Abstract
AIM: To evaluate whether diabetic patients had a higher risk of colon cancer mortality and its associated risk factors.
METHODS: The sex-specific crude and age-standardized (to the 2000 World Health Organization population) mortality rates of colon cancer in the Taiwanese general population were first calculated from 1995 to 2006. The trends were evaluated by linear regression. A total of 113 347 diabetic men and 131 573 diabetic women aged ≥ 25 years at recruitment from 1995 to 1998 were followed up until the end of 2006. Age/sex-specific colon cancer mortality rate ratios were calculated comparing the mortality rates of the diabetic patients with the average mortality rates of the general population within 12 years (1995-2006). A sub-cohort of diabetic patients (42 260 men and 49 405 women) was interviewed using a baseline questionnaire and Cox’s regression was used to evaluate the risk factors for colon cancer mortality in these diabetic patients.
RESULTS: The crude and age-standardized trends of colon cancer mortality from 1995 to 2006 increased significantly for both sexes in the general population. A total of 641 diabetic men and 573 diabetic women died of colon cancer, with a mortality rate of 74.4 and 54.3 per 100 000 person-years, respectively. Mortality rate ratios [95% confidence intervals (CIs)] showed a significantly higher risk of mortality from colon cancer for the diabetic patients compared to the general population, with the magnitude increasing with decreasing age: 1.65 (1.40-1.95), 2.01 (1.78-2.27), 2.75 (2.36-3.21) and 5.69 (4.65-6.96) for ≥ 75, 65-74, 55-64 and 25-54 years old, respectively, for men; and 1.46 (1.24-1.72), 2.09 (1.84-2.38), 2.67 (2.27-3.14) and 3.05 (2.29-4.06), respectively, for women. Among the sub-cohort of diabetic patients who had been interviewed with the baseline questionnaire, including information on age, sex, diabetes duration, diabetes type, body mass index, smoking, insulin use and area of residence, age and smoking were significantly predictive for colon cancer mortality, with respective adjusted hazard ratios (HRs) (95% CIs) of 1.077 (1.066-1.088) and 1.384 (1.068-1.792). Diabetes duration became a significant factor when those who died of colon cancer within 5 years of diabetes diagnosis were excluded to minimize the possible contamination of diabetes caused by incipient colon cancer, with an adjusted hazard ratio of 1.021 (1.007-1.034). Sex, diabetes type, insulin use, body mass index and area of residence were not significant predictors for colon cancer mortality in the diabetic patients. Although insulin use was categorized into subgroups of duration of use (non-users and users < 5 years, 5-9 years and ≥ 10 years), none of the HRs for colon cancer mortality was significant with regards to different durations of insulin use.
CONCLUSION: Colon cancer mortality is increasing in Taiwan. A higher risk is observed in diabetic patients. Smoking, but not insulin use, is a modifiable risk factor.
Keywords: Colon cancer, Diabetes mellitus, Mortality, Secular trend
INTRODUCTION
Colorectal cancer is a major cause of death in developed countries[1]. In Taiwan, it is the third most common cause of cancer-related death[2], and the trend of its age-adjusted mortality showed an increase from 1971 to 1996[3]. A meta-analysis concluded there was a 30% higher risk in diabetic patients[4]. However, most studies were done in western countries, and the only one involving Asians in the meta-analysis was conducted in Korea, which showed a 28% higher risk of mortality in diabetic men, but not women[5]. On the other hand, some studies showed an association in women[6,7].
Most previous studies did not distinguish between type 1 and type 2 diabetes. A recent prospective study in the United States identified patients with type 2 diabetes and nondiabetic subjects aged 50-74 years in 1992-1993 and followed biannually by questionnaires from 1997 to 2007[8]. Diabetes was significantly associated with colorectal cancer in men who were either insulin users or non-users; but diabetes and insulin use were not associated with a higher risk among women[8].
Whether insulin use is associated with colon cancer mortality has rarely been studied. Furthermore, no previous studies have examined prospectively the confounding effects of diabetes duration and age; both being highly associated with insulin use. Therefore, this study evaluated: (1) the trends of colon cancer mortality in the Taiwanese general population; (2) the age/sex-specific mortality rate ratio between diabetic patients and the general population; and (3) the risk factors for colon cancer mortality in diabetic patients, including age, sex, diabetes duration, diabetes type, body mass index, smoking, insulin use/duration of insulin use and area of residence.
MATERIALS AND METHODS
Colon cancer mortality in the general population
The study was approved and supported by the Department of Health, Executive Yuan, Taiwan. In Taiwan, every resident has a unique identification number and events like birth, death, marriage or migration should be registered. If a person dies, a death certificate should be reported to the household registration offices within 30 d as required by law. The death certificate database includes the identification number, date of birth, sex, and date and cause of death. The causes of death coded in the ninth revision of the International Classification of Diseases are used. Colon cancer has a code of 153.
The age/sex-specific population numbers are reported annually by the government. The sex-specific trends of crude and age-standardized (to the 2000 World Health Organization population) mortality rates for colon cancer in the general population were first calculated from 1995 to 2006 for all ages. Linear regression was used to evaluate whether the trends changed with regard to calendar years, where the mortality rate was the dependent variable and the calendar year the independent variable.
Colon cancer is rare in young individuals, therefore, we analyzed the data for those aged ≥ 25 years in the following groups: 25-54 years, 55-64 years, 65-74 years and ≥ 75 years old. Age/sex-specific average mortality rates during 1995-2006 were calculated by dividing the average numbers of colon cancer deaths by the average mid-year population of the specific age and sex within the period.
Colon cancer mortality in diabetic patients
Figure 1 shows a flow chart for the follow-up of diabetic patients. In March 1995, a compulsory and universal National Health Insurance (NHI) program was implemented and covered > 96% of the population. From 1995 to 1998 a cohort of 256 036 diabetic patients (“the original cohort”) using the NHI was established (detailed elsewhere)[9,10].
Figure 1.
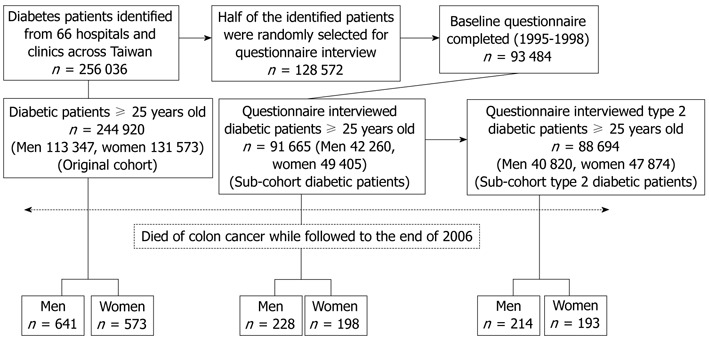
Flow chart showing the procedures in the calculation of colon cancer mortality in the diabetic cohorts.
All patients were followed until 2006. The date and cause of death were obtained from the death certificate database. Mortality rates were computed using a person-years denominator: the duration from enrollment until the end of 2006 for those who were alive or to the date of death. The patients were categorized into age subgroups by their age at enrollment. Age/sex-specific mortality rates and mortality rate ratios were calculated. The mortality rate ratio was calculated using the average mortality rate of that subgroup within the 12 years in the general population as a reference. To reduce the aging effect on age subgroup categorization, analyses for the original cohort were also performed by splitting the follow-up duration into two periods: (1) from enrollment to the end of 2000: age categorized at enrollment and mortality followed from enrollment to 2000; and (2) from 2001 to the end of 2006: those who died before the end of 2000 were excluded, age calculated at 2001 and mortality followed from 2001-2006.
For sub-cohort analyses, we calculated the mortality rates and mortality rate ratios in the patients who had been interviewed with a baseline questionnaire (detailed elsewhere)[11-13]. The number interviewed was 93 484 and among them 91 665 patients (42 260 men and 49 405 women) were aged ≥ 25 years (“sub-cohort diabetic patients”). To evaluate whether an association was found in patients with type 2 diabetes, mortality rates and mortality rate ratios were calculated after excluding patients with type 1 diabetes based on diabetic ketoacidosis at diabetes onset, or the need for insulin injection within 1 year after diabetes diagnosis. There were 40 820 diabetic men and 47 874 diabetic women after this exclusion (“sub-cohort type 2 diabetic patients”). Only 1440 men and 1531 women were excluded for type 1 diabetes, and among them only 14 men and five women died of colon cancer, therefore, we did not analyze the association with type 1 diabetes. To minimize the possibility that diabetes might be caused by incipient colon cancer, analyses were also done by dividing the patients into subgroups with a diabetes duration at enrollment < 10 years and ≥ 10 years.
Risk factors for colon cancer mortality in diabetic patients
The baseline characteristics of the sub-cohort diabetic patients who had been interviewed (Figure 1) were compared between men and women by either the t test for continuous variables or the χ2 test for categorical variables. Cox proportional hazards models were then used to identify the risk factors for colon cancer mortality. Colon cancer mortality was the dependent variable in the models and the independent variables included age, sex (men vs women), diabetes duration, diabetes type (type 2 vs type 1), body mass index, smoking (yes vs no), insulin use (yes vs no) and area of residence (urban vs rural). The area of residence was defined as urban for the Metropolitan Taipei area including Taipei City and Taipei County (New Taipei City) and other administratively named cities across Taiwan; and as rural for administratively named counties and offshore islands. To evaluate whether the duration of insulin use could be associated with colon cancer mortality, Cox models were also created by comparing insulin use at < 5 years, 5-9 years and ≥ 10 years to insulin non-users, before adjustment, at adjustment for age, sex, diabetes type, diabetes duration, body mass index, smoking and area of residence one at a time, and at adjustment for all these factors simultaneously (full model). The analyses were done before and after excluding patients who died of colon cancer within 5 years of diabetes onset, to minimize the possibility that diabetes might be caused by incipient colon cancer or might occur after the diagnosis of colon cancer.
RESULTS
The trends of crude and age-standardized colon cancer mortality showed a significant increase in both sexes in the general population during the period from 1995 to 2006 in Taiwan (Figure 2). The average mortality rates for colon cancer during the period in the general population aged 25-54 years, 55-64 years, 65-74 years and ≥ 75 years were 5.1, 24.75, 76.6 and 160.92 per 100 000 for men, respectively, and 4.51, 18.01, 46.08 and 130.90 for women.
Figure 2.
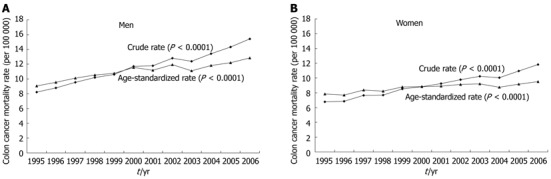
Secular trends of mortality from colon cancer from 1995 to 2006 in the general population of Taiwan in men (A) and women (B), respectively. The 2000 World Health Organization population was used as reference for age standardization.
A total of 113 347 diabetic men and 131 573 diabetic women in the original cohort were followed (Figure 1). Among them, 641 men and 573 women died of colon cancer, with mortality rates of 74.4 and 54.3 per 100 000 person-years, respectively. The age/sex-specific mortality rates in the diabetic patients and their mortality rate ratios compared to the general population are shown in Table 1. Except for those aged ≥ 75 years and with a diabetes duration of ≥ 10 years at enrollment in men, and with a diabetes duration of < 10 years at enrollment in women (Table 1), the mortality rate ratios were all significant, and especially remarkable in those aged 25-54 years. Diabetes was unlikely to be caused by colon cancer, because diabetes diagnosed ≥ 10 years before colon cancer mortality can hardly be a consequence of the carcinogenic process. The aging effect during follow-up on age subgroup categorization was also minimal because the results were similar when the long duration was split into two shorter periods in the original cohort analyses.
Table 1.
Age/sex-specific mortality rates (per 100 000 person-years) for colon cancer in diabetic patients and their mortality rate ratios compared to the average mortality rates in the general population of Taiwan
| Age (yr) |
Men |
Women |
||||||||
| n | N | PY | MR | MRR (95% CI) | n | N | PY | MR | MRR (95% CI) | |
| Original diabetic cohort | ||||||||||
| Followed from enrollment to the end of 2006 | ||||||||||
| 25-54 | 76 | 42107 | 363948.2 | 20.88 | 5.69 (4.65, 6.96)a | 43 | 43749 | 399556.9 | 10.76 | 3.05 (2.29, 4.06)a |
| 55-64 | 158 | 28867 | 225706.1 | 70.00 | 2.75 (2.36, 3.21)a | 146 | 37317 | 314033.6 | 46.49 | 2.67 (2.27, 3.14)a |
| 65-74 | 265 | 30704 | 211811.1 | 125.11 | 2.01 (1.78, 2.27)a | 238 | 35194 | 258155.6 | 92.19 | 2.09 (1.84, 2.38)a |
| ≥ 75 | 142 | 11669 | 59761.6 | 237.61 | 1.65 (1.40, 1.95)a | 146 | 15313 | 83905.5 | 174.01 | 1.46 (1.24, 1.72)a |
| Followed from enrollment to the end of 2000 | ||||||||||
| 25-54 | 23 | 42107 | 143238.6 | 16.06 | 4.38 (3.00, 6.37)a | 16 | 43749 | 153584.7 | 10.42 | 2.95 (1.84, 4.71)a |
| 55-64 | 52 | 28867 | 96078 | 54.12 | 2.12 (1.62, 2.78)a | 55 | 37317 | 129321.2 | 42.53 | 2.44 (1.88, 3.17)a |
| 65-74 | 112 | 30704 | 94488.8 | 118.53 | 1.90 (1.58, 2.29)a | 91 | 35194 | 113687.6 | 80.04 | 1.81 (1.48, 2.23)a |
| ≥ 75 | 70 | 11669 | 30353.6 | 230.61 | 1.60 (1.27, 2.03)a | 75 | 15313 | 42092.9 | 178.18 | 1.49 (1.19, 1.87)a |
| Followed from 2001 to the end of 2006 | ||||||||||
| 25-54 | 30 | 32626 | 186021.3 | 16.13 | 4.39 (3.16, 6.11)a | 22 | 34400 | 201514.2 | 10.92 | 3.09 (2.07, 4.60)a |
| 55-64 | 68 | 22743 | 122446.4 | 55.53 | 2.18 (1.72, 2.76)a | 52 | 29880 | 167205.9 | 31.10 | 1.79 (1.36, 2.34)a |
| 65-74 | 172 | 26548 | 133463.8 | 128.87 | 2.07 (1.78, 2.40)a | 131 | 33591 | 176023.3 | 74.42 | 1.69 (1.42, 2.00)a |
| ≥ 75 | 114 | 13653 | 59608.3 | 191.25 | 1.33 (1.11, 1.60)a | 131 | 17808 | 79994.6 | 163.76 | 1.37 (1.16, 1.63)a |
| Sub-cohort diabetic patients | ||||||||||
| Diabetes of any duration at enrollment | ||||||||||
| 25-54 | 25 | 13837 | 100793.2 | 24.80 | 6.76 (4.81, 9.50)a | 16 | 12768 | 95960.8 | 16.67 | 4.72 (3.02, 7.37)a |
| 55-64 | 62 | 12454 | 84551.8 | 73.33 | 2.88 (2.26, 3.66)a | 53 | 16717 | 118278.5 | 44.81 | 2.57 (1.98, 3.35)a |
| 65-74 | 99 | 12395 | 76349.6 | 129.67 | 2.08 (1.71, 2.53)a | 86 | 15029 | 96058.4 | 89.53 | 2.03 (1.64, 2.50)a |
| ≥ 75 | 42 | 3574 | 17710.4 | 237.15 | 1.65 (1.22, 2.23)a | 43 | 4891 | 25384.8 | 169.39 | 1.42 (1.05, 1.91)a |
| Diabetes duration < 10 yr at enrollment | ||||||||||
| 25-54 | 15 | 11631 | 86016.8 | 17.44 | 4.75 (3.00, 7.53)a | 12 | 10389 | 78983.7 | 15.19 | 4.30 (2.56, 7.23)a |
| 55-64 | 42 | 8892 | 61949.1 | 67.80 | 2.66 (1.99, 3.57)a | 37 | 11636 | 84435.4 | 43.82 | 2.52 (1.84, 3.45)a |
| 65-74 | 62 | 7887 | 49825.0 | 124.44 | 2.00 (1.56, 2.55)a | 47 | 9246 | 61226.8 | 76.76 | 1.74 (1.31, 2.31)a |
| ≥ 75 | 29 | 2138 | 10946.7 | 264.92 | 1.84 (1.29, 2.64)a | 23 | 2814 | 15302.6 | 150.30 | 1.26 (0.84, 1.90) |
| Diabetes duration ≥ 10 yr at enrollment | ||||||||||
| 25-54 | 10 | 2206 | 14773 | 67.69 | 18.44 (11.81, 28.80)a | 4 | 2379 | 16988.5 | 23.55 | 6.66 (2.85, 15.56)a |
| 55-64 | 20 | 3562 | 22608.3 | 88.46 | 3.47 (2.30, 5.25)a | 16 | 5081 | 33862.5 | 47.25 | 2.71 (1.69, 4.35)a |
| 65-74 | 37 | 4508 | 26541.7 | 139.40 | 2.24 (1.63, 3.07)a | 39 | 5783 | 34845.7 | 111.92 | 2.54 (1.87, 3.44)a |
| ≥ 75 | 13 | 1436 | 6785.4 | 191.59 | 1.33 (0.77, 2.29) | 20 | 2077 | 10103.5 | 197.95 | 1.66 (1.07, 2.56)a |
| Sub-cohort type 2 diabetic patients | ||||||||||
| Diabetes of any duration at enrollment | ||||||||||
| 25-54 | 23 | 13239 | 96635.9 | 23.80 | 6.49 (4.54, 9.26)a | 16 | 12260 | 92076.3 | 17.38 | 4.92 (3.16, 7.66)a |
| 55-64 | 60 | 12082 | 82183.2 | 73.01 | 2.87 (2.24, 3.66)a | 51 | 16288 | 115494.5 | 44.16 | 2.54 (1.94, 3.32)a |
| 65-74 | 92 | 12051 | 74413.3 | 123.63 | 1.98 (1.62, 2.43)a | 83 | 14570 | 93223.1 | 89.03 | 2.02 (1.63, 2.50)a |
| ≥ 75 | 39 | 3448 | 17171.3 | 227.12 | 1.58 (1.16, 2.16)a | 43 | 4756 | 24716.7 | 173.97 | 1.46 (1.08, 1.97)a |
| Diabetes duration < 10 yr at enrollment | ||||||||||
| 25-54 | 14 | 11248 | 83333.9 | 16.80 | 4.58 (2.84, 7.38)a | 12 | 10082 | 76622.8 | 15.66 | 4.43 (2.64, 7.44)a |
| 55-64 | 40 | 8730 | 60901.6 | 65.68 | 2.58 (1.91, 3.48)a | 36 | 11453 | 83223.4 | 43.26 | 2.48 (1.81, 3.42)a |
| 65-74 | 59 | 7742 | 48999.2 | 120.41 | 1.93 (1.50, 2.49)a | 46 | 9068 | 60169.4 | 76.45 | 1.73 (1.30, 2.31)a |
| ≥ 75 | 27 | 2088 | 10708.6 | 252.13 | 1.75 (1.21, 2.55)a | 23 | 2760 | 15063.9 | 152.68 | 1.28 (0.85, 1.93) |
| Diabetes duration ≥ 10 yr at enrollment | ||||||||||
| 25-54 | 9 | 1991 | 13298.7 | 67.68 | 18.44 (11.53, 29.50)a | 4 | 2178 | 15465.0 | 25.86 | 7.32 (3.17, 16.88)a |
| 55-64 | 20 | 3352 | 21284.3 | 93.97 | 3.69 (2.45, 5.56)a | 15 | 4835 | 32290.5 | 46.45 | 2.67 (1.64, 4.35)a |
| 65-74 | 33 | 4309 | 25428.1 | 129.78 | 2.08 (1.49, 2.91)a | 37 | 5502 | 33067.7 | 111.89 | 2.53 (1.85, 3.47)a |
| ≥ 75 | 12 | 1360 | 6484.3 | 185.06 | 1.29 (0.73, 2.26) | 20 | 1996 | 9674.1 | 206.74 | 1.73 (1.12, 2.67)a |
P < 0.05 vs general population. PY: Person-years; MR: Mortality rate; MRR: Mortality rate ratio; CI: Confidence interval; n: Case number of colon cancer; N: Case number observed.
All baseline characteristics of the sub-cohort diabetic patients who had been interviewed with a baseline questionnaire differed significantly between the diabetic men and women (Table 2). The unadjusted and mutually-adjusted HRs for different age groups are shown in Table 3. In the adjusted models, age and smoking (especially in those aged < 65 years) were significant. When diabetic patients who died of colon cancer within 5 years of diabetes diagnosis were excluded, diabetes duration was significant (especially in those aged ≥ 65 years). Sex, diabetes type, insulin use, body mass index and area of residence were not significant after adjustment.
Table 2.
Baseline characteristics of the sub-cohort of diabetic men and women who had been interviewed with a baseline questionnaire
| Variable | Men | Women | P value |
| n | 42 260 | 49 405 | |
| Age, yr | 59.8 (11.7) | 61.5 (10.8) | < 0.0001 |
| Diabetes duration, yr | 7.0 (6.6) | 7.5 (6.6) | < 0.0001 |
| Diabetes type, % type 1 | 1440 (3.4) | 1531 (3.1) | 0.0085 |
| Body mass index, kg/m2 | 24.4 (3.4) | 24.7 (3.8) | < 0.0001 |
| Smoking, % yes | 26 522 (62.8) | 1703 (3.5) | < 0.0001 |
| Use of insulin, % yes | 3717 (8.8) | 5059 (10.2) | < 0.0001 |
| Area of residence, % urban | 20 231 (47.9) | 22 264 (45.1) | < 0.0001 |
| Colon cancer mortality | 228 (0.5) | 198 (0.4) | 0.0021 |
Data are expressed as mean (SD) or n (%).
Table 3.
Cox proportional hazards models showing hazard ratios and 95% confidence intervals for colon cancer mortality in diabetic patients by age before and after exclusion of patients with a duration of < 5 years between onset of diabetes and colon cancer mortality
| Variables | Interpretation |
Hazard ratio (95% CI) |
|||||||
|
Age ≥ 25 yr |
Age 25-64 yr |
Age ≥ 65 yr |
|||||||
| Unadjusted | P value | Mutually adjusted | P value | Mutually adjusted | P value | Mutually-adjusted | P value | ||
| Before exclusion | |||||||||
| Age | Every 1-yr increment | 1.077 (1.066-1.088) | < 0.0001 | 1.077 (1.066-1.088) | < 0.0001 | 1.090 (1.062-1.119) | < 0.0001 | 1.072 (1.049-1.096) | < 0.0001 |
| Sex | Men vs women | 1.395 (1.150-1.691) | 0.0007 | 1.256 (0.976-1.618) | 0.0769 | 1.181 (0.758-1.841) | 0.4622 | 1.288 (0.946-1.753) | 0.1081 |
| Diabetes duration | Every 1-yr increment | 1.032 (1.019-1.045) | < 0.0001 | 1.006 (0.992-1.020) | 0.3845 | 0.993 (0.964-1.023) | 0.6652 | 1.009 (0.994-1.025) | 0.2329 |
| Diabetes type | Type 2 vs type 1 | 0.682 (0.430-1.081) | 0.1036 | 0.750 (0.432-1.301) | 0.3057 | 0.914 (0.345-2.425) | 0.8574 | 0.680 (0.348-1.328) | 0.2588 |
| Body mass index | Every 1-kg/m2 increment | 0.956 (0.929-0.984) | 0.0021 | 0.976 (0.948-1.005) | 0.1062 | 0.966 (0.920-1.014) | 0.1616 | 0.981 (0.946-1.018) | 0.3080 |
| Smoking | Yes vs no | 1.484 (1.219-1.808) | < 0.0001 | 1.384 (1.068-1.792) | 0.0140 | 1.746 (1.121-2.720) | 0.0136 | 1.215 (0.881-1.676) | 0.2351 |
| Insulin use | Yes vs no | 1.344 (0.992-1.821) | 0.0564 | 1.235 (0.843-1.809) | 0.2782 | 1.318 (0.686-2.532) | 0.4076 | 1.215 (0.759-1.946) | 0.4168 |
| Area of residence | Urban vs rural | 0.901 (0.743-1.094) | 0.2928 | 0.852 (0.702-1.034) | 0.1046 | 0.734 (0.530-1.016) | 0.0621 | 0.931 (0.731-1.187) | 0.5660 |
| After exclusion | |||||||||
| Age | Every 1-yr increment | 1.082 (1.070-1.094) | < 0.0001 | 1.080 (1.068-1.092) | < 0.0001 | 1.096 (1.064-1.128) | < 0.0001 | 1.070 (1.046-1.095) | < 0.0001 |
| Sex | Men vs women | 1.454 (1.186-1.784) | 0.0003 | 1.281 (0.980-1.676) | 0.0704 | 1.355 (0.836-2.195) | 0.2172 | 1.249 (0.903-1.728) | 0.1787 |
| Diabetes duration | Every 1-yr increment | 1.046 (1.034-1.059) | < 0.0001 | 1.021 (1.007-1.034) | 0.0022 | 1.025 (0.996-1.054) | 0.0883 | 1.020 (1.004-1.035) | 0.0120 |
| Diabetes type | Type 2 vs type 1 | 0.640 (0.398-1.028) | 0.0647 | 0.710 (0.402-1.254) | 0.2386 | 0.637 (0.229-1.771) | 0.3878 | 0.737 (0.371-1.465) | 0.3837 |
| Body mass index | Every 1-kg/m2 increment | 0.960 (0.932-0.989) | 0.0078 | 0.984 (0.955-1.015) | 0.3034 | 0.983 (0.933-1.035) | 0.5160 | 0.984 (0.948-1.022) | 0.4153 |
| Smoking | Yes vs no | 1.555 (1.263-1.914) | < 0.0001 | 1.445 (1.099-1.899) | 0.0083 | 1.814 (1.130-2.913) | 0.0137 | 1.268 (0.905-1.778) | 0.1672 |
| Insulin use | Yes vs no | 1.431 (1.044-1.961) | 0.0258 | 1.159 (0.781-1.722) | 0.4639 | 0.927 (0.440-1.954) | 0.8419 | 1.274 (0.798-2.035) | 0.3106 |
| Area of residence | Urban vs rural | 0.909 (0.741-1.116) | 0.3639 | 0.848 (0.691-1.041) | 0.1158 | 0.815 (0.573-1.158) | 0.2536 | 0.876 (0.680-1.128) | 0.3040 |
CI: Confidence interval.
Table 4 shows the HRs for different durations of insulin use compared to non-users in unadjusted and adjusted models. Before exclusion of patients with a duration of < 5 years between the onset of diabetes and colon cancer mortality, none of the HRs was significant. In models after exclusion, insulin use ≥ 10 years might be associated with a higher risk. However, in the models after respective adjustment for age, diabetes type or diabetes duration, and in the full model, insulin use of any duration was not predictive, suggesting that the association with insulin use might be due to the effects of some confounders.
Table 4.
Cox proportional hazards models for mortality from colon cancer by duration of insulin use (reference group: diabetic patients not using insulin) before and after exclusion of patients with a duration of < 5 years between onset of diabetes and colon cancer mortality
|
Duration of insulin use |
|||||||||
| Variables adjusted |
<5 yr |
5-9 yr |
≥ 10 yr |
||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Before exclusion | |||||||||
| Unadjusted | 1.128 | 0.734-1.735 | 0.5824 | 1.245 | 0.664-2.333 | 0.4947 | 1.539 | 0.918-2.579 | 0.1018 |
| Age | 1.388 | 0.902-2.136 | 0.1355 | 1.487 | 0.793-2.787 | 0.2163 | 1.441 | 0.860-2.416 | 0.1654 |
| Sex | 1.146 | 0.745-1.763 | 0.5339 | 1.267 | 0.676-2.375 | 0.4605 | 1.562 | 0.932-2.618 | 0.0907 |
| Diabetes type | 1.101 | 0.682-1.777 | 0.6931 | 0.817 | 0.362-1.843 | 0.6259 | 1.321 | 0.589-2.964 | 0.4991 |
| Diabetes duration | 1.009 | 0.655-1.556 | 0.9663 | 1.028 | 0.546-1.935 | 0.9330 | 1.018 | 0.590-1.756 | 0.9491 |
| Body mass index | 1.089 | 0.707-1.675 | 0.6995 | 1.210 | 0.645-2.268 | 0.5530 | 1.505 | 0.898-2.523 | 0.1208 |
| Smoking | 1.143 | 0.743-1.759 | 0.5416 | 1.263 | 0.674-2.367 | 0.4666 | 1.549 | 0.924-2.596 | 0.0966 |
| Area of residence | 1.122 | 0.729-1.726 | 0.6005 | 1.237 | 0.660-2.318 | 0.5074 | 1.538 | 0.918-2.578 | 0.1023 |
| Full model1 | 1.217 | 0.764-1.937 | 0.4082 | 0.943 | 0.442-2.010 | 0.8788 | 1.003 | 0.454-2.212 | 0.9948 |
| After exclusion | |||||||||
| Unadjusted | 1.108 | 0.698-1.761 | 0.6630 | 1.416 | 0.755-2.656 | 0.2782 | 1.746 | 1.041-2.931 | 0.0348 |
| Age | 1.384 | 0.871-2.199 | 0.1696 | 1.704 | 0.908-3.197 | 0.0971 | 1.633 | 0.973-2.740 | 0.0635 |
| Sex | 1.129 | 0.710-1.793 | 0.6085 | 1.445 | 0.771-2.711 | 0.2510 | 1.776 | 1.058-2.981 | 0.0297 |
| Diabetes type | 1.089 | 0.651-1.821 | 0.7447 | 1.010 | 0.446-2.287 | 0.9807 | 1.700 | 0.770-3.757 | 0.1893 |
| Diabetes duration | 0.943 | 0.592-1.501 | 0.8045 | 1.079 | 0.573-2.034 | 0.8133 | 0.968 | 0.561-1.669 | 0.9057 |
| Body mass index | 1.072 | 0.674-1.703 | 0.7702 | 1.375 | 0.733-2.581 | 0.3215 | 1.710 | 1.019-2.870 | 0.0424 |
| Smoking | 1.126 | 0.708-1.788 | 0.6164 | 1.440 | 0.768-2.701 | 0.2557 | 1.760 | 1.049-2.954 | 0.0323 |
| Area of residence | 1.103 | 0.694-1.752 | 0.6786 | 1.409 | 0.751-2.643 | 0.2855 | 1.746 | 1.040-2.929 | 0.0349 |
| Full model1 | 1.133 | 0.691-1.857 | 0.6214 | 1.038 | 0.491-2.195 | 0.9221 | 1.003 | 0.462-2.178 | 0.9949 |
Adjusted for age, sex, diabetes type, diabetes duration, body mass index, smoking and area of residence. HR: Hazard ratio; CI: Confidence interval.
DISCUSSION
Contrary to the decreasing trend since the mid-1980s in the United States[14], colon cancer mortality in Taiwan is increasing (Figure 2), and has been since 1971 if the observation of Chen et al[3] is considered simultaneously. Although the etiology of the increasing trend remains to be explored, it may be due to the westernization of the Taiwanese lifestyle in recent decades, with increased fat intake, and the high prevalence of metabolic syndrome and diabetes. The higher risk among diabetic patients of both sexes (Table 1) was also contrary to Korean[5] and United States[8] studies showing an association in men but not in women, and to others showing an association only in women[7].
It is interesting that the increased mortality rate ratio was more remarkable at the youngest age of 25-54 years (Table 1). This has public health importance because the incidence of diabetes is increasing dramatically in the younger generation[9]. One explanation is that age per se is a strong risk factor, and therefore, the impact of diabetes might not be as obvious in the elderly, resulting in a remarkably higher incidence rate ratio and a higher mortality rate ratio in the younger age group. Other explanations include that diabetes has a different impact on colon cancer mortality in different age groups, and that younger diabetic patients with colon cancer would have a poorer prognosis than non-diabetic patients. Mucin production in colorectal cancer has an inverse effect on survival among Taiwanese patients[15]. Taiwanese patients with colon cancer and aged < 40 years also have significantly poorer 5-year survival[16]; age is inversely associated with tumor stage at diagnosis, tumor differentiation and mucin production[17]. In addition, the healthy survivor effect might also lead to a reduced mortality rate ratio in the elderly.
Metabolic syndrome is associated with a 35% higher risk of colon cancer in Taiwan[18]. Similarly, a cluster of three components of the metabolic syndrome (hypertension, body mass index ≥ 25 kg/m2 and high-density lipoprotein cholesterol < 40 mg/dL) was associated with a 58% higher risk in a Finnish study[19]. In Taiwan, metabolic syndrome is present in 76.2% of diabetic patients[20], in contrast to 15% in the general population[21]. Therefore, the higher prevalence rates of hypertension, obesity and dyslipidemia in diabetic patients might explain partly their higher risk of colon cancer.
Contrary to other studies showing an association between body mass index and distal colon adenoma[22] or colorectal cancer[19], body mass index was not predictive for colon cancer mortality in the present study (Table 3). One possibility is that the risk factors for incidence and mortality or for different ethnicities might be different. It is also possible that if diabetes per se incurred a markedly higher risk, the impact of other risk factors might be overshadowed. Therefore, risk factors in diabetic patients might not be the same as those observed in nondiabetic subjects.
The association between colorectal neoplasm and insulin use in patients with type 2 diabetes has been controversial[8,23,24]. Although two retrospective studies suggested a positive link, a recent prospective study concluded a lack of association[8]. A study conducted in Korea suggested a threefold higher risk of colorectal adenoma associated with insulin therapy[23]. However, this study used a retrospective case-control design and evaluated adenoma rather than cancer. Another retrospective cohort study using the General Practice Research Database from the United Kingdom showed a significantly twofold higher risk of colorectal cancer associated with insulin use[24]. On the other hand, a prospective cohort study conducted in the United States did not suggest an association between colorectal cancer and insulin use in either men or women[8]. The finding of the present study was in line with the United States study, suggesting a lack of association between insulin use and colon cancer mortality (Tables 3 and 4). Insulin use is essential in patients with type 1 diabetes and is always seen in older patients with type 2 diabetes who might have prolonged duration of diabetes. Therefore, it is worth mentioning that adjustments should be made simultaneously for age, diabetes duration and diabetes type in the analyses evaluating the risk of cancer associated with insulin use. The present study is probably the longest follow-up study showing that insulin use was not predictive for colon cancer mortality after adjustment for confounders, including all of these factors (Tables 3 and 4).
Consistent with some prior studies[25,26], smoking was significantly predictive, especially in those aged < 65 years (Table 3). A recent Swedish retrospective cohort study evaluating the use of snuff and colorectal and anal cancer found no significant association[27]. We were not able to evaluate the effect of snuff use because of a lack of information. Although we could not evaluate the impact of socioeconomic status, this study did not show an association with area of residence (Table 3).
Incidence and mortality are two different entities, and probably linked to different factors. If diabetic patients with colon cancer had a poorer prognosis, the mortality rate ratio would not properly reflect the incidence rate ratio. A recently published prospective study (Cancer Prevention Study-II Nutrition Cohort) conducted among 2278 patients with colorectal cancer suggested that patients with type 2 diabetes had a higher risk of mortality than those without diabetes; especially a higher risk of death from cardiovascular disease[28]. A study from Taiwan also showed that diabetic patients with colon cancer had an overall 21% higher mortality than nondiabetic patients[29]. However, this was only observed in stage II cancer. It was believed that the 21% higher case-fatality rate could not explain the several-fold higher mortality rate ratios in the diabetic patients (Table 1).
The strengths of this study included a prospective follow-up of a large cohort of diabetic patients over a long duration; the completeness of the ascertainment of vital status by matching with the death certificate database; and the consistency observed in both sexes, and in different age groups, enrollment periods and sub-cohorts of diabetic patients.
There were limitations to the study. First, diabetic patients might have visited their physicians more frequently, resulting in a higher probability of detecting cancer. However, this might only suggest a higher rate of detection of early colon cancer with a better prognosis, which might have attenuated the magnitude of the mortality rate ratios. Second, the use of cause of death on the death certificate as the only source of colon cancer diagnosis might have underestimated the mortality related to colon cancer, because some patients with colon cancer might have died without having colon cancer listed as the cause of death. Therefore the impact of this possible effect awaits further investigation. Third, multiple drug therapy in diabetic patients might have complicated the situation. For example, statin, aspirin and nonsteroidal anti-inflammatory drugs are possibly preventive for colorectal cancer[30,31]. A higher proportion of diabetic patients might have been using these medications for the prevention of cardiovascular diseases. Different oral antidiabetic agents may have different effects on cancer development. For example, metformin has been shown to be preventive for cancer[32], but the use of sulfonylureas may be associated with a higher risk of cancer[33]. We could not evaluate the effects of these medications because such information was not collected. Fourth, this study did not consider confounders identified in Taiwan, including less exercise, less vegetable and fruit consumption, increased meat intake, and alcohol intake[25]. Furthermore, we were not able to adjust for some other confounders, as discussed below. For example, hyperhomocysteinemia has been shown to be a risk factor for type 2 diabetes and is also associated with abnormal DNA methylation, which has the potential to inactivate tumor suppressor genes leading to colorectal cancer[31]. Family history and inflammatory bowel disease are also significant risk factors for colorectal cancer[34-36]. None of these potential confounders were measured and could not be considered for adjustment.
In summary, we have demonstrated an increasing trend in colon cancer mortality in the Taiwanese general population from 1995 to 2006. The risk is increased in diabetic patients, with the magnitude of the mortality rate ratio becoming larger with decreasing age. Smoking is a risk factor, but insulin use is not. Given that the population is aging and the incidence of type 2 diabetes is increasing, the impact of the link between diabetes and colon cancer on the mortality of the population warrants public health attention.
COMMENTS
Background
Diabetic patients may have a higher risk of colon cancer, but whether insulin use in the diabetic patients can be a risk factor is controversial. Studies related to these issues are rarely conducted in Asian populations.
Research frontiers
A meta-analysis suggested a 30% higher risk of colorectal cancer in diabetic patients. However, most studies were done in western countries. In Korea, a 28% higher risk of mortality was observed in diabetic men, but not women. Whether insulin use is associated with colorectal cancer has rarely been studied. A recent prospective United States study showed that diabetes was significantly associated with colorectal cancer in men who were either insulin users or non-users, but diabetes and insulin use were not associated with a higher risk among women.
Innovations and breakthroughs
The author demonstrated an increasing trend in colon cancer mortality in the Taiwanese general population from 1995 to 2006. The risk is increased in diabetic patients, with the magnitude of the mortality rate ratio becoming larger with decreasing age. In patients with diabetes, smoking is a risk factor for colon cancer mortality, but insulin use is not. The strengths of this study included a prospective follow-up of a large cohort of diabetic patients over a long duration of 12 years; the completeness of the ascertainment of vital status by matching with the national death certificate database; the consistency observed in both sexes, and in different age groups, enrollment periods and sub-cohorts of diabetic patients; and consideration of the confounding effects of diabetes duration and age - both being highly associated with insulin use.
Applications
Given that the population is aging and the incidence of diabetes is increasing, the impact of the link between diabetes and colon cancer on the mortality of the population warrants public health attention. Insulin is commonly used in diabetic patients, therefore, clarification of a lack of insulin effect on colon cancer development relieves concern about the use of insulin.
Terminology
Secular trend of mortality: a systematic change in mortality rates over a period of (calendar) time; Mortality rate ratio: ratio of two mortality rates.
Peer review
This was a well-performed study that may be published when some corrections have been done.
Footnotes
Supported by The Department of Health of Taiwan, No. DOH97-TD-D-113-97009
Peer reviewer: Christa Buechler, PhD, Regensburg University Medical Center, Internal Medicine I, Franz Josef Strauss Allee 11, 93042 Regensburg, Germany
S-Editor Cheng JX L-Editor Kerr C E-Editor Zhang DN
References
- 1.Edwards BK, Howe HL, Ries LA, Thun MJ, Rosenberg HM, Yancik R, Wingo PA, Jemal A, Feigal EG. Annual report to the nation on the status of cancer, 1973-1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94:2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 2.Ju JH, Chang SC, Wang HS, Yang SH, Jiang JK, Chen WC, Lin TC, Hung Hsu FM, Lin JK. Changes in disease pattern and treatment outcome of colorectal cancer: a review of 5,474 cases in 20 years. Int J Colorectal Dis. 2007;22:855–862. doi: 10.1007/s00384-007-0293-z. [DOI] [PubMed] [Google Scholar]
- 3.Chen CJ, You SL, Lin LH, Hsu WL, Yang YW. Cancer epidemiology and control in Taiwan: a brief review. Jpn J Clin Oncol. 2002;32 Suppl:S66–S81. doi: 10.1093/jjco/hye138. [DOI] [PubMed] [Google Scholar]
- 4.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 5.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 6.Limburg PJ, Anderson KE, Johnson TW, Jacobs DR, Lazovich D, Hong CP, Nicodemus KK, Folsom AR. Diabetes mellitus and subsite-specific colorectal cancer risks in the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2005;14:133–137. [PubMed] [Google Scholar]
- 7.Nilsen TI, Vatten LJ. Prospective study of colorectal cancer risk and physical activity, diabetes, blood glucose and BMI: exploring the hyperinsulinaemia hypothesis. Br J Cancer. 2001;84:417–422. doi: 10.1054/bjoc.2000.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell PT, Deka A, Jacobs EJ, Newton CC, Hildebrand JS, McCullough ML, Limburg PJ, Gapstur SM. Prospective study reveals associations between colorectal cancer and type 2 diabetes mellitus or insulin use in men. Gastroenterology. 2010;139:1138–1146. doi: 10.1053/j.gastro.2010.06.072. [DOI] [PubMed] [Google Scholar]
- 9.Tseng CH, Tseng CP, Chong CK, Huang TP, Song YM, Chou CW, Lai SM, Tai TY, Cheng JC. Increasing incidence of diagnosed type 2 diabetes in Taiwan: analysis of data from a national cohort. Diabetologia. 2006;49:1755–1760. doi: 10.1007/s00125-006-0314-4. [DOI] [PubMed] [Google Scholar]
- 10.Tseng CH. Mortality and causes of death in a national sample of diabetic patients in Taiwan. Diabetes Care. 2004;27:1605–1609. doi: 10.2337/diacare.27.7.1605. [DOI] [PubMed] [Google Scholar]
- 11.Tseng CH. Diabetes conveys a higher risk of gastric cancer mortality despite an age-standardised decreasing trend in the general population in Taiwan. Gut. 2011;60:774–779. doi: 10.1136/gut.2010.226522. [DOI] [PubMed] [Google Scholar]
- 12.Tseng CH, Chong CK, Tai TY. Secular trend for mortality from breast cancer and the association between diabetes and breast cancer in Taiwan between 1995 and 2006. Diabetologia. 2009;52:240–246. doi: 10.1007/s00125-008-1204-8. [DOI] [PubMed] [Google Scholar]
- 13.Tseng CH. Prostate cancer mortality in Taiwanese men: increasing age-standardized trend in general population and increased risk in diabetic men. Ann Med. 2011;43:142–150. doi: 10.3109/07853890.2010.530683. [DOI] [PubMed] [Google Scholar]
- 14.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 15.You JF, Hsieh LL, Changchien CR, Chen JS, Chen JR, Chiang JM, Yeh CY, Hsieh PS, Fan CW, Liu CT, et al. Inverse effects of mucin on survival of matched hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer patients. Clin Cancer Res. 2006;12:4244–4250. doi: 10.1158/1078-0432.CCR-06-0202. [DOI] [PubMed] [Google Scholar]
- 16.Juang YF, Huang TJ, Huang YS, Huang CJ, Hsieh JS, Chien CH, Lin HJ. Clinicopathologic characteristics in colorectal adenocarcinoma and their relationship to survival. Gaoxiong Yixue Kexue Zazhi. 1990;6:45–57. [PubMed] [Google Scholar]
- 17.Chiang JM, Chen MC, Changchien CR, Chen JS, Tang R, Wang JY, Yeh CY, Fan CW, Tsai WS. Favorable influence of age on tumor characteristics of sporadic colorectal adenocarcinoma: patients 30 years of age or younger may be a distinct patient group. Dis Colon Rectum. 2003;46:904–910. doi: 10.1007/s10350-004-6683-1. [DOI] [PubMed] [Google Scholar]
- 18.Chiu HM, Lin JT, Shun CT, Liang JT, Lee YC, Huang SP, Wu MS. Association of metabolic syndrome with proximal and synchronous colorectal neoplasm. Clin Gastroenterol Hepatol. 2007;5:221–229; quiz 141. doi: 10.1016/j.cgh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 19.Bowers K, Albanes D, Limburg P, Pietinen P, Taylor PR, Virtamo J, Stolzenberg-Solomon R. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am J Epidemiol. 2006;164:652–664. doi: 10.1093/aje/kwj253. [DOI] [PubMed] [Google Scholar]
- 20.Tseng CH, Chong CK, Tseng CP, Shau WY, Tai TY. Hypertension is the most important component of metabolic syndrome in the association with ischemic heart disease in Taiwanese type 2 diabetic patients. Circ J. 2008;72:1419–1424. doi: 10.1253/circj.cj-08-0009. [DOI] [PubMed] [Google Scholar]
- 21.Hwang LC, Bai CH, Chen CJ. Prevalence of obesity and metabolic syndrome in Taiwan. J Formos Med Assoc. 2006;105:626–635. doi: 10.1016/S0929-6646(09)60161-3. [DOI] [PubMed] [Google Scholar]
- 22.Kim MC, Kim CS, Chung TH, Park HO, Yoo CI. Metabolic syndrome, lifestyle risk factors, and distal colon adenoma: a retrospective cohort study. World J Gastroenterol. 2011;17:4031–4037. doi: 10.3748/wjg.v17.i35.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chung YW, Han DS, Park KH, Eun CS, Yoo KS, Park CK. Insulin therapy and colorectal adenoma risk among patients with Type 2 diabetes mellitus: a case-control study in Korea. Dis Colon Rectum. 2008;51:593–597. doi: 10.1007/s10350-007-9184-1. [DOI] [PubMed] [Google Scholar]
- 24.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044–1050. doi: 10.1053/j.gastro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 25.Yeh CC, Hsieh LL, Tang R, Chang-Chieh CR, Sung FC. Risk factors for colorectal cancer in Taiwan: a hospital-based case-control study. J Formos Med Assoc. 2003;102:305–312. [PubMed] [Google Scholar]
- 26.Phipps AI, Baron J, Newcomb PA. Prediagnostic smoking history, alcohol consumption, and colorectal cancer survival: the Seattle Colon Cancer Family Registry. Cancer. 2011;117:4948–4957. doi: 10.1002/cncr.26114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nordenvall C, Nilsson PJ, Ye W, Nyrén O. Smoking, snus use and risk of right- and left-sided colon, rectal and anal cancer: a 37-year follow-up study. Int J Cancer. 2011;128:157–165. doi: 10.1002/ijc.25305. [DOI] [PubMed] [Google Scholar]
- 28.Dehal AN, Newton CC, Jacobs EJ, Patel AV, Gapstur SM, Campbell PT. Impact of diabetes mellitus and insulin use on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;30:53–59. doi: 10.1200/JCO.2011.38.0303. [DOI] [PubMed] [Google Scholar]
- 29.Huang YC, Lin JK, Chen WS, Lin TC, Yang SH, Jiang JK, Chang SC, Lan YT, Wang HS, Liu CY, et al. Diabetes mellitus negatively impacts survival of patients with colon cancer, particularly in stage II disease. J Cancer Res Clin Oncol. 2011;137:211–220. doi: 10.1007/s00432-010-0879-7. [DOI] [PubMed] [Google Scholar]
- 30.García-Rodríguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology. 2001;12:88–93. doi: 10.1097/00001648-200101000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Giouleme O, Diamantidis MD, Katsaros MG. Is diabetes a causal agent for colorectal cancer? Pathophysiological and molecular mechanisms. World J Gastroenterol. 2011;17:444–448. doi: 10.3748/wjg.v17.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aljada A, Mousa SA. Metformin and neoplasia: implications and indications. Pharmacol Ther. 2012;133:108–115. doi: 10.1016/j.pharmthera.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 34.Wei YS, Lu JC, Wang L, Lan P, Zhao HJ, Pan ZZ, Huang J, Wang JP. Risk factors for sporadic colorectal cancer in southern Chinese. World J Gastroenterol. 2009;15:2526–2530. doi: 10.3748/wjg.15.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmadi A, Polyak S, Draganov PV. Colorectal cancer surveillance in inflammatory bowel disease: the search continues. World J Gastroenterol. 2009;15:61–66. doi: 10.3748/wjg.15.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378–389. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]


