in cancers may be inherent (intrinsic) or acquired during treatment. Primary cancer cell culture or use of established cell lines to test sensitivities to chemotherapeutic drugs as well as targeted therapies may help to detect intrinsic resistance to drugs. Clinically, this may generate “assay-assisted” therapeutic regimens which may achieve more successful outcomes, decrease side effects from possible resistance to treatment and reduce the cost [174]. Recently, Villarroel et al. reported establishment of mouse xenografts using fresh human pancreatic cancer samples obtained at surgery and establishment of a PancXenobank of tumor samples maintained in mice. Sensitivities to various drugs were tested using these samples as well as genomic and proteomic analysis. In one patient with advanced, gemcitabine-resistant pancreatic cancer, a personalized regimen with mitomycin C and cisplatin was designed based on these studies. The patient was treated successfully with minimal side effects and this patient has remained symptom free three years after surgery [175]. Many factors have been found to be related to drug resistance in pancreatic cancer, mainly to gemcitabine and 5-FU. Table (3) lists recently reported factors associated with chemotherapy resistance. Most of the investigations were performed in in vitro studies. Although antagonists of these factors achieved some effect in reversing drug resistance, additional efforts are required through pre-clinical and clinical studies. TMS1 demethylation agent, azacitidine, is in phase I clinical trials to treat pancreatic cancer. Notch signal pathway inhibitors MK0752 and RO4929097 in combination with gemcitabine are in current clinical trials to treat pancreatic cancer. Notch signaling is a mechanism that controls cell fate in a broad spectrum of cells [176]. Notch receptors are trans-membrane proteins expressed on the cell surface with four isotypes (Notch 1–4). Binding of Notch receptors to their ligands induces proteolytic cleavage of the receptor by γ-secretase and releases its intracellular domain to the nucleus, which subsequently transactivates target genes. In normal pancreas, Notch signaling is relatively inactive. The signal may be activated if there exists acinar dediffer-entiation and/or epithelial cell proliferation. Moderate to high level expression of Notch receptors, their ligands as well as their target genes has been found in pancreatic intraepithelial neoplasms and pancreatic cancer [177, 178]. Notch signaling plays a substantial role in pancreatic cancer initiation, progression, and maintenance [177]. In gemcitabine-resistant pancreatic cancer cells, acquired epithelial-mesenchymal transition (EMT) was found to be associated with highly upregulated Notch signaling. Down-regulation of Notch signaling with siRNA partially reversed EMT through down-regulation of NF-κB, thus resulting in up regulation of E-cadherin [179]. Among the isotypes of Notch receptors, Notch 2 and 3 are related to gemcitabine resistance [178, 180]. In vitro studies showed down-regulation of Notch 3 with siRNA increased the gemcitabine-induced apoptosis in BxPC-3 and PANC-1 pancreatic cancer cell lines with concomitant down-regulation of PI3K/Akt activity [180]. There are no pancreatic cancer clinical trial results available regarding the application of γ-secretase inhibitors MK-0752 and RO4929097.
Table 3.
List of Factors Related to Chemotherapy Resistance in Pancreatic Cancer
| Factors | Description | Antagonist | References |
|---|---|---|---|
| CXCL12-CXCR4 (chemokine receptor) | Activation of FAK, ERK, Akt, β-catenin and NF-κB | AMD3100 | [181] |
| Multidrug resistance protein 5 (MRP5) | Gemcitabine and 5-FU upregulate MRP5 | SiRNA | [182–184] |
| MUC1 | Overexpressed MUC1 decrease intracellular drug concentration | Genzyl-α, GalNAC | [185] |
| 14-3-3σ | Increases cell cycle arrest upon DNA damage, resistant to drug induced apoptosis | SiRNA | [186] |
| TMS1 | TMS1, a pro-apoptotic gene was silenced by methylation | Azacitidine, decitabine | [187, 188] |
| Notch signaling | Anti-apoptosis through activation of PI3K/Akt | MK0752, RO4929097 | [177, 189–191] |
OTHER TARGETED THERAPIES
Immunotherapy
Algenpantucel-L Immunotherapy: Algenpantucel-L is a preparation of irradiated, live, allogeneic human pancreatic cancer cells expressing the enzyme α-1,3 galactosyl transferase (α-GT). An antibody against the α-GT epitope of algenpantucel-L was induced which might possess an anti-tumor effect. In a recent clinical trial reported by Hardacre et al., algenpantucel-L was used in combination with standard adjuvant therapy in patients with resected pancreatic cancer. Kaplan-Meier estimated survival rates at 12 and 24 months were 91% and 54%, respectively, comparing favorably to 63% and 32% expected [192]. Median progression free survival was 16 months which compared favorably to the 11 months observed in RTOG 9704 [192]. Algenpantucel-L is now being tested in an ongoing phase III clinical trial.
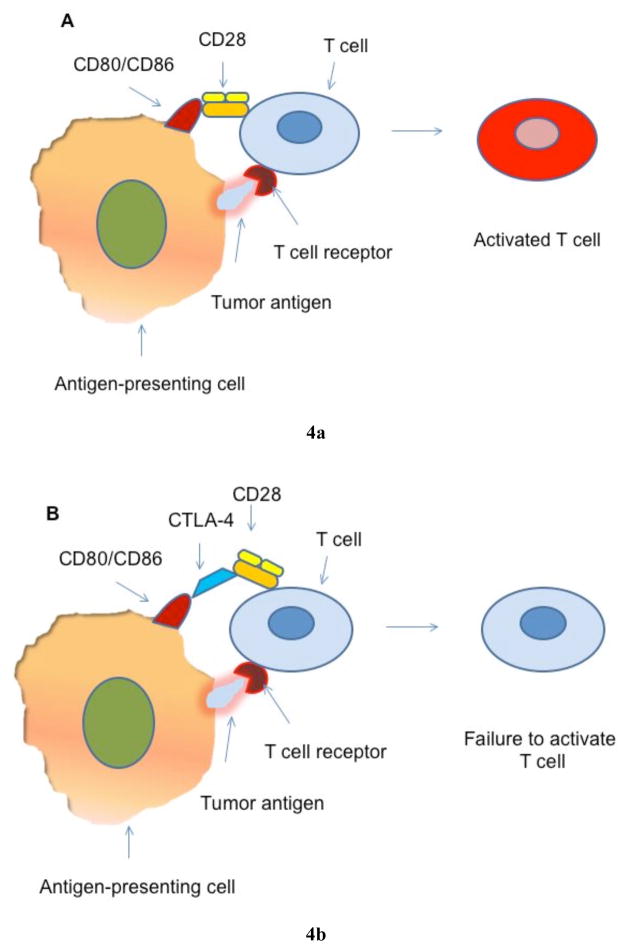
Cytotoxic T-lymphocyte Antigen-4 (CTLA-4): Tumor antigens induce host immunosurveillance and initiate activation of cytotoxic T lymphocytes through the reaction between tumor antigens presented by antigen-presenting cells (APCs) with the T-cell receptor. The further activation of T cells requires binding of CD28 of T cells with CD80 (B7-1) or CD86 (B7-2) from APCs. CTLA-4, a homolog of CD28, can bind to CD80 or CD86 with a much higher affinity than CD28. This binding prevents further activation of T cells and reduces the T cell population to a small pool of memory cells, thus compromising host immunosurveillance (Fig. (4)) [193]. CTLA-4 was found in tumor-infiltrating immune cells in human pancreatic cancer [194]. Blocking CTLA-4 may play an important part in immunotherapy of pancreatic cancer. Ipilimumab and tremelimumab are two human anti-CTLA-4 antibodies that have been tested in clinical trials in pancreatic cancer patients [195]. In a phase II trial reported by Royal et al. recently, one patient showed regression of the primary lesion and 20 hepatic metastases after single agent treatment using ipilimumab, although most of the patients enrolled in this phase II trial did not show responses. The response was evidenced by tumor marker normalization and clinically improved performance status [196].
Pomalidomide (CL-4047): Pomalidomide is an immunomodulatory derivative of thalidomide which has been used to treat multiple myeloma. Its immunomodulatory effects have been discussed in a recently published review [197]. It is now in an ongoing clinical trial with other chemotherapy drugs to treat pancreatic cancer. In twenty evaluable patients with metastatic pancreatic cancer, three patients (15%) showed partial responses, and 10 (50%) showed more than 50% decreased CA19-19 levels [198].
Fig. 4. T cell activation in immunosurveillance.
(A) Tumor antigens are expressed by antigen-presenting cells (APCs). T cells are activated by the binding of expressed tumor antigens to T cell receptors. The activation requires the binding of CD80/CD86 from APCs to CD28 which is expressed on T cell surface. (B) CTLA-4 is a homolog of CD28 expressed on T cells. CTLA-4 can bind to CD80/CD86 with a high affinity. This binding prevents the activation of T cells even in the presence of tumor antigen on APCs. Blocking the binding between CTLA-4 and CD80/CD86 may resume T cell activation and enhance immunosurveillance.
Gene Therapy
BC-819
BC-819 (DTA-H19) is a double stranded DNA plasmid carrying the gene for the A subunit of diphtheria toxin under the regulation of the H19 gene promoter. H19 gene RNA was found in a variety of cancers including pancreatic cancer. Its anti-tumor effects have been shown in pre-clinical xenograft models [199]. This drug is now under investigation in a clinical trial to treat pancreatic cancer [200]. No clinical results are yet available from these trials.
Rexin-G
Rexin-G is a nonreplicative retroviral vector, which encodes a mutant human cyclin G1 gene. Blockade of cyclin G1 activity with Rexin-G induced inhibition of pancreatic cancer cell proliferation. A phase I study with Rexin-G in gemcitabine refractory and metastatic pancreatic cancer patients was carried out [201]. Gordon et al. reported three cases of metastatic pancreatic cancer patients with responses from stable disease to complete remission of primary lesion and decreased size of metastatic lesions in the liver. One patient used Rexin-G as a neo-adjuvant treatment and underwent surgery to remove the primary tumor [202].
Reolysin
Reolysin is a reovirus strain, which inhibits activated Ras kinase activity. In a phase I trial in solid tumors, overall clinical response was seen in 45% of patients enrolled with one partial response and seven stable disease [203]. This drug is now in a phase II clinical trial for pancreatic cancer.
Conditionally replicative adenovirus (CRAd)
A CRAd under control of cyclooxygenase (COX) 2 was produced, which possesses the ability to replicate and spread within the tumor. In a pre-clinical study, administration of CRAd in combination with gemcitabine or 5-FU showed synergistic effects in some pancreatic cancer cell lines. Synergistic effects were also observed in a MIA PaCa-2 pancreatic cancer xenograft model [204].
hTNF-α vector
The vector AdEgr.TNF.11D produces TNF-α which induces apoptosis through the TNF receptor. Intra-tumoral injection of AdEgr.TNF.11D increased TNF-α expression and produced additional effects when combined with gemcitabine in a human pancreatic cancer xenograft model [205]. It is now in an ongoing clinical trial of pancreatic cancer.
X-linked inhibitor of apoptosis protein (XIAP) targeting
Small hairpin RNA (shRNA) targeting XIAP was cloned into a modified pU6.ENTR plasmid to generate pBlockIt.shXIAP plasmid for XIAP knockdown. In a human pancreatic cancer xenograft mouse model, XIAP silencing enhanced the sensitivity of pancreatic cancer cells to apoptosis induced by tumor necrosis factor-related apoptosis (TRAIL), and blocked lymphatic tumor cell dissemination [206].
Targeting telomerase
Imetelstat (GRN163L) is an oligonucleotide which carries a sequence complementary to the template region of human telomerase. Telomerase was reported as a critical enzyme in maintaining infinite replicative potential of cancer cells, especially cancer stem cells. In vitro treatment with imetelstat on pancreatic cancer cell line PANC1 showed reduced tumor engraftment and reduction of cancer stem cell levels [4].
CONCLUSIONS
The long list of investigative treatment methods for pancreatic cancer with low success rates indicates human pancreatic cancer is not a disease controlled by a simple pathological process. Discoveries in pathogenesis of pancreatic cancer, including abnormalities at the gene level and signal transduction pathways, revealed very valuable information. However, targeted therapies based on these discoveries have not changed the overall survival of this malignant disease clinically, despite very promising results in some pre-clinical studies. Pancreatic cancer apparently possesses treatment resistance via multiple pathways. Overcoming this resistance requires further investigation of these pathways. In pancreatic cancer, homeostasis of tissue has been severely altered. Observed pathological phenomenon in pancreatic cancer may relate to oncogenesis or metastasis, or may be the biological responses associated with cancer. The role of molecular targets in pancreatic cancer development need to be evaluated not only with in vitro studies or preclinical studies, but also at different stages of this disease using clinical samples. The importance of molecular targets at different stages during cancer development needs to be analyzed with integration of information collected from multiple pathways. New therapeutic approaches for pancreatic cancer need to be considered based on combined analysis of pathophysiological information obtained from multiple levels and dimensions. Successful molecular targeted and chemotherapy based regimens may be established based on this information. Gene mutation determination, proteomic analysis and integration efforts using advanced informatics may help to uncover the complicated pathological processes and expose the resistance mechanisms in pancreatic cancer patients. Critical pathways may also be defined through examination of tumor samples from patients who responded to targeted therapies, such as the patients treated with the FOLFIRINOX regimen. In this review, we also identified several patients who responded well to ipilimumab and Rexin-G treatment. A thorough analysis of the samples from these patients may help investigators to gain insight into an effective treatment for pancreatic cancer.
FUTURE PERSPECTIVE
Previous investigations in basic research and clinical trials of targeted therapy of pancreatic cancer have built a good platform for further study to treat this dismal disease. Genomic and proteomic studies will be essential in generating useful approaches towards effective targeted therapies. This challenge needs to be accomplished by interdisciplinary efforts from cell biologists, pathologists, oncologists, geneticists, molecular biologists, proteomics investigators, and pharmacologists. Information obtained needs to be analyzed from multiple angles and levels, in order to better understand the network of pancreatic cancer pathogenesis, maintenance and progression. Importantly, the genomic studies need to be correlated with pathological characteristics of pancreatic lesions at different stages of development. An intensive investigation in genomics and proteomics using biopsy or surgical samples may supply valuable information for the design of molecular targeted strategies. In pre-clinical investigations, a cancer environment resembling human pancreatic cancer needs to be created and used to test different therapeutic methods. These efforts might help to establish an effective, combined targeted therapy with acceptable side effects, and eventually to establish a tailored, well tolerated and successful therapeutic regimen to improve the prognosis of pancreatic cancer.
Acknowledgments
Supported by NIH grant 2 P50 CA101955.
ABBREVIATIONS
- ADCC
Antibody-dependent cellular cytotoxicity
- AuNP
Gold nanoparticles
- CEA
Carcinoembryonic antigen
- DFS
Disease-free survival
- DISC
Death-inducing signaling complex
- DR
Death receptor
- GPCR
G protein-coupled receptor
- Hh
Hedgehog
- HSG
Histamine-succinyl-glycine
- IGF-1
Insulin/insulin-like growth factor 1
- IGF-1R
Insulin-like growth factor-1 receptor
- IR
Insulin receptor
- MSLN
Mesothelin gene
- PD
Progressive disease
- PFS
Progression free survival
- PLGF
Placenta growth factor
- PR
Partial response
- PSC
Pancreatic stellate cells
- Ptc
Patched
- RAAG12
N-linked carbohydrate antigen
- SD
stable disease
- SPARC
Secreted protein, acidic and rich in cysteine
- SS1P
Single-chain Fv against mesothelin isolated from a phage display library
- STAT3
Signal transducer and activator of transcription 3
- TNFR
Tumor necrosis factor receptor
- TRAIL
TNF-related apoptosis-inducing ligand
Footnotes
CONFLICT OF INTEREST
Dr. Buchsbaum has intellectual property interests related to the TRA-8 anti-DR5 antibody.
References
- 1.Ellison LF, Wilkins K. An update on cancer survival. Health Rep. 2010;21:55–60. [PubMed] [Google Scholar]
- 2.Alexakis N, Halloran C, Raraty M, et al. Current standards of surgery for pancreatic cancer. Br J Surg. 2004;91:1410–27. doi: 10.1002/bjs.4794. [DOI] [PubMed] [Google Scholar]
- 3.Kim R. FOLFIRINOX: a new standard treatment for advanced pancreatic cancer? Lancet Oncol. 2011;12:8–9. doi: 10.1016/S1470-2045(10)70237-0. [DOI] [PubMed] [Google Scholar]
- 4.Joseph I, Tressler R, Bassett E, et al. The telomerase inhibitor imetelstat depletes cancer stem cells in breast and pancreatic cancer cell lines. Cancer Res. 2010;70:9494–504. doi: 10.1158/0008-5472.CAN-10-0233. [DOI] [PubMed] [Google Scholar]
- 5.Kelleher FC. Hedgehog signalling and therapeutics in pancreatic cancer. Carcinogenesis. 2011;32:445–51. doi: 10.1093/carcin/bgq280. [DOI] [PubMed] [Google Scholar]
- 6.Azmi AS, Wang Z, Philip PA, et al. Proof of concept: network and systems biology approaches aid in the discovery of potent anticancer drug combinations. Mol Cancer Ther. 2010;9:3137–44. doi: 10.1158/1535-7163.MCT-10-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kern SE, Shi C, Hruban RH. The complexity of pancreatic ductal cancers and multidimensional strategies for therapeutic targeting. J Pathol. 2011;223:295–306. doi: 10.1002/path.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham D, Chong I. Optimal treatment of metastatic pancreatic cancer. Gut. 2010;59:1454–5. doi: 10.1136/gut.2010.220954. [DOI] [PubMed] [Google Scholar]
- 9.McClean MN, Mody A, Broach JR, et al. Cross-talk and decision making in MAP kinase pathways. Nat Genet. 2007;39:409–14. doi: 10.1038/ng1957. [DOI] [PubMed] [Google Scholar]
- 10.Cloughesy TF, Mischel PS. New strategies in the molecular targeting of glioblastoma: how do you hit a moving target? Clin Cancer Res. 2011;17:6–11. doi: 10.1158/1078-0432.CCR-09-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhim AD. A combined, rational approach towards inhibition of the MEK-ERK and mTOR pathways in pancreatic ductal adenocarcinoma: promise or deja vu? Cancer Biol Ther. 2009;8:1902–3. doi: 10.4161/cbt.8.20.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res. 2010;16:2505–11. doi: 10.1158/1078-0432.CCR-09-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kisfalvi K, Eibl G, Sinnett-Smith J, et al. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009;69:6539–45. doi: 10.1158/0008-5472.CAN-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez-Otin C, Hunter T. The regulatory crosstalk between kinases and proteases in cancer. Nat Rev Cancer. 2010;10:278–92. doi: 10.1038/nrc2823. [DOI] [PubMed] [Google Scholar]
- 15.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 16.Fujita H, Ohuchida K, Mizumoto K, et al. High EGFR mRNA expression is a prognostic factor for reduced survival in pancreatic cancer after gemcitabine-based adjuvant chemotherapy. Int J Oncol. 2011;38:629–41. doi: 10.3892/ijo.2011.908. [DOI] [PubMed] [Google Scholar]
- 17.Huang Z-q, Buchsbaum DJ, Raisch KP, et al. Differential responses by pancreatic carcinoma cell lines to prolonged exposure to Erbitux (IMC-C225) anti-EGFR antibody. J Surg Res. 2003;111:274–83. doi: 10.1016/s0022-4804(03)00076-3. [DOI] [PubMed] [Google Scholar]
- 18.Morgan MA, Parsels LA, Kollar LE, et al. The combination of epidermal growth factor receptor inhibitors with gemcitabine and radiation in pancreatic cancer. Clin Cancer Res. 2008;14:5142–9. doi: 10.1158/1078-0432.CCR-07-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cascinu S, Berardi R, Labianca R, et al. Cetuximab plus gemcitabine and cisplatin compared with gemcitabine and cisplatin alone in patients with advanced pancreatic cancer: a randomised, multi-centre, phase II trial. Lancet Oncol. 2008;9:39–44. doi: 10.1016/S1470-2045(07)70383-2. [DOI] [PubMed] [Google Scholar]
- 20.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205; J Clin Oncol. 2010;28:3605–10. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philip PA, Goldman BH, Ramanathan RK, et al. SWOG S0727: A randomized phase II trial of combination gemcitabine plus erlotinib plus IMC-A12 (cixutumumab) versus gemcitabine plus erlotinib as first-line treatment in patients (pts) with metastatic pancreatic cancer. J Clin Oncol. 2010;28:Abstract TPS 223. [Google Scholar]
- 22.Berlin J, Catalano P, Feng Y, et al. ECOG 2204: An intergroup randomized phase II study of cetuximab (Ce) or bevacizumab (B) in combination with gemcitabine (G) and in combination with capecitabine (Ca) and radiation (XRT) as adjuvant therapy (Adj Tx) for patients (pts) with completely resected pancreatic adenocarcinoma (PC) J Clin Oncol. 2010;28:Abstract 4034. [Google Scholar]
- 23.Rocha Lima CS, Lin EH, Kim G, et al. Phase II trial of ixabepilone (IXA) plus cetuximab (C) as first-line therapy for advanced pancreatic carcinoma (PC) J Clin Oncol. 2010;28:Abstract 4086. [Google Scholar]
- 24.Kleespies A, Ischenko I, Eichhorn ME, et al. Matuzumab short-term therapy in experimental pancreatic cancer: prolonged antitumor activity in combination with gemcitabine. Clin Cancer Res. 2008;14:5426–36. doi: 10.1158/1078-0432.CCR-07-5245. [DOI] [PubMed] [Google Scholar]
- 25.Saif MW, Peccerillo J, Potter V. Successful re-challenge with panitumumab in patients who developed hypersensitivity reactions to cetuximab: report of three cases and review of literature. Cancer Chemother Pharmacol. 2009;63:1017–22. doi: 10.1007/s00280-008-0831-6. [DOI] [PubMed] [Google Scholar]
- 26.Strumberg D, Schultheis B, Scheulen ME, et al. Phase II study of nimotuzumab, a humanized monoclonal anti-epidermal growth factor receptor (EGFR) antibody, in patients with locally advanced or metastatic pancreatic cancer. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9619-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Tsiambas E, Karameris A, Dervenis C, et al. HER2/neu expression and gene alterations in pancreatic ductal adenocarcinoma: a comparative immunohistochemistry and chromogenic in situ hybridization study based on tissue microarrays and computerized image analysis. JOP. 2006;7:283–94. [PubMed] [Google Scholar]
- 28.Komoto M, Nakata B, Amano R, et al. HER2 overexpression correlates with survival after curative resection of pancreatic cancer. Cancer Sci. 2009;100:1243–7. doi: 10.1111/j.1349-7006.2009.01176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaturvedi P, Singh AP, Chakraborty S, et al. MUC4 mucin interacts with and stabilizes the HER2 oncoprotein in human pancreatic cancer cells. Cancer Res. 2008;68:2065–70. doi: 10.1158/0008-5472.CAN-07-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saeki H, Yanoma S, Takemiya S, et al. Antitumor activity of a combination of trastuzumab (Herceptin) and oral fluoropyrimidine S-1 on human epidermal growth factor receptor 2-overexpressing pancreatic cancer. Oncol Rep. 2007;18:433–9. [PubMed] [Google Scholar]
- 31.Kimura K, Sawada T, Komatsu M, et al. Antitumor effect of trastuzumab for pancreatic cancer with high HER-2 expression and enhancement of effect by combined therapy with gemcitabine. Clin Cancer Res. 2006;12:4925–32. doi: 10.1158/1078-0432.CCR-06-0544. [DOI] [PubMed] [Google Scholar]
- 32.Geissler M, Hofheinz R, Meoehler MH, et al. Trastuzumab and capecitabine in patients with HER2-expressing metastatic pancreatic cancer: A multicenter phase II study of the German AIO Pancreatic Cancer Group (AIO PK-0204) J Clin Oncol. 2010;28:Abstract 4070. [Google Scholar]
- 33.Vidal-pla A, Perez-Torras S, Miquel R, et al. Targeted therapy to EGFR and HER2 combined with gemcitabine in a novel human pancreatic orthotopic model. Journal of Clinical Oncology. 2010;28:Abstract e14601. [Google Scholar]
- 34.Milenic DE, Wong KJ, Baidoo KE, et al. Targeting HER2: a report on the in vitro and in vivo pre-clinical data supporting trastuzumab as a radioimmunoconjugate for clinical trials. MAbs. 2010;2:550–64. doi: 10.4161/mabs.2.5.13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agus DB, Gordon MS, Taylor C, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005;23:2534–43. doi: 10.1200/JCO.2005.03.184. [DOI] [PubMed] [Google Scholar]
- 36.Aggarwal BB, Bhardwaj U, Takada Y. Regulation of TRAIL-induced apoptosis by ectopic expression of antiapoptotic factors. Vitam Horm. 2004;67:453–83. doi: 10.1016/S0083-6729(04)67023-3. [DOI] [PubMed] [Google Scholar]
- 37.Debatin K-M, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23:2950–66. doi: 10.1038/sj.onc.1207558. [DOI] [PubMed] [Google Scholar]
- 38.Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell. 2004;116:491–7. doi: 10.1016/s0092-8674(04)00166-7. [DOI] [PubMed] [Google Scholar]
- 39.Rudner J, Jendrossek V, Lauber K, et al. Type I and type II reactions in TRAIL-induced apoptosis -- results from dose-response studies. Oncogene. 2005;24:130–40. doi: 10.1038/sj.onc.1208191. [DOI] [PubMed] [Google Scholar]
- 40.Buchsbaum DJ, Zhou T, LoBuglio AF. TRAIL receptor-targeted therapy. Future Oncol. 2006;2:493–508. doi: 10.2217/14796694.2.4.493. [DOI] [PubMed] [Google Scholar]
- 41.Yada A, Yazawa M, Ishida S, et al. A novel humanized anti-human death receptor 5 antibody CS-1008 induces apoptosis in tumor cells without toxicity in hepatocytes. Ann Oncol. 2008;19:1060–7. doi: 10.1093/annonc/mdn015. [DOI] [PubMed] [Google Scholar]
- 42.Amm HM, Oliver PG, Lee CH, et al. Combined modality therapy with TRAIL or agonistic death receptor antibodies. Cancer Biol Ther. 2011;11:431–49. doi: 10.4161/cbt.11.5.14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeRosier LC, Vickers SM, Zinn KR, et al. TRA-8 anti-DR5 monoclonal antibody and gemcitabine induce apoptosis and inhibit radiologically validated orthotopic pancreatic tumor growth, Mol. Cancer Ther. 2007;6:3198–207. doi: 10.1158/1535-7163.MCT-07-0299. [DOI] [PubMed] [Google Scholar]
- 44.DeRosier LC, Buchsbaum DJ, Oliver PG, et al. Combination treatment with TRA-8 anti-death receptor-5 antibody and CPT-11 induces tumor regression in an orthotopic model of pancreatic cancer, Clin. Cancer Res. 2007;13:5535s–43s. doi: 10.1158/1078-0432.CCR-07-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajeshkumar NV, Rasheed ZA, Garcia-Garcia E, et al. A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model. Mol Cancer Ther. 2010;9:2582–92. doi: 10.1158/1535-7163.MCT-10-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosevear HM, Lightfoot AJ, Griffith TS. Conatumumab, a fully human mAb against death receptor 5 for the treatment of cancer. Curr Opin Investig Drugs. 2010;11:688–98. [PubMed] [Google Scholar]
- 47.Herbst RS, Kurzrock R, Hong DS, et al. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin Cancer Res. 2010;16:5883–91. doi: 10.1158/1078-0432.CCR-10-0631. [DOI] [PubMed] [Google Scholar]
- 48.Doi T, Murakami H, Ohtsu A, et al. Phase 1 study of conatumumab, a pro-apoptotic death receptor 5 agonist antibody, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol. 2011 doi: 10.1007/s00280-010-1544-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Adams C, Totpal K, Lawrence D, et al. Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5. Cell Death Differ. 2008;15:751–61. doi: 10.1038/sj.cdd.4402306. [DOI] [PubMed] [Google Scholar]
- 50.Punnoose E, Wagner K, Lamler L, et al. Sensitivity to Apomab, an agonistic DR5 specific antibody, is correlated with expression of specific O-glycosyl transfereases in tumor-cell lines of both epithelial and non epithelial origin. Proc. Am. Assoc. Cancer Res; 2009 Apr 18–22; p. abstract 687. [Google Scholar]
- 51.Neid M, Datta K, Stephan S, et al. Role of insulin receptor substrates and protein kinase C-zeta in vascular permeability factor/vascular endothelial growth factor expression in pancreatic cancer cells. J Biol Chem. 2004;279:3941–8. doi: 10.1074/jbc.M303975200. [DOI] [PubMed] [Google Scholar]
- 52.Liu W, Bloom DA, Cance WG, et al. FAK and IGF-IR interact to provide survival signals in human pancreatic adenocarcinoma cells. Carcinogenesis. 2008;29:1096–107. doi: 10.1093/carcin/bgn026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ma J, Sawai H, Matsuo Y, et al. IGF-1 mediates PTEN suppression and enhances cell invasion and proliferation via activation of the IGF-1/PI3K/Akt signaling pathway in pancreatic cancer cells. J Surg Res. 2010;160:90–101. doi: 10.1016/j.jss.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 54.Adachi Y, Li R, Yamamoto H, et al. Insulin-like growth factor-I receptor blockade reduces the invasiveness of gastrointestinal cancers via blocking production of matrilysin. Carcinogenesis. 2009;30:1305–13. doi: 10.1093/carcin/bgp134. [DOI] [PubMed] [Google Scholar]
- 55.Tomizawa M, Shinozaki F, Sugiyama T, et al. Insulin-like growth factor-I receptor in proliferation and motility of pancreatic cancer. World J Gastroenterol. 2010;16:1854–8. doi: 10.3748/wjg.v16.i15.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sprynski AC, Hose D, Kassambara A, Vincent L, Jourdan M, Rossi JF, et al. Insulin is a potent myeloma cell growth factor through insulin/IGF-1 hybrid receptor activation. Leukemia. 2010;24:1940–50. doi: 10.1038/leu.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulanet DB, Ludwig DL, Kahn CR, et al. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proc Natl Acad Sci U S A. 2010;107:10791–8. doi: 10.1073/pnas.0914076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rowinsky EK, Youssoufian H, Tonra JR, et al. IMC-A12, a human IgG1 monoclonal antibody to the insulin-like growth factor I receptor. Clin Cancer Res. 2007;13:5549s–55s. doi: 10.1158/1078-0432.CCR-07-1109. [DOI] [PubMed] [Google Scholar]
- 59.Prewett M, Damoci C, Bassi R, et al. IMC-A12 enhances the efficacy of cetuximab + gemcitabine therapy in human pancreatic carcinoma xenografts. AACR Meeting Abstracts. 2007;2007:652. [Google Scholar]
- 60.Beltran PJ, Mitchell P, Chung YA, et al. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Mol Cancer Ther. 2009;8:1095–105. doi: 10.1158/1535-7163.MCT-08-1171. [DOI] [PubMed] [Google Scholar]
- 61.Tolcher AW, Sarantopoulos J, Patnaik A, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27:5800–7. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 62.Li JC, Li R. RAV12 accelerates the desensitization of Akt/PKB pathway of insulin-like growth factor I receptor signaling in COLO205. Cancer Res. 2007;67:8856–64. doi: 10.1158/0008-5472.CAN-07-0971. [DOI] [PubMed] [Google Scholar]
- 63.Loo D, Pryer N, Young P, et al. The glycotope-specific RAV12 monoclonal antibody induces oncosis in vitro and has antitumor activity against gastrointestinal adenocarcinoma tumor xenografts in vivo. Mol Cancer Ther. 2007;6:856–65. doi: 10.1158/1535-7163.MCT-06-0581. [DOI] [PubMed] [Google Scholar]
- 64.Burris HA, Rosen LS, Rocha-Lima CM, et al. Phase 1 experience with an anti-glycotope monoclonal antibody, RAV12, in recurrent adenocarcinoma. Clin Cancer Res. 2010;16:1673–81. doi: 10.1158/1078-0432.CCR-09-2263. [DOI] [PubMed] [Google Scholar]
- 65.Cohen BD, Baker DA, Soderstrom C, et al. Combination therapy enhances the inhibition of tumor growth with the fully human antitype 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res. 2005;11:2063–73. doi: 10.1158/1078-0432.CCR-04-1070. [DOI] [PubMed] [Google Scholar]
- 66.Runnels HA, Arbuckle JA, Bailey KS, et al. Human monoclonal antibodies to the insulin-like growth factor 1 receptor inhibit receptor activation and tumor growth in preclinical studies. Adv Ther. 2010;27:458–75. doi: 10.1007/s12325-010-0026-5. [DOI] [PubMed] [Google Scholar]
- 67.Watkins DJ, Tabernero J, Schmoll HJ, et al. A phase II study of the anti-IGFR antibody MK-0646 in combination with cetuximab and irinotecan in the treatment of chemorefractory metastatic colorectal cancer. J Clin Oncol. 2009;27:Abstract 4127. [Google Scholar]
- 68.Javie MM, Varadhachary GR, Shroff RT, et al. Phase I/II study of MK-0646; the humanized monoclonal IGF-1R antibody in combination with gemcitabine or gemcitabine plus erlotinib (E) for advanced pancreatic cancer. J Clin Oncol. 2010;28:Abstract 4039. [Google Scholar]
- 69.Carboni JM, Wittman M, Yang Z, et al. BMS-754807; a small molecule inhibitor of insulin-like growth factor-1R/IR. Mol Cancer Ther. 2009;8:3341–9. doi: 10.1158/1535-7163.MCT-09-0499. [DOI] [PubMed] [Google Scholar]
- 70.Hassan R, Laszik ZG, Lerner M, et al. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124:838–45. [PubMed] [Google Scholar]
- 71.Hucl T, Brody JR, Gallmeier E, et al. High cancer-specific expression of mesothelin (MSLN) is attributable to an upstream enhancer containing a transcription enhancer factor dependent MCAT motif. Cancer Res. 2007;67:9055–65. doi: 10.1158/0008-5472.CAN-07-0474. [DOI] [PubMed] [Google Scholar]
- 72.Hassan R, Williams-Gould J, Steinberg SM, et al. Tumor-directed radiation and the immunotoxin SS1P in the treatment of mesothelin-expressing tumor xenografts. Clin Cancer Res. 2006;12:4983–8. doi: 10.1158/1078-0432.CCR-06-0441. [DOI] [PubMed] [Google Scholar]
- 73.Hassan R, Broaddus VC, Wilson S, et al. Anti-mesothelin immunotoxin SS1P in combination with gemcitabine results in increased activity against mesothelin-expressing tumor xenografts. Clin Cancer Res. 2007;13:7166–71. doi: 10.1158/1078-0432.CCR-07-1592. [DOI] [PubMed] [Google Scholar]
- 74.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I, V infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13:5144–9. doi: 10.1158/1078-0432.CCR-07-0869. [DOI] [PubMed] [Google Scholar]
- 75.Kreitman RJ, Hassan R, Fitzgerald DJ, et al. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15:5274–9. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hassan R, Ebel W, Routhier EL, et al. Preclinical evaluation of MORAb-009, a chimeric antibody targeting tumor-associated mesothelin. Cancer Immun. 2007;7:20. [PMC free article] [PubMed] [Google Scholar]
- 77.Feng Y, Xiao X, Zhu Z, et al. A novel human monoclonal antibody that binds with high affinity to mesothelin-expressing cells and kills them by antibody-dependent cell-mediated cytotoxicity. Mol Cancer Ther. 2009;8:1113–8. doi: 10.1158/1535-7163.MCT-08-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levi E, Klimstra DS, Andea A, et al. MUC1 and MUC2 in pancreatic neoplasia. J Clin Pathol. 2004;57:456–62. doi: 10.1136/jcp.2003.013292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patel KN, Maghami E, Wreesmann VB, et al. MUC1 plays a role in tumor maintenance in aggressive thyroid carcinomas. Surgery. 2005;138:994–1001. doi: 10.1016/j.surg.2005.09.030. discussion -2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zaretsky JZ, Barnea I, Aylon Y, et al. MUC1 gene overexpressed in breast cancer: structure and transcriptional activity of the MUC1 promoter and role of estrogen receptor alpha (ERα) in regulation of the MUC1 gene expression. Mol Cancer. 2006;5:57. doi: 10.1186/1476-4598-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamada N, Nishida Y, Tsutsumida H, et al. MUC1 expression is regulated by DNA methylation and histone H3 lysine 9 modification in cancer cells. Cancer Res. 2008;68:2708–16. doi: 10.1158/0008-5472.CAN-07-6844. [DOI] [PubMed] [Google Scholar]
- 82.Swanson BJ, McDermott KM, Singh PK, et al. MUC1 is a counter-receptor for myelin-associated glycoprotein (Siglec-4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res. 2007;67:10222–9. doi: 10.1158/0008-5472.CAN-06-2483. [DOI] [PubMed] [Google Scholar]
- 83.Kohlgraf KG, Gawron AJ, Higashi M, et al. Contribution of the MUC1 tandem repeat and cytoplasmic tail to invasive and metastatic properties of a pancreatic cancer cell line. Cancer Res. 2003;63:5011–20. [PubMed] [Google Scholar]
- 84.Roy LD, Sahraei M, Subramani DB, et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene. 2011;30:1449–59. doi: 10.1038/onc.2010.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ahmad R, Raina D, Trivedi V, et al. MUC1 oncoprotein activates the IκB kinase β complex and constitutive NF-κB signalling. Nat Cell Biol. 2007;9:1419–27. doi: 10.1038/ncb1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agata N, Ahmad R, Kawano T, et al. MUC1 oncoprotein blocks death receptor-mediated apoptosis by inhibiting recruitment of caspase-8. Cancer Res. 2008;68:6136–44. doi: 10.1158/0008-5472.CAN-08-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuan Z, Wong S, Borrelli A, et al. Down-regulation of MUC1 in cancer cells inhibits cell migration by promoting E-cadherin/catenin complex formation. Biochem Biophys Res Commun. 2007;362:740–6. doi: 10.1016/j.bbrc.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 88.Yuan Z, Liu X, Wong S, et al. MUC1 Knockdown With RNA Interference Inhibits Pancreatic Cancer Growth. J Surg Res. 2009;157:e39–46. doi: 10.1016/j.jss.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 89.Gulec SC, Cohen SJ, Zuckier LS, et al. First clinical experience with 90Y-radiolabeled humanized anti-MUC1 antibody (hPAM4) in patients with advanced pancreatic cancer: A phase I study. J Clin Oncol. 2007;25:15034. [Google Scholar]
- 90.Gold DV, Goldenberg DM, Karacay H, et al. A novel bispecific, trivalent antibody construct for targeting pancreatic carcinoma. Cancer Res. 2008;68:4819–26. doi: 10.1158/0008-5472.CAN-08-0232. [DOI] [PubMed] [Google Scholar]
- 91.Karacay H, Sharkey RM, Gold DV, et al. Pretargeted radioimmunotherapy of pancreatic cancer xenografts: TF10–90Y-IMP-288 alone and combined with gemcitabine. J Nucl Med. 2009;50:2008–16. doi: 10.2967/jnumed.109.067686. [DOI] [PubMed] [Google Scholar]
- 92.Glazer ES, Zhu C, Massey KL, et al. Noninvasive radiofrequency field destruction of pancreatic adenocarcinoma xenografts treated with targeted gold nanoparticles. Clin Cancer Res. 2010;16:5712–21. doi: 10.1158/1078-0432.CCR-10-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baranowska-Kortylewicz J, Abe M, Nearman J, et al. Emerging role of platelet-derived growth factor receptor-β inhibition in radioimmunotherapy of experimental pancreatic cancer. Clin Cancer Res. 2007;13:299–306. doi: 10.1158/1078-0432.CCR-06-1702. [DOI] [PubMed] [Google Scholar]
- 94.Mita M, Ricart A, Mita A, et al. A phase I study of a CanAg-targeted immunoconjugate, huC242-DM4, in patients with CanAg-expressing solid tumors. J Clin Oncol. 2007;25:Abstract 3062. [Google Scholar]
- 95.Govindan SV, Cardillo TM, Moon SJ, et al. CEACAM5-targeted therapy of human colonic and pancreatic cancer xenografts with potent labetuzumab-SN-38 immunoconjugates. Clin Cancer Res. 2009;15:6052–61. doi: 10.1158/1078-0432.CCR-09-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Safran H, Miner T, Resnick M, et al. Lapatinib/gemcitabine and lapatinib/gemcitabine/oxaliplatin: a phase I study for advanced pancreaticobiliary cancer. Am J Clin Oncol. 2008;31:140–4. doi: 10.1097/COC.0b013e318145b9a5. [DOI] [PubMed] [Google Scholar]
- 97.Stieler J, Pelzer U, Sinn M, et al. CONKO-008: Oxaliplatin (O)/folinic acid (FA)/5-fluorouracil (5-FU) (24 h) in combination with lapatinib as a second-line therapy in pancreatic cancer after gemcitabine failure: A phase I/II trial. J Clin Oncol. 2010;28:Abstract TPS225. [Google Scholar]
- 98.El-Rayes BF, Philip PA, Sarkar FH, et al. A phase II study of isoflavones, erlotinib, and gemcitabine in advanced pancreatic cancer. Invest New Drugs. 2011;29:694–9. doi: 10.1007/s10637-010-9386-6. [DOI] [PubMed] [Google Scholar]
- 99.Keogh GP, Langdon RM, Stella PJ, et al. APRICoT-P: A phase II randomized placebo controlled study of apricoxib, a potent COX-2 inhibitor in combination with erlotinib and gemcitabine in pancreatic cancer patients. J Clin Oncol. 2010;28:Abstract TPS224. [Google Scholar]
- 100.Boeck SH, Vehling-Kaiser U, Waldshmidt D, et al. Gemcitabine plus erlotinib (GE) followed by capecitabine (C) versus capecitabine plus erlotinib (CE) followed by gemcitabine (G) in advanced pancreatic cancer (APC): A randomized, cross-over phase III trial of the Arbeitsgemeinschaft Internistische Onkologie (AIO) J Clin Oncol. 2010;28:Abstract LBA4011. [Google Scholar]
- 101.Ko AH, Venook AP, Bergsland EK, et al. A phase II study of bevacizumab plus erlotinib for gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2010;66:1051–7. doi: 10.1007/s00280-010-1257-5. [DOI] [PubMed] [Google Scholar]
- 102.Watkins DJ, Starling N, Chau I, et al. The combination of a chemotherapy doublet (gemcitabine plus capecitabine) with a biologic doublet (bevacizumab plus erlotinib) in patients with advanced pancreatic adenocarcinoma: The TARGET study. J Clin Oncol. 2010;28:Abstract 4036. [Google Scholar]
- 103.Manzano J, Rivera F, Galan M, et al. A phase II, open label study to evaluate the relationship between skin rash and survival in patients with unresectable and/or metastatic pancreatic cancer treated with erlotinib combined with gemcitabine. J Clin Oncol. 2010;28:Abstract 4094. [Google Scholar]
- 104.Milella M, Vaccaro V, Sperduti I, et al. Phase II study of erlotinib (E) combined with fixed dose-rate gemcitabine (FDR-Gem) as first-line treatment for advanced adenocarcinoma of the pancreas (PDAC) J Clin Oncol. 2010;28:Abstract e14565. [Google Scholar]
- 105.Vickers MM, Powell ED, Asmis TR, et al. Comorbidity and overall survival (OS) in patients with advanced pancreatic cancer (APC): Results from NCIC CTG PA.3-A phase III trial of erlotinib plus gemcitabine (E+G) versus gemcitabine (G) alone. J Clin Oncol. 2010;28:Abstract 4079. [Google Scholar]
- 106.Stuebs P, Habermann P, Zierau K, et al. First-line therapy for advanced pancreatic cancer with gemcitabine and docetaxel versus gemcitabine and erlotinib: A multivariate matched pair analysis. J Clin Oncol. 2010;28:Abstract e14572. [Google Scholar]
- 107.Jiang Y, Kimchi E, Gusani NJ, et al. Phase I study of capecitabine and erlotinib with radiotherapy in locally advanced nonoperable pancreatic cancer. J Clin Oncol. 2010;28:Abstract e14680. [Google Scholar]
- 108.Javle M, Reddy S, Davis D, et al. Inhibition of mammalian target of rapamycin (mTOR) in advanced pancreatic cancer - the results of two prospective phase II studies. Proc Am Assoc Cancer Res. 2009:Abstract 4363. [Google Scholar]
- 109.Jeldres C, Sun M, Perrotte P, Karakiewicz PI. Pazopanib trial data cannot support first-line use. Nat Rev Urol. 2010;7:307–8. doi: 10.1038/nrurol.2010.65. [DOI] [PubMed] [Google Scholar]
- 110.Tan AR, Dowlati A, Jones SF, et al. Phase I study of pazopanib in combination with weekly paclitaxel in patients with advanced solid tumors. Oncologist. 2010;15:1253–61. doi: 10.1634/theoncologist.2010-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Spano JP, Chodkiewicz C, Maurel J, et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet. 2008;371:2101–8. doi: 10.1016/S0140-6736(08)60661-3. [DOI] [PubMed] [Google Scholar]
- 112.Starling N, Hawkes EA, Chau I, et al. A dose-escalation study of gemcitabine (Gem) plus oxaliplatin (Ox) in combination with imatinib in patients (pts) with gemcitabine-refractory advanced pancreatic adenocarcinoma (PC) J Clin Oncol. 2010;28:Abstract 4155. doi: 10.1093/annonc/mdr317. [DOI] [PubMed] [Google Scholar]
- 113.Garrido-Laguna I, Tan AC, Uson M, et al. Integrated preclinical and clinical development of mTOR inhibitors in pancreatic cancer. Br J Cancer. 2010;103:649–55. doi: 10.1038/sj.bjc.6605819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Javle MM, Shroff RT, Xiong H, et al. Inhibition of the mammalian target of rapamycin (mTOR) in advanced pancreatic cancer: results of two phase II studies. BMC Cancer. 2010;10:368. doi: 10.1186/1471-2407-10-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hong DS, Sebti SM, Newman RA, et al. Phase I trial of a combination of the multikinase inhibitor sorafenib and the farnesyltransferase inhibitor tipifarnib in advanced malignancies. Clin Cancer Res. 2009;15:7061–8. doi: 10.1158/1078-0432.CCR-09-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Eder JP, Shapiro GI, Appleman LJ, et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res. 2010;16:3507–16. doi: 10.1158/1078-0432.CCR-10-0574. [DOI] [PubMed] [Google Scholar]
- 117.Messersmith WA, Nallapareddy S, Arcaroli J, et al. A phase II trial of saracatinib (AZD0530), an oral Src inhibitor, in previously treated metastatic pancreatic cancer, Print this page. J Clin Oncol. 2010;28:Abstract e14515. [Google Scholar]
- 118.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 119.Yuan R, Kay A, Berg WJ, Lebwohl D. Targeting tumorigenesis: development and use of mTOR inhibitors in cancer therapy. J Hematol Oncol. 2009;2:45. doi: 10.1186/1756-8722-2-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.O’Reilly T, McSheehy PMJ, Brueggen J, et al. In vivo antitumor activity of RAD001 (everolimus) in 58 specialized human tumor xenograft models. Proc Am Assoc Cancer Res. 2008:Abstract 2917. [Google Scholar]
- 121.Schmid H, Jensen M. Inhibitory effect of pasireotide (SOM230) in a rat pancreatic tumor model (CA20948) alone and in combination with everolimus (RAD001) Proc Am Assoc Cancer Res. 2009:Abstract 808. [Google Scholar]
- 122.Chang Q, Chapman MS, Miner JN, et al. Antitumour activity of a potent MEK inhibitor RDEA119/BAY 869766 combined with rapamycin in human orthotopic primary pancreatic cancer xenografts. BMC Cancer. 2010;10:515. doi: 10.1186/1471-2407-10-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Clark A, Ma JG, Qiu D, et al. Pharmacokinetics, efficacy and target pathway inhibition in vivo of AS703026; a small molecule inhibitor of MEK. Proc Am Assoc Cancer Res. 2009:Abstract 3694. [Google Scholar]
- 124.Machl A, Ogden j, Romanelli A. Efficacy of MEK inhibitor AS703026 in various primary tumor explants. Proc Am Assoc Cancer Res; 2009 Apr 18–22; Denver, CO. Philadelphia (PA): AACR; 2009. p. Abstract nr 3698. [Google Scholar]
- 125.Ulivi P, Arienti C, Tesei A, et al. Different mechanisms involved in the activity of BAY 43–9006 (Sorafenib) in human pancreatic cancer cell lines. Proc Am Assoc Cancer Res. 2008:Abstract 2809. [Google Scholar]
- 126.Huang S, Sinicrope FA. Sorafenib inhibits STAT3 activation to enhance TRAIL-mediated apoptosis in human pancreatic cancer cells. Mol Cancer Ther. 2010;9:742–50. doi: 10.1158/1535-7163.MCT-09-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shi H, Liu L, Greger J, et al. GSK1363089 inhibits MET and synergizes with HER targeted agents in MET amplified/over-expressed and HER1/HER2 amplified tumor cells. Proc Am Assoc Cancer Res. 2009:Abstract 1746. [Google Scholar]
- 128.Kindler HL, Wroblewski K, Wallace JA, et al. Gemcitabine plus sorafenib in patients with advanced pancreatic cancer: a phase II trial of the University of Chicago Phase II Consortium. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9526-z. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–80. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 130.Nagaraj NS, Smith JJ, Revetta F. Targeted inhibition of SRC kinase signaling attenuates pancreatic tumorigenesis. Mol Cancer Ther. 2010;9:2322–32. doi: 10.1158/1535-7163.MCT-09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nagaraj NS, Washington MK, Merchant NB. Combined Blockade of Src Kinase and Epidermal Growth Factor Receptor with Gemcitabine Overcomes STAT3-Mediated Resistance of Inhibition of Pancreatic Tumor Growth. Clin Cancer Res. 17:483–93. doi: 10.1158/1078-0432.CCR-10-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Evans T, Morton J, Karim S, et al. Dasatinib inhibits the development of metastases in a mouse model of pancreatic cancer. Proc Am Assoc Cancer Res; 2009 Apr 18–22; Denver, CO. Philadelphia (PA): AACR; 2009. p. Abstract nr LB-217. [Google Scholar]
- 133.Chang Q, Jorgensen C, Pawson T, et al. Effects of dasatinib on EphA2 receptor tyrosine kinase activity and downstream signalling in pancreatic cancer. Br J Cancer. 2008;99:1074–82. doi: 10.1038/sj.bjc.6604676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Renouf DJ, Moore MJ, Hedley D, Gill S, Jonker D, Chen E, et al. A phase I/II study of the Src inhibitor saracatinib (AZD0530) in combination with gemcitabine in advanced pancreatic cancer. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9611-3. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 135.Neesse A, Michl P, Frese KK, et al. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–8. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 136.Xu Z, Vonlaufen A, Phillips PA, et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010;177:2585–96. doi: 10.2353/ajpath.2010.090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ikenaga N, Ohuchida K, Mizumoto K, et al. CD10+ pancreatic stellate cells enhance the progression of pancreatic cancer. Gastroenterology. 2010;139:1041–51. 51, e1–8. doi: 10.1053/j.gastro.2010.05.084. [DOI] [PubMed] [Google Scholar]
- 138.Xu D, Matsuo Y, Ma J, et al. Cancer cell-derived IL-1alpha promotes HGF secretion by stromal cells and enhances metastatic potential in pancreatic cancer cells. J Surg Oncol. 2010;102:469–77. doi: 10.1002/jso.21530. [DOI] [PubMed] [Google Scholar]
- 139.Garber K. Stromal depletion goes on trial in pancreatic cancer. J Natl Cancer Inst. 2010;102:448–50. doi: 10.1093/jnci/djq113. [DOI] [PubMed] [Google Scholar]
- 140.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–17. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 141.Steg A, Amm HM, Novak Z, et al. Gli3 mediates cell survival and sensitivity to cyclopamine in pancreatic cancer. Cancer Biol Ther. 2010;10:893–902. doi: 10.4161/cbt.10.9.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Thayer SP, di Magliano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–6. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Walter K, Omura N, Hong SM, et al. Overexpression of smooth-ened activates the sonic hedgehog signaling pathway in pancreatic cancer-associated fibroblasts. Clin Cancer Res. 2010;16:1781–9. doi: 10.1158/1078-0432.CCR-09-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tian H, Callahan CA, DuPree KJ, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci USA. 2009;106:4254–9. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gradishar WJ. Albumin-bound paclitaxel: a next-generation taxane. Expert Opin Pharmacother. 2006;7:1041–53. doi: 10.1517/14656566.7.8.1041. [DOI] [PubMed] [Google Scholar]
- 146.Iglesias J. nab-Paclitaxel (Abraxane®): an albumin-bound cytotoxic exploiting natural delivery mechanisms into tumors. Breast Cancer Res. 2009;11 (Suppl 1):S9. [Google Scholar]
- 147.Clark CJ, Sage EH. A prototypic matricellular protein in the tumor microenvironment--where there’s SPARC, there’s fire. J Cell Biochem. 2008;104:721–32. doi: 10.1002/jcb.21688. [DOI] [PubMed] [Google Scholar]
- 148.Mantoni TS, Schendel RR, Rodel F, et al. Stromal SPARC expression and patient survival after chemoradiation for non-resectable pancreatic adenocarcinoma. Cancer Biol Ther. 2008;7:1806–15. doi: 10.4161/cbt.7.11.6846. [DOI] [PubMed] [Google Scholar]
- 149.Infante JR, Matsubayashi H, Sato N, et al. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–25. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 150.Miyoshi K, Sato N, Ohuchida K, et al. SPARC mRNA expression as a prognostic marker for pancreatic adenocarcinoma patients. Anticancer Res. 2010;30:867–71. [PubMed] [Google Scholar]
- 151.Gao J, Song J, Huang H, et al. Methylation of the SPARC gene promoter and its clinical implication in pancreatic cancer. J Exp Clin Cancer Res. 2010;29:28. doi: 10.1186/1756-9966-29-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hosein PJ, Lopes GD, Jr, Gomez CM, et al. A phase II trial of nab-paclitaxel (NP) in patients with advanced pancreatic cancer (PC) who have progressed on gemcitabine (G)-based therapy. J Clin Oncol. 2010;28:Abstract 4120. [Google Scholar]
- 153.Thapaliya P, Kundranda MN, Curtis KK, et al. Gemcitabine and nab-paclitaxel in patients with unresectable/borderline resectable pancreatic cancer. J Clin Oncol. 2010;28:Abstract e14675. [Google Scholar]
- 154.Isacoff WH, Reber HA, Purcell FM, Clerkin BM, Clerkin KM. Low-dose continuous infusion 5-fluorouracil combined with weekly leucovorin, nab-paclitaxel, oxaliplatin, and bevacizumab for patients with advanced pancreatic cancer: A pilot study. J Clin Oncol. 2010;28:Abstract e14545. [Google Scholar]
- 155.Shojaei F, Ferrara N. Antiangiogenic therapy for cancer: an update. Cancer J. 2007;13:345–8. doi: 10.1097/PPO.0b013e31815a7b69. [DOI] [PubMed] [Google Scholar]
- 156.Beckermann BM, Kallifatidis G, Groth A, et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br J Cancer. 2008;99:622–31. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang B, Zhao WH, Zhou WY, et al. Expression of vascular endothelial growth factors-C and -D correlate with evidence of lymphangiogenesis and angiogenesis in pancreatic adenocarcinoma. Cancer Detect Prev. 2007;31:436–42. doi: 10.1016/j.cdp.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 158.Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA. 2011;305:487–94. doi: 10.1001/jama.2011.51. [DOI] [PubMed] [Google Scholar]
- 159.Lorusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib GDC-0449 in patients with refractory, locally-advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–11. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chien AJ, Illi JA, Ko AH, et al. A phase I study of a 2-day lapatinib chemosensitization pulse preceding nanoparticle albumin-bound Paclitaxel for advanced solid malignancies. Clin Cancer Res. 2009;15:5569–75. doi: 10.1158/1078-0432.CCR-09-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Martin L, Li X, Kim S, et al. A multi-center phase II study of the combination of bevacizumab, fixed dose rate gemcitabine, and infusional 5-fluorouracil in patients with advanced pancreas cancer. Proc Am Assoc Cancer Res. 2009:Abstract 4500. [Google Scholar]
- 162.Garrido C, Brunet M, Didelot C, et al. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 163.Aghdassi A, Phillips P, Dudeja V, et al. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67:616–25. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
- 164.Dudeja V, Chugh RK, Sangwan V, et al. Pro-survival Role of Heat Shock Factor 1 (HSF1) in the Pathogenesis of PancreatoBiliary Tumors. Am J Physiol Gastrointest Liver Physiol. 2011;300:G948–G55. doi: 10.1152/ajpgi.00346.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Joo M, Chi JG, Lee H. Expressions of HSP70 and HSP27 in hepatocellular carcinoma. J Korean Med Sci. 2005;20:829–34. doi: 10.3346/jkms.2005.20.5.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang XP, Qiu FR, Liu GZ, et al. Correlation between clinicopathology and expression of heat shock protein 70 and glucose-regulated protein 94 in human colonic adenocarcinoma. World J Gastroenterol. 2005;11:1056–9. doi: 10.3748/wjg.v11.i7.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Phillips PA, Dudeja V, McCarroll JA, et al. Triptolide Induces Pancreatic Cancer Cell Death via Inhibition of Heat Shock Protein 70. Cancer Res. 2007;67:9407–16. doi: 10.1158/0008-5472.CAN-07-1077. [DOI] [PubMed] [Google Scholar]
- 168.Westerheide SD, Kawahara TL, Orton K, et al. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J Biol Chem. 2006;281:9616–22. doi: 10.1074/jbc.M512044200. [DOI] [PubMed] [Google Scholar]
- 169.Borja-Cacho D, Yokoyama Y, Chugh RK, et al. TRAIL and triptolide: an effective combination that induces apoptosis in pancreatic cancer cells. J Gastrointest Surg. 2010;14:252–60. doi: 10.1007/s11605-009-1065-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nylandsted J, Brand K, Jaattela M. Heat shock protein 70 is required for the survival of cancer cells. Ann N Y Acad Sci. 2000;926:122–5. doi: 10.1111/j.1749-6632.2000.tb05605.x. [DOI] [PubMed] [Google Scholar]
- 171.Sherman M, Multhoff G. Heat shock proteins in cancer. Ann N Y Acad Sci. 2007;1113:192–201. doi: 10.1196/annals.1391.030. [DOI] [PubMed] [Google Scholar]
- 172.Antonoff MB, Chugh R, Borja-Cacho D, et al. Triptolide therapy for neuroblastoma decreases cell viability in vitro and inhibits tumor growth in vivo. Surgery. 2009;146:282–90. doi: 10.1016/j.surg.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 173.Clawson KA, Borja-Cacho D, Antonoff MB, et al. Triptolide and TRAIL combination enhances apoptosis in cholangiocarcinoma. J Surg Res. 2010;163:244–9. doi: 10.1016/j.jss.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lippert TH, Ruoff HJ, Volm M. Current status of methods to assess cancer drug resistance. Int J Med Sci. 2011;8:245–53. doi: 10.7150/ijms.8.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Villarroel MC, Rajeshkumar NV, Garrido-Laguna I, et al. Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. Mol Cancer Ther. 2011;10:3–8. doi: 10.1158/1535-7163.MCT-10-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 177.Mysliwiec P, Boucher MJ. Targeting Notch signaling in pancreatic cancer patients--rationale for new therapy. Adv Med Sci. 2009;54:136–42. doi: 10.2478/v10039-009-0026-3. [DOI] [PubMed] [Google Scholar]
- 178.Mazur PK, Einwachter H, Lee M, et al. Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A. 2010;107:13438–43. doi: 10.1073/pnas.1002423107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wang Z, Li Y, Kong D, et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400–7. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yao J, Qian C. Inhibition of Notch3 enhances sensitivity to gemcitabine in pancreatic cancer through an inactivation of PI3K/Akt-dependent pathway. Med Oncol. 2010;27:1017–22. doi: 10.1007/s12032-009-9326-5. [DOI] [PubMed] [Google Scholar]
- 181.Singh S, Srivastava SK, Bhardwaj A, et al. CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br J Cancer. 103:1671–9. doi: 10.1038/sj.bjc.6605968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hagmann W, Jesnowski R, Lohr JM. Interdependence of gemcitabine treatment, transporter expression, and resistance in human pancreatic carcinoma cells. Neoplasia. 12:740–7. doi: 10.1593/neo.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nambaru PK, Hubner T, Kock K, et al. Drug efflux transporter multidrug resistance-associated protein 5 affects sensitivity of pancreatic cancer cell lines to the nucleoside anticancer drug 5-fluorouracil. Drug Metab Dispos. 39:132–9. doi: 10.1124/dmd.110.033613. [DOI] [PubMed] [Google Scholar]
- 184.Tanaka M, Okazaki T, Suzuki H, et al. Association of multi-drug resistance gene polymorphisms with pancreatic cancer outcome. Cancer. 117:744–51. doi: 10.1002/cncr.25510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kalra AV, Campbell RB. Mucin overexpression limits the effectiveness of 5-FU by reducing intracellular drug uptake and antineoplastic drug effects in pancreatic tumours. Eur J Cancer. 2009;45:164–73. doi: 10.1016/j.ejca.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 186.Li Z, Dong Z, Myer D, Yip-Schneider M, Liu J, Cui P, et al. Role of 14–3-3sigma in poor prognosis and in radiation and drug resistance of human pancreatic cancers. BMC Cancer. 10:598. doi: 10.1186/1471-2407-10-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ramachandran K, Miller H, Gordian E, et al. Methylation-mediated silencing of TMS1 in pancreatic cancer and its potential contribution to chemosensitivity. Anticancer Res. 30:3919–25. [PubMed] [Google Scholar]
- 188.Siraj AK, Hussain AR, Al-Rasheed M, et al. Demethylation of TMS1 gene sensitizes thyroid cancer cells to TRAIL-induced apoptosis. J Clin Endocrinol Metab. 96:E215–24. doi: 10.1210/jc.2010-0790. [DOI] [PubMed] [Google Scholar]
- 189.Mazur PK, Einwachter H, Lee M, et al. Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A. 107:13438–43. doi: 10.1073/pnas.1002423107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang Z, Li Y, Ahmad A, et al. Targeting Notch signaling pathway to overcome drug resistance for cancer therapy. Biochim Biophys Acta. 1806:258–67. doi: 10.1016/j.bbcan.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yao J, Qian C. Inhibition of Notch3 enhances sensitivity to gemcitabine in pancreatic cancer through an inactivation of PI3K/Akt-dependent pathway. Med Oncol. 27:1017–22. doi: 10.1007/s12032-009-9326-5. [DOI] [PubMed] [Google Scholar]
- 192.Smith JK, Ng S, Simons JP, et al. Adjuvant therapy for resectable pancreatic cancer: A simple prediction rule. ASCO - 2010 Gastrointestinal Cancers Symposium; 2010. p. Abstract 236. [Google Scholar]
- 193.O’Day SJ, Hamid O, Urba WJ. Targeting cytotoxic T-lymphocyte antigen-4 (CTLA-4): a novel strategy for the treatment of melanoma and other malignancies. Cancer. 2007;110:2614–27. doi: 10.1002/cncr.23086. [DOI] [PubMed] [Google Scholar]
- 194.Loos M, Giese NA, Kleeff J, et al. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268:98–109. doi: 10.1016/j.canlet.2008.03.056. [DOI] [PubMed] [Google Scholar]
- 195.Aglietta M, Barone C, Muliello M, et al. A phase I dose escalation trial of CP-675206 (tremelimumab) in combination with gemcitabine in patients with chemotherapy-naive metastatic pancreatic cancer. J Clin Oncol. 2010;28:Abstract 4134. [Google Scholar]
- 196.Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33:828–33. doi: 10.1097/CJI.0b013e3181eec14c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Li S, Gill N, Lentzsch S. Recent advances of IMiDs in cancer therapy. Curr Opin Oncol. 2010;22:579–85. doi: 10.1097/CCO.0b013e32833d752c. [DOI] [PubMed] [Google Scholar]
- 198.Infante JR, Jones SF, Bendell JC, et al. A phase I, dose-escalation study of pomalidomide (CC-4047) in combination with gemcitabine in metastatic pancreas cancer. Eur J Cancer. 2011;47:199–205. doi: 10.1016/j.ejca.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 199.Scaiewicz V, Sorin V, Fellig Y, et al. Use of H19 Gene Regulatory Sequences in DNA-Based Therapy for Pancreatic Cancer. J Oncol. 2010;2010:178174. doi: 10.1155/2010/178174. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Smaldone MC, Davies BJ. BC-819, a plasmid comprising the H19 gene regulatory sequences and diphtheria toxin A, for the potential targeted therapy of cancers. Curr Opin Mol Ther. 2010;12:607–16. [PubMed] [Google Scholar]
- 201.Galanis E, Carlson SK, Foster NR, et al. Phase I trial of a pathotropic retroviral vector expressing a cytocidal cyclin G1 construct (Rexin-G) in patients with advanced pancreatic cancer. Mol Ther. 2008;16:979–84. doi: 10.1038/mt.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gordon EM, Hall FL. Noteworthy clinical case studies in cancer gene therapy: tumor-targeted Rexin-G advances as an efficacious anti-cancer agent. Int J Oncol. 2010;36:1341–53. doi: 10.3892/ijo_00000619. [DOI] [PubMed] [Google Scholar]
- 203.Gollamudi R, Ghalib MH, Desai KK, et al. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs. 2010;28:641–9. doi: 10.1007/s10637-009-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nelson AR, Davydova J, Curiel DT, et al. Combination of conditionally replicative adenovirus and standard chemotherapies shows synergistic antitumor effect in pancreatic cancer. Cancer Sci. 2009;100:2181–7. doi: 10.1111/j.1349-7006.2009.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Murugesan SR, King CR, Osborn R, et al. Combination of human tumor necrosis factor-alpha (hTNF-alpha) gene delivery with gemcitabine is effective in models of pancreatic cancer. Cancer Gene Ther. 2009;16:841–7. doi: 10.1038/cgt.2009.32. [DOI] [PubMed] [Google Scholar]
- 206.Mohr A, Albarenque SM, Deedigan L, et al. Targeting of XIAP combined with systemic mesenchymal stem cell-mediated delivery of sTRAIL ligand inhibits metastatic growth of pancreatic carcinoma cells. Stem Cells. 2010;28:2109–20. doi: 10.1002/stem.533. [DOI] [PubMed] [Google Scholar]