Abstract
The skin provides an anatomical barrier to physical, chemical and biological agents. Hence, it is not surprising that it has well developed innate immunity. What we find surprising is that the CD49f+/CD34+ hair follicle stem cells should have an enriched expression profile of so many genes involved in innate immunity. Do these stem cells require extra protection from environmental insults? Or, could there be a new role for these genes? To probe these questions, we first summarize the roles of some key players in epidermal innate immunity. We next focus on their expression in CD49f+/CD34+ hair follicle stem cells. Then we consider recent data suggesting a new role for these “old players” in the regulation and mobilization of hematopoietic and mesenchymal stem cells. Finally, we hypothesize that the “old players” in these hair follicle stem cells, may be playing a “new game”.
Keywords: Skin, innate immunity, keratinocyte, Toll-like receptor, stem cells, mobilization
Introduction
The epidermis is a renewing tissue characterized by lifelong proliferation in the basal layer and loss through terminal differentiation from the suprabasal layers. The epidermis consists primarily of keratinocytes, although melanocytes, Langerhans cells, and Merkel cells are also present (1). The hair follicles (HFs)are appendages of the epidermis and undergo cyclic growth, regression, and rest. The HFs are associated with the sebaceous glands and the arrector pili muscle. Keratinocytes provide the barrier against environmental damage from pathogens, heat, UV-radiation, and water loss, etc. Keratinocyte stem cells (KSCs) have an important role in maintaining the normal structure and function of both the epidermis and HFs, and are important players in inherited and acquired skin disease. Additionally, adult KSCs are also implicated in carcinogenesis, epidermal regeneration, and maintenance via their stem cell properties of self-renewal and long-term persistence (2–4). Several stem cell populations have been discovered in the HFs of mice. One of them, the alpha 6-integrin+/CD34+ (or CD49f+/CD34+)population, is known to contribute to HF cycling, wound healing, and carcinogenesis (4).
Skin serves as a first line of defense against microbes. Many types of cells take part in the process of immune surveillance that involves the detection, identification, and elimination of pathogens from the skin. However, fighting invasive microorganisms or allergens requires a mechanism for identifying the pathogens or chemicals and their molecular patterns. Interestingly, the mammalian defense system inherited Toll like receptors (Tlrs)for this purpose. Tlrs respond against endogenous and exogenous signals by recognizing molecular patterns and are therefore also known as “pattern recognition receptors” (or PRRs) (5, 6). The Tlr signaling cascade is initiated by the recruitment of adaptor proteins associated with the cytoplasmic domains of Tlrs such as MYD88 (myeloid differentiation primary response protein 88), TIRAP (TIR domain containing adaptor protein), and TRIF (TIR domain containing adaptor protein) among others (7). Tlrs are remarkably well-conserved in evolution (8). Because activation of innate immunity leads to development of antigen-specific adaptive immunity, Tlrs are key players in adaptive immune responses as well as those innate. In addition to Tlrs, there are other additional skin sensors such as Nod (nucleotide-binding oligomerization domain)-like receptors or NLRs (ex. Nod1 and Nod2),(reviewed in 9), and c-type lectin receptors (ex. dectin-1) and RIG-like helicase receptors (RLRs) (10).
In the this Viewpoint review, we will discuss the expression patterns of Tlrs in keratinocytes, the cytokine profiles of KSCs and their association with stem cell survival, and propose a mechanism for KSC regulation and mobilization using HSCs and MSCs as a model.
Toll like receptors and their expression in skin: Players in the “old game”
The response of the skin to Tlr ligands depends on the involvement of the cell type in the immune response. In principle, three major cell populations react to microbial stimuli within the epidermis: keratinocytes, antigen-presenting cells, and melanocytes Tlr expression, however, varies among the cells. Tlrs are also expressed in dermal cells such as neutrophils, mast cells, and macrophages involved in host defense (11–15). Tlrs are well studied in mature human epidermal cells in connection with immunological stimuli and their roles in skin diseases.
Human keratinocytes express Tlr1, -2, -3, -5, -6, and -10. Although expression of Tlr4 and -9 has been controversial in the past (16–18), more recently, expression of these Tlrs has been reported in human keratinocytes (19). The roles of some Tlrs in skin defense are poorly understood. Several Tlrs,-2,-3, -4, -8, and -10, are also expressed in Langerhans cells (20). Tlr4 is expressed in cutaneous melanocytes (21), human primary keratinocytes (22), and rat epithelial cells (RTE2) (23), whereas Tlr2 is expressed in CD49f+CD34+ mouse KSCs (24). A comprehensive report on Tlrs, their expression, and evolutionary relatedness is discussed elsewhere (25).
There is evidence for involvement of innate immunity in skin carcinogenesis in mice. Tlr4 signaling has a protective effect against 7, 12-dimethylbenz(a)anthracene (DMBA) induced skin cancer in certain strains of mice that develop a cell mediated immune response to DMBA. Tlr4 can also play an important role during skin tumor promotion (26–28).
Keratinocytes as a reservoir of, and targets for, cytokines
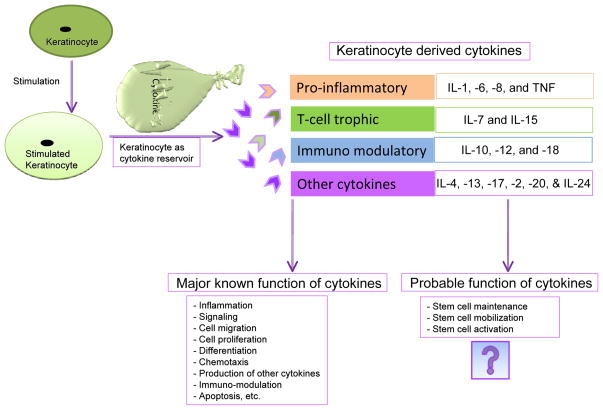
In addition to Tlrs, other molecules termed cytokines are actively and abundantly expressed. Cytokines are produced by keratinocytes, either constitutively or upon induction. These cytokines includes interleukins such as IL-1, -6, -7, -8, -10, -12, -15, -18, and -20, tumor necrosis factor-α (TNF), TGFβ1, and interferon (IFN) α, β, and γ(reviewed in 29, 30). The cytokines can be categorized on the basis of their function in keratinocytes such as pro-inflammatory, T-cell trophic, or immunomodulatory, or as ligands for the cytokine receptors (See Figure 1& Figure 2) (reviewed in 29). The effects of these cytokines are diverse. For example, IL-1 is chemotactic for keratinocytes, induces expression of keratin 6, decreases adherence of some bacteria to keratinocytes, and protects transformed keratinocytes against apoptosis induced by exposure to TNF-related apoptosis inducing ligands (TRAIL) (31–34). Healthy human keratinocytes are able to produce IL6, IL-8, CCL-20, MMP-9 (35), and thymic stromal lymphopoietin (TSLP) following Tlr3 stimulation (36). Similarly, human keratinocyte cell lines such as the HaCaT, CCD 1106 KERTr and HEK001 (CRL-2404) express chemokines namely IL-8, CCL20, CXCL9 and CXCL10 following many different treatments (37).
Figure 1. Keratinocytes as reservoir of cytokines.
This figure illustrates some of the known and plausible unknown function of keratinocyte derived cytokines. The cartoon also depicts some of the various cytokines released from keratinocytes.
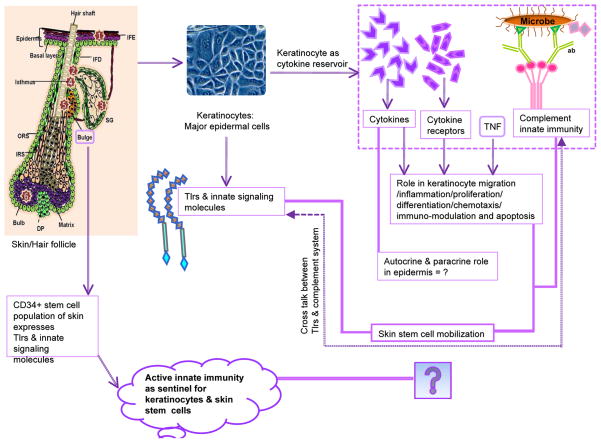
Figure 2. This figure illustrates the involvement of Tlrs, cytokines, and other innate signatures of stem cells use to boost the innate immunity of keratinocytes in mammalian skin.
The left side of the figure depicts skin and its CD34+ stem cells (located in bulge region), and their connection to the innate immune system via Tlrs and other associated molecules. The numbers inside the stars indicates the locations of known stem cell populations within the hair follicle. The middle portion of the figure shows the presence of Tlrs in keratinocytes, whereas the right portions show cytokines and their receptors, and their role in keratinocytes (inside the dotted box). The far right of the figure illustrates the complement cascade and it’s connection in HSC mobilization. The bold lines represent the Viewpoint question related to paracrine or autocrine role of cytokines, and propose a role for innate immunity and its working arms such as Tlrs and the complement system in epidermal stem cell mobilization and stabilization. A divided double line indicates probable cross-talk between the Tlrs and the complement system.
Abbreviations:(TNF= Tumor Necrosis Factor, c= complement molecule, ab = antibody, SG = Sebaceous gland, IM = Arrector pili muscle, DP = Dermal papillae, IFE = inter-follicular epidermis, IFD = Infundibulum, ORS =Outer root sheath, IRS = Inner root sheath).
Cytokines play important role during the migration of inflammatory cells, in keratinocyte proliferation and differentiation, and in induction of other cytokines (reviewed in 29). Similarly, the IL-10 from keratinocytes can modulate the function of antigen presenting cells (38). Moreover, IL-10 deficient mice show impaired immunosuppressive response to UV (39). Production of cytokines and their roles in keratinocytes biology justify the name “Cytokinocyte” given by 1995 by G. F. Murphy. The cytokines are also implemented in hematopoietic stem cell (HSC) mobilization in various in vivo and clinical settings for their ability to enhance HSC trafficking (40).
Endotoxins and cytokines as mobilizers of stem cells: Essence of a new game
The HSCs and MSCs have the ability to move from site of origin in the bone marrow to distant organs during physiological and pathological conditions. Such trafficking of HSCs or progenitor cells into peripheral blood is known as mobilization and is orchestrated by players of the innate immune system (41–43). Multiplelines of evidence implicate HSC mobilization during many different clinical conditions (44); however, regulation and mobilization of KSCs is poorly understood.
Stem cell mobilization has an important role during wounding (45), inflammation, therapy, and development (46, 47). Collective evidence suggests that stem cell mobilization is influenced by physiological and pathological conditions that involve innate immunity (40). Interestingly, endotoxins from pathogenic sources, and some cytokines, can mobilize HSCs. Interleukin-1, IL-3, IL-8, thrombopoietin, granulocyte colony-stimulating factor (GCSF), granulocyte-macrophage colony-stimulating factor (GMCSF), stem cell factor as well as flt3 ligand (FL) when administered either alone, or in combination, are capable of mobilizing HSCs (48–53). Interestingly, the mobilization response to IL-8, GCSF, or FL is significantly reduced in germ free OF-1 mice (or mice without the microbial endotoxin) (54). This finding suggests a role for endotoxin as a cofactor in cytokine-induced HSC mobilization. However, it also indicates that there is some role for Tlrs as they are the primary receptors for endotoxins and their ultimate downstream signaling. The exact mechanism of the observed phenomenon is not known; however, endotoxins are also known as potent inducers of stem cell mobilization following systemic administration (55, 56).
Lipopolysaccharide (LPS) of the aerobic Gram negative bacterial wall can damage the endothelial cells (57) and induces the release of pro-inflammatory cytokines such as TNF, IL-1, IL-6, and IL-8 from macrophages (58) (see figure 3). Similarly, low doses of LPS (Tlr4 ligand)induce emigration of monocytes from bone marrow to the peripheral blood of mice (59). Moreover, CpG-oligodeoxynucleotides induce mobilization of HSCs into peripheral blood in association with the keratinocyte-derived chemokine IL-8 production in mice (44). Additionally, mast cells express Tlrs and produce a number of cytokines, and may be an additional player in stem cell mobilization (See Figure 3).
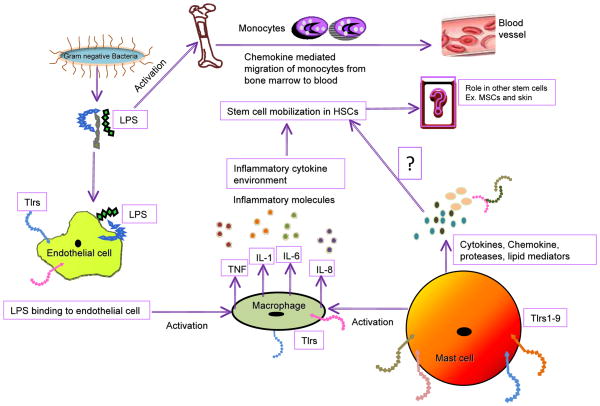
Figure 3. This figure illustrates one of the probable mechanisms of HSC mobilization via involvement of endothelial cells, macrophages, cytokines and bacterial LPS.
The LPS of the aerobic Gram-negative bacteria damages the endothelial cells and activates macrophages to releases pro-inflammatory cytokines such as TNF, IL-1 (Interleukin-1), IL-6, and IL-8 in response to LPS. LPS also induces monocyte migration from bone marrow to blood. It is noteworthy that the macrophage and endothelial cells express Tlrs. The mast cells also express Tlrs and releases cytokines, histamine, chemokines, proteases and lipid mediators against diverse signals; however, their involvement in stem cell mobilization has not yet been determined. Involvement of cytokines, interleukins and LPS induced HSCs activation is reported in literature.
Recently, a role for Tlrs and other inflammatory mediators was reported in hematopoiesis (60), and in HSC activation (61)and regulation (62). However, the question still remains the same: are there positive links between innate immunity and stem cell populations in skin?
Evidence of innate immunity via differentially expressed genes in CD49f+/CD34+ KSCs: an innate link or “Old Players on a New Field”
In one of our microarray studies of CD49f+/CD34+ HF stem cells, we observed many differentially expressed genes in CD34+ versus CD34 depleted (CD34−) keratinocytes of mice (24). The FACS sorted CD34+ cells possess the characteristics of KSCs, including restricted expression in the HFs regardless of stage, and their ability to reconstitute the epidermis (3, 63, 64).
Differentially expressed genes from the Tlr pathway are listed for CD49f+/CD34+ KSCs (see Supplementary Table 1, Figure 2). Of these, TNF genes are associated with regulation of immune cells and their modulation (65). Additionally, mitogen activated protein kinases (MAPKs) that respond to extracellular stimuli (osmotic stress, heat shock, mitogens and pro-inflammatory cytokines), and regulate cellular activities, such as gene expression, proliferation, mitosis, differentiation, and cell survival/apoptosis (66) are also differentially expressed. TNF is associated with LPS induced shock in mice and acts as a primary mediator of inflammation (67).
The differentially expressed Pellino 1 (Peli 1) protein is required for interleukin-1 (a major inflammatory cytokine) mediated signaling through its interaction with interleukin-1 receptor associated kinase 4 (IRAK4) - IRAK-tumor necrosis factor receptor associated factor 6 complex (68). The Pellino proteins are novel players in Tlr and IL-1R signaling (69). The only differentially expressed Tlr family gene was Tlr2 in human epidermal keratinocytes (70). Such observations indicate that these cells play a functional role during the process of immunomodulation, proliferation, differentiation, cell survival and pattern recognition. However, the link between the skin innate immunity and KSCs may be associated via multiple pathways and possibly through the disease connections (71). One such disease connection is alopecia areata.
Innate and adaptive immunity signatures in alopecia areata: A new battlefield
Recently, in a genome wide association study of alopecia areata (AA), the signatures of both adaptive and innate immunity were observed (72)indicating a complex interaction of these responses during this cutaneous autoimmune disease. The genes found to be significantly associated with AA are listed in Supplementary Table 2. Of these, PRDX5 and STX17 genes are expressed in HF itself. In accordance with the common cause hypothesis, this GWAS revealed that several risk loci are common with other form of autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, coeliac disease, type I diabetes, multiple sclerosis and psoriasis (72). Recently, the HF stem cells, their specific markers (73, 74), and immune, and hormonal systems have been found to be associated with the AA (71). Additionally, the possibility of cross-talk among different KSC populations cannot be ignored.
Signaling innate immunity in HSCs and MSCs: Old players take on a new game
Tlrs, their co-receptors, and associated molecules (Tlr2, Tlr4, MD-2, and CD14) are expressed by HSCs (75). CD14 contributes lipopolysaccharide recognition by HSCs whereas the Tlr signaling drives their differentiation (75). Moreover, CD34+ cord blood stem cells express functional Tlr9 (76). However, KSC populations have not been investigated regarding their potential for innate and adaptive immunity. Therefore, it is important to study the Tlrs in stem cell populations because they may be the key players in skin pathologies and cancers.
Similarly, innate immunity and its role in orchestrating HSC mobilization in peripheral blood is currently under study (41, 77, 78). Recent evidence implicates innate immunity and its major arms including granulocytes, the complement cascade, naturally occurring antibodies (NA-Ig), and the network of Tlrs. These players are thought to coordinate a mechanism for HSCs to move from the bone marrow into the peripheral blood(reviewed in 40). This process of HSC mobilization seems to be a conserved mechanism and related to the immune response, inflammation, and tissue damage. HSC mobilization in mice is also facilitated by activation of Tlrs expressed on bone marrow myeloid cells in response to LPS released by intestinal anerobic bacterial flora. Moreover, the mice were found to be poor mobilizers if the bacteria lack LPS (54).
Bone marrow MSCs have the ability to differentiate into cell of multiple germ layers and show promise for regenerative medicine in various diseases. Immunomodulation is one of the important properties of MSCs demonstrating their importance as versatile multipotent cells. MSCs can modulate T cell proliferation, NK-cell proliferation and cytotoxicity, B cell proliferation and differentiation, and dendritic cell differentiation and maturation (79). There are many in vitro and in vivo reports of MSCs expressing molecules that can interact with both innate and adaptive immune systems (79, 80). MSCs express many immunomodulatory molecules such as B7-H1 (CD274), B7-H4, CD54 (ICAM-1), CD58 (LFA3), CD106 (VCAM-1), CD166 (ALCAM), IL-1a, IL-6, FGF, HO-1, nitric oxide (NO), indoleaminedeoxygenase (IDO-1), PGE2, Factor H, TGFβ 1, HLA-A, HLA-B, HLA-C, HLA-E, HLA-G, HLA-G5, Galectin-1, Galectin-3 and TSG-6 (reviewed in 81). Additionally, after systemic infusion MSCs migrate and home to sites of tissue injury or inflammation (82). Many other factors (summarized in Figure 4)are involved in the mobilization of MSCs(reviewed in 83). We postulate that such immunomodulatory behavior and innate immunity signature are of prime importance during migration through the vasculature to distant organs including skin following tissue injury or inflammation (84–86).
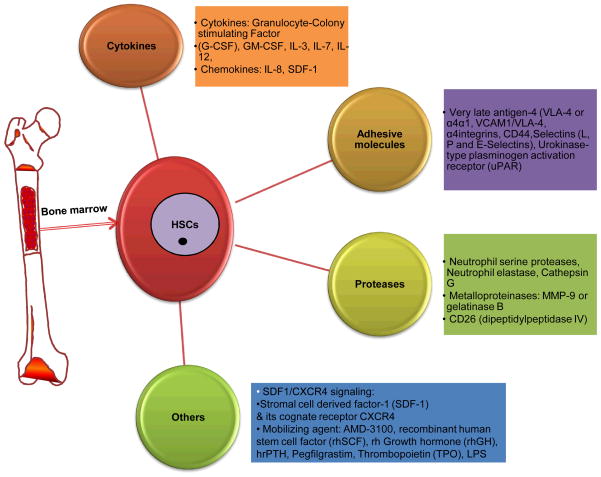
Figure 4.
This figure illustrates involvement of various cytokines, adhesive molecules, proteases and other factors during the process of HSC mobilization.
Moreover, CD49f+/CD34+ HF stem cells have been studied for their ability to form skin and its appendages (3, 4); however, the association of Tlrs with their regulation and mobilization has not yet been studied. Nevertheless, there are some innate immunity related players expressed in common by MSCs, HSCs, and KSCs such as Tlr2, Tlr4 and CD14 (22, 24). In our analysis of microarray data from the Trempus paper (24), we found that MSC stemness markers (Lif, Wnt3a), MSC-specific markers (Alcam, Anpep, Itga6, Nt5e), MSC differentiation markers (Rhoa, Bmp4, Pcaf, Notch1), and other genes (Bdnf, Bmp7, Icam1, Kitl, Mmp2), are also found to be differentially expressed in CD34+ KSCs. This shared expression profile might simply be a genetic fingerprint of stem cells, or it might have some other novel link. However, based on expression of Tlrs and other innate immunity-related molecules in KSCs, there may be a plausible role of Tlrs in their mobilization or maintenance.
Major outstanding questions in the field
What are the roles of various cytokine, chemokines and Tlrs in KSC populations?
Are there particular cytokines or Tlrs more important for stem cell regulation?
Does a HSC mobilization model also exist for KSCs?
What relevant regulatory pathways are associated with KSC mobilization?
Conclusions and prospects for the future
Profiling of the CD49f+/CD34+ HF stem cells has indicated that these cells have enhanced expression of genes involved in innate immunity. This enhanced expression suggests that this population could be protected with a mechanism for extra surveillance. In this regard, the expression of Tlrs on HF stem cells could make them more sensitive towards pathogen-specific molecular patterns that would stimulate protective downstream signaling pathways. Such a mechanism might be protective for stem cell self-renewal. Moreover, Tlrs, and Tlr4 in particular, are thought to play a protective role following exposure to carcinogens. Although the detailed mechanism of this protection is not yet known, it may be due to the higher expression of the anti-apoptotic protein, Bcl-2 and/or accelerated DNA repair in the CD34+ KSCs (87). Presently, Tlrs are linked with the initial phase of immune defense; however, we hypothesize that they are potentially associated with some basic, as yet unknown mechanisms of KSC regulation and mobilization.
A rationale for the second mechanism, mobilization of KSCs, is that the other arms of innate immunity such as the complement cascade, granulocytes, naturally occurring antibodies, as well as Tlrs, have been recently implicated in mobilization of HSCs from the bone marrow. Similarly, MSCs are also mobilized to enter the circulation via a Tlr mechanism. Such immunomodulatory behavior may be an additional property of stem cells for their survival during their migration from one organ to another. However, whether Tlrs and innate immune mechanisms are involved in the regulation and mobilization of CD49f+/CD34+ HF stem cells is not yet known. Their expression of the players in innate immunity and keratinocyte ability to modulate the immune system in general, suggest the possibility of their participation in a new game of mobilization. Furthermore, the signatures of innate and adaptive immunity found in AA and other autoimmune diseases also highlights the importance of the CD49f+/CD34+ HF stem cells and their relationship with the immune system. This association results in the HF bulge becoming a battleground leading to the devastating hair loss in this disease.
In conclusion, we have considered the enhanced expression of genes involved in innate immunity on CD49f+/CD34+ HF stem cells and the possible roles played by these genes. One scenario is that these stem cells require extra protection from environmental insults due to their stem cell status. We have presented the evidence that this could be the case. The other enticing possibility hypothesized here is that the players in classical innate immune responses may play a new role in regulating and mobilizing KSCs for migratory behaviors associated with wound healing, carcinogenesis, and other cutaneous battlefields when thing go wrong. Therefore, we suggest that the “old players” might be playing a “new game”.
Supplementary Material
Acknowledgments
This work was supported by NIAMSNIH grant R01 AR052713 (RJM). AS and RJM design the manuscript outline, interpreted the data, and wrote the manuscript. We thank Carol Trempus for carefully reading the manuscript and for helpful suggestions.
Footnotes
Conflict of interests:
We declare no conflict of interest.
References
- 1.Tizard I. Immunity at body surface. Philadelphia, PA: Saunders; 2000. [Google Scholar]
- 2.Trempus CS, Morris RJ, Ehinger M, et al. CD34 expression by hair follicle stem cells is required for skin tumor development in mice. Cancer Res. 2007;67:4173–4181. doi: 10.1158/0008-5472.CAN-06-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito M, Liu Y, Yang Z, et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 4.Morris RJ, Liu Y, Marles L, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 5.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Barton GM, Kagan JC. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat Rev Immunol. 2009;9:535–542. doi: 10.1038/nri2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 9.Le Bourhis L, Benko S, Girardin SE. Nod1 and Nod2 in innate immunity and human inflammatory disorders. Biochemical Society transactions. 2007;35:1479–1484. doi: 10.1042/BST0351479. [DOI] [PubMed] [Google Scholar]
- 10.de Koning HD, Rodijk-Olthuis D, van Vlijmen-Willems IM, et al. A comprehensive analysis of pattern recognition receptors in normal and inflamed human epidermis: upregulation of dectin-1 in psoriasis. The Journal of investigative dermatology. 2010;130:2611–2620. doi: 10.1038/jid.2010.196. [DOI] [PubMed] [Google Scholar]
- 11.Becker MN, Diamond G, Verghese MW, Randell SH. CD14-dependent lipopolysaccharide-induced beta-defens in-2 expression in human tracheobronchial epithelium. J Biol Chem. 2000;275:29731–29736. doi: 10.1074/jbc.M000184200. [DOI] [PubMed] [Google Scholar]
- 12.Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol. 2000;164:966–972. doi: 10.4049/jimmunol.164.2.966. [DOI] [PubMed] [Google Scholar]
- 13.Faure E, Thomas L, Xu H, Medvedev A, Equils O, Arditi M. Bacterial lipopolysaccharide and IFN-gamma induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-kappa B activation. J Immunol. 2001;166:2018–2024. doi: 10.4049/jimmunol.166.3.2018. [DOI] [PubMed] [Google Scholar]
- 14.Muzio M, Bosisio D, Polentarutti N, et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 15.Visintin A, Mazzoni A, Spitzer JH, Wyllie DH, Dower SK, Segal DM. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–255. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- 16.Miller LS, Sorensen OE, Liu PT, et al. TGF-alpha regulates TLR expression and function on epidermal keratinocytes. J Immunol. 2005;174:6137–6143. doi: 10.4049/jimmunol.174.10.6137. [DOI] [PubMed] [Google Scholar]
- 17.Kollisch G, Kalali BN, Voelcker V, et al. Various members of the Toll-like receptor family contribute to the innate immune response of human epidermal keratinocytes. Immunology. 2005;114:531–541. doi: 10.1111/j.1365-2567.2005.02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mempel M, Voelcker V, Kollisch G, et al. Toll-like receptor expression in human keratinocytes: nuclear factor kappaB controlled gene activation by Staphylococcus aureus is toll-like receptor 2 but not toll-like receptor 4 or platelet activating factor receptor dependent. The Journal of investigative dermatology. 2003;121:1389–1396. doi: 10.1111/j.1523-1747.2003.12630.x. [DOI] [PubMed] [Google Scholar]
- 19.Pivarcsi A, Bodai L, Rethi B, et al. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int Immunol. 2003;15:721–730. doi: 10.1093/intimm/dxg068. [DOI] [PubMed] [Google Scholar]
- 20.Renn CN, Sanchez DJ, Ochoa MT, et al. TLR activation of Langerhans cell-like dendritic cells triggers an antiviral immune response. J Immunol. 2006;177:298–305. doi: 10.4049/jimmunol.177.1.298. [DOI] [PubMed] [Google Scholar]
- 21.Mempel M, Kalali BN, Ollert M, Ring J. Toll-Like Receptors in Dermatology. Dermatologic Clinics. 2007;25:531–540. doi: 10.1016/j.det.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Song PI, Park YM, Abraham T, et al. Human keratinocytes express functional CD14 and toll-like receptor 4. The Journal of investigative dermatology. 2002;119:424–432. doi: 10.1046/j.1523-1747.2002.01847.x. [DOI] [PubMed] [Google Scholar]
- 23.Muller-Decker K, Manegold G, Butz H, et al. Inhibition of cell proliferation by bacterial lipopolysaccharides in TLR4-positive epithelial cells: independence of nitric oxide and cytokine release. The Journal of investigative dermatology. 2005;124:553–561. doi: 10.1111/j.0022-202X.2004.23598.x. [DOI] [PubMed] [Google Scholar]
- 24.Trempus CS, Dang H, Humble MM, et al. Comprehensive microarray transcriptome profiling of CD34-enriched mouse keratinocyte stem cells. The Journal of investigative dermatology. 2007;127:2904–2907. doi: 10.1038/sj.jid.5700917. [DOI] [PubMed] [Google Scholar]
- 25.Leulier F, Lemaitre B. Toll-like receptors--taking an evolutionary approach. Nature reviews Genetics. 2008;9:165–178. doi: 10.1038/nrg2303. [DOI] [PubMed] [Google Scholar]
- 26.Naseemuddin M, Iqbal A, Nasti TH, Ghandhi JL, Kapadia AD, Yusuf N. Cell mediated immune responses through TLR4 prevents DMBA-induced mammary carcinogenesis in mice. Int J Cancer. 2011 doi: 10.1002/ijc.26100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yusuf N, Nasti TH, Long JA, et al. Protective role of Toll-like receptor 4 during the initiation stage of cutaneous chemical carcinogenesis. Cancer Res. 2008;68:615–622. doi: 10.1158/0008-5472.CAN-07-5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittal D, Saccheri F, Venereau E, Pusterla T, Bianchi ME, Rescigno M. TLR4-mediated skin carcinogenesis is dependent on immune and radio resistant cells. EMBO J. 2010;29:2242–2252. doi: 10.1038/emboj.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grone A. Keratinocytes and cytokines. Vet Immunol Immunopathol. 2002;88:1–12. doi: 10.1016/s0165-2427(02)00136-8. [DOI] [PubMed] [Google Scholar]
- 30.Kocic J, Bugarski D, Santibanez JF. SMAD3 is essential for transforming growth factor-beta1-induced urokinase type plasminogen activator expression and migration in transformed keratinocytes. European journal of cancer (Oxford, England : 1990) 2011 doi: 10.1016/j.ejca.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 31.Sauder DN, Orr FW, Matic S, et al. Human interleukin-1 alpha is chemotactic for normal human keratinocytes. Immunol Lett. 1989;22:123–127. doi: 10.1016/0165-2478(89)90178-8. [DOI] [PubMed] [Google Scholar]
- 32.Kothny-Wilkes G, Kulms D, Poppelmann B, Luger TA, Kubin M, Schwarz T. Interleukin-1 protects transformed keratinocytes from tumor necrosis factor-related apoptosis-inducing ligand. J Biol Chem. 1998;273:29247–29253. doi: 10.1074/jbc.273.44.29247. [DOI] [PubMed] [Google Scholar]
- 33.Darmstadt GL, Fleckman P, Rubens CE. Tumor necrosis factor-alpha and interleukin-1alpha decrease the adherence of Streptococcus pyogenes to cultured keratinocytes. J Infect Dis. 1999;180:1718–1721. doi: 10.1086/315066. [DOI] [PubMed] [Google Scholar]
- 34.Komine M, Rao LS, Freedberg IM, Simon M, Milisavljevic V, Blumenberg M. Interleukin-1 induces transcription of keratin K6 in human epidermal keratinocytes. The Journal of investigative dermatology. 2001;116:330–338. doi: 10.1046/j.1523-1747.2001.01249.x. [DOI] [PubMed] [Google Scholar]
- 35.Niebuhr M, Heratizadeh A, Wichmann K, Satzger I, Werfel T. Intrinsic alterations of pro-inflammatory mediators in unstimulated and TLR-2 stimulated keratinocytes from atopic dermatitis patients. Experimental dermatology. 2011;20:468–472. doi: 10.1111/j.1600-0625.2011.01277.x. [DOI] [PubMed] [Google Scholar]
- 36.Lee KH, Cho KA, Kim JY, Baek JH, Woo SY, Kim JW. Filaggrin knockdown and Toll-like receptor 3 (TLR3) stimulation enhanced the production of thymic stromal lymphopoietin (TSLP) from epidermal layers. Experimental dermatology. 2011;20:149–151. doi: 10.1111/j.1600-0625.2010.01203.x. [DOI] [PubMed] [Google Scholar]
- 37.Olaru F, Jensen LE. Chemokine expression by human keratinocyte cell lines after activation of Toll-like receptors. Experimental dermatology. 2010;19:e314–316. doi: 10.1111/j.1600-0625.2009.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ullrich SE. Mechanism involved in the systemic suppression of antigen-presenting cell function by UV irradiation. Keratinocyte-derived IL-10 modulates antigen-presenting cell function of splenic adherent cells. J Immunol. 1994;152:3410–3416. [PubMed] [Google Scholar]
- 39.Beissert S, Hosoi J, Kuhn R, Rajewsky K, Muller W, Granstein RD. Impaired immunosuppressive response to ultraviolet radiation in interleukin-10-deficient mice. The Journal of investigative dermatology. 1996;107:553–557. doi: 10.1111/1523-1747.ep12582809. [DOI] [PubMed] [Google Scholar]
- 40.Ratajczak MZ, Kim CH, Wojakowski W, Janowska-Wieczorek A, Kucia M, Ratajczak J. Innate immunity as orchestrator of stem cell mobilization. Leukemia. 2010;24:1667–1675. doi: 10.1038/leu.2010.162. [DOI] [PubMed] [Google Scholar]
- 41.Lee HM, Wysoczynski M, Liu R, et al. Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia. 2010;24:573–582. doi: 10.1038/leu.2009.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ratajczak MZ, Lee H, Wysoczynski M, et al. Novel insight into stem cell mobilization–plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–985. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee HM, Wu W, Wysoczynski M, et al. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23:2052–2062. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nardini E, Morelli D, Aiello P, et al. CpG-oligodeoxynucleotides induce mobilization of hematopoietic progenitor cells into peripheral blood in association with mouse KC (IL-8) production. Journal of cellular physiology. 2005;204:889–895. doi: 10.1002/jcp.20360. [DOI] [PubMed] [Google Scholar]
- 45.Wong SY, Reiter JF. Wounding mobilizes hair follicle stem cells to form tumors. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4093–4098. doi: 10.1073/pnas.1013098108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ratajczak MZ, Kim C. The use of chemokine receptor agonists in stem cell mobilization. Expert opinion on biological therapy. 2012;12:287–297. doi: 10.1517/14712598.2012.657174. [DOI] [PubMed] [Google Scholar]
- 47.Keating GM. Plerixa for: a review of its use in stem-cell mobilization in patients with lymphoma or multiple myeloma. Drugs. 2011;71:1623–1647. doi: 10.2165/11206040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 48.Lieschke GJ, Burgess AW. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor (2) N Engl J Med. 1992;327:99–106. doi: 10.1056/NEJM199207093270207. [DOI] [PubMed] [Google Scholar]
- 49.Tong J, Gordon MS, Srour EF, et al. In vivo administration of recombinant methionyl human stem cell factor expands the number of human marrow hematopoietic stem cells. Blood. 1993;82:784–791. [PubMed] [Google Scholar]
- 50.Brasel K, McKenna HJ, Morrissey PJ, et al. Hematologic effects of flt3 ligand in vivo in mice. Blood. 1996;88:2004–2012. [PubMed] [Google Scholar]
- 51.Fibbe WE, Pruijt JF, Velders GA, et al. Biology of IL-8-induced stem cell mobilization. Ann N Y Acad Sci. 1999;872:71–82. doi: 10.1111/j.1749-6632.1999.tb08454.x. [DOI] [PubMed] [Google Scholar]
- 52.Ganser A, Lindemann A, Seipelt G, et al. Effects of recombinant human interleukin-3 in aplastic anemia. Blood. 1990;76:1287–1292. [PubMed] [Google Scholar]
- 53.Laterveer L, Lindley IJ, Heemskerk DP, et al. Rapid mobilization of hematopoietic progenitor cells in rhesus monkeys by a single intravenous injection of interleukin-8. Blood. 1996;87:781–788. [PubMed] [Google Scholar]
- 54.Velders GA, van Os R, Hagoort H, et al. Reduced stem cell mobilization in mice receiving antibiotic modulation of the intestinal flora: involvement of endotoxins as cofactors in mobilization. Blood. 2004;103:340–346. doi: 10.1182/blood-2002-07-2270. [DOI] [PubMed] [Google Scholar]
- 55.Cline MJ, Golde DW. Mobilization of hematopoietic stem cells (CFU-C) into the peripheral blood of man by endotoxin. Exp Hematol. 1977;5:186–190. [PubMed] [Google Scholar]
- 56.Vos O, Wilschut IJ. Further studies on mobilization of CFUs. Cell Tissue Kinet. 1979;12:257–267. doi: 10.1111/j.1365-2184.1979.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 57.Meyrick BO, Ryan US, Brigham KL. Direct effects of E coli endotoxin on structure and permeability of pulmonary endothelial monolayers and the endothelial layer of intimal explants. Am J Pathol. 1986;122:140–151. [PMC free article] [PubMed] [Google Scholar]
- 58.Schletter J, Heine H, Ulmer AJ, Rietschel ET. Molecular mechanisms of endotoxin activity. Arch Microbiol. 1995;164:383–389. doi: 10.1007/BF02529735. [DOI] [PubMed] [Google Scholar]
- 59.Shi C, Jia T, Mendez-Ferrer S, et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boiko JR, Borghesi L. Hematopoiesis sculpted by pathogens: Toll-like receptors and inflammatory mediators directly activate stem cells. Cytokine. 2011 doi: 10.1016/j.cyto.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32:57–65. doi: 10.1016/j.it.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118:635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 65.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 66.Pearson G, Robinson F, Beers Gibson T, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 67.Beutler B, Greenwald D, Hulmes JD, et al. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985;316:552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- 68.Jiang Z, Johnson HJ, Nie H, Qin J, Bird TA, Li X. Pellino 1 is required for interleukin-1 (IL-1)-mediated signaling through its interaction with the IL-1 receptor-associated kinase 4 (IRAK4)-IRAK-tumor necrosis factor receptor-associated factor 6 (TRAF6) complex. J Biol Chem. 2003;278:10952–10956. doi: 10.1074/jbc.M212112200. [DOI] [PubMed] [Google Scholar]
- 69.Schauvliege R, Janssens S, Beyaert R. Pellino proteins: novel players in TLR and IL-1R signalling. J Cell Mol Med. 2007;11:453–461. doi: 10.1111/j.1582-4934.2007.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawai K, Shimura H, Minagawa M, Ito A, Tomiyama K, Ito M. Expression of functional Toll-like receptor 2 on human epidermal keratinocytes. Journal of dermatological science. 2002;30:185–194. doi: 10.1016/s0923-1811(02)00105-6. [DOI] [PubMed] [Google Scholar]
- 71.Ito T. Hair follicle is a target of stress hormone and autoimmune reactions. Journal of dermatological science. 2010;60:67–73. doi: 10.1016/j.jdermsci.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 72.Petukhova L, Duvic M, Hordinsky M, et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature. 2010;466:113–117. doi: 10.1038/nature09114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sellheyer K, Atanaskova-Mesinkovska N, Nelson P, Bergfeld WF. Differential expression of stem cell markers in lichen planopilaris and alopecia areata. The British journal of dermatology. 2011;165:1149–1151. doi: 10.1111/j.1365-2133.2011.10491.x. [DOI] [PubMed] [Google Scholar]
- 74.Amoh Y, Maejima H, Niiyama S, et al. Hair follicle stem cell marker nestin expression in regenerating hair follicles of patients with alopecia areata. European journal of dermatology : EJD. 2011;21:209–212. doi: 10.1684/ejd.2011.1306. [DOI] [PubMed] [Google Scholar]
- 75.Nagai Y, Garrett KP, Ohta S, et al. Toll-like receptors on hematopoietic progenitor cells stimulate innate immune system replenishment. Immunity. 2006;24:801–812. doi: 10.1016/j.immuni.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim JM, Kim NI, Oh YK, Kim YJ, Youn J, Ahn MJ. CpG oligodeoxynucleotides induce IL-8 expression in CD34+ cells via mitogen-activated protein kinase-dependent and NF-kappaB-independent pathways. Int Immunol. 2005;17:1525–1531. doi: 10.1093/intimm/dxh345. [DOI] [PubMed] [Google Scholar]
- 77.Ratajczak MZ, Liu R, Ratajczak J, Kucia M, Shin D-M. The role of pluripotent embryonic-like stem cells residing in adult tissues in regeneration and longevity. Differentiation. 2011;81:153–161. doi: 10.1016/j.diff.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 78.Reca R, Mastellos D, Majka M, et al. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101:3784–3793. doi: 10.1182/blood-2002-10-3233. [DOI] [PubMed] [Google Scholar]
- 79.Vija L, Farge D, Gautier JF, et al. Mesenchymal stem cells: Stem cell therapy perspectives for type 1 diabetes. Diabetes Metab. 2009;35:85–93. doi: 10.1016/j.diabet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 80.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 81.Anzalone R, Lo Iacono M, Loria T, et al. Wharton’s jelly mesenchymal stem cells as candidates for beta cells regeneration: extending the differentiative and immunomodulatory benefits of adult mesenchymal stem cells for the treatment of type 1 diabetes. Stem Cell Rev. 2011;7:342–363. doi: 10.1007/s12015-010-9196-4. [DOI] [PubMed] [Google Scholar]
- 82.Pittenger M. Sleuthing the source of regeneration by MSCs. Cell Stem Cell. 2009;5:8–10. doi: 10.1016/j.stem.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 83.Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–705. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- 84.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 85.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 86.Deng W, Han Q, Liao L, et al. Engrafted bone marrow-derived flk-(1+) mesenchymal stem cells regenerate skin tissue. Tissue Eng. 2005;11:110–119. doi: 10.1089/ten.2005.11.110. [DOI] [PubMed] [Google Scholar]
- 87.Sotiropoulou PA, Candi A, Mascre G, et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat Cell Biol. 2010;12:572–582. doi: 10.1038/ncb2059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.