Abstract
AIM: To investigate whether suspected blood indicator (SBI) in capsule endoscopy (CE) is affected by background color and capsule passage velocity.
METHODS: Experimental models of the small intestine constructed from paper in a variety of colors were used to simulate the background colors observed in CE images. The background colors studied included very pale yellow, yellow, very pale magenta, light grayish pink, burnt sienna, and deep and dark brown, and red spots were attached inside them. An endoscopic capsule was manually passed through the models. The rate of detection of the red spots by the SBI was evaluated based on the colors of the models and the capsule passage velocities (0.5 cm/s, 1 cm/s, and 2 cm/s).
RESULTS: The rate of detection of the red spots by the SBI differed significantly according to the background color of the model (P < 0.001). Detection rates were highest for backgrounds of very pale magenta, burnt sienna, and yellow, in that order. They were lowest for backgrounds of dark brown and very pale yellow. The rate of detection of red spots by the SBI tended to decrease at rapid capsule passage velocities (1-2 cm/s) compared to slow velocities (0.5 cm/s) for backgrounds of very pale yellow (P = 0.042), yellow (P = 0.001), very pale magenta (P = 0.002), and burnt sienna (P = 0.001). No significant differences in the rate of detection were observed according to velocity for light grayish pink (P = 0.643) or dark brown (P = 0.396).
CONCLUSION: SBI sensitivity was affected by background color and capsule passage velocity in the models. These findings may facilitate the rapid detection of bleeding lesions by CE.
Keywords: Capsule endoscopy, Suspected blood indicator, Sensitivity, Background color, Passage velocity
INTRODUCTION
Capsule endoscopy (CE) is a useful method for the diagnosis of small bowel diseases, such as gastrointestinal bleeding of an unknown cause[1-7]. However, it is relatively time consuming to examine and interpret the results. To reduce the reading time of this procedure, the RAPID software (Given Imaging Ltd., Duluth, GA, United States) contains a suspected blood identification system, which identifies hemorrhages and suspicious vascular lesions by recognizing red-colored pixels against different colored backgrounds that may be encountered in the small intestine[8].
Reports on the usefulness of the suspected blood indicator (SBI) generally show a low and variable overall sensitivity for lesions, ranging from 20% to 56.4%[8-12]. Positive predictive values are also observed to be variable, from 24% to 90.3%. Therefore, SBI generates false-positive and false-negative results, and its clinical usefulness has not been verified.
Active bleeding is the most important factor affecting SBI sensitivity. The sensitivity increases to a range of 58.3% to 93% in cases of active bleeding. Additionally, the sensitivity of SBI may also be affected by other factors. However, not all of the factors that can affect SBI sensitivity have been fully assessed.
The RAPID software includes a tissue color bar function that represents the average color of the region of interest in the intestine and provides information to help determine the anatomical location of a lesion[13]. When the small intestine is observed via CE, on-screen images are primarily composed of the intestinal mucosa and liquid present in the lumen. The background color behind a lesion may vary according to the color of the small intestinal mucosa, which is affected by patient factors, such as hemoglobin and bilirubin levels. Thus, very pale magenta, which is the normal mucous membrane color, can appear very pale yellow in patients with anemia or burnt sienna or deep brown in patients with jaundice. The background color can also be affected by the presence of intestinal fluid. The color and the degree of transparency of the fluid can vary depending on the presence of bile, debris, stool residue, and blood in the intestine. Therefore, a combination of these elements may produce many different colors that can be presented on screen during CE. The various background colors of lesions may influence the detection of SBI in CE images.
The velocity of the passage of a capsule though the small intestine can vary depending on the presence of underlying disease, such as diabetes and disorders of intestinal peristalsis[14]. Furthermore, the passage velocity can vary in different sections of the small intestine. Capsule movement may be influenced regionally by the gastric emptying time, chronic intestinal motility disorders, small bowel obstruction, intestinal diverticulosis, and other factors[15]. These differences in capsule passage velocity according to the clinical circumstances may also affect SBI sensitivity. It would be useful to examine the different factors that influence the sensitivity of the SBI for shortening the time required to interpret CE-generated video.
Therefore, we investigated the rate of detection of red spots using SBI according to the background colors of the screen image and the capsule passage velocity in experimental models of the small intestine to determine the factors that affect SBI sensitivity in diverse clinical situations.
MATERIALS AND METHODS
Experimental small intestine model
To represent a variety of background colors that may be encountered in clinical situations, experimental small intestine models prepared with seven colors of paper were produced. The colors included very pale yellow, yellow, very pale magenta, light grayish pink, burnt sienna, deep brown, and dark brown (Figure 1). Very pale magenta corresponds to the color of a normal intestinal mucous membrane. Lighter colors can be observed in conditions such as anemia, and darker colors are visible in mucosae with concentrated bile. The Hue and Tone 120-color system that was developed by the Nippon Color and Design Institute was used to define the colors and hues used in this study, and all color saturation levels were uniform[16]. Small bowel models that were 3 cm in diameter and 50 cm in length were constructed, and ten 6-mm red spots were attached inside the models at 5-cm intervals to simulate angiodysplasia.
Figure 1.
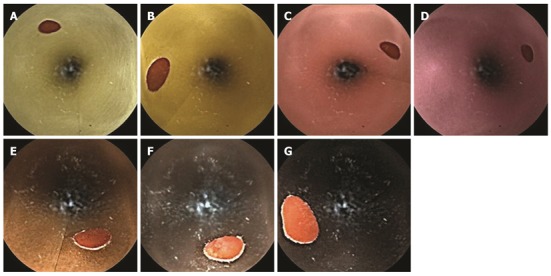
Endoscopic findings on backgrounds of different colors. A: Very pale yellow; B: Yellow; C: Very pale magenta; D: Light grayish pink; E: Burnt sienna; F: Deep brown; G: Dark brown.
Investigation of capsule passage
A CE device (M2A capsule; Given Imaging Ltd., Yoqneam, Israel) was fixed to a solid-line cardiac catheter and passed inside the cylinder of each small intestine model at a constant speed (0.5 cm/s). The screen image from the CE instrument as it passed through model was observed using a real-time viewer (Figure 2). To detect differences in the SBI sensitivity according to the background color, 150 red dots were required to achieve a power of 80% (α = 5%) according to a preliminary pilot study. Therefore, through repetition, the CE instrument was guided past 150 red spots on each of the background colors, and the number of red tags on the scroll bar was examined during the application of SBI.
Figure 2.

Experimental settings used for this study. A capsule endoscopy (CE) device was manually passed through models of the small bowel. The CE screen was observed using a real-time viewer.
Differences in the SBI sensitivity were examined when the CE was passed through the intestinal models at speeds of 0.5 cm/s, 1 cm/s, and 2 cm/s.
Statistical analysis
For all statistical analysis in this study, SPSS version 12.0 (SPSS Inc., Chicago, IL, United States) was used. Continuous variables were expressed as the mean ± SD or as the median. For comparative analysis of continuous variables, the Student’s t-test, the Mann-Whitney U test, or the Kruskall-Wallis test were used depending on the normality of the data. For the comparison of nominal variables, Fisher’s exact test and a χ2 test were used. A post-hoc Bonferroni’s correction was applied for comparative analysis of the groups. All values with a P < 0.05 were considered statistically significant.
RESULTS
Detection according to background color
We compared the number of red tags recognized by the SBI in the small intestine model for each background color. A significant difference was observed in the SBI detection rates on different background colors (P < 0.001, Table 1). The detection rates were highest for very pale magenta, burnt sienna, and yellow, in that order, and they were lowest for dark brown. Thus, the sensitivity of the SBI was the highest for a background of very pale magenta, which represented a normal mucosal color with good bowel cleansing, and the sensitivity was the lowest for dark brown, which represented concentrated bile. For a background of very pale yellow, which represented light bile, the detection rate of the red spots was low.
Table 1.
Detection rates of red spots by suspected blood indicator according to the background color
| Color | Detection rate (%) | Rank |
| Very pale yellow | 16/150 (10.7) | 6 |
| Yellow | 28/150 (18.7) | 3 |
| Very pale magenta | 64/150 (42.7) | 1 |
| Light grayish pink | 19/150 (12.7) | 4 |
| Burnt sienna | 36/150 (24.0) | 2 |
| Deep brown | 18/150 (12.0) | 5 |
| Dark brown | 5/150 (3.3) | 7 |
| Total | 186/1050 (17.7) | P < 0.001 |
The sensitivity of the SBI for different background colors, analyzed with Bonferroni’s correction, showed a significant difference for very pale magenta, for which the detection rate was the highest, compared to that of the other colors. The detection rate with a burnt sienna background was different from that with a very pale yellow and dark brown background, and the detection rate with a yellow background was different from that when a dark brown background was used (Table 2).
Table 2.
Differences in detection rate among background colors using Bonferroni’s correction
| Color | Detection rate (%) | Dark brown | Very pale yellow | Deep brown | Light grayish pink | Yellow | Burnt sienna | Very pale magenta |
| Dark brown | 3.3 | 1 | 0.0130 | 0.0049 | 0.0029 | 0.0000 | 0.0000 | 0.0000 |
| Very pale yellow | 10.7 | 1 | 0.7161 | 0.5901 | 0.0506 | 0.0023 | 0.0000 | |
| Deep brown | 12.0 | 1 | 0.8609 | 0.1097 | 0.0069 | 0.0000 | ||
| Light grayish pink | 12.7 | 1 | 0.1535 | 0.0113 | 0.0000 | |||
| Yellow | 18.7 | 1 | 0.2603 | 0.0000 | ||||
| Burnt sienna | 24.0 | 1 | 0.0006 | |||||
| Very pale magenta | 42.7 | 1 | ||||||
| Adjusted α | 0.002381 |
Detection according to capsule passage velocity
Generally, statistically significant differences were observed in the SBI detection rates of the red spots according to the capsule passage velocity in the small intestine models of some colors (Figure 3). Detection of the red spots by SBI tended to decrease at rapid capsule passage velocities (1-2 cm/s) compared to slow velocities (0.5 cm/s) for very pale yellow (P = 0.042), yellow (P = 0.001), very pale magenta (P = 0.002), and burnt sienna (P = 0.001) backgrounds. No significant differences were observed according to velocity for light grayish pink (P = 0.643) or dark brown (P = 0.396). Thus, differences according to velocity were highly pronounced in models constructed from colors that showed high SBI detection rates, and differences in sensitivity were not present for models with colors that had lower detection rates.
Figure 3.
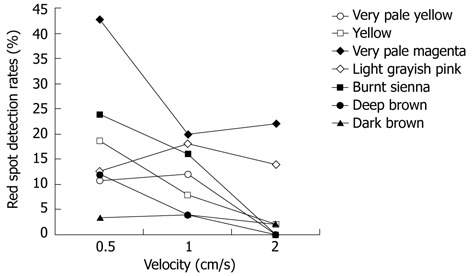
Detection rates of red spots by the suspected blood indicator according to the capsule endoscopy passage velocity.
DISCUSSION
CE was developed to diagnose lesions of the small intestine, and it is a useful method for diagnosing obscure gastrointestinal bleeding, Crohn’s disease, polyposis syndrome, and small bowel tumors[2-4,17-19]. CE has a higher diagnostic rate than radiological diagnosis methods and is less invasive and easier to perform than enteroscopy[2-4,7,20]. However, CE has the disadvantage of requiring time to interpret multiple images[21,22]. Usually, 45 min to two hours of viewing are required, although the reading time can be shortened using the multiview function, which allows the simultaneous observation of several images[22]. To solve these problems, particularly in patients with suspected small intestine bleeding, SBI was developed[10,23,24].
SBI can display frames that depict red zones during CE. The technique is activated in the first image frame of the duodenum and operates only in frames corresponding to the small intestine. The function is activated by an SBI view button within the software, resulting in the automatic identification of red pixels on the screen as red hash marks on the scroll bar. Frame with suspected bleeding lesions can be selected in this way. Therefore, SBI helps physicians to review the video quickly, and bleeding lesions can be found easily. SBI provides supplementary information, but it does not replace the analysis of the video image. All frames recognized using the SBI feature should be later examined by a clinician in more detail.
In the first report on the accuracy of SBI in 24 patients by Liangpunsakul et al[8] in 2003, the sensitivity of the SBI was 25.7% (Table 3). However, when only active bleeding lesions were targeted, the sensitivity increased to 81.2%. In another study by Signorelli et al[12], the sensitivity was only 40.9% in 100 patients, although it increased to 60.9% in the presence of red blood or actively bleeding lesions. In another study, the overall sensitivity was 45%, with 83% for active bleeding[10]. In a study by Buscaglia et al[9], the overall sensitivity was 56.4% and that in patients with active bleeding was 58.3%. According to a Korean report by Kim et al[11], SBI sensitivity of was as low as 20%; however, for actively bleeding lesions, such as angiodysplasia, a much higher sensitivity was observed (93%). SBI sensitivity showed large differences in these studies, ranging from 20% to 56.4%. The positive predictive value also varied from 24% to 90.3%. Even with active bleeding, sensitivity still differed from 58.3% to 93%. Therefore, SBI is thought to be useful as an adjunct method to screen for bleeding lesions using CE. Still, SBI cannot completely replace the reading of the CE-generated video.
Table 3.
Diagnostic accuracy of suspected blood indicator according to the literature (%)
|
Liangpunsakul et al[8] |
Signorelli et al[12] |
D’Halluin et al[10] |
Buscaglia et al[9] |
Kim et al[11] |
||||||
| OL | AB | OL | AB | OL | AB | OL | AB | OL | AB | |
| Sensitivity | 25.7 | 81.2 | 40.9 | 60.9 | 45 | 83 | 56.4 | 58.3 | 20 | 93 |
| PPV | 90.3 | 81.3 | 69.2 | 53.8 | 52 | 23 | 24 | 70 | 44 | 21 |
PPV: Positive predictive value; OL: Overall lesion; AB: Active bleeding.
Cases with no significant bleeding, but in which bleeding was still suspected, are problematic and clinically important[25]. The cause of the variability in SBI sensitivity is likely to be due to the differences in CE images. The presence of bile, debris, stool residue, and blood in the small intestine can vary depending on the patient’s condition and bowel preparation. The intestinal fluid may be yellowish due to bile juices, light grayish pink due to blood, brownish due to stool residue, or dark brownish due to discolored stool. It can also vary according to the concentrations of bile and debris. That is, the intestinal fluid may be very pale yellow or yellow when clear of bile, or it may be burnt sienna or deep brown when thick bile is present. Therefore, the background colors may vary within the same patient depending on the area investigated. Additionally, the color of the intestinal mucosa may be different according to the underlying diseases of patients, such as anemia or jaundice.
The color contrast indicates that the presence of a combination of different colors can influence the detection of of individual colors[26]. The contrast is greatest when a color is combined with its complementary color in the color circle. The color contrast includes contrast related to lightness, hue, saturation, and square. In the color circle, the complementary color of red is blue-green, for which the contrast is greatest. Saturation contrast means that if other vivid colors are located close to the color of interest, the saturation of the color will be reduced. In this study, we investigated whether the ability of SBI to detect red pixels was affected by color contrast, especially in conditions of similar color saturation. We found that the detection rate of red spots was 42.7% for a very pale magenta color, significantly decreasing to 3.3% for dark brown and 10.7% for very pale yellow. A decreased detection rate of 12% was observed when a deep brown background was used. Therefore, significant differences in the SBI detection rates were observed for the different background colors. For colors commonly observed during CE, such as very pale magenta, yellow, and burnt sienna, the SBI sensitivity was similar to that of the clinical data. The sensitivity was decreased for background colors such as dark brown or very pale yellow, which may be infrequently observed in clinical settings. These results suggest that the SBI sensitivity may vary depending on the amount of concentrated bile, food or debris present in the small intestinal lumen and the underlying diseases of patients.
The average time of capsule passage though the intestine is 217 ± 90 min[14]. However, this can vary from 48 min to more than eight hours depending on the underlying disease, such as diabetes, and on intestinal peristalsis. Significant differences in the sensitivity of SBI were observed at different capsule passage velocities in the experimental intestine models: the faster the capsule moved, the lower the sensitivity.
The results of our experiments suggest that SBI has low detection rates at sites that have colors that are significantly different from those of the normal intestine and in the areas where the capsule passed through relatively quickly. That is, even when the SBI technique fails to detect red pixels, a bleeding spot (i.e., angiodysplasia) may exist in those regions. Physicians must examine the images from these sites to detect false-negatives. The different background colors of the screen image can be easily located by using the tissue color bar in the software. Therefore, the results of our study may assist physicians in the interpretation of SBI results and reduce the time required for detection of bleeding sites within the small intestine.
This study may be helpful for improving the diagnostic accuracy of SBI. To improve SBI sensitivity, technical improvements should be made. Additionally, the results of this study suggest clinically correctable factors for SBI. Bowel preparation before CE will improve the SBI sensitivity by minimizing the presence of bile and debris in the small intestine. To confirm this hypothesis, further studies investigating the factors that affect SBI sensitivity in different clinical situations are necessary.
The limitation of this study is that experimental models were used. Although the clinical situations of patients vary, if the capsule passes through an average of 6-7 m of small intestine in four hours, the actual average velocity is approximately 0.04 cm/s. The velocities used in this study of 0.5 cm/s, 1 cm/s, and 2 cm/s are not typically experienced in the clinic. Because it is technically difficult to artificially replicate an average capsule passage velocity, we randomly selected a velocity that was easy to replicate. Therefore, our results are difficult to apply directly to a clinical setting. However, it was shown that the sensitivity of SBI can differ depending on the velocity of the capsule.
In summary, the SBI sensitivity was significantly lower for some background colors on CE images, and the sensitivity decreased with faster capsule passage velocities in experimental models of the small intestine. Therefore, physicians should consider these factors when using SBI in the evaluation of CE images.
COMMENTS
Background
It is relatively time consuming to examine and interpret the results of capsule endoscopy (CE). The suspected blood indicator (SBI) is used for rapid screening of gastrointestinal bleeding, and it automatically recognizes frames that include red-colored pixels. However, reports on the usefulness of a SBI generally show low and variable overall sensitivity for lesions.
Research frontiers
SBI generates false-positive and false-negative results, and its clinical usefulness has not been verified. The color of the small intestinal mucosa and luminal fluid, which are the backgrounds on which lesions are detected, and the capsule passage velocity may vary according to bowel preparation and patient factors.
Innovations and breakthroughs
The detection by SBI differed significantly according to the background colors used (P < 0.001). The sensitivities on very pale magenta, burnt sienna and yellow backgrounds were significantly higher than on the other colors, such as very pale yellow or dark brown. The sensitivity tended to decrease at rapid capsule velocities in experimental models of the small intestine.
Applications
The results of this study may assist physicians in the interpretation of SBI results and reduce the time required for detecting bleeding sites in the small intestine. Physicians must examine the images that contain sites that could have false negative results more carefully than other sites. Bowel preparation before CE is useful for improving SBI sensitivity by minimizing bile or debris in the small intestine.
Peer review
The paper seeks to assess the automatic diagnostic yield using the suspected blood identification system from GIVEN Imaging Ltd., designed to reduce the time spent reviewing CE images in patients with intermediate hemorrhage. The authors developed an experimental method to assess the sensitivity of SBI in different conditions.
Footnotes
Peer reviewer: Dr. Josep M Bordas, Gastroenterology Unit, Hospital Clinic, Llusanes 11-13, 08022 Barcelona, Spain
S- Editor Gou SX L- Editor A E- Editor Zhang DN
References
- 1.Ben Soussan E, Antonietti M, Hervé S, Savoye G, Ramirez S, Lecleire S, Ducrotté P, Lerebours E. Diagnostic yield and therapeutic implications of capsule endoscopy in obscure gastrointestinal bleeding. Gastroenterol Clin Biol. 2004;28:1068–1073. doi: 10.1016/s0399-8320(04)95183-4. [DOI] [PubMed] [Google Scholar]
- 2.Costamagna G, Shah SK, Riccioni ME, Foschia F, Mutignani M, Perri V, Vecchioli A, Brizi MG, Picciocchi A, Marano P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002;123:999–1005. doi: 10.1053/gast.2002.35988. [DOI] [PubMed] [Google Scholar]
- 3.Eliakim R, Fischer D, Suissa A, Yassin K, Katz D, Guttman N, Migdal M. Wireless capsule video endoscopy is a superior diagnostic tool in comparison to barium follow-through and computerized tomography in patients with suspected Crohn’s disease. Eur J Gastroenterol Hepatol. 2003;15:363–367. doi: 10.1097/00042737-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Ell C, Remke S, May A, Helou L, Henrich R, Mayer G. The first prospective controlled trial comparing wireless capsule endoscopy with push enteroscopy in chronic gastrointestinal bleeding. Endoscopy. 2002;34:685–689. doi: 10.1055/s-2002-33446. [DOI] [PubMed] [Google Scholar]
- 5.Ladas SD, Triantafyllou K, Spada C, Riccioni ME, Rey JF, Niv Y, Delvaux M, de Franchis R, Costamagna G. European Society of Gastrointestinal Endoscopy (ESGE): recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy. 2010;42:220–227. doi: 10.1055/s-0029-1243968. [DOI] [PubMed] [Google Scholar]
- 6.Pennazio M, Santucci R, Rondonotti E, Abbiati C, Beccari G, Rossini FP, De Franchis R. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology. 2004;126:643–653. doi: 10.1053/j.gastro.2003.11.057. [DOI] [PubMed] [Google Scholar]
- 7.Saurin JC, Delvaux M, Gaudin JL, Fassler I, Villarejo J, Vahedi K, Bitoun A, Canard JM, Souquet JC, Ponchon T, et al. Diagnostic value of endoscopic capsule in patients with obscure digestive bleeding: blinded comparison with video push-enteroscopy. Endoscopy. 2003;35:576–584. doi: 10.1055/s-2003-40244. [DOI] [PubMed] [Google Scholar]
- 8.Liangpunsakul S, Mays L, Rex DK. Performance of Given suspected blood indicator. Am J Gastroenterol. 2003;98:2676–2678. doi: 10.1111/j.1572-0241.2003.08731.x. [DOI] [PubMed] [Google Scholar]
- 9.Buscaglia JM, Giday SA, Kantsevoy SV, Clarke JO, Magno P, Yong E, Mullin GE. Performance characteristics of the suspected blood indicator feature in capsule endoscopy according to indication for study. Clin Gastroenterol Hepatol. 2008;6:298–301. doi: 10.1016/j.cgh.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 10.D’Halluin PN, Delvaux M, Lapalus MG, Sacher-Huvelin S, Ben Soussan E, Heyries L, Filoche B, Saurin JC, Gay G, Heresbach D. Does the “Suspected Blood Indicator” improve the detection of bleeding lesions by capsule endoscopy? Gastrointest Endosc. 2005;61:243–249. doi: 10.1016/s0016-5107(04)02587-8. [DOI] [PubMed] [Google Scholar]
- 11.Kim JY, Chun HJ, Kim CY, Jang JS, Kwon YD, Park S, Keum B, Seo YS, Kim YS, Jeen YT, et al. The Usefulness of a Suspected Blood Identification System (SBIS) in Capsule Endoscopy according to Various Small Bowel Bleeding Lesions. Korean J Gastrointest Endosc. 2008;37:253–258. [Google Scholar]
- 12.Signorelli C, Villa F, Rondonotti E, Abbiati C, Beccari G, de Franchis R. Sensitivity and specificity of the suspected blood identification system in video capsule enteroscopy. Endoscopy. 2005;37:1170–1173. doi: 10.1055/s-2005-870410. [DOI] [PubMed] [Google Scholar]
- 13.Gerber J, Bergwerk A, Fleischer D. A capsule endoscopy guide for the practicing clinician: technology and troubleshooting. Gastrointest Endosc. 2007;66:1188–1195. doi: 10.1016/j.gie.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 14.de Franchis R, Lewis BS. Procedure and Evaluation. In: Keuchel M, Hagenmueller F, Fleischer DE, editors. Atlas of video capsule endoscopy. 1st ed. Hamburg: Springer; 2006. pp. 8–23. [Google Scholar]
- 15.Buscaglia JM, Kapoor S, Clarke JO, Bucobo JC, Giday SA, Magno P, Yong E, Mullin GE. Enhanced diagnostic yield with prolonged small bowel transit time during capsule endoscopy. Int J Med Sci. 2008;5:303–308. doi: 10.7150/ijms.5.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi S. The aim and method of the color image scale. Color Res Appl. 1981;6:93–107. [Google Scholar]
- 17.Bardan E, Nadler M, Chowers Y, Fidder H, Bar-Meir S. Capsule endoscopy for the evaluation of patients with chronic abdominal pain. Endoscopy. 2003;35:688–689. doi: 10.1055/s-2003-41520. [DOI] [PubMed] [Google Scholar]
- 18.Gupta R, Lakhtakia S, Tandan M, Banerjee R, Ramchandani M, Anuradha S, Ramji C, Rao GV, Pradeep R, Reddy DN. Capsule endoscopy in obscure gastrointestinal bleeding--an Indian experience. Indian J Gastroenterol. 2006;25:188–190. [PubMed] [Google Scholar]
- 19.Herrerías JM, Caunedo A, Rodríguez-Téllez M, Pellicer F, Herrerías JM. Capsule endoscopy in patients with suspected Crohn’s disease and negative endoscopy. Endoscopy. 2003;35:564–568. doi: 10.1055/s-2003-40241. [DOI] [PubMed] [Google Scholar]
- 20.Adler DG, Knipschield M, Gostout C. A prospective comparison of capsule endoscopy and push enteroscopy in patients with GI bleeding of obscure origin. Gastrointest Endosc. 2004;59:492–498. doi: 10.1016/s0016-5107(03)02862-1. [DOI] [PubMed] [Google Scholar]
- 21.Lewis BS. How to read wireless capsule endoscopic images: tips of the trade. Gastrointest Endosc Clin N Am. 2004;14:11–16. doi: 10.1016/j.giec.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Lim YJ, Moon JS, Chang DK, Jang BI, Chun HJ, Choi MG. Korean Society of Gastrointestinal Endoscopy (KSGE) Guidelines for Credentialing and Granting Previleges for Capsule Endoscopy. Korean J Gastrointest Endosc. 2008;37:393–402. [Google Scholar]
- 23.Fischer D, Schreiber R, Levi D, Eliakim R. Capsule endoscopy: the localization system. Gastrointest Endosc Clin N Am. 2004;14:25–31. doi: 10.1016/j.giec.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Melmed GY, Lo SK. Capsule endoscopy: practical applications. Clin Gastroenterol Hepatol. 2005;3:411–422. doi: 10.1016/s1542-3565(05)00019-4. [DOI] [PubMed] [Google Scholar]
- 25.Apostolopoulos P, Papanikolaou IS, Kalantzis N. Capsule endoscopy in obscure occult vs. obscure overt GI bleeding. Gastrointest Endosc. 2005;61:187–188. doi: 10.1016/s0016-5107(04)02465-4. [DOI] [PubMed] [Google Scholar]
- 26.Itten J. The Art of Color: the subjective experience and objective rationale of color. New York: John Wiley and Sons Inc; 1974. [Google Scholar]


