Abstract
AIM: To evaluate the impact of surgical volume on nationwide hospital mortality after pancreaticoduodenectomy (PD) for periampullary tumors in South Korea.
METHODS: Periampullary cancer patients who underwent PD between 2005 and 2008 were analyzed from the database of the Health Insurance Review and Assessment Service of South Korea. A total of 126 hospitals were divided into 5 categories, each similar in terms of surgical volume for each category. We used hospital mortality as a quality indicator, which was defined as death during the hospital stay for PD, and calculated adjusted mortality through multivariate logistic models using several confounder variables.
RESULTS: A total of eligible 4975 patients were enrolled in this study. Average annual surgical volume of hospitals was markedly varied, ranging from 215 PDs in the very-high-volume hospital to < 10 PDs in the very-low-volume hospitals. Admission route, type of medical security, and type of operation were significantly different by surgical volume. The overall hospital mortality was 2.1% and the observed hospital mortality by surgical volume showed statistical difference. Surgical volume, age, and type of operation were independent risk factors for hospital death, and adjusted hospital mortality showed a similar difference between hospitals with observed mortality. The result of the Hosmer-Lemeshow test was 5.76 (P = 0.674), indicating an acceptable appropriateness of our regression model.
CONCLUSION: The higher-volume hospitals showed lower hospital mortality than the lower-volume hospitals after PD in South Korea, which were clarified through the nationwide database.
Keywords: Hospital mortality, Pancreaticoduodenectomy, South Korea, Databases, Factual, Logistic models, Risk factors
INTRODUCTION
Nowadays pancreaticoduodenectomy (PD) is considered a common and feasibly-performed abdominal surgery for periampullary tumors, but it is still a high-risk surgical procedure with potential morbidity and mortality rates. Reducing the morbidity and mortality of this formidable operation is therefore imperative. Although several acceptable results after PD in low-volume hospitals have been reported[1-4], most studies on volume-outcome correlation in performing PD have purported better outcomes in high-volume hospitals, suggesting centralization or regionalization of PD[5-13].
Centralization of PD can be affected by the national healthcare system, information on hospital quality, or patient hospital preference. The Korean healthcare system is based on compulsory insurance of the whole population and free selection of medical care and hospital under stepwise referral to tertiary hospital without regional restriction. Although we have no governmental guidelines for distribution of cancer treatment service or information on hospital quality officially provided by the government, PD tends to be centralized in the large and well-equipped hospitals in Seoul, the capital city of South Korea.
Analyzing the present state of centralization of PD and providing information on hospital quality can help facilitate the government to develop nationwide guidelines and help patients to select good-quality hospitals for themselves. Hospital quality can be representatively appraised through PD-related morbidity or mortality. However, perioperative morbidity may vary from institution to institution according to the criteria or definitions of particular complications, making it difficult to obtain reliable nationwide morbidity data. Therefore, more definitive and objective data, such as standardized or risk-adjusted mortality rates, are requisite as an indicator for hospital quality.
With the implication toward public reporting, we performed this study to evaluate the impact of surgical volume on nationwide hospital mortality after PD for periampullary tumors and to validate the utility of surgical volume as a quality indicator of hospitals in South Korea. To date, just a few nationwide studies have been carried out to assess the effect of surgical volume on outcomes after PD. We hope the present study can contribute to nationwide evidence for the volume-outcome correlation in performing PD.
MATERIALS AND METHODS
Data sources and subjects
Data were obtained from the Health Insurance Review and Assessment Service (HIRA), whose database was constructed through the process of medical fee claims. After providing medical treatment, the medical institutions submit treatment details and file medical fee claims through an electronic billing system in the form of diskettes, compact discs or electronic data interchange (EDI). The EDI system, which was developed to review medical fees electronically by converting claim information into an EDI file and automatically reviewing items such as medical and drug fees within the software, occupies 99.7% of all medical claims in South Korea. Each claim contains information on demographic data, diagnoses, procedures, comorbidity, route of admission, length of stay, discharge status, source of payment, hospital charges, etc. Diagnostic data were coded using the International Classification of Diseases, 10th Revision (ICD-10), and procedural data were coded using the health insurance claims code developed by the Ministry of Health and Welfare. From the HIRA database, we obtained anonymous data on patients who underwent PD for periampullary cancers during the period from January 2005 to December 2008. Only the primary cancers were included, and cancers originating at the adjacent organs such as the colon, stomach, or gallbladder were excluded. Benign diseases, including trauma, were also excluded. Additionally, patients with other combined operations which could affect the surgical outcomes such as hepatectomy, gastrectomy, or colectomy were not analyzed.
Categorization of hospitals
A total of 126 hospitals performed at least one PD from 2005 to 2008 in South Korea. Four-year surgical volume of each hospital showed a large gap, ranging from 1 to 861. Therefore, we divided the hospitals into quintiles; very-low, low, medium, high, and very-high categories. For this fractionation, the hospitals were sorted in descending order by total surgical volume, and cut-off points were decided to categorize hospitals into five similarly-sized groups.
Assessment of outcome
For clarification of volume-outcome correlation of PD, we adopted hospital mortality as an outcome indicator, which was defined as death during the hospital stay for PD. Hospital mortality had to be calculated in the form of adjusted mortality, because the hospital and patient characteristics of each category were different. For this adjustment of hospital mortality, we selected several risk factors for death from the HIRA database and the literature; age, sex, admission route as a surrogate for patient’s general condition [outpatient department vs emergency room (ER)], Charlson comorbidity score[14] as an index for current comorbid status (≥ 3 vs < 3), type of medical security as a surrogate for socioeconomic status (medical aid for the destitute vs health insurance for the others), and operation type [classical pancreaticoduodenectomy (CPD) vs pylorus-preserving pancreaticoduodenectomy (PPPD)]. However, we were unable to obtain more detailed information on preoperative treatment, tumor node metastasis stage, PD-specific complications, or radicality of PD from the HIRA database, which was a major limitation of the nationwide data. Observed mortality was first obtained according to surgical volume and patient characteristics. Risk-adjusted mortality was then calculated through: (observed hospital deaths/predicted hospital deaths) × overall mortality rate. The predicted mortality of each category could be produced by summing the probability of death of each patient in that category, and the probability of death was determined by adjustment with significant confounder variables validated through multivariate logistic regression.
Statistical analysis
Statistical analysis was carried out using the SAS statistical package version 9.1 (SAS System for Windows, SAS Institute, Cary, NC, United States). Descriptive statistics were used to obtain patient characteristics and hospital mortality in each surgical volume category. Continuous variables were compared with Student’s t test for two groups and with analysis of variance for multiple groups. Categorical variables were assessed with χ2 tests. Multivariate logistic regression was used to assess the correlation between surgical volume and hospital mortality, with risk-adjusted mortality as the dependent variable and surgical volume and other risk factors for death as covariates. The result of the Hosmer-Lemeshow test was 5.76 (P = 0.674), indicating an acceptable appropriateness of our regression model. Statistical significance was set at P values < 0.05.
RESULTS
Hospital and patient characteristics by surgical volume
Of the patients who underwent PD for periampullary cancers during the period from 2005 to 2008 in South Korea, a total of 4975 patients were eligible for the inclusion criteria and enrolled in this study. Pancreatic cancer (1800, 36.2%) occupied the most common indication for PD and was followed by common bile duct cancer (1433, 28.8%), ampulla of Vater cancer (1280, 25.7%), duodenal cancer (238, 4.8%), and other periampullary cancers (227, 4.5%).
PD patients of each category were arranged to be similar in number; 1021 (20.5%) in the very-low-volume hospitals, 1005 (20.2%) in the low-volume hospitals, 1020 (20.5%) in the medium-volume hospitals, 1068 (21.5%) in the high-volume hospitals and 861 (17.3%) in the very-high-volume hospitals. Only one hospital corresponded to the very-high-volume hospital, whereas as many as 92 (73.0%) hospitals belonged to the very-low-volume hospitals. Average annual surgical volume of the total 126 participating hospitals was markedly varied; 215 PDs in the very-high-volume hospital and fewer than 10 PDs in the very-low-volume hospitals (Table 1). Even worse, 33 of the very-low-volume hospitals performed less than 1 PD per year.
Table 1.
Patient and hospital characteristics by surgical volume
| Characteristics | Very-low (n = 92) | Low (n = 20) | Medium (n = 10) | High (n = 3) | Very-high (n = 1) | P value |
| Age (mean ± SD) (yr) | 62.2 ± 10.7 | 62.1 ± 10.2 | 62.1 ± 10.5 | 61.2 ± 9.9 | 59.9 ± 10.3 | 0.077 |
| Sex ratio (M:F) | 1.5 | 1.3 | 1.5 | 1.6 | 1.6 | 0.282 |
| Admission route | < 0.001 | |||||
| Outpatient department (%) | 803 (78.6) | 774 (77.0) | 788 (77.3) | 901 (84.4) | 593 (68.9) | |
| Emergency room (%) | 218 (21.4) | 231 (23.0) | 232 (22.7) | 167 (15.6) | 268 (31.1) | |
| Charlson comorbidity score | 0.193 | |||||
| < 3 (%) | 683 (66.9) | 651 (64.8) | 680 (66.7) | 667 (62.5) | 554 (64.3) | |
| ≥ 3 (%) | 338 (33.1) | 354 (35.2) | 340 (33.3) | 401 (37.5) | 307 (35.7) | |
| Type of medical security | < 0.001 | |||||
| Health insurance (%) | 919 (90.0) | 925 (92.0) | 955 (93.6) | 1048 (98.1) | 841 (97.7) | |
| Medical aid (%) | 102 (10.0) | 80 (8.0) | 65 (6.4) | 20 (1.9) | 20 (2.3) | |
| Type of operation | < 0.001 | |||||
| CPD (%) | 604 (59.2) | 420 (41.8) | 361 (35.4) | 325 (30.4) | 301 (35.0) | |
| PPPD (%) | 417 (40.8) | 585 (58.2) | 659 (64.6) | 743 (69.6) | 560 (65.0) | |
| Average annual volume | < 10 | 10-18 | 19-35 | 54-111 | 215 | 9.91 |
Denotes average annual surgical volume of all hospitals. SD: Standard deviation; M: Male; F: Female; CPD: Classical pancreaticoduodenectomy; PPPD: Pylorus-preserving pancreaticoduodenectomy.
The mean age of the PD patients was 61.5 years and there were 1.5 times more males than females. Admission via ER was significantly more frequent and the percentage of payment by medical aid was significantly lower in the higher-volume hospitals (both P < 0.001). However, the Charlson comorbidity score didn’t show any association with the surgical volume category. PPPD was performed in 59.6% (2964/4975) and this proportion increased with an increase in surgical volume, ranging from 40.8% in the very-low-volume hospitals to 69.6% in the high-volume hospitals (P < 0.001, Table 1).
Observed hospital mortality
The overall hospital mortality rate after PD was 2.1% during the study period. The observed hospital mortality rates were higher in lower-volume hospitals, the medical aid group, and CPD patients (P < 0.001, P = 0.015, P < 0.001, respectively). The mean age of both the mortality and survival group was also significantly different (66.0 vs 61.4, P < 0.001). Other risk factors didn’t affect hospital mortality (Table 2).
Table 2.
Observed hospital mortality by patient characteristics
| Characteristics | No. of patients | Mortality (%) | P value |
| Age | < 0.001 | ||
| Live (%) | 4869 (97.9) | 61.4 (10.4)1 | |
| Dead (%) | 106 (2.1%) | 66.0 (8.6)1 | |
| Sex | 0.193 | ||
| Male (%) | 2 989 (60.1) | 1.9 | |
| Female (%) | 1 986 (39.9) | 2.5 | |
| Admission route | 0.291 | ||
| Outpatient department (%) | 3 859 (77.6) | 2.3 | |
| Emergency room (%) | 1 116 (22.4) | 1.7 | |
| Charlson comorbidity score | 0.291 | ||
| < 3 (%) | 3 235 (65.0) | 2.0 | |
| ≥ 3 (%) | 1 740 (35.0) | 2.4 | |
| Type of medical security | 0.015 | ||
| Health insurance (%) | 4 688 (94.2) | 2.0 | |
| Medical aid (%) | 287 (5.8) | 4.9 | |
| Operation type | < 0.001 | ||
| CPD (%) | 2 011 (40.4) | 3.0 | |
| PPPD (%) | 2 964 (59.6) | 1.5 |
Indicates average age (standard deviation) of survival group and mortality group. No: Number; CPD: Classical pancreaticoduodenectomy; PPPD: Pylorus-preserving pancreaticoduodenectomy.
Adjusted hospital mortality
All the risk factors with P < 0.25 in univariate logistic regression were included for multivariate analysis. These were: age, sex, type of medical security and operation type. Table 3 shows the results of multivariate logistic regression. Hospital mortality had a significant correlation with surgical volume (P < 0.001). Although there was no statistical difference in hospital mortality between the very-low-volume and medium-volume hospitals, or just a small statistical difference between the very-low-volume and low-volume hospitals, adjusted odds ratios (ORs) for hospital death of the high-volume and very-high-volume hospitals were significant lower than those of the very-low-volume hospitals (both P < 0.001). Age and operation type were the only significant confounder variables (P < 0.001 and P = 0.025, respectively) and were used for adjustment to produce the predicted mortality. Risk-adjusted hospital mortality rates according to surgical volume are depicted in Figure 1, ranging from 0.5% to 3.9%.
Table 3.
Logistic regression for hospital mortality
| Characteristics | Odds ratio (95% CI) | P value |
| Surgical volume | ||
| Very-low | 1.00 | |
| Low | 0.59 (0.36-0.98) | 0.042 |
| Medium | 0.61 (0.37-1.01) | 0.056 |
| High | 0.13 (0.05-0.32) | < 0.001 |
| Very-high | 0.16 (0.06-0.41) | < 0.001 |
| Age1 | 1.04 (1.02-1.06) | < 0.001 |
| Sex | 0.329 | |
| Male | 1.00 | |
| Female | 1.22 (0.82-1.80) | |
| Type of medical security | 0.120 | |
| Health insurance | 1.00 | |
| Medical aid | 1.60 (0.89-2.88) | |
| Operation type | 0.025 | |
| CPD | 1.00 | |
| PPPD | 0.64 (0.43-0.96) |
Per year increase in age. CI: Confidence Interval; CPD: Classical pancreaticoduodenectomy; PPPD: Pylorus-preserving pancreaticoduodenectomy.
Figure 1.
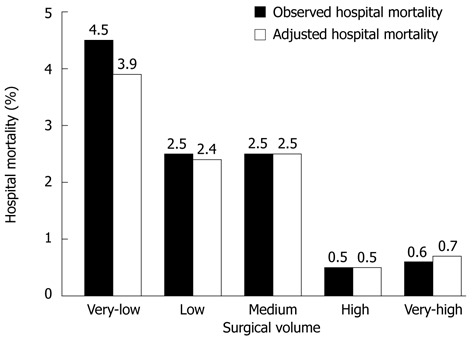
Observed and adjusted hospital mortality according to surgical volume. Observed hospital mortality rates showed a significant decreasing trend as surgical volume increased (P < 0.001). Adjusted hospital mortality rates were then calculated through multivariate logistic regression with hospital mortality rates as a dependent variable and age and operation type as significant confounder variables.
DISCUSSION
We used the nationwide database to validate the association of surgical volume and hospital mortality. Nationwide data has the power to yield reliable and objective results by itself, mainly due to a large number of subjects and the least influence of selection bias. Of the nationwide reports, however, there have been just a few studies on only PD[5,8,10,11,13,15,16], whereas the majority included all types of pancreatic resections, including PD[17-21]. It is known that PD is different from left-sided pancreatectomy, at least in postoperative morbidity and mortality. Nationwide hospital mortality results on PD would be reliable evidence and a base to support governmental or administrative guidelines for the establishment of policy on medical service, to select high-quality hospitals for patients, and to compare quality of care providers within and beyond South Korea.
The inverse relationship between surgical volume and hospital mortality in performing PD has been clarified in South Korea through this study. The risk-adjusted hospital mortality rates of the high-volume and very-high-volume hospitals were very low (0.5% and 0.7%) compared to those of the very-low-volume, low-volume and medium-volume hospitals (3.9%, 2.4% and 2.5%, respectively). This difference in the adjusted hospital mortality rates was found to be similar to that in the observed hospital mortality rates (0.5% and 0.6% vs 4.5%, 2.5% and 2.5%). In other words, the ORs for hospital mortality in the low-volume and medium-volume hospitals vs the very-low-volume hospitals were around 0.6 (a 40% decrease in the probability of hospital mortality), whereas the ORs in the high-volume and very-high-volume hospitals vs the very-low-volume hospitals were as low as 0.13 and 0.16 (a decrease of more than 80%).
The overall hospital mortality rate after PD in South Korea between 2005 and 2008 was 2.1%. This value was much lower than mortality rates from other statewide[12,22,23] or nationwide[5,8,10,11,13,15] databases, but a slightly higher than those from high-volume single institutions[7,24-27]. Hospital mortality after PD is affected by many independent variables. Significant risk factors for hospital mortality, other than surgical volume as mentioned above, were age and operation type in this study. Type of medical security showed statistical significance in univariate analysis but not in multivariate analysis. Significant confounder variables for hospital mortality in PD were similar within the literature. Age, gender, body mass index, and urgent admission were advocated in other studies[8,11,28].
Regionalization and centralization in severe medical illnesses and high-risk surgical procedures are worldwide trends which may occur naturally by patients’ free selection, or intentionally by governmental policy across the world. About 40% of PDs were undertaken in the high-volume and very-high-volume hospitals, or the big 4 hospitals in South Korea. Again, more than 50% of PDs were performed only in 14 (11.1%) out of 126 hospitals. With these data, South Korea can be said to show a typical example of centralization for PD. Despite trends toward regionalization of care[5,9,12,13,29], not a few PDs were safely performed in community hospitals by surgeons with varying degrees of experience[28], as evidenced by a comparably low mortality rate and a high one-year survival rate[1-4]. About 20% of patients still received PD in as many as 92 very-low-volume hospitals (73.0%) performing fewer than 10 PDs per year in South Korea. There could be several community hospitals with comparably low mortality rates, because the overall mortality rate of the very-low-volume hospitals in South Korea was not so high.
Hospital quality can be assessed with diverse outcome indicators. These are divided into short-term and long-term outcomes, with short-term outcomes including mortality, morbidity, hospital cost, and postoperative hospital stay. Survival outcome represents the long-term outcome. Considering that PD is a very complicated surgical procedure, postoperative morbidity results are very useful in comparing short-term outcomes between hospitals. However, our nationwide study didn’t include morbidity results due to difficulty in data collection through the medical fee claims system of the HIRA. Additional drawbacks of this study were as follows; no long-term outcome, total hospital stay not postoperative hospital stay, no pathology-related data, or no surgeon volume. Of these drawbacks, surgeon volume is worthy of note. Surgeon volume[19,22,28,30-32] or experience[30], could be a more exact and detailed indicator of hospital outcome after PD than total hospital surgical volume. The HIRA database does not yet have the surgeon identifier which was used in the study by Eppsteiner et al[33]; therefore we couldn’t analyze the correlation of surgeon volume and hospital outcome. In one study[30], an experienced surgeon was defined as one performing 50 or more PDs, and experienced surgeons had comparable outcomes irrespective of annual volume. Learning curves also projected that less experienced surgeons would achieve morbidity and mortality rates equivalent to those of experienced surgeons when they reached 20 and 60 PDs, respectively. In other studies, a high-volume surgeon was defined as having an average of 10 or more PDs per year[28], or 5 or more PDs per year[33]. Like stratification of hospitals by surgical volume, defining experienced or high-volume surgeons is difficult and varies according to the medical situation or surgeon training system of each country.
Quality indicators other than surgical volume have been introduced. Some researchers focused on the importance of surgery residency training programs, reporting a greater impact on outcomes after PD than hospital volume or surgeon frequency[34]. Similarly, Joseph et al[35] put emphasis on hospital clinical resources, such as the Leapfrog Safe Practice Score, HealthGrades 5-star rating, or interventional radiology services, as well as surgical volume for lower operative mortality after PD. A pathologic indicator was also proposed by reporting that patients undergoing PD at low-volume centers were more likely to have margin-positive resections[36].
Categorization of hospitals was carried out by two methods in the previous reports; by cut-off points of hospital similar in size in each category like our study and by cut-off points of surgical volume that were arbitrarily determined. These cutoff points of surgical volume were varying according to the medical situation and total surgical volume of states or countries. For example, Birkmeyer et al[10] defined > 5/year for high-volume hospitals in United States between 1992 and 1995, and Topal et al[5] did > 10/year for high-volume and > 20/year for very-high-volume hospitals in Belgium between 2000 and 2004, while Balzano et al[11] used a cut-off point of 14-51/year for high-volume hospitals and 89-104/year for very-high-volume hospitals in Italy in 2003. For this stratification of hospitals by surgical volume, the size of each category was uneven according to the cut-off points. In our study, the high-volume hospitals corresponded to 54-111/year and the very-high-volume hospital did 215/year between 2005 and 2008, as a result of dividing hospitals into 5 similar-sized categories. In addition, quintile division[5,17] was rarely used in the previous studies, with the majority being performed in three or four stratifications. Accordingly it is not easy to reach an international consent on established stratification of hospitals by surgical volume.
In conclusion, the nationwide database clarified the impact of surgical volume on hospital mortality after PD in South Korea. The higher-volume hospitals had a better mortality outcome than the lower-volume hospitals. PD performance showed centralization in South Korea and the overall hospital mortality rate was comparable among countries. Further nationwide studies with surgeon volume, morbidity data, and long-term survival results after PD are warranted for more detailed information, and for a domestic and international comparison.
COMMENTS
Background
Pancreaticoduodenectomy (PD), performed for various diseases around the duodenal ampulla, is one of the high-risk surgical procedures which tend to be centralized in high-volume hospitals across the world. Previous studies have reported that high-volume hospitals show better surgical outcomes than low-volume hospitals. Up to now, however, there have been no reports on surgical outcomes after PD, or whether surgical volume is a good quality indicator of care providers in South Korea.
Research frontiers
The correlation of surgical volume and hospital outcome after PD is well known between individual institutions or in a limited area. For research into clarifying this relationship, comprehensive results from nationwide databases are important for patients and government, as well as medical personnel.
Innovations and breakthroughs
Although there have been many studies on the relationship between surgical volume and hospital outcome after all types of pancreatic surgery from nationwide databases or after only PD from databases of institutions or states, nationwide results on only PD, which is still an operation with high morbidity and mortality rates, are very rare. Moreover, this is the first study of its type performed in South Korea and having a recent study period of four years.
Applications
With the information on the relationship between surgical volume and nationwide hospital mortality after PD in South Korea, reference guidelines for establishing medical policy and selecting good-quality hospitals could be supported.
Peer review
This is a frontier study on the relationship between surgical volume and hospital outcome after one type of major surgery in South Korea. This is a well-analyzed and clear manuscript that describes the impact of hospital volume on mortality following pancreaticoduodenectomy.
Footnotes
Peer reviewers: Dr. Yasuhiro Fujino, Department of Surgery, Hyogo Cancer Center, 13-70 Kitaoji-cho, Akashi 673-8558, Japan; Dr. Tsutomu Fujii, Department of Surgery II, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 4668550, Japan
S- Editor Gou SX L- Editor Rutherford A E- Editor Zhang DN
References
- 1.Afsari A, Zhandoug Z, Young S, Ferguson L, Silapaswan S, Mittal V. Outcome analysis of pancreaticoduodenectomy at a community hospital. Am Surg. 2002;68:281–284. [PubMed] [Google Scholar]
- 2.Schwartz GS, Swan RZ, Ruangvoravat L, Attiyeh FF. Morbidity and mortality after hepatic and pancreatic resections: results from one surgeon at a low-volume urban hospital over thirty years. Am J Surg. 2011;201:438–444. doi: 10.1016/j.amjsurg.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Chew DK, Attiyeh FF. Experience with the Whipple procedure (pancreaticoduodenectomy) in a university-affiliated community hospital. Am J Surg. 1997;174:312–315. doi: 10.1016/s0002-9610(97)00110-4. [DOI] [PubMed] [Google Scholar]
- 4.Akhtar K, Perricone V, Chang D, Watson RJ. Experience of pancreaticoduodenectomy in a district general hospital. Br J Surg. 2000;87:362–373. doi: 10.1046/j.1365-2168.2000.01383-15.x. [DOI] [PubMed] [Google Scholar]
- 5.Topal B, Van de Sande S, Fieuws S, Penninckx F. Effect of centralization of pancreaticoduodenectomy on nationwide hospital mortality and length of stay. Br J Surg. 2007;94:1377–1381. doi: 10.1002/bjs.5861. [DOI] [PubMed] [Google Scholar]
- 6.Birkmeyer JD, Warshaw AL, Finlayson SR, Grove MR, Tosteson AN. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery. 1999;126:178–183. [PubMed] [Google Scholar]
- 7.Mukherjee S, Kocher HM, Hutchins RR, Bhattacharya S, Abraham AT. Impact of hospital volume on outcomes for pancreaticoduodenectomy: a single UK HPB centre experience. Eur J Surg Oncol. 2009;35:734–738. doi: 10.1016/j.ejso.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Kotwall CA, Maxwell JG, Brinker CC, Koch GG, Covington DL. National estimates of mortality rates for radical pancreaticoduodenectomy in 25,000 patients. Ann Surg Oncol. 2002;9:847–854. doi: 10.1007/BF02557520. [DOI] [PubMed] [Google Scholar]
- 9.Gordon TA, Burleyson GP, Tielsch JM, Cameron JL. The effects of regionalization on cost and outcome for one general high-risk surgical procedure. Ann Surg. 1995;221:43–49. doi: 10.1097/00000658-199501000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birkmeyer JD, Finlayson SR, Tosteson AN, Sharp SM, Warshaw AL, Fisher ES. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery. 1999;125:250–256. [PubMed] [Google Scholar]
- 11.Balzano G, Zerbi A, Capretti G, Rocchetti S, Capitanio V, Di Carlo V. Effect of hospital volume on outcome of pancreaticoduodenectomy in Italy. Br J Surg. 2008;95:357–362. doi: 10.1002/bjs.5982. [DOI] [PubMed] [Google Scholar]
- 12.Gordon TA, Bowman HM, Tielsch JM, Bass EB, Burleyson GP, Cameron JL. Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Ann Surg. 1998;228:71–78. doi: 10.1097/00000658-199807000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, Obertop H. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232:786–795. doi: 10.1097/00000658-200012000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.van Heek NT, Kuhlmann KF, Scholten RJ, de Castro SM, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781–788. doi: 10.1097/01.sla.0000188462.00249.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nordback L, Parviainen M, Räty S, Kuivanen H, Sand J. Resection of the head of the pancreas in Finland: effects of hospital and surgeon on short-term and long-term results. Scand J Gastroenterol. 2002;37:1454–1460. doi: 10.1080/003655202762671350. [DOI] [PubMed] [Google Scholar]
- 17.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, Welch HG, Wennberg DE. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 18.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003;138:721–725; discussion 726. doi: 10.1001/archsurg.138.7.721. [DOI] [PubMed] [Google Scholar]
- 19.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 20.McPhee JT, Hill JS, Whalen GF, Zayaruzny M, Litwin DE, Sullivan ME, Anderson FA, Tseng JF. Perioperative mortality for pancreatectomy: a national perspective. Ann Surg. 2007;246:246–253. doi: 10.1097/01.sla.0000259993.17350.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CG, Kwak EK, Lee SI. The relationship between hospital volume and outcome of gastrointestinal cancer surgery in Korea. J Surg Oncol. 2011;104:116–123. doi: 10.1002/jso.21946. [DOI] [PubMed] [Google Scholar]
- 22.Rosemurgy AS, Bloomston M, Serafini FM, Coon B, Murr MM, Carey LC. Frequency with which surgeons undertake pancreaticoduodenectomy determines length of stay, hospital charges, and in-hospital mortality. J Gastrointest Surg. 2001;5:21–26. doi: 10.1016/s1091-255x(01)80009-3. [DOI] [PubMed] [Google Scholar]
- 23.Ho V, Heslin MJ. Effect of hospital volume and experience on in-hospital mortality for pancreaticoduodenectomy. Ann Surg. 2003;237:509–514. doi: 10.1097/01.SLA.0000059981.13160.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balcom JH, Rattner DW, Warshaw AL, Chang Y, Fernandez-del Castillo C. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–398. doi: 10.1001/archsurg.136.4.391. [DOI] [PubMed] [Google Scholar]
- 26.Büchler MW, Wagner M, Schmied BM, Uhl W, Friess H, Z’graggen K. Changes in morbidity after pancreatic resection: toward the end of completion pancreatectomy. Arch Surg. 2003;138:1310–1314; discussion 1315. doi: 10.1001/archsurg.138.12.1310. [DOI] [PubMed] [Google Scholar]
- 27.Yeo CJ. The Johns Hopkins experience with pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma. J Gastrointest Surg. 2000;4:231–232. doi: 10.1016/s1091-255x(00)80070-0. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy TJ, Cassera MA, Wolf R, Swanstrom LL, Hansen PD. Surgeon volume versus morbidity and cost in patients undergoing pancreaticoduodenectomy in an academic community medical center. J Gastrointest Surg. 2010;14:1990–1996. doi: 10.1007/s11605-010-1280-1. [DOI] [PubMed] [Google Scholar]
- 29.Birkmeyer JD, Lucas FL, Wennberg DE. Potential benefits of regionalizing major surgery in Medicare patients. Eff Clin Pract. 1999;2:277–283. [PubMed] [Google Scholar]
- 30.Schmidt CM, Turrini O, Parikh P, House MG, Zyromski NJ, Nakeeb A, Howard TJ, Pitt HA, Lillemoe KD. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg. 2010;145:634–640. doi: 10.1001/archsurg.2010.118. [DOI] [PubMed] [Google Scholar]
- 31.Nathan H, Cameron JL, Choti MA, Schulick RD, Pawlik TM. The volume-outcomes effect in hepato-pancreato-biliary surgery: hospital versus surgeon contributions and specificity of the relationship. J Am Coll Surg. 2009;208:528–538. doi: 10.1016/j.jamcollsurg.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Rosemurgy A, Cowgill S, Coe B, Thomas A, Al-Saadi S, Goldin S, Zervos E. Frequency with which surgeons undertake pancreaticoduodenectomy continues to determine length of stay, hospital charges, and in-hospital mortality. J Gastrointest Surg. 2008;12:442–449. doi: 10.1007/s11605-007-0442-2. [DOI] [PubMed] [Google Scholar]
- 33.Eppsteiner RW, Csikesz NG, McPhee JT, Tseng JF, Shah SA. Surgeon volume impacts hospital mortality for pancreatic resection. Ann Surg. 2009;249:635–640. doi: 10.1097/SLA.0b013e31819ed958. [DOI] [PubMed] [Google Scholar]
- 34.Clark W, Hernandez J, McKeon BA, Kahn A, Morton C, Toomey P, Mullinax J, Ross S, Rosemurgy A. Surgery residency training programmes have greater impact on outcomes after pancreaticoduodenectomy than hospital volume or surgeon frequency. HPB ( Oxford) 2010;12:68–72. doi: 10.1111/j.1477-2574.2009.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joseph B, Morton JM, Hernandez-Boussard T, Rubinfeld I, Faraj C, Velanovich V. Relationship between hospital volume, system clinical resources, and mortality in pancreatic resection. J Am Coll Surg. 2009;208:520–527. doi: 10.1016/j.jamcollsurg.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Bilimoria KY, Talamonti MS, Sener SF, Bilimoria MM, Stewart AK, Winchester DP, Ko CY, Bentrem DJ. Effect of hospital volume on margin status after pancreaticoduodenectomy for cancer. J Am Coll Surg. 2008;207:510–519. doi: 10.1016/j.jamcollsurg.2008.04.033. [DOI] [PubMed] [Google Scholar]


