Abstract
The capacity to associate neutral stimuli with affective value is an important survival strategy that can be accomplished by cell assemblies obeying Hebbian learning principles. In the neuroscience laboratory, classical fear conditioning has been extensively used as a model to study learning related changes in neural structure and function. Here, we review the effects of classical fear conditioning on electromagnetic brain activity in humans, focusing on how sensory systems adapt to changing fear-related contingencies. By considering spatio-temporal patterns of mass neuronal activity we illustrate a range of cortical changes related to a retuning of neuronal sensitivity to amplify signals consistent with fear-associated stimuli at the cost of other sensory information. Putative mechanisms that may underlie fear-associated plasticity at the level of the sensory cortices are briefly considered and several avenues for future work are outlined.
Introduction
Evolutionary biologists have long recognized that behavioral programs exhibit differential susceptibility to experience-dependent modification (Mayr, 1976). For instance, innate behavioral routines depend on phylogenetic memory traces (instantiated in the genetic wiring of neural circuits) and environmental predictability, as in the case of a sign stimulus eliciting a fixed action pattern in the absence of learning. However, complex organisms inhabiting uncertain and unpredictable environments must remain highly sensitive to changes in ongoing sensory feedback such that an organism’s internal dispositions influence the perception of stimuli according to their momentary relevance for controlling behavior. The capacity to associate a range of stimuli with motivationally relevant outcomes represents an important survival strategy that enables the organization of adaptive and flexible responses in accordance with the unique history of contingencies encountered by an individual (e.g., Edelman, 1992; Sporns, Almássy, & Edelman, 2000). Since the meaning and value of stimuli in the environment undergo constant change (e.g., when a prey animal recognizes that a specific arrangement of trees and rocks at a previously visited watering hole signals the increased likelihood of predator attack), there is a need for physiological mechanisms with sufficient plasticity to capture that dynamism.
Enhanced perceptual processing of sensory signals associated with danger or safety constitutes the initial step in a cascade of changes that occur when organisms adapt their behavior to important contingencies in their environment. In a non-trivial sense, the experience-and value-dependent process of extracting statistical regularities within the environment is accompanied by structural and functional changes within the substrate of the sensory cortices themselves. Indeed, there is increasing appreciation within neuroscience that the sensory cortex of animals is an ‘adaptive processor’ rather than a set of inflexible modules engaged in passive feedforward conduction of input (Gilbert & Sigman, 2007). Perceptual acts may be best described as emerging from a process of dynamic resonance between a bottom-up stream of sensory information and stored internal representations derived from past experience (Roelfsema, 2006).
The focus of this review is on the changes occurring in large-scale sensory systems when human participants learn to associate previously innocuous stimuli with aversive outcomes. Unlike previous reviews of human fear conditioning which have focused largely on regional activations observed using PET/fMRI measures (Sehlmeyer et al., 2009), our aim is to provide an overview of the insights afforded by taking the temporal dimension of brain function into consideration. Consistent with previous conceptualizations (e.g., Freeman, 1995) we suggest that the spatio-temporal properties of en masse neuronal activity reflect relevant aspects of external stimuli as well as internal dispositions directed toward these stimuli. The perspective adopted in this article is that the macroscopic system states indexed in scalp-recorded electroencephalography (EEG) and magnetoencephalography (MEG) are uniquely suited to examining the dynamic changes of sensory function that accompany fear conditioning.
We begin with a brief introduction to basic principles of association that govern fear-conditioning induced changes in neuroarchitecture and function. We then provide an exhaustive overview of human EEG/MEG studies, with a focus on the effects of classical fear conditioning on cortical sensory processing. Finally, we end with a brief consideration of the potential mechanisms that underlie the effects of fear conditioning on sensory responses and outline several avenues for future investigation.
Section 1: Associative Principles Of Fear Conditioning
Fear conditioning depends on establishing an association between a neutral stimulus, the conditional stimulus (CS), and another, innately aversive stimulus, the unconditional stimulus (US). Following repeated CS-US pairings, the CS that initially elicited no emotional response becomes capable of activating fear-related responses on its own. Simple conditioning involves conditioning of a single, isolated CS, while differential conditioning depends on two distinct cues, one of which (the CS+) is reliably paired with the US while another (the CS−) comes to signal US absence. Differential conditioning is the most common procedure employed by human researchers, as it allows one to control the physical appearance of stimuli while minimizing the effects of non-associative learning (such as dishabituation and sensitization) on subsequent responses. Extinction refers to a form of inhibitory fear learning that occurs when a previously established CS+ is repeatedly presented without the US, leading to a decrement of conditioned responding as the CS-US contingency becomes revised (Bouton, 2004).
The brain’s ability to link a CS with a US depends on principles of association initially formalized by Donald Hebb (1949). Briefly summarized, these rules state that (i) a growth process is initiated when one cell persistently contributes to firing another one, and (ii) that the temporally synchronous activation of two cells or cell systems strengthens the connection between them. Theoretical and methodological advances in the neurosciences, including the discovery of long-term potentiation (Bliss & Lomo, 1973), whereby the responses of a post-synaptic cell are facilitated by tetanic stimulation have largely validated Hebb’s prescient insights, while offering more detailed analyses of the physiological substrates that underlie associative learning (see Johansen, Cain, Ostroff & LeDoux, 2011 for a review). Today, the ability of nerve cells to detect temporal coincidence of firing patterns can be explained on a molecular level (Huber, Mauk, & Kelly, 1995), by reference to the unique kinetic properties of NMDA (N-Methyl-D-Aspartate) glutamate receptors, which require both initial depolarization and ligand activation to initiate intracellular ionic entry. Additional physiological mechanisms for coincidence detection have been elucidated, such as the dual sensitivity of L-type calcium channels to back-propagating action potentials and pre-synaptic excitatory currents (Blair, Schafe, Bauer, Rodrigues, & LeDoux, 2001).
At the level of neuronal populations, establishing an associative fear trace depends on the coordination of activity between neural representations of the conditional stimuli and the motive systems that code their relative value. Although conditioning-induced changes in neuroarchitecture and function occur across multiple spatio-temporal scales (Maren & Quirk, 2004), ranging from individual neurons to cortical sheets, and from minutes to days, the notion of a cell assembly (Hebb, 1949) provides the necessary conceptual framework for bridging across these multi-scale phenomena. A cell assembly refers to a distributed network of neurons that are bound together by the temporal synchronization of their sub-threshold membrane potentials and/or firing rates (Singer, Engel, Kreiter, Munk, Neuenschwander, & Roelfsema, 1997; Varela, Lachaux, Rodriguez, & Martinerie, 2001). Importantly, neuronal cell assemblies may be synchronized at local scales, separated in the millimeter range, but also at distances that span distinct cortical lobes (Varela et al., 2001). Hebb’s original suggestion (1949) held that reverberating activity within cell assemblies constituted a transient percept or memory trace that can become more consolidated with the passage of time.
Recent findings suggest that neuronal oscillations are ideally suited for the flexible formation of cell assemblies (Buzsáki & Draghun, 2004; Varela et al., 2001), including those that support the acquisition and extinction of fear memories (Paré, Collins, & Pelletier, 2002). Complimenting this literature, computational models with simulated agents situated in artificial evolutionary environments have demonstrated that network oscillations can help to promote rapid switches in perception and attention for affectively laden stimuli, thereby permitting greater flexibility in responding to environments with fluctuating threats and opportunities (Heerebout & Phaf, 2010). Oscillatory signals may therefore play an important role in the critical changes that occur when a previously innocuous stimulus acquires relevance for controlling behavior (Headley & Weinberger, 2011; Miltner, Braun, Arnold, Witte, & Taub, 1999). In this review, we argue that macroscopic oscillatory neuronal activity, as reflected in EEG and MEG measures, provides a rich avenue for exploring learning-induced reorganization of human cortical networks.
Section 2: Macroscopic Correlates Of Fear Conditioning: Human EEG/MEG Studies
2.1 EEG/MEG Measures Of Neuronal Activity
As previously mentioned, associative learning depends on cell assemblies obeying basic Hebbian principles. In terms of brain function, this type of learning is reflected by changes in neural communication between nearby and distant brain loci, which dynamically sculpt neural signaling pathways at multiple levels of analysis. As might be expected for a complex non-linear system such as the brain, the levels involved range from the micro-level of synaptic communication to the macro-level of neural masses. It is particularly the latter level of analysis that is highly accessible using scalp-recorded EEG or MEG in humans, allowing real-time reconstruction of the changes in widespread cortical assemblies that occur with the establishment of new associative links. Importantly, unlike the blood oxygen level dependent (BOLD) signal obtained in the fMRI modality, EEG/MEG oscillations provide a direct index of neuronal activity. EEG and MEG represent the synchronized activity of significant numbers of cortical pyramidal cells. Estimates of this number range from thousands to millions of neurons and vary between EEG and MEG, with MEG generally considered more sensitive to smaller neuronal populations, although this latter observation needs to be qualified by the geometry and intensity of current dipoles (see Barkley, 2004). Since communication between neurons embedded in larger populations involves excitatory as well as inhibitory components, the global network activity over time is oscillatory in nature. In terms of neuronal events, researchers agree that the majority of electromagnetic events measured outside the skull are due to synaptic activity at the dendrites (Olejniczak, 2006).
There are a number of methods for interrogating conditioning-induced changes in the function of sensory brain regions using electromagnetic recordings. In this review we will cover evidence derived from event-related potential (ERP) and event-related field (ERF) time averaged responses to the transient delivery of conditioned sensory stimuli. Coverage will also be extended to studies that have relied on frequency-domain analyses, including ones that have relied on repetitive steady-state driving of the sensory cortices and others that have examined changes in the amplitude or phase connectivity of transient stimulus-related oscillations. While a direct comparison of these findings with previous summaries of fMRI and PET imaging (Sehlmeyer et al., 2009; Mechias, Etkin, & Kalisch, 2010) is made difficult by differences in recording methodology and review focus, our aim is to provide additional insight into how acquired fears alter the time dynamics of cortical sensory processing. Inferences about the spatial localization of sensory activations reported here are necessarily limited by the wide variety of recording montages, ranging from the use of a single channel to dense-array whole head coverage.
2.2 Existing Studies
Fear conditioning has a long history within human neuroscience research, covering more than five decades of work beginning with scalp and intracranial recordings conducted by Lesse and collaborators (1957), that demonstrated conditioning-induced increases in amygdalar beta and gama oscillations. Although a considerable amount of work in the field has focused on measures such as the contingent negative variation and slow cortical potentials (e.g., Flor et al., 1996; Hellwig et al., 2008; Regan & Howard, 1998; Wong, Shevrin, & Williams, 1994), presumed to index states of expectation and preparatory motor action, our primary concern for this review relates to sensory processing of conditioned stimuli. With the increased availability of sophisticated averaging and signal processing techniques along with improved precision of stimulation devices, application of the ERP and ERF techniques has enabled analyses of time-locked cortical responses to conditioned and unconditioned stimuli in healthy humans (see Begleiter & Platz, 1969 for an early example of this approach). In general, human research has converged with an extensive body of findings in laboratory animals, dating back several decades (Galambos, Sheatz, & Vernier, 1956) that has documented the plastic modification of sensory cortices by affective experience.
2.3 Unimodal Plasticity
Conditioning-induced plasticity of sensory systems may be either unimodal or cross-modal in nature. Unimodal plasticity refers to experience-dependent changes occurring in the sensory cortex that matches the modality of the conditioned stimulus (e.g., visuocortical plasticity in response to a visual CS+). The earliest enhancement of processing in the associative sensory cortex (with a broad occipito-parieto-temporal distribution) in response to a visual CS+ (human faces paired with noxious odor) has been observed around 50 to 80 ms post-stimulus in a study that tracked ERFs in a novel experimental design using multiple conditioned stimuli (Steinberg et al., 2012). A learning-related amplitude amplification was obtained at similar latencies over regions covering the right orbital and lateral prefrontal cortex, arguing for a relatively fast sweep of initial activation. Although this early extrastriate enhancement was observed following only several CS-US pairings, it was less consistent in subsequent studies that employed electric shock and aversive sounds as unconditioned stimuli (Steinberg, Bröckelmann, Rehbein, Dobel, & Junghöfer, in press). However, a subsequent phase of CS+ selective enhancement in the extrastriate regions during mid-latency intervals (>120 ms) appears to be more robust, as reported by several research groups using pictures of human faces as the conditioned stimuli and measures of magnetic and electrical cerebral activity as conditioned responses (Dolan, Heinze, Hurlemann, & Hinrichs, 2006; Pizzagalli, Greischar, & Davidson, 2003; Steinberg et al., 2012). An EEG study that employed simple visual stimuli (filled and open Landoldt rings) discovered learning-related increases in the field strength of a visual component evoked by the CS+ at comparatively late (> 300 ms) stages of sensory processing (Skrandies & Jedynak, 2000).
Although to date most studies have relied on fear conditioning to neutral visual cues, there is increasing evidence that sensory enhancements are also observed with auditory conditioned stimuli at overlapping (but somewhat earlier) response latencies. For example, Bröckelmann and colleagues (2012) have recently demonstrated greater CS+ vs. CS− evoked field amplitude for components occurring very early (25 to 65 ms) in the sensory processing stream. Notably, conditioning-related effects on these early auditory cortex responses seemed to depend more on response diminution for CS− features than enhancement for the CS+ ones, per se (Steinberg et al., in press). Somewhat confusingly, another recent MEG study actually found decreased amplitude for a CS+ relative to CS− evoked early (30 to 50 ms) auditory component (Kluge, Bauer, Leff, Heinze, Dolan, & Driver, 2011). The discrepancy in outcomes may relate to considerable differences in methodology between the two studies, including differences in both CS (multiple vs. classical differential) and US cues (affective sounds vs. electric shock). More traditional CS+ enhancements are observed at subsequent auditory processing stages, including the N1m (~100 ms) and P2m (~200 ms). Following contingency reversal (i.e., when the CS− acquires CS+ status in a subsequent learning phase, and vice versa) each of the auditory evoked field components (P1m, N1M, P2m) expresses unique constraints, with later responses (P2m) generally showing more response flexibility for the updated CS+/CS− status compared to earlier activations along the auditory processing hierarchy (Kluge et al., 2011).
In another study with more complex auditory stimuli, namely speech syllables presented along a/ba/-/da/continuum, Heim and Keil (2006) found an enhancement of N2 (248 to 312 ms) amplitude for the intermittently conditioned CS+ in the vicinity of the right hemispheric supra-temporal plane and prefrontal areas. Interestingly, this was one of the few studies to also examine learning-related changes in behavioral discrimination of conditioned stimuli. The neurophysiological evidence for perceptual response enhancement was not mirrored in a performance advantage for discriminating the CS+ cue.
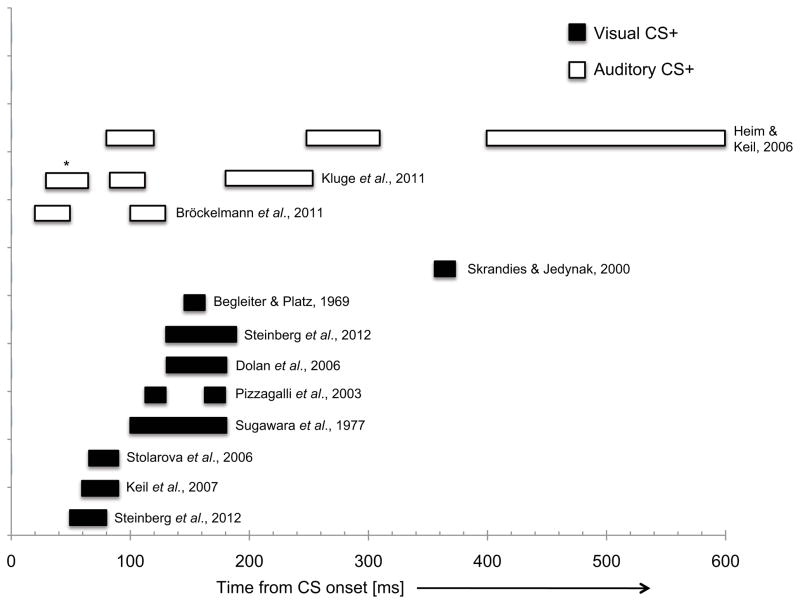
Figure 1 provides a visual summary of the evidence for CS+ vs. CS− enhancements across multiple experiments that have used transient delivery of stimuli. When considered together, these findings suggest that sensory systems are able to rapidly resolve motivational significance leading to response enhancement for emotion-associated stimuli, regardless of stimulus modality. Generally speaking, modulations associated with auditory CS+ cues seem to occur more rapidly than those associated with visual CS+ cues. Given the wide disparity in CS (simple vs. complex) and US (electric shock, aversive loud noise, noxious odors) types between studies, it remains difficult to draw clear conclusions regarding the effects exerted by these parameters on conditioned response outcomes. Similarly, the precise cortical mapping of the conditioned responses is precluded by current evidence, although it appears that many response enhancements obtained using within-session conditioning designs with limited CS-US pairings, occur within secondary or associative sensory regions.
Figure 1.
An illustration summarizing effects related to an enhancement of CS+ (vs. CS−) responses in sensory regions, gathered from studies that employed transient presentations of the CS cues. The x-axis depicts time from CS-onset and the width of the bars depicts the approximate temporal window of significant effects, as described in the published studies. Black bars depict visual CSs and white bars depict auditory CSs. The asterisk notes an example of a response reversal (i.e., CS− eliciting a greater response than the CS+).
On a neurophysiological level, the changes in sensory function reviewed above are consistent with re-entrant modulation of sensory cortical networks by other brain regions, through which the sensory gain for CS+ related features is gradually amplified. The source of reentrant modulation most likely derives from brain regions that neuroimaging studies have identified as forming a network of areas that are reliably activated during fear conditioning, such as the amygdala, insula and anterior cingulate cortex (Mechias et al., 2010; Sehlmeyer et al., 2009). Cortico-cortical modulations appear highly likely given evidence of rapid recruitment of orbital and lateral prefrontal cortex regions during fear conditioning (Steinberg et al., in press). The rapid latencies with which these re-entrant signals reach sensory cortical regions in conditioning experiments is generally consistent with other findings demonstrating that influences from recurrent processing are already evident within the initial 100 ms of visual stimulus processing in non-human primate (Lamme & Roelfsema, 2000) and human (Foxe & Simpson, 2002) brains. These data seem most consistent with a ‘multiple-waves’ model (Pessoa & Adolphs, 2010) in which the influence of biologically significant stimuli on sensory processing is exerted in a series of parallel cascades, rather than originating from a single neurobiological source such as the amygdala. Indeed, recent evidence from a single-case experiment suggests that the modulation of ERP components as a result of fear conditioning was intact when the stimuli were subliminally presented to a patient with a right amygdala lesion (Heutnik, Brouwer, de Jong, & Bouma, 2011).
On each trial, the CS undergoes an initial sweep of visual analysis, which may result in its identification as a threat cue (the CS+), accompanied by engagement of the relevant network of sub-cortical and/or cortical structures such as the amygdala, insula, and frontal cortices. It has been suggested that these structures then initiate re-entrant bias signals (Keil et al., in press; Keil, Sabatinelli, Ding, Lang, Ihssen, & Heim, 2009; Sabatinelli, Lang, Bradley, Costa, & Keil, 2009), providing a source of feedback that affects ongoing processing cascades within and across multiple tiers of the visual cortex, ultimately resulting in an amplified sensory representation of the relevant stimuli (Bradley, Sabatinelli, Lang, Fitzsimmons, King, & Desai, 2003). These mechanisms parallel the processes that have been proposed to underlie endogenous forms of attention, in which re-entrant bias signals originate from frontal or fronto-parietal cortical networks (Kastner & Ungerleider, 2000; Yantis, 2008) and facilitate the representation of selected objects in sensory regions.
2.3.1 Sensory dynamics over blocks of trials: sharpening of networks in primary sensory cortex
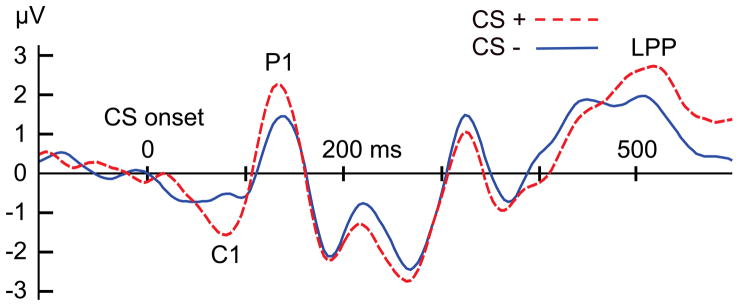
A critical phenomenon may occur when conditioning is maintained over longer periods of time (e.g., over consecutive testing days), or when several hundreds of pairings between the CS+ and US are performed. We have reported that observers then acquire sensory enhancement for simple grating stimuli signaling threat (i.e., the CS+) compared to the CS−, in regions of the primary visual cortex (Stolarova, Keil, & Moratti, 2006). In ERP data, this sensory modulation can be observed in the earliest cortical visual component, often called the C1, which has a latency around 55 ms, shows pronounced retinotopy, and is thought to originate in the primary visual cortex. Although some authors have questioned the degree to which the C1 component depends solely on V1 sources, it remains the only reliable index of primary visual cortex activity that can be obtained using non-invasive measures of brain electric activity (see Rauss, Schwartz, & Pourtois, 2011). The fact that enhancement of the sensory C1 component is found in the laboratory only after massive numbers of conditioning trials parallels findings from perceptual learning experiments (Bao, Yang, Rios, He, & Engel, 2010), which involve extensive training of new visual skills across thousands of trials. Figure 2 shows the response to a CS+ and CS− gratings after 120 trials of differential conditioning, in which all of the 60 CS+ stimuli were followed by an aversive sound blast whereas the 60 CS− stimuli were not. The C1 visual component is markedly enhanced for the CS+, compared to the CS−. Although they are not directly comparable, such findings are broadly consistent with studies of experimental animals where conditioning is associated with specific changes in the tuning of receptive fields in lower-tier sensory cortices resulting in increased CS+ sensitivity (see Weinberger, 2004 for an authoritative review).
Figure 2.
ERP responses to the CS+ (dashed, red line) and CS− (dashed, blue line) experimental conditions averaged across 18 participants, after 160 trials of differential conditioning. Note the amplitude enhancement associated with CS+ processing, beginning with the C1 component, and continuing for the P1 and late positive potential (LPP). Data are taken from Stolarova et al. (2006).
We hypothesize that in the course of fear learning, extraction of simple threat features (e.g., the orientation of a Gabor patch) may occur more rapidly, with increasing recruitment of lower-tier visual cortical regions. Rather than encompassing a complete feedforward and feedback sweep, the processing of recurring threat features may come to be embedded in primary sensory networks, aiding fast and efficient discrimination of threat cues. As a short-term mechanism, oscillatory re-entrant modulation of visual cortex may change network thresholds to selectively enhance responses specifically related to the CS+. These results suggest that an observer’s learning history continuously shapes the processing of incoming sensory information by retuning the sensitivity of sensory neuronal populations to specific threat features, even at the earliest stages of processing hierarchies. Given the increasing spatio-temporal resolution of neuroimaging and electrophysiological techniques, it is now possible to more closely describe the cortical dynamics underlying these changes.
2.3.2 The evolution of cortical retuning over time: single-trial analyses and steady-state driving of sensory networks
The findings reported above raise questions about the temporal evolution of fear conditioned effects in visual cortex. Insights into the trial-by-trial evolution of associative plasticity have been difficult to obtain in human studies to date, due largely to methodological limitations. However, recent advances in signal processing algorithms (e.g., Xu, Stoica, Li, Bressler, Shao, & Ding, 2009) have enabled researchers to begin charting the trial-by-trial dynamics of conditioned changes in brain electrical potentials. Work from our group (Liu, Keil, & Ding, in press) has focused on the visual P1 component of the ERP, which is generated in the extrastriate cortex (Di Russo, Martínez, Sereno, Pitzalis, & Hillyard, 2001). Using a machine-learning algorithm (Analysis of Single Trial ERP and Ongoing Activity [ASEO]) to extract single trial amplitudes during fear conditioning, distinct phases of sensory changes were identified during conditioning, involving (i) a phase of sustained relative increase in CS+ amplitude, and (ii) a final adaptation phase in which amplitudes for both CSs decreased, near pre-conditioning levels. These stages can be related to the (i) initial formation of an associative sensory network that embeds aspects of the threat cue through ongoing re-entrant amplification, and (ii) its subsequent consolidation into a more efficient mode, where highly connected and effective connectivity ensures reliable threat processing at lower energy expense (cf. Wieser & Keil, 2011).
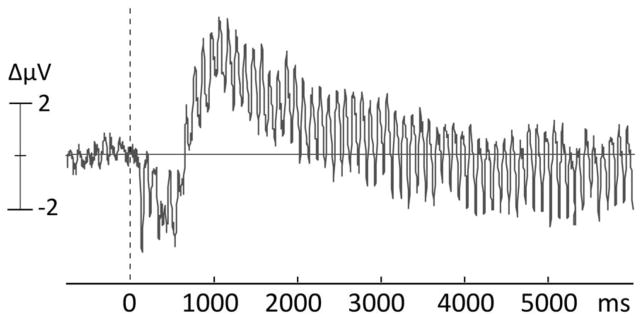
The effects of sustained re-entrant modulation of lower-tier visual cortices by afferent input from other regions, proximal and distal, can also be examined using a variant of ERPs, the steady-state visual evoked potential (ssVEP) or its magnetic counterpart, the steady-state visual evoked field (ssVEF). Steady-state methodology has contributed to the understanding of conditioning-related changes of sensory function, beginning with early research on instrumental conditioning in non-human animal subjects (John & Killam, 1959). As illustrated in Figure 3, the ssVEP is an oscillatory brain response to stimuli modulated in luminance (i.e., flickered), in which the frequency of the electrocortical response recorded from the scalp equals that of the driving stimulus (Regan, 1989). Of significant advantage, the oscillatory cortical response manifests at a known frequency and can thus be reliably separated from noise and quantified in the frequency domain (Wang, Clementz, & Keil, 2007), even on the level of single learning trials (Keil et al., 2008). Generators of the ssVEP have been localized to extended visual cortex (Müller, Teder, & Hillyard, 1997), with strong contributions from V1 and higher-order visual regions (DiRusso et al., 2007). Importantly, ssVEPs reflect multiple excitations of the visual system with the same stimulus over a discrete epoch. Thus, changes in ssVEP amplitude can be affected both by initial sensory processing and by re-entrant, top-down modulation of sensory activity by higher order processes, such as spatial attention (e.g., Müller, Teder-Salejarvi, & Hillyard, 1998) and emotional arousal (e.g., Keil et al., 2003).
Figure 3.
Example of an evoked oscillatory response, elicited by flickering visual stimuli, brightness-modulated at a fixed rate of 10 cycles/second. The resulting scalp-recorded waveform is referred to as steady-state visual evoked potential and represents primarily activity from early sensory areas that are repeatedly engaged by the same stimulus.
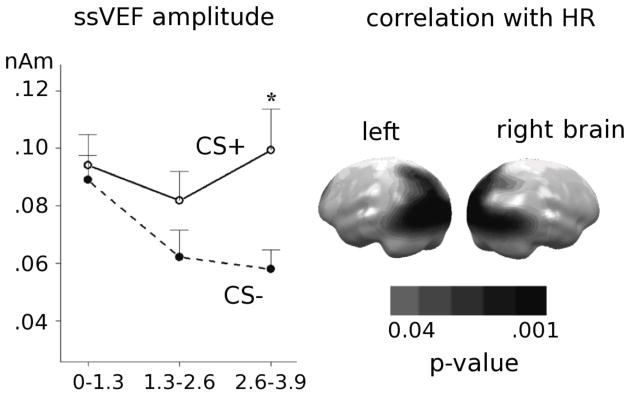
In differential classical conditioning, the ssVEP amplitude over trials is enhanced for the CS+ compared to the CS−, and these changes have been apparent with as few as 10 CS+/US pairings (Moratti & Keil, 2005; Moratti, Keil, & Miller, 2006), although an earlier form of plasticity is observed when using compound conditional stimuli with dissociable perceptual components (Weisz, Kostadinov, Dohrmann, Hartmann, & Schlee, 2007). Across studies, results have suggested that the enhancement of the sensory ssVEP/ssVEF for the CS+ occurs in a late time interval preceding US delivery, suggesting increased perceptual processing of features that predict a temporally imminent aversive event (see Figure 4). Moreover, this response enhancement in sensory regions depends on the activation of a systemic fear response as measured by autonomic arousal, i.e., heart rate acceleration in response to the conditioned threat cue.
Figure 4.
On the right, mean amplitudes (n=20) across significant source clusters in occipito-parietal regions. ssVEF amplitude is greater for the CS+ than the CS− in a late temporal window, that precedes US onset. Left panel depicts the distribution of correlation coefficients for the relationship between heart rate change (CS+ acceleration) and increased cortical activation for the CS+. Redrawn from data in Moratti et al., 2006. Bars depict SEM.
A question left unanswered by the studies mentioned above is the extent to which enhanced visual processing of the CS+ represents the outcome of a declarative attention process that involves cognitive anticipation of the US rather than a true contingency-based effect driven by CS-US associative strength. A simple conditioning paradigm developed by Perruchet (1985) can disambiguate among these possibilities by using extended runs of nonreinforced and reinforced CS sequences in which participants are asked to rate their expectancy of the US being presented on the next trial. The rationale behind Perruchet’s design is that a sequence of nonreinforced trial runs leads participants to increase their expectancy of the US on the upcoming trial, as might be predicted on the basis of gambler’s fallacy. By contrast, associative strength of the CS should decrease after an extended run of nonreinforced trials. The opposite pattern obtains during runs of reinforced trials (i.e., diminished expectancy and stronger associative strength). In a recent report, Moratti and Keil (2009) conducted a steady-state MEG experiment based on the Perruchet paradigm. A key finding to emerge from this study was that ssVEF amplitude evoked by the CS+ in occipital and supplementary motor areas was linearly related to the associative strength (number of previous CS+/US pairings), but decreased as a function of expectancy of the US.
As with the ERP findings reported above, work with ssVEPs suggests that the early cortical facilitation for fear cue processing is determined by associative strength and previous exposure to CS+/US contingencies. Based on this body of work, we have proposed that with repeated exposure to the CS+ and CS−, the temporal dynamics of perception and attention adapt to optimize perception of the threat stimulus.
2.3.3 Oscillatory correlates of cortical CS+ representations
An important property of electrocortical signals concerns the pronounced shifts in spectra and amplitude composition that occur when an individual is engaged in different cognitive, affective and behavioral states. Many state-dependent oscillatory changes are readily observable when inspecting the ongoing electrophysiological time series. One example of such a macroscopic, qualitative change is provided by the well-known slowing of the dominant EEG rhythm down to the 1 to 2 Hz range when entering deeper stages of sleep (Axmacher, Mormann, Fernandez, Elger, & Fell, 2006). Another robust phenomenon is the spectral transition from predominantly high-frequency EEG activity observed during states of focused alertness (e.g., attentive with eyes open) to large (8 to 12 Hz, so-called alpha band) oscillations when the individual is in a state of relaxed wakefulness, with closed eyes (Berger, 1969).
It has been shown that macro-states such as alpha wave activity are not just a passive integral of the individual units but that this macroscopic state in turn affects the individual units, changing their firing thresholds (Rajagovindan & Ding, 2011). This “enslavement” of individual units by the macroscopic control state has been identified in many biological systems and is considered a hallmark feature of dynamic interactions (Jirsa, Friedrich, Haken, & Kelso, 1994). In the present context, it is the ability to link EEG and MEG-derived oscillations to the formation of large-scale neuronal assemblies (Fingelkurts & Fingelkurts, 2001) that makes these measures ideal for monitoring functional adaptations during learning and experience in general, and during experimentally controlled fear conditioning in particular.
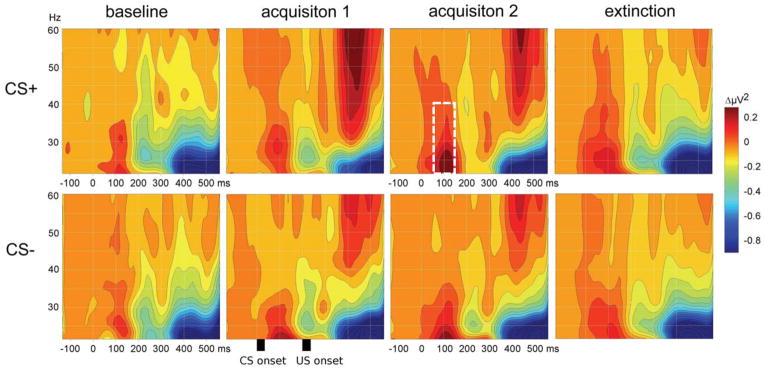
Synchronized neuronal activity in the high frequency (> 20 Hz) EEG gamma band range is a reliable oscillatory macro-signature associated with the formation of a stable cortical object representation (Keil, Gruber, & Müller, 2001; Tallon-Baudry & Bertrand, 1999). Supporting this notion, Keil and colleagues (2007) found a gradual increase of early (60–90 ms) EEG gamma oscillations evoked by the CS+ in the visual cortex, across two consecutive days of conditioning. As illustrated in Figure 5, this gamma band increase was most evident during the second block of acquisition. Accompanying the increase in gamma band activity, there was an enhancement of phase synchrony within regions of the visual cortex. The degree of phase synchrony between neuronal populations captures changes in the functional connectivity of human cortical networks (Lachaux, Rodriguez, Martinerie, & Varela, 1999), including those involved in perception (Elbert & Keil, 2000). Other research groups (Miltner et al., 1999) likewise observed increases in gamma-band power and coherence between pairs of electrodes covering the visual (corresponding to the CS+) and somatosensory (corresponding to the tactile US) cortical regions during fear conditioning, which the authors interpreted as signatures of cell assembly formation. In a reanalysis of EEG data from a fear conditioning study with simple Landoldt rings, Klein and colleagues (2006) were able to discriminate learners from non-learners by the presence of increased gamma-band coherence (by ~120 ms post-stimulus) over posterior electrodes in the former group only.
Figure 5.
The evolutionary spectrum of visual electrocortical responses during classical conditioning. Graphs show a grand mean (n=23) sequence of time-frequency representations of visual responses to the CS+ (top) and CS− (bottom) during 4 stages of classical conditioning, derived using a Morlet wavelet transform (redrawn from data in Keil et al., 2007 and Stolarova et al., 2006). Visible spectral events include an early response in the lower gamma band range (around 80–120 ms at 20–35 Hz), in response to the CS; late high-frequency gamma response to the US onset (at 600 ms post-CS and above 40 Hz); pronounced lower band reduction in response to the US (starting at 400 ms). Note the increase of the early gamma band response during acquisition for the CS+, but not the CS− (white dashed box, top, acquisition 2).
Paralleling the findings with visual conditioned stimuli, a study that involved conditioning of speech syllables found increased gamma-band activity for an intermittently reinforced CS+ during the extinction learning phase, during both an early (80–120 ms) and a late (400–600 ms) processing stage (Heim & Keil, 2006). It is interesting to note that in vivo work with non-human animals has produced parallel findings of increased high-frequency neuronal synchronization during cortical cell assembly formation in both Pavlovian (Headley & Weinberger, 2011) and operant (Dumenko, 1995) forms of conditioning.
2.3.4 Interim Summary: A Cascade of Cortical Changes
The process of learning that particular stimuli in one’s environment are reliably associated with danger is associated with adaptive changes in the structure and function of sensory cortices, leading to enhanced processing of CS+ related features. Analogous structural (expansion) and functional (synaptic strengthening) reorganization of the cortices occurs for representation of other forms of behaviorally significant information, not necessarily related to fear conditioning (Elbert & Rockstroh, 2004). We suggest that sensory amplification of CS+ features in fear conditioning represents an adaptive response insofar as it aids the organism in prioritizing behaviorally significant elements of the environment at the cost of competing information. The degree to which sensory gains generalize to stimuli that are perceptually similar to the CS+ remains an unexplored question that might be addressed by future studies examining generalization gradients of conditioned responses, similar to previous work using measures of fear-potentiated startle in humans (Lissek et al., 2008) and receptive field mapping in non-human animals (Ohl & Scheich, 1997).
As suggested by the data reviewed above, the amplitude of CS-evoked sensory responses may be influenced both by (i) the amount of re-entry that originates from higher order cortical and/or sub-cortical structures to influence processing within the sensory cortices and (ii) the local sensitivity of sensory neurons within the early visual cortex to the specific features that distinguish the CS+ from the CS−. These two factors most likely operate at different time scales (e.g., re-entrant processes may dominate during the early phases of learning, while changes in local sensitivity become operational through extended learning opportunities) and are probably interactive at some level of analysis. For example, persisting top-down modulation by coherently oscillating networks can have long-term effects on network architecture, including lower-tier sensory areas with small and narrowly tuned receptive fields. If motivational relevance remains high across time (as occurs when the CS-US associative strength is high), oscillatory top-down regulation may induce lasting plastic changes of network architecture and/or synaptic connectivity (Gilbert, Sigman, & Crist, 2001). Such effects may be involved in maintaining perceptual vigilance for learned fear cues despite potentially decreasing modulation of visual areas by amygdalo-fugal (Zald, 2003) or cortico-cortical re-entrant connections. Hence, a reasonable hypothesis is that a cortical cell assembly emerges over time, which is specifically sensitive to arousing features in a given context, even at the earliest levels of perception as indexed by a retinotopic ERP component such as the C1.
Functionally, fear conditioning-induced sensory amplification bears many similarities to what previous experiments have demonstrated when using stimuli whose motivational value does not depend on instruction by experience (Lang & Bradley, 2010; Vuilleumier, 2005) or for stimuli that cue potential threat by virtue of verbal instruction (Shackman, Maxwell, McMenamin, Greischar, & Davidson, 2011; Weymar, Bradley, Hamm, Lang, in press). Moreover, the putative neuronal mechanisms that drive increases in sensory processing (e.g., reentry from brain regions such as the prefrontal cortex, insula, amygdala) appear to share a considerable degree of overlap regardless of whether the stimuli possess inherent or acquired value.
While most of the evidence reviewed above discusses quantitative changes in the amplification of sensory responses, the process of fear acquisition may be associated with other, more qualitative changes of CS+ sensory representation. The latter possibility is suggested by the evidence reviewed in Section 2.3.3 regarding shifts in large-scale oscillatory power and phase connectivity that result from fear conditioning regimes.
2.4 Cross-Modal Plasticity
Another, less studied, form of conditioning-induced sensory plasticity occurs when a CS+ presented in one modality elicits a conditioned response of the sensory cortex matching the US modality, presuming that these are different. For example, when a visual CS+ is presented in the absence of an auditory US, it elicits stronger activation of the primary auditory cortex as rapidly as 30–61 ms following US omission, compared to the CS− or the CS+ before conditioning or during extinction (Moses, Martin, Houck, Ilmoniemi, & Tesche, 2005). Likewise, Pizzagalli and colleagues (2003) found amplitude enhancements over primary auditory cortex occurring within 100 ms following presentation of a visual CS+ that was previously paired with an auditory US. In other studies, a visual (Wik, Elbert, Fredrikson, Hoke, & Ross, 1996) or an auditory (Moses, Bardouille, Brown, Ross, & McIntosh, 2010) CS+, compared to the CS−, increased activation of the primary somatosensory cortex when the electrodermal US was omitted. Cross-modal conditioned brain responses disappear following extinction learning (Wik, Elbert, Fredrikson, Hoke, & Ross, 1997). Cross-modal plasticity reflects the scalable nature of neuronal assemblies formed during associative learning, suggesting that they can integrate activity across distinct cortical lobes. Despite the intriguing nature of these findings, the omission-related cross-modal conditioned responses may represent a different phenomenon from the unimodal forms of plasticity reviewed in the previous section, being more closely related to expectancy or preparation in the face of an imminent aversive event.
Section 3: Potential Mechanisms Of Fear-Associated Plasticity
What are some of the potential mechanistic pathways that underlie enhanced sensory responses for fear-conditioned stimuli? Such questions are notoriously difficult to address in human studies, where researchers are usually limited to correlational evidence. Given the cross-species relevance of fear learning, however, this work can be informed by non-human animal research. The past several decades have witnessed significant progress in mapping the cellular and molecular circuitry of fear conditioning (Johansen et al., 2011). A combination of invasive techniques including neurosurgery, neurochemistry and cell electrophysiology, have made considerable contributions to our understanding of the brain pathways involved in aversive learning, especially with respect to the model system of classical auditory conditioning in rodents. More recently, the combination of techniques for manipulating specific neuronal cell types or circuits (optogenetics), along with the development of tools for in vivo imaging at unprecedented spatial resolutions (two-photon microscopy), has further expanded causal understanding of the networks that underlie fear and safety conditioning (Johansen, Wolff, Luthi, & LeDoux, in press).
While processes endogenous to the sensory cortices may underlie some, transient, forms of plasticity (Armony, Quirk, & LeDoux, 1998), subcortical nuclei such as the amygdala and thalamus represent the initial storage sites of CS-US association and their input on downstream targets seems to be necessary for the consolidation of fear-related associations at the cortical level. Conditioned responses in the lateral amygdala (Quirk, Armony, & LeDoux, 1997) and thalamus (Weinberger, 2011) precede those that are observed in the primary sensory cortices further suggesting that the subcortical centers are necessary for the induction of sustained fear-related plasticity in the cortex.
The precise physiological mechanisms by which subcortical regions might promote plasticity at cortical target sites remain to be discovered, although two important factors involve the synchrony of neuronal oscillations and cholinergic neurotransmission. Oscillatory synchronization between distributed neuronal assemblies in theta (4 to 8 Hz) and gamma bandwidths represents a highly plausible substrate for synaptic plasticity transfer (Pape & Paré, 2010; Paré, Collins, & Pelletier, 2002). Periods of large-scale oscillatory synchrony may create well-defined temporal windows for fear-related associations, initially stored in subcortical nuclei, to influence neuronal activity in other regions. In particular, increased synchrony between subcortical and cortical phase-locked networks during the consolidation of fear learning may be crucial in facilitating interregional interactions by which conditioned cues increase their perceptual and attentional sensitivity. By contrast, the recall of conditioned fear and extinction is disrupted when interfering with inter-areal theta-band coupling (Lesting et al., 2011). Most of our current knowledge about changes in cross-regional phase synchronization during fear conditioning has been derived from rodent studies. One might expect such interactions to gain increasing importance among primates, where the physical connections between the amygdala and neocortex, especially the sensory neocortical regions, are considerably enlarged (Freese & Amaral, 2006).
Cholinergic neurotransmission has emerged as an important chemical factor that influences associative plasticity within the sensory cortices (Weinberger, 1998, 2004). When a CS is paired with contingent stimulation of the nucleus basalis magnocellularis (the principal source of diffuse cholinergic input to the cerebral cortex), instead of a motivationally engaging US, receptive fields show associative changes in tuning that favor the paired CS frequency (e.g., Bakin & Weinberger, 1996). One influential model of auditory fear conditioning (Weinberger, 2004) proposes a two-stage model, involving (i) the convergence of CS-US information within subcortical nuclei (namely, the magnocellular division of the thalamic medial geniculate nucleus and the basolateral nucleus of the amygdala), through which trained stimuli acquire their value, followed by (ii) activation of the nucleus basalis, with corticopetal cholinergic projections to the primary auditory regions. In turn, cholinergic influx into the sensory cortex acts to enhance attention and perceptual sensitivity for afferent sensory stimuli and diminish the spontaneous background activity of neurons (Hasselmo & Giacomo, 2006). Since cholinergic bursts of firing are temporally coincident with CS+, but not CS−, presentations, Hebbian learning principles explain why subsequent changes within the sensory cortex express fear-associated specificity.
In humans, it is know that pharmacological treatment with the anticholinergic drug, scopolamine, abolishes experience-dependent increases in CS-evoked BOLD activity (Thiel, Friston, & Dolan, 2002). On the other hand, administration of a cholinergic agonist (physostigmine) during a spatial attention task, speeds behavioral performance and influences oscillatory synchrony within the visual cortex in ways that increase target sensitivity (Bauer et al., 2012). Clearly, there are many opportunities for combined neuropharmacological and EEG/MEG studies to provide insights into putative mechanisms that may underlie increases in cortical sensory acuity for fear conditioned signals.
Section 4: Conclusions And Future Directions
We have provided a review of EEG/MEG studies of classical fear conditioning in humans, with a special focus on sensory regions that amplify signals consistent with fear-associated stimuli at the cost of other sensory information. One framework for the interpretation of these findings refers to a temporal cascade of effects, with both short- and long-term adaptations for fear-associated stimuli. Table 1 includes a brief summary of the main points that have been covered in this review.
Table 1.
Summary Points
|
Inspection of Table 1 would seem to suggest that, in the future, studies that are restricted to reporting only conditioning related enhancements in the response amplitude of electric or magnetic field components will have limited additional significance in terms of expanding extant theories. However there are numerous gaps in our current knowledge that await experimental evidence. One unresolved question concerns whether there exists a direct link between increased activation of the sensory cortices due to conditioning and psychophysical measures of perceptual sensitivity. A recent fMRI study found a positive relation between increased early visual cortex activity in response to the CS+ and a greater hit rate on a challenging visual detection task (Padmala & Pessoa, 2008). Others have reported that mirror-image odor molecules which are initially indiscriminable become dissociated following aversive conditioning, leading to spatial divergence of ensemble neuronal activity in the olfactory cortex as well as an improvement in perceptual indices of discrimination (Li, Howard, Parrish, & Gottfried, 2008). Additional evidence, however, points to dissociations between neurophysiological measures of sensory enhancement and behavioral measures of perceptual discrimination (Heim & Keil, 2006).
A second branch of questions concerns the impact of acute stress and individual differences in anxiety on conditioned sensory biases, given the high relevance of fear conditioning to the onset and maintenance of anxiety disorders (Mineka & Oehlberg, 2008). An important contribution stemming from this area of inquiry concerns the conceptual and empirical distinctions that are currently being drawn between phasic forms of fear to specific conditioned cues and sustained anxiety in the presence of non-specific ambient environmental contexts where fear conditioning takes place (e.g., Davis, Walker, Miles, & Grillon, 2010).
Other outstanding questions for the field are related to disentangling how contextual variables modulate cortical responses for fear-associated cues and whether the expression of cortical responses differs depending on the method of initial fear acquisition, whether through Pavlovian learning or more indirect methods, such as symbolic instruction or observation of conspecifics (Olsson & Phelps, 2007). A direct comparison of conditioning findings with those derived from similar experimental paradigms, such as symbolically cued threat of shock (e.g., Shackman et al., 2011), could provide valuable insights into the overlap of neural processes that are involved. Considered together, the outcomes of this program of research stand to make notable contributions to both basic and clinical domains of psychophysiology.
Acknowledgments
This research was supported by a fellowship from the Canadian Institutes of Health Research (CIHR) awarded to VM and by grants from NIMH (R01 MH084932 – 02 and R01 MH097320) awarded to AK.
References
- Armony JL, Quirk GJ, LeDoux JE. Differential effects of amygdala lesions on early and late plastic components of auditory cortex spike trains during fear conditioning. The Journal of Neuroscience. 1998;18:2592–2601. doi: 10.1523/JNEUROSCI.18-07-02592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends in Neurosciences. 2002;25:621–625. doi: 10.1016/S0166-2236(02)02264-6. [DOI] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernandez G, Elger CE, Fell J. Memory formation by neuronal synchronization. Brain Research Reviews. 2006;52:170–182. doi: 10.1016/j.brainresrev.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Bao M, Yang L, Rios C, He B, Engel SA. Perceptual learning increases the strength of the earliest signals in visual cortex. The Journal of Neuroscience. 2010;30:15080–15084. doi: 10.1523/JNEUROSCI.5703-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley GL. Controversies in neurophysiology. MEG is superior to EEG in localization of interictal epileptiform activity: Pro. Clinical Neurophysiology. 2004;115:1001–1009. doi: 10.1016/j.clinph.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Bauer M, Kluge C, Bach D, Bradbury D, Heinze HJ, Dolan RJ, Driver J. Cholinergic enhancement of visual attention and neural oscillations in the human brain. Current Biology. 2012;22:397–402. doi: 10.1016/j.cub.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Begleiter H, Platz A. Evoked potentials: Modification by classical conditioning. Science. 1969;166:769–771. doi: 10.1126/science.166.3906.769. [DOI] [PubMed] [Google Scholar]
- Berger H. On the electroencephalogram of man. Electroencephalography and Clinical Neurophysiology. 1969;(Suppl 28):37. [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: A cellular hypothesis of fear learning. Learning & Memory. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. The Journal of Physiology. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning & Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behavioral Neuroscience. 2003;117:369–380. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- *.Bröckelmann AK, Steinberg C, Elling L, Zwanzger P, Pantev C, Junghöfer M. Emotion-associated tones attract enhanced attention at early auditory processing: Magnetoencephalographic correlates. The Journal of Neuroscience. 2011;31:7801–7810. doi: 10.1523/JNEUROSCI.6236-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Draghun A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- Conrad N, Giabbiconi CM, Muller MM, Gruber T. Neuronal correlates of repetition priming of frequently presented objects: Insights from induced gamma band responses. Neuroscience Letters. 2007;429:126–130. doi: 10.1016/j.neulet.2007.09.065. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: Role of the extended amygdala in fear vs. anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRusso F, Martínez A, Sereno MI, Pitzalis S, Hillyard SA. Cortical sources of the early components of the visual evoked potential. Human Brain Mapping. 2001;15:95–111. doi: 10.1002/hbm.10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiRusso F, Pitzalis S, Aprile T, Spitoni G, Patria F, Stella A, et al. Spatiotemporal analysis of the cortical sources of the steady-state visual evoked potential. Human Brain Mapping. 2007;28:323–334. doi: 10.1002/hbm.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Dolan RJ, Heinze HJ, Hurlemann R, Hinrichs H. Magnetoencephalography (MEG) determined temporal modulation of visual and auditory sensory processing in the context of classical conditioning to faces. NeuroImage. 2006;32:778–789. doi: 10.1016/j.neuroimage.2006.04.206. [DOI] [PubMed] [Google Scholar]
- Dumenko VN. Dynamic shifts in the parameters of the traditional frequency range of the EEG during learning in dogs. Neuroscience and Behavioral Physiology. 1995;25:403–412. doi: 10.1007/BF02359597. [DOI] [PubMed] [Google Scholar]
- Edelman G. Bright air, brilliant fire: On the matter of mind. New York: Basic Books; 1992. [Google Scholar]
- Elbert T, Keil A. Imaging in the fourth dimension. Nature. 2000;404:29–31. doi: 10.1038/35003682. [DOI] [PubMed] [Google Scholar]
- Elbert T, Rockstroh B. Reorganization of human cerebral cortex: The range of changes following use and injury. The Neuroscientist. 2004;10:129–141. doi: 10.1177/1073858403262111. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV. Flow of activation from V1 to frontal cortex in humans: A framework for defining “early” visual processing. Experimental Brain Research. 2002;142:139–150. doi: 10.1007/s00221-001-0906-7. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron. 2001;31:681–697. doi: 10.1016/S0896-6273(01)00424-X. [DOI] [PubMed] [Google Scholar]
- Gruber T, Müller MM, Keil A. Modulation of induced gamma band responses in a perceptual learning task in the human EEG. Journal of Cognitive Neuroscience. 2002;14:732–744. doi: 10.1162/08989290260138636. [DOI] [PubMed] [Google Scholar]
- Gruber T, Müller MM, Keil A. Modulation of induced gamma band responses and phase synchrony in a paired associate learning task in the human EEG. Neuroscience Letters. 2001;316:29–32. doi: 10.1016/S0304-3940(01)02361-8. [DOI] [PubMed] [Google Scholar]
- Fingelkurts AA, Fingelkurts AA. Operational architectonics of the human brain biopotential field: Towards solving the mind-brain problem. Brain and Mind. 2001;2:261–296. [Google Scholar]
- Flor H, Birbaumer N, Roberts LE, Feige B, Lutzenberger W, Hermann C, Kopp B. Slow potentials, event-related potentials, “gamma-band” activity, and motor responses during aversive conditioning in humans. Experimental Brain Research. 1996;112:298–312. doi: 10.1007/BF00227648. [DOI] [PubMed] [Google Scholar]
- Freeman WJ. The creation of perceptual meanings in cortex through chaotic itinerancy and sequential state transitions induced by sensory stimuli. In: Kruse P, Stadler M, editors. Ambiguity in Mind and Nature. Vol. 64. Berlin: Springer; 1995. pp. 421–440. [Google Scholar]
- Freese JL, Amaral DG. Synaptic organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. Journal of Comparative Neurology. 2006;496:655–667. doi: 10.1002/cne.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos R, Sheatz G, Vernier V. Electrophysiological correlates of a conditioned response in cats. Science. 1956;123:376–377. doi: 10.1126/science.123.3192.376. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M. Brain states: Top-down influences in sensory processing. Neuron. 2007;54:677–696. doi: 10.1016/j.neuron.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Giacomo LM. Cholinergic modulation of cortical function. Journal of Molecular Neuroscience. 2006;30:133–135. doi: 10.1385/JMN/30:1–2:133. [DOI] [PubMed] [Google Scholar]
- Headley DB, Weinberger NM. Gamma-band activation predicts both associative memory and cortical plasticity. The Journal of Neuroscience. 2011;31:12748–12758. doi: 10.1523/JNEUROSCI.2528-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D. The organization of behavior: A neuropsychological theory. New York: Wiley; 1949. [Google Scholar]
- *.Heim S, Keil A. Effects of classical conditioning on identification and cortical processing of speech syllables. Experimental Brain Research. 2006;175:411–424. doi: 10.1007/s00221-006-0560-1. [DOI] [PubMed] [Google Scholar]
- Hellwig S, Weisbrod M, Jochum V, Rentrop M, Unger J, Walther S, Haefner K, Roth A, Fiedler P, Bender S. Slow cortical potentials in human aversive trace conditioning. International Journal of Psychophysiology. 2008;69:41–51. doi: 10.1016/j.ijpsycho.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Heerebout BT, Phaf RH. Good vibrations switch attention: An affective function for network oscillations in evolutionary simulations. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:217–229. doi: 10.3758/CABN.10.2.217. [DOI] [PubMed] [Google Scholar]
- Hermann C, Ziegler S, Birbaumer N, Flor H. Pavlovian aversive and appetitive odor conditioning in humans: Subjective, peripheral, and electrocortical changes. Experimental Brain Research. 2000;132:203–215. doi: 10.1007/s002210000343. [DOI] [PubMed] [Google Scholar]
- Heutnik J, Brouwer WH, de Jong BM, Bouma A. Conscious and unconscious processing of fear after right amygdala damage: A single case ERP-study. Neurocase. 2011;17:297–312. doi: 10.1080/13554794.2010.504730. [DOI] [PubMed] [Google Scholar]
- Huber KM, Mauk MD, Kelly PT. Distinct LTP induction mechanisms: Contribution of NMDA receptors and voltage-dependent calcium channels. Journal of Neurophysiology. 1995;73:270–279. doi: 10.1152/jn.1995.73.1.270. [DOI] [PubMed] [Google Scholar]
- Jirsa VK, Friedrich R, Haken H, Kelso JA. A theoretical model of phase transitions in the human brain. Biological Cybernetics. 1994;71:27–35. doi: 10.1007/BF00198909. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Wolff SB, Luthi A, LeDoux JE. Controlling the elements: An optogenetic approach to understanding the neural circuits of fear. Biological Psychiatry. doi: 10.1016/j.biopsych.2011.10.023. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- John ER, Killam KF. Electrophysiological correlates of avoidance conditioning in the cat. The Journal of Pharmacology and Experimental Therapeutics. 1959;125:252–274. [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Keil A, Costa V, Smith JC, Sabatinelli D, McGinnis EM, Bradley MM, Lang PJ. Tagging cortical networks in emotion: A topographical analysis. Human Brain Mapping. doi: 10.1002/hbm.21413. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Gruber T, Müller MM. Functional correlates of macroscopic high-frequency brain activity in the human visual system. Neuroscience & Biobehavioral Reviews. 2001;25:527–534. doi: 10.1016/S0149-7634(01)00031-8. [DOI] [PubMed] [Google Scholar]
- Keil A, Gruber T, Müller MM, Moratti S, Stolarova M, Bradley MM, et al. Early modulation of visual perception by emotional arousal: Evidence from steady-state visual evoked brain potentials. Cognitive, Affective, & Behavioral Neuroscience. 2003;3:195–206. doi: 10.3758/cabn.3.3.195. [DOI] [PubMed] [Google Scholar]
- Keil A, Sabatinelli D, Ding M, Lang PJ, Ihssen N, Heim S. Re-entrant projections modulate visual cortex in affective perception: Directional evidence from Granger causality analysis. Human Brain Mapping. 2009;30:532–540. doi: 10.1002/hbm.20521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil A, Smith JC, Wangelin BC, Sabatinelli D, Bradley MM, Lang PJ. Electrocortical and electrodermal responses covary as a function of emotional arousal: A single-trial analysis. Psychophysiology. 2008;45:516–523. doi: 10.1111/j.1469-8986.2008.00667.x. [DOI] [PubMed] [Google Scholar]
- *.Keil A, Stolarova M, Moratti S, Ray WJ. Adaptation in human visual cortex as a mechanism for rapid discrimination of aversive stimuli. NeuroImage. 2007;36:472–479. doi: 10.1016/j.neuroimage.2007.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Sauer T, Jedynak A, Skrandies W. Conventional and wavelet coherence applied to sensory-evoked electrical brain activity. IEEE Transactions on Biomedical Engineering. 2006;53:266–272. doi: 10.1109/TBME.2005.862535. [DOI] [PubMed] [Google Scholar]
- *.Kluge C, Bauer M, Leff AP, Heinze HC, Dolan RJ, Driver J. Plasticity of human auditory-evoked fields induced by shock conditioning and contingency reversal. Proceedings of the National Academy of Sciences USA. 2011;108:12545–12550. doi: 10.1073/pnas.1016124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaChaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Human Brain Mapping. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamme VA, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends in Neurosciences. 2000;23:571–579. doi: 10.1016/S0166-2236(00)01657-X. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM. Emotion and the motivational brain. Biological Psychology. 2010;84:437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesse H. Amygdoloid electrical activity during a conditioned response. Paper presented at the International Congress of Electroencephalography and clinical Neurophysiology; Brüssel. 1957. [Google Scholar]
- Lesting J, Narayanan RT, Kluge C, Sangha S, Seidenbecher T, Pape HC. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS One. 2011;6:e21714. doi: 10.1371/journal.pone.0021714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Howard JD, Parrish TB, Gottfried JA. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science. 2008;319:1842–1845. doi: 10.1126/science.1152837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, Grillon C. Generalization of conditioned fear-potentiated startle in humans: Experimental validation and clinical relevance. Behaviour Research and Therapy. 2008;46:678–687. doi: 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Keil A, Ding M. Effects of emotional conditioning on early visual processing: Temporal dynamics revealed by ERP single-trial analyses. Human Brain Mapping. doi: 10.1002/hbm.21259. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signaling of fear memory. Nature Reviews Neuroscience. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Mayr E. Behavior programs and evolutionary strategies. In: Mayr E, editor. Evolution and the Diversity of Life: Selected Essays. Cambridge, MA: Harvard University Press; 1976. [Google Scholar]
- Mechias ML, Etkin A, Kalisch R. A meta-analysis of instructed fear studies: Implications for conscious appraisal of threat. NeuroImage. 2010;49:1760–1768. doi: 10.1016/j.neuroimage.2009.09.040. [DOI] [PubMed] [Google Scholar]
- Miltner WH, Braun C, Arnold M, Witte H, Taub E. Coherence of gamma-band EEG activity as a basis for associative learning. Nature. 1999;397:434–436. doi: 10.1038/17126. [DOI] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychologica (Amst) 2008;127:567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Moratti S, Keil A. Cortical activation during Pavlovian fear conditioning depends on heart rate response patterns: An MEG study. Cognitive Brain Research. 2005;25:459–471. doi: 10.1016/j.cogbrainres.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Moratti S, Keil A. Not what you expect: Experience but not expectancy predicts conditioned responses in human visual and supplementary cortex. Cerebral Cortex. 2009;19:2803–2809. doi: 10.1093/cercor/bhp052. [DOI] [PubMed] [Google Scholar]
- Moratti S, Keil A, Miller GA. Fear but not awareness predicts enhanced sensory processing in fear conditioning. Psychophysiology. 2006;43:216–226. doi: 10.1111/j.1464-8986.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- Moses SN, Bardouille T, Brown TM, Ross B, McIntosh AR. Learning related activation of somatosensory cortex by an auditory stimulus recorded with magnetoencephalography. NeuroImage. 2010;53:275–282. doi: 10.1016/j.neuroimage.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Moses SN, Martin T, Houck JM, Ilmoniemi R, Tesche CD. The C50m response: Conditioned magnetocerebral activity recorded from the human brain. NeuroImage. 2005;27:778–788. doi: 10.1016/j.neuroimage.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Müller MM, Teder W, Hillyard SA. Magnetoencephalographic recording of steady-state visual evoked cortical activity. Brain Topography. 1997;9:163–168. doi: 10.1007/BF01190385. [DOI] [PubMed] [Google Scholar]
- Müller MM, Teder-Salejarvi W, Hillyard SA. The time course of cortical facilitation during cued shifts of spatial attention. Nature Neuroscience. 1998;1:631–634. doi: 10.1038/2865. [DOI] [PubMed] [Google Scholar]
- Ohl FW, Scheich H. Learning-induced dynamic receptive field changes in primary auditory cortex of the unanaesthetized Mongolian gerbil. Journal of Comparative Physiology A. 1997;181:685–696. doi: 10.1007/s003590050150. [DOI] [PubMed] [Google Scholar]
- Olejniczak P. Neurophysiologic basis of EEG. Journal of Clinical Neurophysiology. 2006;23:186–189. doi: 10.1097/01.wnp.0000220079.61973.6c. [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA. Social learning of fear. Nature Neuroscience. 2007;10:1095–1102. doi: 10.1038/nn1968. [DOI] [PubMed] [Google Scholar]
- Padmala S, Pessoa L. Affective learning enhances visual detection and responses in primary visual cortex. The Journal of Neuroscience. 2008;28:6202–6210. doi: 10.1523/JNEUROSCI.1233-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Paré D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiological Reviews. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Collins DR, Pelletier JG. Amygdala oscillations and the consolidation of emotional memories. Trends in Cognitive Sciences. 2002;6:306–314. doi: 10.1016/S1364-6613(02)01924-1. [DOI] [PubMed] [Google Scholar]
- Perruchet P. A pitfall for the expectancy theory of human eyelid conditioning. Pavlovian Journal of Biological Sciences. 1985;20:163–170. doi: 10.1007/BF03003653. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: From a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience. 2010;11:773–783. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Pizzagalli DA, Greischar LL, Davidson RJ. Spatio-temporal dynamics of brain mechanisms in aversive classical conditioning: High-density event-related potential and brain electrical tomography analyses. Neuropsychologia. 2003;41:184–194. doi: 10.1016/S0028-3932(02)00148-3. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19:613–24. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Rajagovindan R, Ding M. From prestimulus alpha oscillation to visual-evoked response: an inverted-U function and its attentional modulation. Journal of Cognitive Neuroscience. 2011;23:1379–1394. doi: 10.1162/jocn.2010.21478. [DOI] [PubMed] [Google Scholar]
- Rauss K, Schwartz S, Pourtois G. Top-down effects on early visual processing in humans: A predictive coding framework. Neuroscience and Biobehavioral Reviews. 2011;35:1237–1253. doi: 10.1016/j.neubiorev.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Regan D. Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine. New York: Elsevier; 1989. [Google Scholar]
- Regan M, Howard R. Fear conditioning, preparedness, and the contingent negative variation. Psychophysiology. 1995;32:208–214. doi: 10.1111/j.1469-8986.1995.tb02950.x. [DOI] [PubMed] [Google Scholar]
- Roelfsema PR. Cortical algorithms for perceptual grouping. Annual Review of Neuroscience. 2006;29:203–227. doi: 10.1146/annurev.neuro.29.051605.112939. [DOI] [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Bradley MM, Costa VC, Keil A. The timing of emotional discrimination in human amygdala and ventral visual cortex. Journal of Neuroscience. 2009;29:14864–8. doi: 10.1523/JNEUROSCI.3278-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. Human fear conditioning and extinction in neuroimaging: A systematic review. PLoS ONE. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman AJ, Maxwell JS, McMenamin BW, Greischar LL, Davidson RJ. Stress potentiates early and attenuates late stages of visual processing. The Journal of Neuroscience. 2011;31:1156–1161. doi: 10.1523/JNEUROSCI.3384-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W, Engel AK, Kreiter AK, Munk MHJ, Neuenschwander S, Roelfsema PR. Neuronal assemblies: Necessity, signature and detectability. Trends in Cognitive Sciences. 1997;1:252–261. doi: 10.1016/S1364-6613(97)01079-6. [DOI] [PubMed] [Google Scholar]
- *.Skrandies W, Jedynak A. Associative learning in humans—conditioning of sensory evoked brain activity. Behavioural Brain Research. 2000;107:1–8. doi: 10.1016/s0166-4328(99)00096-0. [DOI] [PubMed] [Google Scholar]
- Sporns O, Almassy N, Edelman GM. Plasticity in value systems and its role in adaptive behavior. Adaptive Behavior. 2000;8:129–148. [Google Scholar]
- Steinberg C, Bröckelmann A-K, Rehbein M, Dobel C, Junghöfer M. Rapid and highly resolving associative affective learning: Convergent electro- and magnetoencephalographic evidence from vision and audition. Biological Psychology. doi: 10.1016/j.biopsycho.2012.02.009. (In press) [DOI] [PubMed] [Google Scholar]
- *.Steinberg C, Dobel C, Schupp HT, Kissler J, Elling L, Pantev C, Junghöfer M. Rapid and highly resolving: Affective evaluation of olfactorily conditioned faces. Journal of Cognitive Neuroscience. 2012;24:17–27. doi: 10.1162/jocn_a_00067. [DOI] [PubMed] [Google Scholar]
- *.Stolarova M, Keil A, Moratti S. Modulation of the C1 visual event-related component by conditioned stimuli: Evidence for sensory plasticity in early affective perception. Cerebral Cortex. 2006;16:876–887. doi: 10.1093/cercor/bhj031. [DOI] [PubMed] [Google Scholar]
- *.Sugawara M, Kitajima S, Kanoh M. Enhancement and dimunition of the evoked responses to conditioned stimuli during discrimination conditioning. Neuropsychologia. 1977;15:243–248. doi: 10.1016/0028-3932(77)90032-x. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends in Cognitive Sciences. 1999;3:151–162. doi: 10.1016/S1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Tesche CD, Moses SN, Houck JM, Martin T, Hanlon FM, Jackson E, Kicic D. Dynamics of frontal and cerebellar activation during aversive conditioning: An MEG study. International Congress Series. 2007;1300:437–440. doi: 10.1016/j.ics.2007.02.057. [DOI] [Google Scholar]
- Thiel CM, Friston KJ, Dolan RJ. Cholinergic modulation of experience-dependent plasticity in human auditory cortex. Neuron. 2002;35:567–574. doi: 10.1016/S0896-6273(02)00801-2. [DOI] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J. The brainweb: Phase synchronization and large-scale integration. Nature Reviews Neuroscience. 2001;2:229–239. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: Neural mechanisms of emotional attention. Trends in Cognitive Sciences. 2005;9:585–594. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- Wang J, Clementz BA, Keil A. The neural correlates of feature-based selective attention when viewing spatially and temporally overlapping images. Neuropsychologia. 2007;45:1393–1399. doi: 10.1016/j.neuropsychologia.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. The medial geniculate, not the amygdala, as the root of auditory fear conditioning. Hearing Research. 2011;274:61–74. doi: 10.1016/j.heares.2010.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nature Reviews Neuroscience. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz N, Kostadinov B, Dohrmann K, Hartmann T, Schlee W. Tracking short-term auditory cortical plasticity during classical conditioning using frequency-tagged stimuli. Cerebral Cortex. 2007;17:1867–1876. doi: 10.1093/cercor/bhl095. [DOI] [PubMed] [Google Scholar]
- Weymar M, Bradley MM, Hamm AO, Lang PJ. When fear forms memories: Threat of shock and brain potentials during encoding and recognition. Cortex. doi: 10.1016/j.cortex.2012.02.012. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser MJ, Keil A. Temporal trade-off effects in sustained attention: Dynamics in visual cortex predict the target detection performance during distraction. Journal of Neuroscience. 2011;31:7784–7790. doi: 10.1523/JNEUROSCI.5632-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wik G, Elbert T, Fredrikson M, Hoke M, Ross B. Magnetic brain imaging of extinction processes in human classical conditioning. Neuroreport. 1997;8:1789–1792. doi: 10.1097/00001756-199705060-00044. [DOI] [PubMed] [Google Scholar]
- Wik G, Elbert T, Fredrikson M, Hoke M, Ross B. Magnetic imaging in human classical conditioning. Neuroreport. 1996;7:737–740. doi: 10.1097/00001756-199602290-00014. [DOI] [PubMed] [Google Scholar]
- Wong PS, Shevrin H, Williams WJ. Conscious and nonconscious processes: An ERP index of an anticipatory response in a conditioning paradigm using visually masked stimuli. Psychophysiology. 1994;31:87–101. doi: 10.1111/j.1469-8986.1994.tb01028.x. [DOI] [PubMed] [Google Scholar]
- Xu L, Stoica P, Li J, Bressler SL, Shao X, Ding M. ASEO: a method for the simultaneous estimation of single-trial event-related potentials and ongoing brain activities. IEEE Transactions on Biomedical Engineering. 2009;56:111–121. doi: 10.1109/TBME.2008.2008166. [DOI] [PubMed] [Google Scholar]
- Yantis S. Neural basis of selective attention: Cortical sources and targets of attentional modulation. Current Directions in Psychological Science. 2008;17:86–90. doi: 10.1111/j.1467-8721.2008.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003;41:88–123. doi: 10.1016/S0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]