Abstract
Background
Xerostomia (dry mouth) after head/neck radiation is a common problem among cancer patients and available treatments are of little benefit. The objective of this trial was to determine if acupuncture can prevent xerostomia among head/neck patients undergoing radiotherapy.
Methods
A randomized, controlled trial among patients with nasopharyngeal carcinoma was conducted comparing acupuncture to standard care. Participants were treated at Fudan University Shanghai Cancer Center, Shanghai, China. Forty patients were randomized to acupuncture treatment and 46 to standard care. Patients were treated 3 times/week on the same days they received radiotherapy. Subjective measures included the Xerostomia Questionnaire (XQ) and MD Anderson Symptom Inventory for Head/Neck (MDASI-HN). Objective measures were unstimulated and stimulated whole salivary flow rates (UWSFR; SSFR). Patients were followed for 6 months after the end of radiotherapy.
Results
XQ scores for acupuncture were statistically significantly lower than controls starting in week 3 through the 6-months(P=0.003 at week3, all other P’s < 0.0001), with clinically significant differences as follows: week 11- RR 0.63 [95% CI, 0.45, 0.87]; 6 months - RR 0.38 [95% CI, 0.19, 0.76]. Similar findings were seen for MDASI-HN scores. Group differences emerged as early as 3 weeks into treatment for saliva (UWSFR, P = 0.0004), with greater saliva flow in the acupuncture group at week 7 (UWSFR, P < 0.0001; SSFR, P = 0.002) and 11 (UWSFR, P < 0.02; SSFR, P < 0.03) and at 6 months (SSFR, P < 0.003).
Conclusions
Acupuncture given concurrently with radiotherapy significantly reduced xerostomia and improved QOL.
Keywords: Acupuncture, xerostomia, head and neck cancer, quality of life
Introduction
The majority of patients with head/neck cancer undergo radiotherapy and most develop xerostomia (dry mouth). Xerostomia often severely impairs quality of life (QOL)1 with patients experiencing taste aberrations, dysphagia, odynophagia, difficulty sleeping and speaking, and loss of appetite. Changes in salivary components can reduce the natural inhibition of bacterial growth in the oral cavity, resulting in increases of caries-forming microbes that can cause bone infection and irreversible nutritional deficits.2 The pathology and course of xerostomia are not well described, but symptoms rarely spontaneously improve.
Salivary glands are particularly sensitive to radiation, and at doses higher than 50 Gy, damage may be irreversible.3 Reduced salivary flow begins during the first few days of radiotherapy with an 80% decrease by 6 weeks. Other changes include decreased salivary pH, increased viscosity, and reduced levels of salivary constituents (i.e., immunoglobulins, buffers, small organic molecules).4, 5
One approach used to help prevent xerostomia is intensity modulated radiation therapy (IMRT). IMRT has the potential to reduce salivary radiation dose-volume intensity by allowing the delivery of high dose radiation to tumor while minimizing the dose to surrounding normal structures. 6–9 Its use, however, is limited by several factors. First, IMRT only partly spares the parotid glands and minor salivary glands within the oral cavity; thus, the development of xerostomia remains high. 10–14 Additional potential disadvantages of IMRT as compared with conventional RT include increased homogeneity of the dose distribution, increased total body dose, increased planning time, increased cost, increased difficulty in interpretation of treatment verification films, and most importantly, potentially increased risk of a marginal miss.7 Although IMRT has been shown to reduce the incidence of late grade 2 xerostomia,15, 16 not all patients receive IMRT, and it is not commonly available in many areas of China. In a pilot trial at MD Anderson investigating the use of acupuncture to treat radiation-induced xerostomia, 17 most participants had received IMRT, yet these patients still benefited from the acupuncture treatment.
Treatment for radiation-induced xerostomia is primarily palliative. Several approaches including saliva substitutes, chewing gum, sialogogue lozenges, and pilocarpine, have been attempted with limited benefit.18–27 Amifostine has been approved by the Food and Drug Administration (FDA) to reduce the incidence and severity of radiation-induced xerostomia, but it requires parenteral administration, has potential side-effects, and is not universally available.23 Electrical stimulation of the tongue and palate28, 29 and hyperbaric oxygen therapy30 have also been tried with little benefit.
Published reports indicate acupuncture can stimulate saliva flow in patients experiencing xerostomia after the end of radiotherapy. Several studies conducted by different investigators have shown positive results.31–43 Some have demonstrated relief of symptoms in as few as 5 treatments31 with benefits lasting up to 3 years.32 Previous randomized trials, however, have not explored whether acupuncture can prevent xerostomia when given concurrently with radiotherapy.
We report results from a randomized trial designed to determine if acupuncture delivered during radiotherapy, relative to standard care, could prevent the development of xerostomia among cancer patients undergoing radiotherapy to the head/neck. Secondarily, we evaluated if acupuncture could reduce xerostomia severity and improve aspects of quality of life. In planning for a subsequent multi-center, phase III trial, a second smaller trial was conducted to examine the same acupuncture procedures compared to a placebo control group for patients undergoing IMRT for head and neck cancer. Results from that study are reported elsewhere.
MATERIALS AND METHODS
This study was approved by Institutional Review Boards at MD Anderson Cancer Center and Shanghai University Cancer Center. Participants were identified by faculty in the Department of Radiation Oncology at Fudan and referred for assessment of eligibility and to obtain informed consent. All patients were treated at Fudan. The trial was registered at ClinicalTrials.gov: NCT00430378. Patients were recruited between March, 2007 and December, 2008.
Patients
Inclusion criteria were: age greater than 18 years; nasopharyngeal carcinoma (NPC); anatomically intact parotid and submandibular glands; and Zubrod performance status of 0, 1, or 2. Patients were excluded if they had a history of xerostomia; planned intensity-modulated radiation therapy (IMRT); suspected or confirmed physical closure of salivary gland ducts on either side; known bleeding disorders and taking heparin or warfarin; contraindications for the use of acupuncture at any acupoints; history of cerebrovascular accident or spinal cord injury; or if they had taken any drug or herbal medicine in the past 30 days that could affect salivary function or were planning to or ended up taking such a substance during the study.
Procedures
After collecting baseline measures, patients were randomly assigned to one of two groups using a random number table: the acupuncture group (G1) and the standard care control group (G2). Patients assigned to G1 received acupuncture on the same day but prior to radiation 3 days/week during a 7-week course of radiotherapy. Patients in G2 did not receive acupuncture or any special education to prevent xerostomia except standard oral hygiene. Self-report questionnaires and sialometry were collected at baseline, weekly during the course of radiotherapy, and 1 and 6 months after the end of radiotherapy.
Acupuncture Treatment
All treatments were given by a hospital credentialed acupuncturist with over 35 years of experience. Patients were treated in a comfortable sitting position. Standardized techniques for point location were used,44, 45 and needle insertion endpoints were standard recommended depth of insertion44, 45 or achievement of De Qi sensation.
Points were selected based on: (1) previously published reports,31–42 (2) point indications according to traditional Chinese medicine (TCM) theory,44, 45 and (3) current understanding of anatomy and neurovascular tissues associated with each point.46 Although many combinations could be used, we attempted to identify those that integrate TCM theory and biomedicine, with a focus on using a minimal number of needles. Body points included Ren 24, Lung 7 (LU 7), and Kidney 6 (K 6), and ear points were Shenmen, Point Zero, Salivary Gland 2-prime (SG 2′), and Larynx. Except for Ren 24 located in the midline, all points were treated bilaterally. Needles remained in place for 20 minutes.
Measures
The Xerostomia Questionnaire (XQ) is an 8-item survey previously validated in several cohorts. 9, 12 The item scores are added and the sum is transformed linearly to produce a final summary score between 0 and 100. Higher scores represent more xerostomia. Clinical relevance of the XQ has been suggested by Eisbruch12 and Pacholke,9 with XQ scores ≤ 30 corresponding to mild to no symptoms of xerostomia.
The MD Anderson Symptom Inventory-Head and Neck (MDASI-HN) measures, on a 0–10 numeric rating scale, both the severity of symptoms and interference with patients’ daily activities. One of the head/neck questions is specific to dry mouth. This was removed in the symptom total score in order to have a measure that assessed symptoms separate from mouth dryness. The 13 core items evaluate symptoms common to cancer patients. It also includes 9 head/neck-specific items. The instrument was validated in a cohort of more than 200 patients and found to be highly reliable.47
Salivary flow was measured using both unstimulated whole salivary flow rates (UWSFR) and stimulated salivary flow rates (SSFR). Sialometry procedures have been previously reported.17
Statistical Analyses
The primary outcome was the XQat end of radiotherapyand 1 month later. The FDA uses subjective outcomes as the standard for drug approval in xerostomia treatment. Secondary outcomes were the MDASI-HN and changes in salivary flow. We estimated 90% of patients in the control group and 60% of patients in the acupuncture group would develop xerostomia, based on the frequent occurrence of xerostomia in this population and the estimated benefits of acupuncture. Given 38 patients per group, we estimated differences could be detected statistically with a two-sided significance level of 0.05 and 80% power. We increased this number to a maximum of 50 per group to allow up to a 24% drop out rate. Recruitment was stopped after we randomized 86 patients as attrition by the end of radiotherapy and 1 month later was less than 10%.
We used SAS PROC MIXED48 procedure to run linear mixed models with the longitudinal data from week 1 to week 11. In these models, the intercept was random effect and covariance was unstructured. Time effect (week) was treated as a continuous variable. We examined the curvature of the outcomes over time by adding the quadratic term for time to the model as necessary. We also tested the least square mean difference between groups by week using the LSMEAN command In reply to: the PROC MIXED procedure. To explore the long-term effect of acupuncture, we used the PROC GLM procedure in SAS to run analysis of covariance for the same outcome variables collected 6 months after week 11, using the baseline measurements as covariates. We also conducted sub-analyses of XQ scores >30 (signifying clinically significant levels of xerostomia) at 7 and 11 weeks and at the 6-month follow-up using chi-square tests.
RESULTS
Enrollment
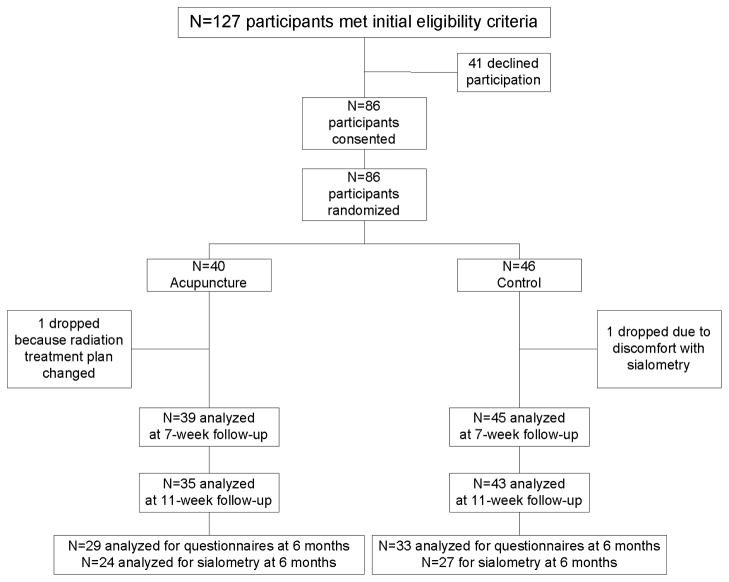
Of 127 eligible patients approached, 86 consented and were randomly assigned to G1 or G2 (68% acceptance rate, Figure 1a). As can be seen in Table 1, the groups were balanced on medical and demographic characteristics. Compliance with acupuncture treatments (3/week for 7 weeks of radiotherapy) among the 39 patients in G1 was very high (82%; N = 32), with the remaining patients receiving 20, 19, 15, 10, 6, 5, or 4 treatments. Among the 86 patients randomized, 62 finished the 6 month follow up. Five patients died prior to follow-up and 19 dropped out of the study due to not being able to come back to do follow up at Fudan University Shanghai Cancer Center.
Figure 1.
Patient flow diagram*
* Five patients died before the 6-month follow-up and the other 19 dropped out because they could not return to the hospital for the assessments.
Table 1.
Demographic and medical characteristics
| Characteristic | Group
|
p-value | |
|---|---|---|---|
| Acupuncture | Control | ||
| Age in years | |||
| Mean (SD) | 45.6 (10.8) | 48.9 (10.5) | 0.16 |
| Gender n (%) | |||
| Male | 30 (76.9) | 29 (64.4) | 0.21 |
| Female | 9 (23.1) | 16 (35.6) | |
| Stage n (%) | |||
| I | 4 (10.2) | 1 (2.2) | 0.14 |
| II | 12 (30.8) | 15 (33.3) | |
| III | 12 (30.8) | 22 (48.9) | |
| IV | 11 (28.2) | 7 (15.6) | |
| Radiation tumor dose (Gy) | |||
| Mean (SD) | 70.8 (1.9) | 70.9 (2.1) | 0.71 |
Patient Characteristics
Participant characteristics are shown in Table 1. There were no group differences in age, sex, NPC stage, or mean tumor dose. Fifteen patients received only radiation, 32 patients underwent induction chemotherapy prior to radiation, 19 patients underwent induction and concurrent chemotherapy with radiation, and 20 patients underwent concurrent chemotherapy with radiation. There were no group differences on chemotherapy regimen. There were also no group differences for any of the outcomes measures at baseline. Differences between the patients who provided data at the 6-month follow-up versus those who did revealed there were fewer patients with stage IV disease and fewer women providing 6-month follow-up data. However, there were no differences on the questionnaire or salivary outcomes at baseline and all other time points between those with and without missing data.
Patient-Reported Outcomes
Xerostomia
Analyses of the XQ scores revealed a significant main effect of time (P<0.001), a group by time interaction (P<0.0001), a quadratic time effect (P<0.0001), and a group by quadratic time interaction effect (quadratic term; P=0.0005, see Figure 2). In the post-hoc analysis, mean comparisons by week showed the control group had significantly higher XQ scores starting in week 3 that remained through week 11. The absolute differences between groups increased over time, with the greatest difference at week 7 (group difference = 10.3) (Figure 2a). In the analysis of covariance at the 6-month follow-up, the control group continued to have significantly higher XQ scores than the acupuncture group (acupuncture 21.9; control 34.0, P = 0.0006; group difference 12.1 [95% CI, 5.4, 18.8]). Examination of XQ scores > 30 by group using chi-square analyses showed by week 7, the majority of patients in both groups had scores > 30, with no significant group differences (acupuncture 89.7%; control 97.8%, P = 0.17; RR 0.92 [95% CI, 0.82, 1.03]). However, by week 11 and 6 months later, the acupuncture group had significantly fewer patients with scores > 30 (week 11- acupuncture 54.3%; control 86.1%, P =0.0019; RR 0.63 [95% CI, 0.45, 0.87]; 6 months - acupuncture 24.14%; control 63.6%, P = 0.0018; RR 0.38 [95% CI, 0.19, 0.76]).
Figure 2.
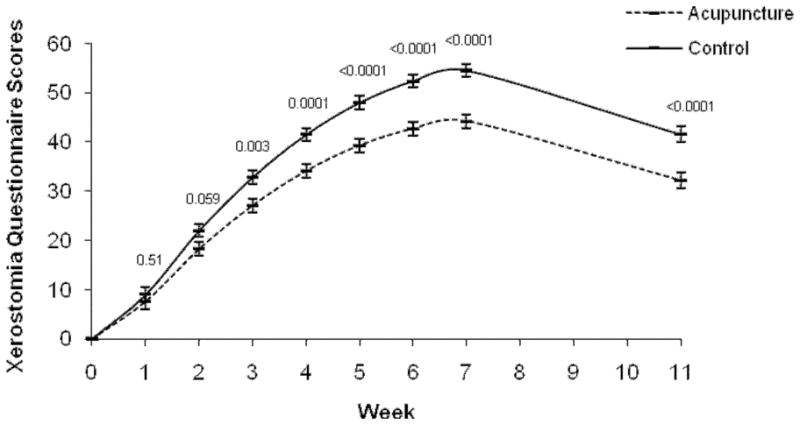
Xerostomia Questionnaire mean scores over time. *
* Week 0 is baseline raw mean. Means at weeks 1 through 11 are the least square means adjusted for baseline score. The perpendicular lines at each time point represent the standard error.
Symptom burden
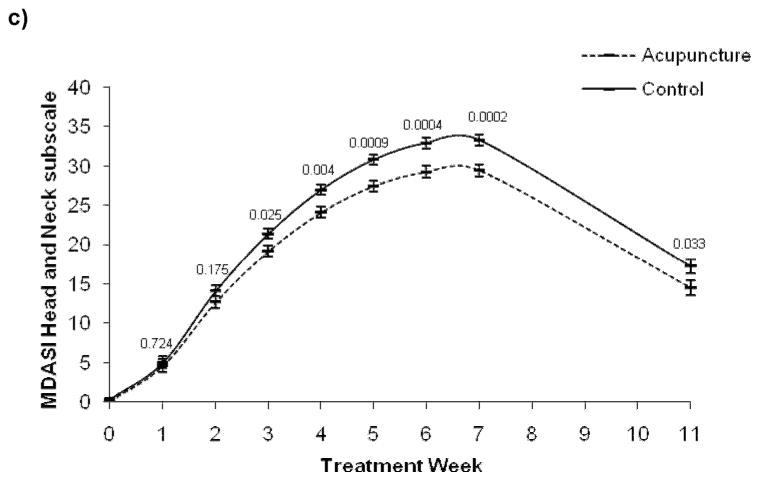
Outcomes were similar for the MDASI-HN questionnaire in weeks 1–11 for general cancer Symptom Severity, Interference, and the Head/Neck subscales (Figure 3); there was a significant main effect of time (P<0.001 for all three subscales), a group by time interaction (symptom severity: P=0.029; interference: P=0.003; head and neck: P=0.0009), a quadratic time effect ( P<0.0001 for all three subscales), and a group by quadratic time interaction effect (Symptom Severity: P=0.046; Interference: P=0.021; Head/Neck: P=0.0057). In the post-hoc analysis, mean comparisons by week showed the control group had significantly higher Symptom Severity and Head/Neck scores starting in week 3 that remained through week 11 for head and neck scores and became non-significant for Symptom Severity. Group differences for Interference emerged at week 4 and remained through week 11. At the 6-month follow-up, there were significant group differences for Interference and Head/Neck Symptoms: Interference: acupuncture 2.6; control 4.6, P < 0.02; group difference 2.0 [95% CI, 0.4, 3.7]; Head/Neck symptoms:acupuncture 7.0; control 10.6, P < 0.02; group difference 3.6 [95% CI, 0.8, 6.5]; and marginally significant differences for Symptom Severity scores: acupuncture 2.6; control 4.9, P = 0.07; group difference 2.3 [95% CI, −0.2, 4.8].
Figure 3.
MD Anderson Symptom Inventory Head and Neck Questionnaire mean scores over time.*
* Week 0 is baseline raw mean. Means at weeks 1 through 11 are the least square means adjusted for baseline score. The perpendicular lines at each time point represent the standard error.
Sialometry Outcomes
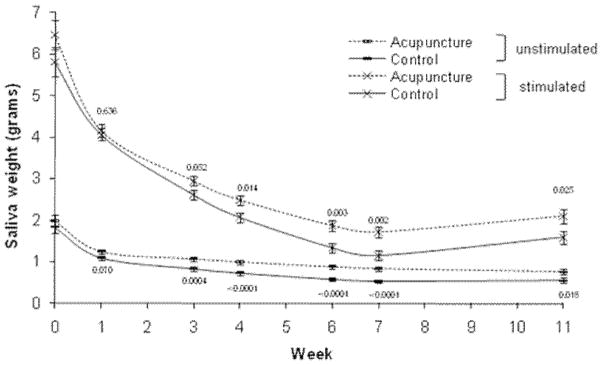
Analyses of UWSFR data revealed a significant main effect of time (P<0.0001), a group by time interaction (P=0.0425), a quadratic time effect (P<0.0001), and a marginally significant group by quadratic time interaction effect (P=0.0559; see Figure 4). In the post-hoc analysis, the two-by-two least square mean comparison by week showed the acupuncture group had significantly higher saliva flow rates starting at week 3 that remained through week 11, with the greatest group differences at week 7 (group difference = 0.3) (Figure 4). In the analysis of covariance at the 6-month follow-up, groups were not significantly different (UWSFR score: acupuncture 0.50; control 0.46, P = 0.60; group difference 0.04 [95% CI, −0.11, 0.19]).
Figure 4.
Acupuncture versus Control (standard of care) saliva flow outcomes over time.*
* Week 0 is baseline raw mean. Means at weeks 1 through 11 are the least square means adjusted for baseline score. The perpendicular lines at each time point represent the standard error.
Analyses of SSFR revealed a significant main effect of time (P<0.0001), a marginally significant group by time interaction (P=0.0657), a quadratic timeeffect (P<0.0001), but the group by quadratic time interaction effectwas not significant (P=0.16). In the post-hoc analysis, the two-by-two least square mean comparison by week showed the acupuncture group had significantly higher saliva flow rates starting at week 4 that remained through week 11, with the greatest group differences at week 7 (group difference = 0.56) (Figure 4). In the analysis of covariance at the 6-month follow-up, the acupuncture group continued to have significantly higher SSFR than the control group (acupuncture 1.57; control 0.95, P < 0.003; group difference 0.62 [95% CI, 0.22, 1.01]).
DISCUSSION
This is the first randomized-controlled trial to demonstrate that acupuncture during a course of radiotherapy can reduce the development and severity of xerostomia. For self-report measures and sialometry, group differences emerged as early as week 3 and remained significant at 1 and 6 months after the end of radiotherapy, even without additional acupuncture. Less than one third of patients in the acupuncture group reported clinically significant symptoms at 6 months versus more than two thirds in the control group. Other cancer-related symptoms and whether symptoms interfered with QOL remained significantly different between groups even 6 months after the end of radiotherapy and undergoing acupuncture. Although not all patients provided 6-month follow-up data, there were no differences between those who did and did not provide data based on QOL outcomes or sialometry, suggesting that the data is missing at random.
In the United States, the FDA uses subjective outcomes as the standard for drug approval in xerostomia treatment because both basal and stimulated salivary flow rates vary significantly among individuals, and there is no minimum salivary flow rate associated with xerostomia symptoms. Subjective sensations of oral dryness are not reliable indicators of flow rate, and impaired salivary gland function can exist with or without the sensation of oral dryness.13 Thus, it is noteworthy that objective results supported subjective results, and significant differences in saliva flow between the two groups emerged as early as 3 weeks into treatment and remained 6-months later.
Putative biological mechanisms involved in the treatment of xerostomia with acupuncture are not well understood, but in 1993, Blom and colleagues 34 showed that local blood flux increased significantly in the skin overlying the parotid glands following acupuncture. Later studies38, 39 demonstrated that acupuncture caused increased concentrations of both vasoactive intestinal polypeptide and calcitonin gene-related peptide in the saliva of xerostomia sufferers. Involved neuronal substrates have also been investigated by functional magnetic resonance imaging (fMRI).49 In a randomized, sham-controlled, cross-over, neuroimaging trial of 20 healthy volunteers, Deng and colleagues found that acupuncture stimulation of the point Large Intestine 2 (LI 2) was associated with bilateral activation of areas of the brain where gustatory, olfactory, visual stimuli and signals from expectation/suggestion are integrated and these areas were not activated during sham acupuncture. These was also a positive correlation between the amount of saliva flow and changes in the brain regions of interest.49 Further exploration of these potential mechanisms is greatly needed.
Acupuncture is cost-effective, minimally invasive, and has a very low incidence of adverse effects. No adverse effects related to the treatment, outside of mild discomfort from needle insertion, were reported by participants. One limitation is that this was a single-center trial, and although representative of patients seen at Fudan, the study population was somewhat select. Thus, generalizability of findings is limited.
The current trial also did not use a placebo comparison arm. Placebo-controlled trials in acupuncture remain controversial. Identifying an inert sham treatment that cannot be distinguished from real acupuncture is difficult. The FDA uses subjective outcomes as the standard for drug approval in xerostomia treatment, thus, comparison to a placebo group is important. However, there were significant group differences on subjective outcomes of saliva flow. After we completed the current trial, we designed a pilot trial utilizing a sham procedure. Results from that trial are reported elsewhere (ref pending). It should be noted, although patients in the initial trial were excluded if they had received IMRT,14 in one of our other previous studies patients did receive IMRT.17 Subset analyses did not show any significant relationship between the amount of radiation received and response to acupuncture with patients who underwent IMRT deriving similar benefit.
This trial demonstrated acupuncture can reduce the occurrence and severity of xerostomia as early as 3 weeks into radiotherapy, with benefits remaining 6 months after the end of treatment. Based on these findings, future large-scale, multi-center, randomized placebo-controlled trials that follow patients out to 12 months and include exploration of putative mechanisms are indicated.
Acknowledgments
Support was provided in part by the United States National Cancer Institute (NCI)grant CA121503 (PI L Cohen), the NCI Cancer Center Support Grant CA016672, and the Chinese Science and Technology Commission of Shanghai Municipality grant 05DZ19747 (PI Z Meng). We thank Drs. Peiying Yang, Zongxing Liao, and Jennifer McQuade for all their support with language, culture, and politics. Thank you to the Department of Scientific Publications, The University of Texas MD Anderson Cancer Center for their helpful editorial comments on this article.
Footnotes
There are no financial disclosures from any of the authors.
References
- 1.Chambers MS, Rosenthal DI, Weber RS. Radiation-induced xerostomia. Head Neck. 2007 Jan;29(1):58–63. doi: 10.1002/hed.20456. [DOI] [PubMed] [Google Scholar]
- 2.Bertram U. Xerostomia. Clinical aspects, pathology and pathogenesis. Acta Odontol Scand. 1967;25(Suppl 49):41–126. [PubMed] [Google Scholar]
- 3.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991 May 15;21(1):109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 4.Chambers MS, Toth BB, Martin JW, Fleming TJ, Lemon JC. Oral and dental management of the cancer patient: prevention and treatment of complications. Support Care Cancer. 1995 May;3(3):168–175. doi: 10.1007/BF00368886. [DOI] [PubMed] [Google Scholar]
- 5.Chambers MS. Clinical commentary on prophylactic treatment of radiation-induced xerostomia. Arch Otolaryngol Head Neck Surg. 2003 Feb;129(2):251–252. doi: 10.1001/archotol.129.2.251. [DOI] [PubMed] [Google Scholar]
- 6.Mendenhall WM, Amdur RJ, Morris CG, Kirwan JM, Li JG. Intensity-modulated radiotherapy for oropharyngeal squamous cell carcinoma. Laryngoscope. Nov;120(11):2218–2222. doi: 10.1002/lary.21144. [DOI] [PubMed] [Google Scholar]
- 7.Mendenhall WM, Amdur RJ, Palta JR. Intensity-modulated radiotherapy in the standard management of head and neck cancer: promises and pitfalls. J Clin Oncol. 2006 Jun 10;24(17):2618–2623. doi: 10.1200/JCO.2005.04.7225. [DOI] [PubMed] [Google Scholar]
- 8.Pacholke HD, Amdur RJ, Louis DA, Yang H, Mendenhall WM. The role of intensity modulated radiation therapy for favorable stage tumor of the nasal cavity or ethmoid sinus. Am J Clin Oncol. 2005 Oct;28(5):474–478. doi: 10.1097/01.coc.0000182600.51019.de. [DOI] [PubMed] [Google Scholar]
- 9.Pacholke HD, Amdur RJ, Morris CG, et al. Late xerostomia after intensity-modulated radiation therapy versus conventional radiotherapy. Am J Clin Oncol. 2005 Aug;28(4):351–358. doi: 10.1097/01.coc.0000158826.88179.75. [DOI] [PubMed] [Google Scholar]
- 10.Eisbruch A, Rhodus N, Rosenthal D, et al. The prevention and treatment of radiotherapy -induced xerostomia. Semin Radiat Oncol. 2003 Jul;13(3):302–308. doi: 10.1016/S1053-4296(03)00027-4. [DOI] [PubMed] [Google Scholar]
- 11.Amosson CM, Teh BS, Van TJ, et al. Dosimetric predictors of xerostomia for head-and-neck cancer patients treated with the smart (simultaneous modulated accelerated radiation therapy) boost technique. Int J Radiat Oncol Biol Phys. 2003 May 1;56(1):136–144. doi: 10.1016/s0360-3016(03)00093-2. [DOI] [PubMed] [Google Scholar]
- 12.Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001 Jul 1;50(3):695–704. doi: 10.1016/s0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- 13.Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577–587. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 14.Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006 Nov 15;66(4):981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 15.Chao KS, Majhail N, Huang CJ, et al. Intensity-modulated radiation therapy reduces late salivary toxicity without compromising tumor control in patients with oropharyngeal carcinoma: a comparison with conventional techniques. Radiother Oncol. 2001 Dec;61(3):275–280. doi: 10.1016/s0167-8140(01)00449-2. [DOI] [PubMed] [Google Scholar]
- 16.Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002 May 1;53(1):12–22. doi: 10.1016/s0360-3016(02)02724-4. [DOI] [PubMed] [Google Scholar]
- 17.Garcia MK, Chiang JS, Cohen L, et al. Acupuncture for radiation-induced xerostomia in patients with cancer: a pilot study. Head Neck. 2009 Oct;31(10):1360–1368. doi: 10.1002/hed.21110. [DOI] [PubMed] [Google Scholar]
- 18.Fox PC, Van der Ven PF, Baum BJ, Mandel ID. Pilocarpine for the treatment of xerostomia associated with salivary gland dysfunction. Oral Surg Oral Med Oral Pathol. 1986;61(3):243–248. doi: 10.1016/0030-4220(86)90369-5. [DOI] [PubMed] [Google Scholar]
- 19.Greenspan D, Daniels TE. Effectiveness of pilocarpine in postradiation xerostomia. Cancer. 1987 Mar 15;59(6):1123–1125. doi: 10.1002/1097-0142(19870315)59:6<1123::aid-cncr2820590614>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 20.Johnson JT, Ferretti GA, Nethery WJ, et al. Oral pilocarpine for post-irradiation xerostomia in patients with head and neck cancer. N Engl J Med. 1993 Aug 5;329(6):390–395. doi: 10.1056/NEJM199308053290603. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman RP, Mark RJ, Tran LM, Juillard GF. Concomitant pilocarpine during head and neck irradiation is associated with decreased posttreatment xerostomia. Int J Radiat Oncol Biol Phys. 1997 Feb 1;37(3):571–575. doi: 10.1016/s0360-3016(96)00557-3. [DOI] [PubMed] [Google Scholar]
- 22.Horiot JC, Lipinski F, Schraub S, et al. Post-radiation severe xerostomia relieved by pilocarpine: a prospective French cooperative study. Radiother Oncol. 2000 Jun;55(3):233–239. doi: 10.1016/s0167-8140(99)00018-3. [DOI] [PubMed] [Google Scholar]
- 23.Brizel DM, Wasserman TH, Henke M, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000 Oct 1;18(19):3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- 24.Rudat V, Meyer J, Momm F, et al. Protective effect of amifostine on dental health after radiotherapy of the head and neck. Int J Radiat Oncol Biol Phys. 2000 Dec 1;48(5):1339–1343. doi: 10.1016/s0360-3016(00)00768-9. [DOI] [PubMed] [Google Scholar]
- 25.Wasserman T, Mackowiak JI, Brizel DM, et al. Effect of amifostine on patient assessed clinical benefit in irradiated head and neck cancer. Int J Radiat Oncol Biol Phys. 2000 Nov 1;48(4):1035–1039. doi: 10.1016/s0360-3016(00)00735-5. [DOI] [PubMed] [Google Scholar]
- 26.Lindegaard JC, Grau C. Has the outlook improved for amifostine as a clinical radioprotector? Radiother Oncol. 2000 Nov;57(2):113–118. doi: 10.1016/s0167-8140(00)00235-8. [DOI] [PubMed] [Google Scholar]
- 27.Jha N, Seikaly H, Harris J, et al. Phase III randomized study: oral pilocarpine versus submandibular salivary gland transfer protocol for the management of radiation-induced xerostomia. Head Neck. 2009 Feb;31(2):234–243. doi: 10.1002/hed.20961. [DOI] [PubMed] [Google Scholar]
- 28.Weiss WW, Jr, Brenman HS, Katz P, Bennett JA. Use of an electronic stimulator for the treatment of dry mouth. J Oral Maxillofac Surg. 1986 Nov;44(11):845–850. doi: 10.1016/0278-2391(86)90219-3. [DOI] [PubMed] [Google Scholar]
- 29.Steller M, Chou L, Daniels TE. Electrical stimulation of salivary flow in patients with Sjogren’s syndrome. J Dent Res. 1988 Oct;67(10):1334–1337. doi: 10.1177/00220345880670101701. [DOI] [PubMed] [Google Scholar]
- 30.Fontanesi J, Golden EB, Cianci P. Hyperbaric oxygen therapy can reverse radiation-induced xerostomia. J Hyperbaric Med. 1991;6:215–221. [Google Scholar]
- 31.Rydholm M, Strang P. Acupuncture for patients in hospital-based home care suffering from xerostomia. J Palliat Care. 1999 Winter;15(4):20–23. [PubMed] [Google Scholar]
- 32.Blom M, Lundeberg T. Long-term follow-up of patients treated with acupuncture for xerostomia and the influence of additional treatment. Oral Dis. 2000 Jan;6(1):15–24. doi: 10.1111/j.1601-0825.2000.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 33.Blom M, Dawidson I, Angmar-Mansson B. The effect of acupuncture on salivary flow rates in patients with xerostomia. Oral Surg Oral Med Oral Pathol. 1992 Mar;73(3):293–298. doi: 10.1016/0030-4220(92)90124-9. [DOI] [PubMed] [Google Scholar]
- 34.Blom M, Lundeberg T, Dawidson I, Angmar-Mansson B. Effects on local blood flux of acupuncture stimulation used to treat xerostomia in patients suffering from Sjogren’s syndrome. J Oral Rehabil. 1993 Sep;20(5):541–548. doi: 10.1111/j.1365-2842.1993.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 35.Blom M, Dawidson I, Fernberg JO, Johnson G, Angmar-Mansson B. Acupuncture treatment of patients with radiation-induced xerostomia. Eur J Cancer B Oral Oncol. 1996 May;32B(3):182–190. doi: 10.1016/0964-1955(95)00085-2. [DOI] [PubMed] [Google Scholar]
- 36.Dawidson I, Blom M, Lundeberg T, Angmar-Mansson B. The influence of acupuncture on salivary flow rates in healthy subjects. J Oral Rehabil. 1997 Mar;24(3):204–208. [PubMed] [Google Scholar]
- 37.Dawidson I, Angmar-Mansson B, Blom M, Theodorsson E, Lundeberg T. The influence of sensory stimulation (acupuncture) on the release of neuropeptides in the saliva of healthy subjects. Life Sci. 1998;63(8):659–674. doi: 10.1016/s0024-3205(98)00317-8. [DOI] [PubMed] [Google Scholar]
- 38.Dawidson I, Angmar-Mansson B, Blom M, Theodorsson E, Lundeberg T. Sensory stimulation (acupuncture) increases the release of vasoactive intestinal polypeptide in the saliva of xerostomia sufferers. Neuropeptides. 1998 Dec;32(6):543–548. doi: 10.1016/s0143-4179(98)90083-x. [DOI] [PubMed] [Google Scholar]
- 39.Dawidson I, Angmar-Mansson B, Blom M, Theodorsson E, Lundeberg T. Sensory stimulation (acupuncture) increases the release of calcitonin gene-related peptide in the saliva of xerostomia sufferers. Neuropeptides. 1999 Jun;33(3):244–250. doi: 10.1054/npep.1999.0759. [DOI] [PubMed] [Google Scholar]
- 40.Johnstone PA, Peng YP, May BC, Inouye WS, Niemtzow RC. Acupuncture for pilocarpine-resistant xerostomia following radiotherapy for head and neck malignancies. Int J Radiat Oncol Biol Phys. 2001 Jun 1;50(2):353–357. doi: 10.1016/s0360-3016(00)01530-3. [DOI] [PubMed] [Google Scholar]
- 41.Johnstone PA, Niemtzow RC, Riffenburgh RH. Acupuncture for xerostomia: clinical update. Cancer. 2002 Feb 15;94(4):1151–1156. [PubMed] [Google Scholar]
- 42.Johnstone PA, Polston GR, Niemtzow RC, Martin PJ. Integration of acupuncture into the oncology clinic. Palliat Med. 2002 May;16(3):235–239. doi: 10.1191/0269216302pm540oa. [DOI] [PubMed] [Google Scholar]
- 43.Cho JH, Chung WK, Kang W, Choi SM, Cho CK, Son CG. Manual acupuncture improved quality of life in cancer patients with radiation-induced xerostomia. J Altern Complement Med. 2008 Jun;14(5):523–526. doi: 10.1089/acm.2007.0793. [DOI] [PubMed] [Google Scholar]
- 44.Deng L, Gan Y, He S, Ji X, et al. Chinese Acupuncture and Moxibustion. Beijing: Foreign Languages Press; 1997. [Google Scholar]
- 45.Deadman P, Al-Khafaji M, Baker K. A Manual of Acupuncture. East Sussex, England: Journal of Chinese Medicine Publications; 1999. [Google Scholar]
- 46.Helms JM. Acupuncture Energetics: A Clinical Approach for Physicians. Berkeley, CA: Medical Acupuncture Publishers; 1997. [Google Scholar]
- 47.Rosenthal DI, Mendoza TR, Chambers MS, et al. Measuring head and neck cancer symptom burden: the development and validation of the M. D. Anderson symptom inventory, head and neck module. Head Neck. 2007 Oct;29(10):923–931. doi: 10.1002/hed.20602. [DOI] [PubMed] [Google Scholar]
- 48.Littell R, Milliken G, Stroup W, Wolfinger R, Schabenberger O. SAS for Mixed Models. 2. Cary, NC: SAS Institute, Inc; 2006. [Google Scholar]
- 49.Deng G, Hou BL, Holodny AI, Cassileth BR. Functional magnetic resonance imaging (fMRI) changes and saliva production associated with acupuncture at LI-2 acupuncture point: a randomized controlled study. BMC Complement Altern Med. 2008;8:37. doi: 10.1186/1472-6882-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]