Abstract
AIM: To investigate the hepatoprotective effect of MK615, a Japanese apricot extract, in an animal model, and its clinical therapeutic effect.
METHODS: Wistar rats were administered physiological saline (4 mL/kg) or MK615 solution (4 mL/kg) for 7 d. On the sixth d, acute hepatic injury was induced by administering a single intraperitoneal injection (ip) of D-galactosamine hydrochloride (D-GalN) (600 mg/kg). Plasma levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined, and liver tissues were used for histopathological analysis. Fifty-eight patients with liver disorders [hepatitis C (n = 40), non-alcoholic fatty liver disease (n = 15), and autoimmune liver disease (n = 3)] were orally administered commercially available Misatol ME-containing MK615 (13 g/d) daily for 12 wk. Blood and urine were sampled immediately before and 6 wk, 12 wk, and 16 wk after the start of intake to measure various biochemical parameters. The percentage change in ALT and AST levels after 12 wk from the pre-intake baseline served as a primary endpoint.
RESULTS: D-GalN effectively induced acute hepatic injury in the rats. At 48 h after the ip injection of D-GalN, the plasma levels of ALT (475.6 ± 191.5 IU/L vs 225.3 ± 194.2 IU/L, P < 0.05) and AST (1253.9 ± 223.4 IU/L vs 621.9 ± 478.2 IU/L, P < 0.05) in the MK615 group were significantly lower than the control group. Scattered single cell necrosis, loss of hepatocytes, and extensive inflammatory cell infiltration were observed in hepatic tissue samples collected from the control group. However, these findings were less pronounced in the group receiving MK615. At the end of the clinical study, serum ALT and AST levels were significantly decreased compared with pre-intake baseline levels from 103.5 ± 58.8 IU/L to 71.8 ± 39.3 IU/L (P < 0.05) and from 93.5 ± 55.6 IU/L to 65.5 ± 34.8 IU/L (P < 0.05), respectively. A reduction of ≥ 30% from the pre-study baseline ALT level was observed in 26 (45%) of the 58 patients, while 25 (43%) patients exhibited similar AST level reductions. The chronic hepatitis C group exhibited significant ALT and AST level reductions from 93.4 ± 51.1 IU/L to 64.6 ± 35.1 IU/L (P < 0.05) and from 94.2 ± 55.5 IU/L to 67.2 ± 35.6 IU/L (P < 0.05), respectively. A reduction of ≥ 30% from the pre-study baseline ALT level was observed in 20 (50%) of the 40 patients. ALT levels in both the combined ursodeoxycholic acid (UDCA) treatment and the UDCA uncombined groups were significantly lower after Misatol ME administration. MK615 protected hepatocytes from D-GalN-induced cytotoxicity in rats. Misatol ME decreased elevated ALT and AST levels in patients with liver disorders.
CONCLUSION: These results suggest that MK615 and Misatol ME are promising hepatoprotective agents for patients with liver disorders.
Keywords: Prunus mume, MK615, Liver damage, Hepatitis C, Non-alcoholic fatty liver disease
INTRODUCTION
Japanese apricot (Prunus mume Sieb. et Zucc.), hereinafter referred to as ume, was brought to Japan from China around the eighth century. The flesh of this fruit has been used not only as food but also as medicine. Ishinho, the oldest medical monograph in Japan, which was written in AD 984, indicates that both umeboshi (pickled ume) and ubai (smoke-dried ume) were used as medicines (e.g., as anti-diarrheal agents and for detoxification in food or drug poisoning). Shokokukodenhiho, published in 1817, also refers to the effectiveness of ume extracts. It is thus evident that ume was used extensively as a folk remedy in Japan. Syringaresinol, a lignan in ume, was recently shown to control infection by inhibiting the migration of Helicobacter pylori[1]. MK615, an extract from Japanese apricot, contains triterpenoids such as ursolic acid (UA)[2], oleanolic acid (OA)[2-4], lupeol[2,4], α-amyrin[2], and β-sitosterol[4]. These substances have been shown to exert various biological actions. Reports have described diverse effects, including anti-tumor activity (against tumor cell lines such as those of gastric cancer[5], leukemia[5], breast cancer[4,6], hepatocellular carcinoma[7,8], colon cancer[9], pancreatic cancer[10], and malignant melanoma[11]) and immunopotentiation in experimental animals exposed to X-rays[4]. MK615 was previously reported to inhibit the release of high-mobility group box 1 (HMGB1) from lipopolysaccharide (LPS)-stimulated macrophage-like RAW264.7 cells and to activate the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2), resulting in the induction of heme oxygenase-1 (HO-1). MK615 was also shown to suppress the formation of inflammation-inducing cytokines [tumor necrosis factor α (TNF-α) and interleukin-6 (IL-6)] by inactivating mitogen-activated protein kinases (MAPKs) and the transcription factor nuclear factor-κB (NF-κB)[3,12]. It is thus evident that ume extracts exert anti-inflammatory and antioxidative actions. However, the significance of these actions in the liver has not been adequately clarified.
Given the anti-inflammatory and antioxidative actions of MK615, we investigated the hepatoprotective effects of MK615. In addition, the effects of Misatol ME, a beverage containing MK615 that is approved as a health food product in Japan, were clinically evaluated in patients with liver disorders that included hepatitis C, chronic inflammation of the liver, as well as fatty liver disease, which is closely involved in oxidative stress.
MATERIALS AND METHODS
Effect of MK615 on D-galactosamine hydrochloride-induced acute hepatic injury in rats
Preparation of MK615 solution: MK615 solution was prepared from a condensed extract of ume. In brief, ume were squeezed using a press, and the ume juice was then heated and concentrated 20-fold[5]. The condensed extract was neutralized using NaOH and was then heat-sterilized. The MK615 solution contained the neutral, condensed ume extract.
D-galactosamine hydrochloride-induced hepatic injury in rats: Seven-week-old male Wistar rats (Crlj:WI) weighing 200-240 g were purchased from Charles River Laboratories Japan (Yokohama, Japan). All rats were maintained under controlled temperature and lighting conditions (12/12-h dark/light cycle), and water and standard diet were provided ad libitum in accordance with the institute’s guidelines for care and use of laboratory animals in research.
Acute hepatic injury was induced by administering a single intraperitoneal (ip) injection of D-galactosamine hydrochloride (D-GalN) (600 mg/kg; Wako Pure Chemical Industries, Osaka, Japan). In this study, rats were divided into 3 experimental groups. In group I (the vehicle control group), rats were administered physiological saline (4 mL/kg per day) via gavage for 7 d and injected with D-GalN (ip) 2 h after the sixth oral administration of saline (6 d from the first oral administration). In group II (the MK615 group), rats received MK615 solution (4 mL/kg per day) via gavage for 7 d and were injected with D-GalN (ip) 2 h after the sixth oral administration of MK615 solution. In group III, rats were administered the neutral MK615 solution (4 mL/kg per day) via gavage for 7 d and were injected with saline (ip) 2 h after the sixth oral administration of MK615 solution. group III served as a negative experimental control without D-GalN-induced hepatic injury (Figure 1). Treatments involving oral administration by gavage were conducted between 9:00 and 10:00 AM and ip injections were administered between 11:00 AM and 12:00 noon. All rats were sacrificed by exsanguination under anesthesia 48 h after the ip injection of D-GalN or saline (8 d after the first oral administration). Blood samples from the abdominal aorta were immediately heparinized, and plasma samples were isolated by centrifugation. Plasma samples were frozen and stored at -80 °C until used, and subsequently analyzed to determine the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Liver tissue samples were also obtained from each rat and used for histopathological analysis. The plasma levels of ALT and AST were determined using a commercially available analytical kit (Transaminase CII-Test; Wako Pure Chemical Industries).
Figure 1.

Experiment protocol of D-galactosamine hydrochloride-induced acute hepatic injury in rats.
Evaluation of the effects of MK615 in patients with liver disorder
Subjects: This study involved patients who were definitively diagnosed with a liver disorder at the Jikei University School of Medicine Hospital, the St. Marianna University School of Medicine Hospital, or the Kurihara Clinic between December 2007 and December 2009 and who met the following requirements: (1) ALT level exceeding reference limits when tested within 3 mo before the start of this study, indicating the presence of hepatopathy; (2) serum hepatitis C virus (HCV)-RNA positivity (determined by real-time polymerase chain reaction) in patients with chronic hepatitis C; and (3) presence of fatty liver confirmed by diagnostic imaging in cases of non-alcoholic fatty liver disease (NAFLD). The following patients were excluded from the study: (1) those receiving treatment for liver cirrhosis, hepatocellular carcinoma, or other malignant tumors; (2) patients receiving treatment with Stronger Neo-Minophagen C; (3) those receiving treatment with interferon (IFN); and (4) habitual drinkers (alcohol consumption, > 30 g/d) or occasional heavy drinkers. Concomitant use of drugs or any treatment with antiviral, immunomodulating, or marrow-suppressive activity was prohibited during the study period, but continued use of drugs that had been initiated before the study was permitted. No patients were heavy drinkers. The ethics committee of each participating facility approved the study protocol. Informed consent to participate in the study was obtained in writing from all patients.
Methods: In Japan, MK615 solution is commercially available as Misatol ME (AdaBio Co. Ltd., Takasaki, Japan). For the clinical study, Misatol ME was used as the MK615 solution and was ingested orally every d (2 × 6.5 g packs/d) for 12 wk. Blood and urine were sampled immediately before and 6 wk, 12 wk, and 16 wk after the start of MK615 intake to measure the following parameters: white blood cell (WBC) count, differential leukocyte count, red blood cell (RBC) count, hemoglobin, hematocrit, platelet count, ALT, AST, γ-glutamyl transpeptidase (γ-GTP), alkaline phosphatase (ALP), total protein, albumin, total cholesterol, cholinesterase, and total bilirubin, as well as urinalysis parameters. The percentage change in ALT and AST levels after 12 wk of intake from the pre-intake baseline served as primary and secondary endpoints, respectively. In the analysis of these endpoints, an improvement of ≥ 50% from the pre-intake baseline was regarded “markedly effective”, ≥ 30% was regarded “effective”, ≤ 30% as “ineffective”, and an aggravation of ≥ 30% as “worsened”. The response rate was defined as the percentage of “markedly effective” plus “effective” cases.
Statistical analysis
Data are expressed as mean ± SD. Statistical analyses were performed using Stat View for Windows Version 5.0 (SAS Institute Inc., North Carolina, United States). Differences between 2 groups were analyzed using the Mann-Whitney U test. Comparisons between baseline and each time point were performed using Dunnett’s test. P < 0.05 was considered significant.
RESULTS
The effect of MK615 on D-galactosamine hydrochloride-induced acute hepatic injury in rats
ALT and AST plasma levels in control rats were elevated 48 h after D-GalN induction, with mean values of 475.6 ± 191.5 IU/L (n = 8) and 1253.9 ± 223.4 IU/L (n = 8), respectively. In the MK615 group, the ALT and AST levels were 225.3 ± 194.2 IU/L (n = 9) and 621.9 ± 478.2 IU/L (n = 9), respectively. The levels of ALT and AST in the MK615 group rats were significantly lower than in those of the control group (P = 0.0433 for ALT, P = 0.0124 for AST by Mann-Whitney U test) (Figure 2A and B).
Figure 2.

Effect of MK615 in D-galactosamine hydrochloride-induced acute hepatic injury in rats. A: AST plasma levels; B: ALT plasma levels; C: Control group (liver); D: MK615 group (liver). AST:Aspartate aminotransferase; ALT: Alanine aminotransferase.
Liver tissues were obtained from both control group rats and MK615 group rats at 48 h after D-GalN injection. Scattered single cell necrosis (swollen eosinophilic hepatocytes) and loss of hepatocytes was observed in hepatic tissue samples from the control group. Extensive inflammatory cell infiltration was also noted (Figure 2C). Figure 2D shows that these features of D-GalN-induced hepatic injury were reduced in the treatment group receiving the MK615 solution.
The effects of MK615 in patients with liver disorders
We enrolled 58 patients in this clinical study (mean age, 61.4 ± 12.7 years; range: 29-82 years; 40 men and 18 women). The diagnosis was chronic hepatitis C in 40 patients, NAFLD in 15 patients, and autoimmune liver disease in 3 patients (2 with autoimmune hepatitis and 1 with primary sclerosing cholangitis). Table 1 lists the background variables in relation to the diseases diagnosed.
Table 1.
Background of patients with liver disorders
| Chronic hepatitis C | NAFLD | Autoimmune liver disease | |
| Number | 40 | 15 | 3 |
| Gender (M/F) | 25/15 | 14/1 | 1/2 |
| Age (yr) | 64.4 ± 11.3 | 52.5 ± 13.7 | 65.7 ± 4.0 |
| HCV viral load(10n/mL) | 6.2 ± 0.8 | ||
| ≥ 5log/< 5log/ND | 35/3/2 | ||
| WBC count (/μL) | 4153 ± 994 | 6800 ± 1578 | 3967 ± 723 |
| RBC count (104/μL) | 415 ± 59 | 490 ± 51 | 448 ± 48 |
| Hemoglobin (g/dL) | 13.1 ± 1.8 | 15.5 ± 1.1 | 12.5 ± 1.7 |
| Platelet count (104/μL) | 13.8 ± 5.7 | 20.3 ± 8.4 | 17.2 ± 5.1 |
| AST (IU/L) | 94.2 ± 55.5 | 84.5 ± 50.0 | 129.3 ± 90.5 |
| ALT (IU/L) | 93.4 ± 51.1 | 131.9 ± 72.5 | 96.7 ± 50.1 |
| γ-GTP (IU/L) | 72.9 ± 60.5 | 181.9 ± 197.5 | 120.3 ± 74.2 |
| LDH (IU/L) | 237.8 ± 54.8 | 228.9 ± 44.4 | 270 ± 44.3 |
| ALP (IU/L) | 318.1 ± 116.8 | 303.4 ± 106.8 | 391 ± 293.1 |
| Total bilirubin (mg/dL) | 0.84 ± 0.29 | 0.8 ± 0.44 | 0.67 ± 0.15 |
| Total cholesterol (mg/dL) | 162 ± 35.2 | 188.5 ± 49.1 | 174.7 ± 40.1 |
| Total protein (g/dL) | 7.5 ± 0.6 | 7.7 ± 0.3 | 7.9 ± 0.9 |
| Albumin (g/dL) | 3.9 ± 0.4 | 4.4 ± 0.3 | 3.9 ± 0.9 |
| BUN (mg/dL) | 16.2 ± 4.0 | 13.6 ± 3.2 | 15.3 ± 3.1 |
| Creatinine (mg/dL) | 0.76 ± 0.16 | 0.75 ± 0.1 | 0.62 ± 0.06 |
Data are expressed as the mean ± standard deviation. NAFLD: Non-alcoholic fatty liver disease; ND: Not done; M: Male; F: Female; HCV: Hepatitis C virus; WBC: White blood cell; RBC: Red blood cell; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; γ-GTP: γ guanosine triphosphate; LDH: Lactate dehydrogenase; ALP: Alkaline phosphatase; BUN: Blood urea nitrogen.
Analysis of the entire study population determined that ALT levels had decreased significantly from 103.5 ± 58.8 IU/L before the start of the study to 81.3 ± 45.7 IU/L (P < 0.05) at 6 wk, 71.8 ± 39.3 IU/L (P < 0.05) at 12 wk, and 72.3 ± 40.3 IU/L (P < 0.05) at 16 wk (Figure 3A). AST levels decreased significantly from 93.5 ± 55.6 IU/L before the start of the study to 77.6 ± 47.1 IU/L (P < 0.05) at 6 wk, 65.5 ± 34.8 IU/L (P < 0.05) at 12 wk, and 68.3 ± 37.8 IU/L (P < 0.05) at 16 wk (Figure 3B). A reduction of ≥ 30% from pre-study baseline ALT levels was observed in 26 (45%) of the 58 patients, whereas 25 (43%) patients exhibited a similar reduction in AST levels (Table 2).
Figure 3.
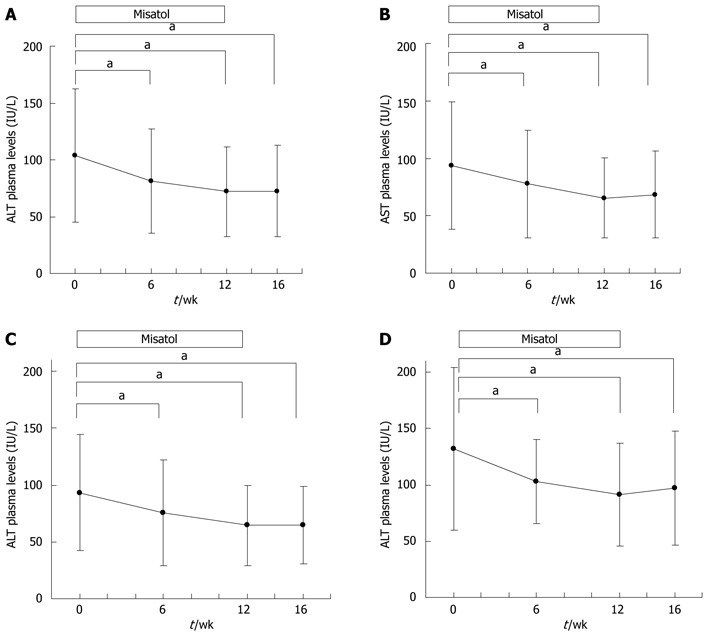
Effects of MK615 in patients with liver disorder, chronic hepatitis C and non-alcoholic fatty liver disease. A: Alanine aminotransferase (ALT); B: Aspartate aminotransferase (AST); C: Chronic hepatitis C group (ALT); D: Non-alcoholic fatty liver disease group (ALT). aP < 0.05 vs 0 wk group. Dunnett’s test.
Table 2.
Response rate of MK615 therapy in patients with liver disorder (%)
| ALT | AST | |
| Chronic hepatitis C | 20/40 (50) | 16/40 (40) |
| NAFLD | 5/15 (33) | 6/15 (40) |
| Autoimmune liver disease | 1/3 (33) | 3/3 (100) |
| Total | 26/58 (45) | 25/58 (43) |
NAFLD: Non-alcoholic fatty liver disease; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase.
When the effects of Misatol ME were analyzed in relation to the disease diagnosed, the chronic hepatitis C group exhibited significant ALT level reductions from the pre-study baseline of 93.4 ± 51.1 IU/L to 75.3 ± 46.6 IU/L (P < 0.05) at 6 wk, 64.6 ± 35.1 IU/L (P < 0.05) at 12 wk, and 64.6 ± 33.8 IU/L (P < 0.05) at 16 wk (Figure 3C). This same group of patients exhibited significant AST level reductions from the pre-study baseline of 94.2 ± 55.5 IU/L to 78.8 ± 49.5 IU/L (P < 0.05) at 6 wk, 67.2 ± 35.6 IU/L (P < 0.05) at 12 wk, and 66.6 ± 33.7 IU/L (P < 0.05) at 16 wk. In the chronic hepatitis C group, a reduction of ≥ 30% from the pre-study baseline ALT level was observed in 20 (50%) of the 40 patients, while 16 (40%) patients exhibited similar AST level reductions (Table 2). Among the patients with chronic hepatitis C, ALT data before the start of test beverage intake (24 wk before starting intake) were available for 32 patients. These patients were subdivided into combined ursodeoxycholic acid (UDCA) treatment (n = 20) (Figure 4A) and UDCA uncombined (n = 12) groups (Figure 4B). In both the combined UDCA treatment and UDCA uncombined groups, ALT levels were significantly lower after the intake of Misatol ME compared with those before intake.
Figure 4.
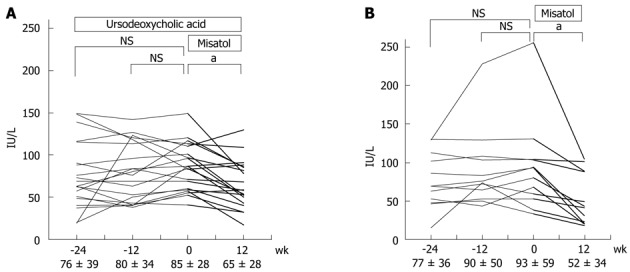
Effects of MK615 in patients with chronic hepatitis C (alanine aminotransferase). A: Misatol was added on ursodeoxycholic acid; B: Only Misatol was used. aP < 0.05 vs 0 wk group. Dunnett’s test. NS: Not significant.
The NAFLD group exhibited significant ALT level reductions from 131.9 ± 72.5 IU/L before the start of the study to 102.8 ± 37.6 IU/L (P < 0.05) at 6 wk, 90.9 ± 45.6 IU/L (P < 0.05) at 12 wk, and 96.9 ± 50.8 IU/L (P < 0.05) at 16 wk (Figure 3D). This group also exhibited significant AST level reductions during the Misatol ME intake period compared with the pre-start baseline level; levels were 84.5 ± 50.0 IU/L before the start of the study, 66.7 ± 24.2 IU/L (P < 0.05) at 6 wk, 58.1 ± 26.0 IU/L (P < 0.05) at 12 wk, and 69.8 ± 41.9 IU/L (NS) at 16 wk. In the NAFLD group, a reduction of ≥ 30% from the pre-study baseline ALT level was observed in 5 (33%) of the 15 patients, whereas similar AST level reductions were observed in 6 (40%) patients (Table 2).
The levels of γ-GTP in the entire study population also decreased significantly after Misatol ME intake compared with pre-intake baseline levels (data not shown).
Table 3 presents the hematological and biochemical data obtained for the clinical study. No change associated with Misatol ME intake was noted in any hematological or biochemical parameter other than in the indicators of liver function, which improved after MK615 intake. An unexplained eruption was observed in 1 patient with NAFLD, which was the only adverse event observed during this study, and was not found to have a causal relationship with the intake of Misatol ME.
Table 3.
Changes of serum level during MK615 therapy in patients with liver disorders
| Before therapy | During therapy 12 wk | P value1 | |
| WBC count (/μL) | 4828 ± 1640 | 4977 ± 1855 | NS |
| RBC count (104/μL) | 436 ± 65 | 435 ± 65 | NS |
| Hemoglobin (g/dL) | 13.7 ± 2.0 | 13.8 ± 1.9 | NS |
| Platelet count (104/μL) | 15.7 ± 7.0 | 15.6 ± 6.6 | NS |
| AST (IU/L) | 94 ± 56 | 66 ± 35 | < 0.05 |
| ALT (IU/L) | 104 ± 59 | 72 ± 39 | < 0.05 |
| γ-GTP (IU/L) | 104 ± 121 | 74 ± 93 | < 0.05 |
| LDH (IU/L) | 237 ± 52 | 227 ± 52 | < 0.05 |
| ALP (IU/L) | 318 ± 124 | 298 ± 126 | < 0.05 |
| Total bilirubin (mg/dL) | 0.8 ± 0.3 | 0.8 ± 0.3 | NS |
| Total cholesterol (mg/dL) | 170 ± 40 | 171 ± 43 | NS |
| Total protein (g/dL) | 7.6 ± 0.6 | 7.6 ± 0.5 | NS |
| Albumin (g/dL) | 4.0 ± 0.5 | 4.1 ± 0.4 | NS |
| BUN (mg/dL) | 15.5 ± 3.9 | 14.7 ± 3.5 | NS |
| Creatinine (mg/dL) | 0.75 ± 0.15 | 0.74 ± 0.15 | NS |
Data are expressed as the mean ± standard deviation.
Dunnett’s test. NS: Not significant; WBC: White blood cell; RBC: Red blood cell; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; γ-GTP: γ-guanosine triphosphate; LDH: Lactate dehydrogenase; ALP: Alkaline phosphatase; BUN: Blood urea nitrogen; NS: Not significant.
DISCUSSION
This is the first study demonstrating that Misatol ME (a beverage containing MK615, an ume extract) lowers blood transaminase levels in patients with liver disorders such as chronic hepatitis C and NAFLD. ume has been used as traditional medicine and food in Japan since ancient times[5]. The clinical effects of ume have been attributed to the biological activity of MK615. MK615 contains triterpenoids such as OA, UA, lupeol, α-amyrin, and β-sitosterol[2-4], and has been shown to exert anti-tumor activity against various tumor cell lines, including those of gastric cancer[5], leukemia[5], breast cancer[4,6], hepatocellular carcinoma[7,8], colorectal cancer[9], pancreatic cancer[10], esophageal cancer[2], and malignant melanoma[11]. The possible mechanisms underlying the anti-tumor activity of MK615 include induction of apoptosis[2,6,9,11], induction of autophagy[9], cell cycle arrest[2,6,7,10], reduced expression of receptors for advanced glycation end products (RAGE) on membrane surfaces of cancer cells[8,11], and immunopotentiation following exposure to X-rays[4]. MK615 inhibits the release of HMGB1 from mouse macrophage-like RAW264.7 cells[3]. This inhibitory activity is mediated by Nrf2 activation and HO-1 induction, suggesting that MK615 possesses antioxidative activity[3]. The authors also previously demonstrated that MK615 suppressed the release of the inflammatory cytokines TNF-α and IL-6 in RAW264.7 cells[12]. This suppression was mediated by the inactivation of MAPKs and NF-κB, thus indicating an anti-inflammatory effect of MK615[12].
The present study reveals that MK615 also exerts hepatoprotective activity in a rat model of D-GalN-induced hepatopathy, given that treatment with MK615 resulted in lower plasma ALT and AST levels accompanied by histological evidence of suppressed destruction of hepatic parenchymal cells when compared with untreated controls. Therefore, MK615 protected the rats from D-GalN-induced hepatopathy.
Previous studies using animal models of D-GalN-induced hepatopathy revealed the activation of MAPKs in the liver[13], suggesting that liver protection might be achieved by the induction of HO-1[14] or by the inhibition of NF-κB in Kupffer cells[15]. In the present study, the effects of MK615 in suppressing MAPK phosphorylation, inducing HO-1, and inhibiting NF-κB activation may have protected the rats from D-GalN-induced hepatopathy.
Additionally, it was shown that the intake of Misatol ME, which contains MK615, lowered the elevated levels of AST and ALT in patients with hepatic impairment. This effect was observed in patients with etiologically different hepatic diseases, i.e., those with hepatitis C and those with NAFLD. No adverse event was associated with the intake of Misatol ME during this study. Furthermore, add-on Misatol ME in combination with UDCA, which had been initiated before the start of Misatol ME intake, resulted in further AST and ALT level reductions in patients with hepatitis C. Moreover, the reduction in ALT levels was also noted in patients who were previously resistant to UDCA therapy.
A major approach to treating HCV infection is antiviral therapy using a combination of IFN and ribavirin[16]. In cases in which the virus cannot be eradicated or IFN is not indicated, it is important to prevent the progression of HCV infection to liver cirrhosis or liver cancer[17]. In practice, the progression of HCV infection to liver fibrosis is accelerated by higher levels of ALT[18-21]. Therefore, when dealing with cases in which virus eradication is difficult, therapeutic interventions that result in lower ALT levels are important for delaying disease progression. In the present study, Misatol ME was shown to significantly reduce ALT levels in patients with chronic hepatitis C, and further reductions in ALT levels were also observed in patients refractory or poorly responsive to UDCA. Given the significance of these findings, Misatol ME warrants further evaluation as a potential treatment for liver disease, including an evaluation of its efficacy during prolonged use. Because Misatol ME is a functional food, conducting the same controlled study to investigate its potential as a medicine was difficult. Nevertheless, the usefulness of Misatol ME as a functional food was clarified. A future investigation is required in which a detailed analysis of the active principal component of Misatol ME should be conducted to elucidate the mechanism underlying its effectiveness as a functional food.
The mechanism underlying the hepatoprotective activity of Misatol ME in patients with chronic hepatitis C appears to involve the anti-inflammatory and antioxidative actions of the MK615 component of Misatol ME. Patients with chronic hepatitis C have high levels of inflammatory cytokines such as TNF-α and IL-6[22-25]. MK615 inhibits the phosphorylation of MAPKs in LPS-stimulated macrophage-like RAW264.7 cells and suppresses the formation of TNF-α and IL-6 by inhibiting NF-κB activation[12]; these findings suggest that the effect of MK615 in suppressing cytokine formation contributes to the suppression of hepatocyte damage in patients with hepatic impairment. Given that Nrf2 activation[26-29] and HO-1 induction[14,30-32] are known to be hepatoprotective, the authors previously demonstrated that MK615 and its component OA activate the transcription factor Nrf2 in LPS-stimulated macrophage-like RAW264.7 cells and induce HO-1, one of the target genes[3]. Whether MK615 also activates Nrf2 and induces HO-1 in clinical cases is unknown. However, it appears highly probable that the antioxidative action of MK615 protects the liver.
MK615 was also effective in patients with NAFLD, reducing serum AST and ALT levels in these patients, as well as in those with hepatitis C. The involvement of factors such as oxidative stress, insulin resistance, and TNF-α in the progression of NAFLD into non-alcoholic steatohepatitis (NASH) has been suggested[33-35]. Diet and exercise are the standard therapies for the treatment of such cases[36,37]. However, the outcomes of these treatments are often unsatisfactory. The effects of MK615 on oxidative stress and insulin resistance in patients with NAFLD are most likely based on the antioxidative effect and the inflammatory cytokine-suppressive action of MK615. Therefore, MK615 therapy may be a promising new means of treating such cases clinically. Obesity is considered a major factor associated with NAFLD. The livers of obese individuals display disturbances in autophagy, with upregulation of autophagy reducing insulin resistance[38]. Since MK615 has been demonstrated to induce autophagy in colorectal carcinoma cell lines[9], this effect is also expected to be useful for treatment[39]. More recently, it was reported that a rat model of NASH exhibited increased expression of RAGE in the liver, suggesting that inhibiting RAGE expression can protect the liver[40]. MK615 reduces the expression of RAGE on the cell membranes of the high-RAGE expression hepatocellular carcinoma cell line HuH7[8]. This RAGE suppression may also play a role in the hepatoprotective effects of Misatol ME.
In the present study, MK615 and Misatol ME, which contains MK615, were shown to potentially alleviate various types of hepatic impairment caused by different factors. MK615 contains multiple triterpenoids (OA, UA, lupeol, etc.); previous in vitro and in vivo studies have shown that these triterpenoids protect the liver from various hepatotoxic substances, such as D-galactosamine, acetaminophen, carbon tetrachloride, and ethanol[27-29,41-47]. As a result of these diverse actions, Misatol ME may exert extensive hepatoprotective effects in patients with hepatic impairments of differing etiologies. Therefore, further studies are required to elucidate the diverse actions of Misatol ME and to assess the significance of its long-term use and its clinical efficacy in suppressing the onset and progression of cancer, as previously demonstrated at experimental level.
COMMENTS
Background
MK615, an extract from Japanese apricot, contains triterpenoids. These substances have been shown to exert various biological actions. In the present study, MK615 (a beverage containing MK615, an ume extract) was found to protect hepatocytes from D-galactosamine hydrochloride-induced cytotoxicity in rats. MK615 decreased the elevated alanine aminotransferase (ALT) and aspartate aminotransferase levels in the patients with liver disorder.
Research frontiers
The mechanism underlying the hepatoprotective activity of MK615 in patients with chronic hepatitis C appears to involve the anti-inflammatory and antioxidative actions of the MK615 component of MK615.
Innovations and breakthroughs
This is the first study to indicate that MK615 lowers blood transaminase levels in patients with liver disorders such as chronic hepatitis C and non-alcoholic fatty liver disease.
Applications
In treating hepatitis C virus infection, therapeutic interventions that result in lower ALT levels are important for delaying disease progression. In the present study, MK615 was shown to significantly reduce the ALT levels in the patients with chronic hepatitis C, and further reductions in ALT levels were observed in the patients refractory or poorly responsive to ursodeoxycholic acid. Given the significance of these findings, MK615 warrants further evaluation as a potential treatment for liver disease, including an evaluation of its efficacy during prolonged use.
Terminology
MK615, an extract from Japanese apricot, contains triterpenoids such as ursolic acid, oleanolic acid, lupeol, α-amyrin, and -sitosterol. Ume extracts exert anti-inflammatory and antioxidative actions.
Peer review
The strongest point of this study should be the histological comparison of the rat livers with galactosamine-induced injury pretreated with MK615 and those not pretreated with MK615. The result is interesting and suggest that MK615 are promising hepatoprotective agents for patients with liver disorders.
Footnotes
Peer reviewer: Marek Hartleb, Department of Gastroenterology and Hepatology, Medical University of Silesia, 70-111 Szczecin, Poland
S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Miyazawa M, Utsunomiya H, Inada K, Yamada T, Okuno Y, Tanaka H, Tatematsu M. Inhibition of Helicobacter pylori motility by (+)-Syringaresinol from unripe Japanese apricot. Biol Pharm Bull. 2006;29:172–173. doi: 10.1248/bpb.29.172. [DOI] [PubMed] [Google Scholar]
- 2.Yamai H, Sawada N, Yoshida T, Seike J, Takizawa H, Kenzaki K, Miyoshi T, Kondo K, Bando Y, Ohnishi Y, et al. Triterpenes augment the inhibitory effects of anticancer drugs on growth of human esophageal carcinoma cells in vitro and suppress experimental metastasis in vivo. Int J Cancer. 2009;125:952–960. doi: 10.1002/ijc.24433. [DOI] [PubMed] [Google Scholar]
- 3.Kawahara K, Hashiguchi T, Masuda K, Saniabadi AR, Kikuchi K, Tancharoen S, Ito T, Miura N, Morimoto Y, Biswas KK, et al. Mechanism of HMGB1 release inhibition from RAW264.7 cells by oleanolic acid in Prunus mume Sieb. et Zucc. Int J Mol Med. 2009;23:615–620. doi: 10.3892/ijmm_00000172. [DOI] [PubMed] [Google Scholar]
- 4.Al-Jahdari WS, Sakurai H, Yoshida Y, Mobaraki A, Suzuki Y, Nakano T. MK615, a prospective anti-proliferative agent, enhances CD4/CD8 ratio after exposure to irradiation. Int J Radiat Biol. 2011;87:81–90. doi: 10.3109/09553002.2010.518202. [DOI] [PubMed] [Google Scholar]
- 5.Adachi M, Suzuki Y, Mizuta T, Osawa T, Adachi T, Osaka K, Suzuki K, Shiojima K, Arai Y, Masuda K, et al. The “Prunus mume Sieb. et Zucc” (Ume) is a Rich Natural Source of Novel Anti-Cancer Substance. Int J Food Prop. 2007;10:375–384. [Google Scholar]
- 6.Nakagawa A, Sawada T, Okada T, Ohsawa T, Adachi M, Kubota K. New antineoplastic agent, MK615, from UME (a Variety of) Japanese apricot inhibits growth of breast cancer cells in vitro. Breast J. 2007;13:44–49. doi: 10.1111/j.1524-4741.2006.00361.x. [DOI] [PubMed] [Google Scholar]
- 7.Okada T, Sawada T, Osawa T, Adachi M, Kubota K. A novel anti-cancer substance, MK615, from ume, a variety of Japanese apricot, inhibits growth of hepatocellular carcinoma cells by suppressing Aurora A kinase activity. Hepatogastroenterology. 2007;54:1770–1774. [PubMed] [Google Scholar]
- 8.Sakuraoka Y, Sawada T, Okada T, Shiraki T, Miura Y, Hiraishi K, Ohsawa T, Adachi M, Takino J, Takeuchi M, et al. MK615 decreases RAGE expression and inhibits TAGE-induced proliferation in hepatocellular carcinoma cells. World J Gastroenterol. 2010;16:5334–5341. doi: 10.3748/wjg.v16.i42.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mori S, Sawada T, Okada T, Ohsawa T, Adachi M, Keiichi K. New anti-proliferative agent, MK615, from Japanese apricot “Prunus mume” induces striking autophagy in colon cancer cells in vitro. World J Gastroenterol. 2007;13:6512–6517. doi: 10.3748/wjg.v13.i48.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada T, Sawada T, Osawa T, Adachi M, Kubota K. MK615 inhibits pancreatic cancer cell growth by dual inhibition of Aurora A and B kinases. World J Gastroenterol. 2008;14:1378–1382. doi: 10.3748/wjg.14.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsushita S, Tada K, Kawahara K, Kawai K, Hashiguchi T, Maruyama I, Kanekura T. Advanced malignant melanoma responds to Prunus mume Sieb. Et Zucc (Ume) extract: Case report and in vitro study. Exp and Ther Med. 2010;1:569–574. doi: 10.3892/etm_00000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morimoto Y, Kikuchi K, Ito T, Tokuda M, Matsuyama T, Noma S, Hashiguchi T, Torii M, Maruyama I, Kawahara K. MK615 attenuates Porphyromonas gingivalis lipopolysaccharide-induced pro-inflammatory cytokine release via MAPK inactivation in murine macrophage-like RAW264.7 cells. Biochem Biophys Res Commun. 2009;389:90–94. doi: 10.1016/j.bbrc.2009.08.103. [DOI] [PubMed] [Google Scholar]
- 13.Nishioka H, Kishioka T, Iida C, Fujii K, Ichi I, Kojo S. Activation of mitogen activated protein kinase (MAPK) during D-galactosamine intoxication in the rat liver. Bioorg Med Chem Lett. 2006;16:3019–3022. doi: 10.1016/j.bmcl.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 14.Wen T, Wu ZM, Liu Y, Tan YF, Ren F, Wu H. Upregulation of heme oxygenase-1 with hemin prevents D-galactosamine and lipopolysaccharide-induced acute hepatic injury in rats. Toxicology. 2007;237:184–193. doi: 10.1016/j.tox.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann F, Sass G, Zillies J, Zahler S, Tiegs G, Hartkorn A, Fuchs S, Wagner J, Winter G, Coester C, et al. A novel technique for selective NF-kappaB inhibition in Kupffer cells: contrary effects in fulminant hepatitis and ischaemia-reperfusion. Gut. 2009;58:1670–1678. doi: 10.1136/gut.2008.165647. [DOI] [PubMed] [Google Scholar]
- 16.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Häussinger D, Diago M, Carosi G, Dhumeaux D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 17.Fassio E. Hepatitis C and hepatocellular carcinoma. Ann Hepatol. 2010;9 Suppl:119–122. [PubMed] [Google Scholar]
- 18.Tarao K, Rino Y, Ohkawa S, Shimizu A, Tamai S, Miyakawa K, Aoki H, Imada T, Shindo K, Okamoto N, et al. Association between high serum alanine aminotransferase levels and more rapid development and higher rate of incidence of hepatocellular carcinoma in patients with hepatitis C virus-associated cirrhosis. Cancer. 1999;86:589–595. doi: 10.1002/(sici)1097-0142(19990815)86:4<589::aid-cncr7>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 19.Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Hayashi K, Honda T, Kuzuya T. Influence of age, sex, and degree of liver fibrosis on the association between serum alanine aminotransferase levels and liver inflammation in patients with chronic hepatitis C. Dig Dis Sci. 2004;49:295–299. doi: 10.1023/b:ddas.0000017454.46589.66. [DOI] [PubMed] [Google Scholar]
- 20.Rino Y, Tarao K, Morinaga S, Ohkawa S, Miyakawa K, Hirokawa S, Masaki T, Tarao N, Yukawa N, Saeki H, et al. Reduction therapy of alanine aminotransferase levels prevent HCC development in patients with HCV-associated cirrhosis. Anticancer Res. 2006;26:2221–2226. [PubMed] [Google Scholar]
- 21.Kurokawa M, Hiramatsu N, Oze T, Mochizuki K, Yakushijin T, Kurashige N, Inoue Y, Igura T, Imanaka K, Yamada A, et al. Effect of interferon alpha-2b plus ribavirin therapy on incidence of hepatocellular carcinoma in patients with chronic hepatitis. Hepatol Res. 2009;39:432–438. doi: 10.1111/j.1872-034X.2008.00477.x. [DOI] [PubMed] [Google Scholar]
- 22.Nelson DR, Lim HL, Marousis CG, Fang JW, Davis GL, Shen L, Urdea MS, Kolberg JA, Lau JY. Activation of tumor necrosis factor-alpha system in chronic hepatitis C virus infection. Dig Dis Sci. 1997;42:2487–2494. doi: 10.1023/a:1018804426724. [DOI] [PubMed] [Google Scholar]
- 23.Gochee PA, Jonsson JR, Clouston AD, Pandeya N, Purdie DM, Powell EE. Steatosis in chronic hepatitis C: association with increased messenger RNA expression of collagen I, tumor necrosis factor-alpha and cytochrome P450 2E1. J Gastroenterol Hepatol. 2003;18:386–392. doi: 10.1046/j.1440-1746.2003.02984.x. [DOI] [PubMed] [Google Scholar]
- 24.Malaguarnera M, Di Fazio I, Romeo MA, Restuccia S, Laurino A, Trovato BA. Elevation of interleukin 6 levels in patients with chronic hepatitis due to hepatitis C virus. J Gastroenterol. 1997;32:211–215. doi: 10.1007/BF02936370. [DOI] [PubMed] [Google Scholar]
- 25.Oyanagi Y, Takahashi T, Matsui S, Takahashi S, Boku S, Takahashi K, Furukawa K, Arai F, Asakura H. Enhanced expression of interleukin-6 in chronic hepatitis C. Liver. 1999;19:464–472. doi: 10.1111/j.1478-3231.1999.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 26.Okada K, Shoda J, Taguchi K, Maher JM, Ishizaki K, Inoue Y, Ohtsuki M, Goto N, Takeda K, Utsunomiya H, et al. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G735–G747. doi: 10.1152/ajpgi.90321.2008. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Wu Q, Lu YF, Pi J. New insights into generalized hepatoprotective effects of oleanolic acid: key roles of metallothionein and Nrf2 induction. Biochem Pharmacol. 2008;76:922–928. doi: 10.1016/j.bcp.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 28.Reisman SA, Aleksunes LM, Klaassen CD. Oleanolic acid activates Nrf2 and protects from acetaminophen hepatotoxicity via Nrf2-dependent and Nrf2-independent processes. Biochem Pharmacol. 2009;77:1273–1282. doi: 10.1016/j.bcp.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X, Ye XL, Liu R, Chen HL, Bai H, Liang X, Zhang XD, Wang Z, Li WL, Hai CX. Antioxidant activities of oleanolic acid in vitro: possible role of Nrf2 and MAP kinases. Chem Biol Interact. 2010;184:328–337. doi: 10.1016/j.cbi.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Z, Wilson AT, Mathahs MM, Wen F, Brown KE, Luxon BA, Schmidt WN. Heme oxygenase-1 suppresses hepatitis C virus replication and increases resistance of hepatocytes to oxidant injury. Hepatology. 2008;48:1430–1439. doi: 10.1002/hep.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roller J, Laschke MW, Scheuer C, Menger MD. Heme oxygenase (HO)-1 protects from lipopolysaccharide (LPS)-mediated liver injury by inhibition of hepatic leukocyte accumulation and improvement of microvascular perfusion. Langenbecks Arch Surg. 2010;395:387–394. doi: 10.1007/s00423-010-0603-8. [DOI] [PubMed] [Google Scholar]
- 32.Immenschuh S, Baumgart-Vogt E, Mueller S. Heme oxygenase-1 and iron in liver inflammation: a complex alliance. Curr Drug Targets. 2010;11:1541–1550. doi: 10.2174/1389450111009011541. [DOI] [PubMed] [Google Scholar]
- 33.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 34.Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 35.Tomita K, Tamiya G, Ando S, Ohsumi K, Chiyo T, Mizutani A, Kitamura N, Toda K, Kaneko T, Horie Y, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki A, Lindor K, St Saver J, Lymp J, Mendes F, Muto A, Okada T, Angulo P. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J Hepatol. 2005;43:1060–1066. doi: 10.1016/j.jhep.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Omagari K, Morikawa S, Nagaoka S, Sadakane Y, Sato M, Hamasaki M, Kato S, Masuda J, Osabe M, Kadota T, et al. Predictive factors for the development or regression of Fatty liver in Japanese adults. J Clin Biochem Nutr. 2009;45:56–67. doi: 10.3164/jcbn.08-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R. Autophagy in liver diseases. J Hepatol. 2010;53:1123–1134. doi: 10.1016/j.jhep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 39.Amir M, Czaja MJ. Autophagy in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. 2011;5:159–166. doi: 10.1586/egh.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J, Zhao MY, Zheng H, Zhang H, Jiang Y. Pentoxifylline alleviates high-fat diet-induced non-alcoholic steatohepatitis and early atherosclerosis in rats by inhibiting AGE and RAGE expression. Acta Pharmacol Sin. 2010;31:1367–1375. doi: 10.1038/aps.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Liu Y, Klaassen CD. Protective effect of oleanolic acid against chemical-induced acute necrotic liver injury in mice. Zhongguo Yao Li Xue Bao. 1995;16:97–102. [PubMed] [Google Scholar]
- 42.Liu Y, Kreppel H, Liu J, Choudhuri S, Klaassen CD. Oleanolic acid protects against cadmium hepatotoxicity by inducing metallothionein. J Pharmacol Exp Ther. 1993;266:400–406. [PubMed] [Google Scholar]
- 43.Liu J, Liu Y, Madhu C, Klaassen CD. Protective effects of oleanolic acid on acetaminophen-induced hepatotoxicity in mice. J Pharmacol Exp Ther. 1993;266:1607–1613. [PubMed] [Google Scholar]
- 44.Tang XH, Gao J, Fang F, Chen J, Xu LZ, Zhao XN, Xu Q. Hepatoprotection of oleanolic acid is related to its inhibition on mitochondrial permeability transition. Am J Chin Med. 2005;33:627–637. doi: 10.1142/S0192415X05003223. [DOI] [PubMed] [Google Scholar]
- 45.Saravanan R, Viswanathan P, Pugalendi KV. Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci. 2006;78:713–718. doi: 10.1016/j.lfs.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 46.Martin-Aragón S, de las Heras B, Sanchez-Reus MI, Benedi J. Pharmacological modification of endogenous antioxidant enzymes by ursolic acid on tetrachloride-induced liver damage in rats and primary cultures of rat hepatocytes. Exp Toxicol Pathol. 2001;53:199–206. doi: 10.1078/0940-2993-00185. [DOI] [PubMed] [Google Scholar]
- 47.Sunitha S, Nagaraj M, Varalakshmi P. Hepatoprotective effect of lupeol and lupeol linoleate on tissue antioxidant defence system in cadmium-induced hepatotoxicity in rats. Fitoterapia. 2001;72:516–523. doi: 10.1016/s0367-326x(01)00259-3. [DOI] [PubMed] [Google Scholar]


