Abstract
AIM: To determine the effect of body mass index (BMI) on the characteristics and overall outcome of colon cancer in Taiwan.
METHODS: From January 1995 to July 2003, 2138 patients with colon cancer were enrolled in this study. BMI categories (in kg/m2) were established according to the classification of the Department of Health of Taiwan. Postoperative morbidities and mortality, and survival analysis including overall survival (OS), disease-free survival (DFS), and cancer-specific survival (CSS) were compared across the BMI categories.
RESULTS: There were 164 (7.7%) underweight (BMI < 18.5 kg/m2), 1109 (51.9%) normal-weight (BMI = 18.5-23.9 kg/m2), 550 (25.7%) overweight (BMI = 24.0-26.9 kg/m2), and 315 (14.7%) obese (BMI ≥ 27 kg/m2) patients. Being female, apparently anemic, hypoalbuminemic, and having body weight loss was more likely among underweight patients than among the other patients (P < 0.001). Underweight patients had higher mortality rate (P = 0.007) and lower OS (P < 0.001) and DFS (P = 0.002) than the other patients. OS and DFS did not differ significantly between normal-weight, overweight, and obese patients, while CSS did not differ significantly with the BMI category.
CONCLUSION: In Taiwan, BMI does not significantly affect colon-CSS. Underweight patients had a higher rate of surgical mortality and a worse OS and DFS than the other patients. Obesity does not predict a worse survival.
Keywords: Body mass index, Colon cancer, Survival, Morbidity, Outcome
INTRODUCTION
The prevalence of obesity and the incidence of colon cancer continue to increase in Taiwan as in other developed countries. The findings of many studies support the idea that obesity is a risk factor for the development of colon cancer[1-3]. However, the few studies that have investigated the influence of body mass index (BMI) on the outcomes of colon cancer patients have produced inconsistent findings[4-7]. These previous reports were mostly from Western countries and based on clinical trial data. We investigated herein the role of BMI in general colon cancer patients in Taiwan; the population of this study may represent both Chinese people and Asians in general, in terms of the clinical characteristics, postoperative morbidities and mortalities, and long-term outcome defined by overall survival (OS), disease-free survival (DFS), and cancer-specific survival (CSS).
MATERIALS AND METHODS
Study population
From January 1995 to July 2003, 2138 patients from a total population of 2765 patients with histologically confirmed adenocarcinoma of the colon were enrolled in this study; 14 nonmetastatic patients without available BMI data and 613 patients with metastatic disease were excluded. The included patients had regular follow-up visits with a healthcare professional until December 2008 (i.e., a postoperative follow-up period of at least 5 years) or until death.
The demographic and clinicopathologic data evaluated for each patient included age, gender, body height and weight, tumor stage (as defined according to the American Joint Committee on Cancer TNM staging system, sixth edition, New York: Springer-Verlag, 2002), tumor location, degree of tumor differentiation, timing of surgery (i.e., elective or emergent), medical illness, and preoperative laboratory data.
BMI was calculated as the weight in kilograms divided by the height in meters squared. The following BMI categories were established according to the classification of the Department of Health (DOH) of Taiwan:[8] underweight (BMI < 18.5 kg/m2), normal weight (BMI = 18.5-23.9 kg/m2), overweight (BMI = 24.0-26.9 kg/m2), and obese (BMI ≥ 27 kg/m2). For long-term outcome, BMI was also categorized according to the World Health Organization (WHO) classification[9] as follows: underweight (BMI < 18.5 kg/m2), normal weight (BMI = 18.5-24.9 kg/m2), overweight (BMI = 25-29.9 kg/m2), and obese (BMI ≥ 30 kg/m2) to enable comparison with the Taiwanese classification, since The Expert Consultation on BMI in Asian populations recommended that the current WHO BMI cut-off points be retained as the international classification[10].
Local recurrence or distant metastasis was confirmed histologically or radiographically, while postoperative mortality was defined as death within 30 d of the primary surgery. The patients were divided into three age groups: younger than 40 years (younger group), 40-75 years (middle age group), and older than 75 years (elderly group). The carcinoembryonic antigen (CEA) level was considered to be abnormal at > 5 ng/mL. Tumor location was categorized as right (from the cecum to the transverse colon) or left (from the splenic flexure to the sigmoid colon). Hypoalbuminemia was defined as a serum albumin level of < 35 g/L and apparent anemia was defined as a hemoglobin level of < 10 g/dL.
Statistical analysis
Survival curves were constructed using the Kaplan-Meier method and then compared using the log-rank test. OS was calculated as the number of years from primary surgery to the date of death. DFS was calculated as the number of years from primary surgery to either the first disease recurrence or death. CSS was calculated as the number of years from primary surgery to the first of disease recurrence. The two arms were compared by Pearson χ2 test and independent-samples t tests to detect any differences in proportions and means. A Cox regression model was used for multivariate analysis. All P values were two-tailed, and they were considered to be statistically significant at < 0.05.
RESULTS
Disease and patient characteristics
The study population comprised 2138 patients with colon cancer, of whom 1109 were males and 1029 were females. BMI in this study ranged from 12.2 kg/m2 to 49.0 kg/m2, with a mean of 23.4 kg/m2. Of the 2138 patients, 164 (7.7%) were underweight, 1109 (51.9%) were normal weight, 550 (25.7%) were overweight, and 315 (14.7%) were obese.
The characteristics of the patients and tumors are presented stratified according to BMI category in Table 1. The age of the entire study population was 61.4 ± 13.9 years (mean ± SD); those of the underweight, normal-weight, overweight, and obese patients were 63.5 ± 17.3 years, 61.8 ± 14.0 years, 61.9 ± 12.4 years, and 61.7 ± 12.3 years, respectively. The mean age did not differ significantly with the BMI category (P = 0.828). However, the distribution of age groups differed significantly with the BMI category, with the proportions of younger and elderly patients being higher in underweight-patients group than in the other groups (P < 0.001). The gender distribution also differed significantly between the underweight patients and the other groups, with underweight patients being more likely to be female. The tumor location also differed significantly, with the proportion of right colon cancer decreasing as the BMI category increased (P < 0.001).
Table 1.
Patient and tumor characteristics stratified according to body mass index category
|
Percentage of patients within each BMI category |
|||||
| Under- weight (n = 164) | Normal weight (n = 1109) | Over- weight (n = 550) | Obese (n = 315) | P value | |
| Age at diagnosis (yr) | < 0.001 | ||||
| ≤ 40 | 12.8 | 8.7 | 5.8 | 4.8 | |
| 41-75 | 57.3 | 75.5 | 81.6 | 83.2 | |
| > 75 | 29.9 | 15.9 | 12.5 | 12.1 | |
| Gender (%) | |||||
| Female | 65.9 | 47.2 | 44.7 | 48.3 | < 0.001 |
| Tumor location | |||||
| Right | 47.0 | 41.6 | 35.5 | 29.5 | < 0.001 |
| Tumor grade | |||||
| High-to-moderate differentiation | 82.9 | 83.7 | 85.6 | 87.9 | 0.239 |
| Tumor stage | < 0.001 | ||||
| I | 9.1 | 9.2 | 15.3 | 19.4 | |
| II | 48.2 | 50.9 | 44.7 | 45.1 | |
| III | 42.7 | 39.9 | 40.0 | 35.6 | |
| Emergent operation | 3.7 | 3.4 | 2.5 | 2.2 | 0.584 |
| Body weight loss | 57.9 | 45.5 | 33.3 | 20.8 | < 0.001 |
| CEA > 5 ng/mL | 44.2 | 34.6 | 36.3 | 36.0 | 0.150 |
| Albumin < 35 g/L | 42.3 | 21.0 | 13.5 | 11.6 | < 0.001 |
| Hemoglobin < 10 g/dL | 40.9 | 32.9 | 23.2 | 21.3 | < 0.001 |
| Comorbidities | |||||
| None | 57.3 | 51.7 | 50.1 | 41.6 | < 0.001 |
| Hypertension | 11.0 | 19.7 | 26.2 | 34.9 | < 0.001 |
| Heart disease | 8.5 | 6.8 | 7.6 | 12.7 | 0.008 |
| Previous stroke | 3.0 | 3.4 | 4.5 | 4.8 | 0.527 |
| Asthma | 3.7 | 3.3 | 3.6 | 2.9 | 0.936 |
| Diabetes mellitus | 4.9 | 10.6 | 12.4 | 20.6 | < 0.001 |
| Peptic ulcer disease | 14.0 | 10.8 | 9.1 | 8.9 | 0.229 |
| Chronic hepatitis | 1.2 | 4.3 | 3.3 | 4.1 | 0.225 |
| Liver cirrhosis | 1.8 | 1.4 | 1.1 | 0.6 | 0.650 |
| Renal insufficiency | 5.2 | 8.9 | 6.8 | 9.4 | 0.202 |
| Others | 16.6 | 13.8 | 12.0 | 15.1 | 0.555 |
Except where stated otherwise, data are the percentage of the particular body mass index (BMI) category population. CEA: Carcinoembryonic antigen.
With respect to the distribution of tumor stage among the BMI categories, the number of stage I tumors was lower in the underweight and normal-weight patients than in the overweight and obese patients. The obese patients had the lowest number of stage III tumors.
The proportion of emergent operations did not differ significantly with the BMI category: 3.7% of underweight, 3.4% of normal-weight, 2.5% of overweight, and 2.2% of obese patients (P = 0.584).
Underweight patients were the most likely to exhibit apparent anemia (hemoglobin < 10 g/dL) and have hypoalbuminemia and body weight loss (P < 0.001). The proportion of patients with body weight loss, hypoalbuminemia, and apparent anemia decreased as the BMI category increased. The percentage of patients with an abnormal preoperative CEA level did not differ significantly with the BMI category.
Finally, with regard to associated medical illnesses, the percentage of patients with hypertension or diabetes mellitus increased with the BMI category. Obese patients were the most likely to have heart disease. The other associated comorbidities including previous stroke, asthma, peptic ulcer disease, chronic hepatitis, renal insufficiency, and others (e.g., gall stones and thyroid disease) did not differ significantly with the BMI category.
Short-term outcome
The postoperative morbidity, anastomostic leakage, and mortality rates did not differ significantly between the underweight, normal-weight, overweight, and obese patients: 12.8%, 12.9%, 13.3% and 15.6%, respectively (P = 0.667); 1.2%, 1.4%, 1.6%, and 1.9%, respectively (P = 0.880); and 3.7%, 1.3%, 1.1%, and 0%, respectively (P = 0.007).
Long-term outcome
Data from all patients who received surgery were used to analyze the long-term outcome. Univariate analysis was performed using the Kaplan-Meier method, along with the log-rank test and Cox proportional-hazards model. The results of our statistical analysis are presented in Table 2, and the survival curves are shown in Figure 1A (OS), Figure 1B (DFS), and Figure 1C (CCS).
Table 2.
Univariate analysis of survival of colon cancer patients stratified according to body mass index category (Taiwan vs World Health Organization)
| Underweight (n = 164) | Normal weight (n = 1109) | Overweight (n = 550) | Obese (n = 315) | |
| BMI category in Taiwan | ||||
| Overall survival | ||||
| 5-yr survival rate | 60.0 | 73.6 | 78.2 | 75.4 |
| HR (95% CI) | 1.67 (1.31-2.13) | Reference | 0.83 (0.69-0.99) | 0.93 (0.74-1.16) |
| P value | < 0.001 | 0.046 | 0.500 | |
| Disease-free survival | ||||
| 5-yr survival rate | 57.4 | 70.8 | 73.4 | 71.0 |
| HR (95% CI) | 1.60 (1.26-2.04) | Reference | 0.86 (0.72-1.04) | 0.97 (0.79-1.20) |
| P value | < 0.001 | 0.103 | 0.807 | |
| Cancer-specific survival | ||||
| 5-yr survival rate | 71.6 | 78.5 | 78.9 | 77.3 |
| HR (95% CI) | 1.31 (0.94-1.82) | Reference | 0.95 (0.77-1.19) | 1.01 (0.78-1.32) |
| P value | 0.107 | 0.657 | 0.932 | |
| BMI categories of WHO | Underweight (n = 164) | Normal-weight (n = 1331) | Overweight (n = 553) | Obese (n = 90) |
| Overall survival | ||||
| 5-yr survival rate | 60.0 | 74.0 | 78.2 | 75.0 |
| HR (95% CI) | 1.70 (1.34-2.15) | Reference | 0.85 (0.71-1.02) | 0.99 (0.66-1.39) |
| P value | < 0.001 | 0.072 | 0.833 | |
| Disease-free survival | ||||
| 5-yr survival rate | 57.4 | 71.0 | 73.2 | 70.4 |
| HR ( 95% CI) | 1.62 (1.28-2.06) | Reference | 0.89 (0.74-1.05) | 1.05 (0.73-1.49) |
| P value | < 0.001 | 0.169 | 0.808 | |
| Cancer-specific survival | ||||
| 5-yr survival rate | 71.6 | 78.2 | 79.5 | 75.0 |
| HR (95% CI) | 1.30 (0.94-1.80) | Reference | 0.92 (0.74-1.13) | 1.10 (0.72-1.70) |
| P value | 0.113 | 0.418 | 0.661 |
Except where stated otherwise, data are the percentage of the particular body mass index (BMI) category population. WHO: World Health Organization; HR: Hazard ratio; CI: Confidence interval.
Figure 1.
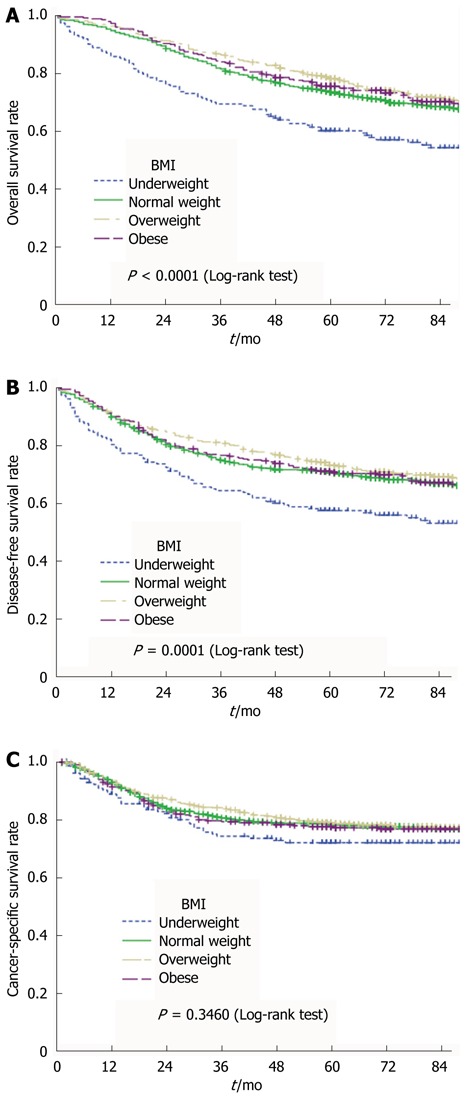
Overall survival curves (A) , disease-free survival curves (B) and cancer-specific survival curves (C) relative to body mass index category using the Kaplan-Meier method, and comparison using the log-rank test. BMI: Body mass index.
Univariate analysis revealed that OS (P < 0.001) and DFS (P = 0.001) were lowest in the underweight patients. The OS was marginally higher (P = 0.046) in overweight patients than in normal-weight patients, but the DFS (P = 0.103) did not differ significantly between the two groups. No significant difference in OS (P = 0.500) or DFS (P = 0.807) was observed between normal-weight and obese patients.
CSS did not differ significantly with the BMI category (P = 0.346). The results were similar when these patients were categorized according to the WHO BMI classifications.
Adjuvant chemotherapy was offered to 58.0%, 70.1%, 73.9%, and 70.8% of underweight, normal-weight, overweight, and obese patients with stage III tumors, respectively (P = 0.096). In total, 70.2% of patients with stage III tumors received adjuvant chemotherapy.
Since the compositions of patients and tumors differed with the BMI category, multivariate analysis was used to determine the effect of BMI along with other confounding factors on the long-term outcome. The variables of the Cox regression model included BMI, TNM stage, age group, gender, comorbidities (patients were divided into three groups: without, with one or two kinds, and with more than two kinds of comorbidity), CEA (normal vs abnormal), hemoglobin (< 10 g/L vs ≥ 10 g/L), albumin (normal vs hypoalbuminemia), timing of surgery (elective vs emergent), postoperative morbidity (with vs without), tumor location (right vs left), histologic type (adenocarcinoma vs mucinous vs signet-ring type), and histologic grade (high vs moderate vs low differentiation). The hazard ratio (HR) of each BMI category was compared with that of the normal-weight patients. The results of multivariate analysis for OS, DFS, and CSS are listed in Table 3.
Table 3.
Multivariate analysis of colon cancer survival (overall, disease-free, and cancer-specific) by Cox regression model
| Variable | Overall survival | Disease-free survival | Cancer-specific survival | |||
| P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | |
| BMI group | 0.000 | 0.002 | 0.369 | |||
| Underweight vs normal | 0.000 | 1.58 (1.23-2.05) | 0.002 | 1.49 (1.16-1.93) | 0.107 | 1.33 (0.94-1.87) |
| Overweight vs normal | 0.060 | 0.83 (0.68-1.01) | 0.123 | 0.86 (0.71-1.04) | 0.751 | 0.96 (0.76-1.22) |
| Obese vs normal | 0.578 | 0.94 (0.74-1.18) | 0.972 | 1.00 (0.79-1.25) | 0.677 | 1.06 (0.80-1.41) |
| TNM stage | 0.000 | 0.000 | 0.000 | |||
| II vs I | 0.721 | 1.06 (0.77-1.45) | 0.981 | 1.00 (0.74-1.35) | 0.025 | 1.78 (1.07-2.95) |
| III vs I | 0.000 | 1.90 (1.39-2.60) | 0.000 | 1.80 (1.34-2.42) | 0.000 | 3.94 (2.39-6.48) |
| Age group (yr) | 0.000 | 0.000 | 0.876 | |||
| 41-75 vs ≤ 40 | 0.054 | 1.45 (0.99-2.11) | 0.052 | 1.44 (0.99-2.09) | 0.672 | 1.09 (0.74-1.58) |
| > 75 vs ≤ 40 | 0.000 | 3.14 (2.11-4.67) | 0.000 | 2.88 (1.94-4.25) | 0.607 | 1.12 (0.72-1.74) |
| Gender (male vs female) | 0.329 | 1.08 (0.92-1.27) | 0.220 | 1.10 (0.94-1.29) | 0.734 | 1.03 (0.85-1.26) |
| Medical illness (comorbidity) | 0.003 | 0.003 | 0.769 | |||
| One or two kinds vs none | 0.358 | 1.09 (0.91-1.32) | 0.442 | 1.08 (0.89-1.29) | 0.927 | 0.99 (0.79-1.24) |
| > two kinds vs none | 0.001 | 1.40 (1.16-1.71) | 0.001 | 1.39 (1.15-1.68) | 0.478 | 0.91 (0.70-1.18) |
| CEA (> 5 vs ≤ 5 ng/mL) | 0.000 | 1.86 (1.58-2.18) | 0.000 | 1.93 (1.65-2.25) | 0.000 | 2.15 (1.77-2.61) |
| Hemoglobin (< 10 vs ≥ 10 g/dL) | 0.913 | 1.01 (0.84-1.22) | 0.760 | 1.03 (0.86-1.23) | 0.835 | 1.03 (0.81-1.29) |
| Albumin (normal vs hypoalbuminemia) | 0.000 | 0.65 (0.54-0.79) | 0.000 | 0.68 (0.57-0.83) | 0.297 | 0.87 (0.67-1.13) |
| Operation timing (emergent vs elective) | 0.562 | 1.21 (0.64-2.26) | 0.416 | 1.28 (0.70-2.34) | 0.687 | 1.18 (0.53-2.66) |
| Morbidity (yes vs no) | 0.000 | 1.55 (1.26-1.91) | 0.000 | 1.50 (1.23-1.84) | 0.279 | 1.17 (0.88-1.56) |
| Tumor location (left vs right) | 0.785 | 1.03 (0.86-1.22) | 0.666 | 1.04 (0.88-1.23) | 0.583 | 1.06 (0.86-1.32) |
| Histologic type | 0.079 | 0.144 | 0.055 | |||
| Signet ring cell vs adenocarcinoma | 0.039 | 2.19 (1.04-4.63) | 0.065 | 2.03 (0.96-4.30) | 0.017 | 2.68 (1.19-6.01) |
| Mucinous vs adenocarcinoma | 0.323 | 1.14 (0.88-1.49) | 0.465 | 1.10 (0.85-1.43) | 0.690 | 1.07 (0.77-1.48) |
| Histologic grade (differentiation) | 0.282 | 0.213 | 0.173 | |||
| Moderate vs high | 0.363 | 1.11 (0.89-1.37) | 0.143 | 1.17 (0.95-1.45) | 0.061 | 1.32 (0.99-1.76) |
| Low vs high | 0.114 | 1.34 (0.93-1.94) | 0.121 | 1.33 (0.93-1.92) | 0.403 | 1.24 (0.75-2.04) |
HR: Hazard ratio; CI: Confidence interval; BMI: Body mass index; TNM: Tumor, nodes, metastasis; CEA: Carcinoembryonic antigen.
As documented in Table 4, underweight patients had a significantly worse OS [HR, 1.58; 95% confidence interval (CI), 1.23-2.05; P < 0.001] and DFS (HR, 1.49; 95% CI, 1.16-1.93; P = 0.002), but their CSS did not differ significantly (HR, 1.33; 95% CI, 0.94-1.87; P = 0.107) when compared with the normal-weight patients. OS, DFS, or CSS did not differ significantly between the normal-weight, overweight, and obese patients.
Table 4.
Results of multivariate analysis of colon cancer survival stratified according to body mass index category
| Underweight | Normal weight | Overweight | Obese | |
| All patients (n = 2138) | ||||
| Overall survival | ||||
| HR (95% CI)1 | 1.58 (1.23-2.05) | Reference | 0.83 (0.68-1.01) | 0.94 (0.74-1.18) |
| P value2 | < 0.001 | 0.060 | 0.578 | |
| Disease-free survival | ||||
| HR (95% CI)1 | 1.49 (1.16-1.93) | Reference | 0.86 (0.71-1.04) | 1.00 (0.79-1.25) |
| P value2 | 0.002 | 0.123 | 0.972 | |
| Cancer-specific survival | ||||
| HR (95% CI)1 | ||||
| P value2 | 1.33 (0.94-1.87) | Reference | 0.96 (0.76-1.22) | 1.06 (0.80-1.41) |
| 0.107 | 0.751 | 0.677 | ||
| Males (n = 1109) | ||||
| Overall survival | ||||
| HR (95% CI) | 1.55 (1.03-2.35) | Reference | 0.77 (0.58-1.01) | 0.91 (0.66-1.25) |
| P value2 | 0.036 | 0.056 | 0.565 | |
| Disease-free survival | ||||
| HR (95% CI)1 | 1.60 (1.04-2.44) | Reference | 0.84 (0.65-1.09) | 1.01 (0.75-1.37) |
| P value2 | 0.031 | 0.192 | 0.937 | |
| Cancer-specific survival | ||||
| HR (95% CI)1 | ||||
| P value2 | 1.46 (0.84-2.52) | Reference | 0.96 (0.69-1.32) | 1.21 (0.83-1.77) |
| 0.179 | 0.789 | 0.328 | ||
| Females (n = 1029) | ||||
| Overall survival | ||||
| HR (95% CI)1 | 1.55 (1.11-2.16) | Reference | 0.95 (0.71-1.27) | 0.99 (0.69-1.41) |
| P value2 | 0.011 | 0.724 | 0.945 | |
| Disease-free survival | ||||
| HR (95% CI)1 | 1.41 (1.01-1.97) | Reference | 0.91 (0.68-1.22) | 1.01 (0.71-1.43) |
| P value2 | 0.042 | 0.544 | 0.957 | |
| Cancer-specific survival | ||||
| HR (95% CI)1 | ||||
| P value2 | 1.16 (0.75-1.82) | Reference | 0.96 (0.69-1.36) | 0.93 (0.60-1.43) |
| 0.508 | 0.839 | 0.727 | ||
HR was calculated using the Cox regression model; the variables in the multivariate analysis included TNM stage, age, gender (when analyzing all patients), comorbidities, CEA, hemoglobin, albumin, operative timing, postoperative morbidity, tumor location, histologic type, and histologic grade;
Each category compared to normal-weight patients. HR: Hazard ratio; CI: Confidence interval; BMI: Body mass index; TNM: Tumor, nodes, metastasis; CEA: Carcinoembryonic antigen.
To determine whether the prognostic effect of BMI was related to patient gender, a multivariate analysis was applied to separated data from male and female patients. The results of this statistical analysis yielded the same findings between the genders: CSS did not differ significantly with the BMI category for all patients or for male or female patients.
DISCUSSION
BMI is a simple and easy-to-determine index used to classify individuals as being underweight, overweight, or obese. The classification of BMI was established to evaluate health risks such as type 2 diabetes and cardiovascular disease[9,10]. The associations between BMI, percentage of body fat, and distribution of body fat differ across populations, which has resulted in different cut-off points for classifications being used in different countries. Furthermore, the cut-off points for certain risks vary between different Asian populations[10]. The Expert Consultation on BMI in Asian populations recommended that the current WHO BMI cut-off points be retained as the international classification[10]. In the present study, BMI classifications were based on the definition of the DOH, Taiwan. In addition, the WHO BMI classifications were used to evaluate the long-term outcome of colon cancer survivors and to compare with the Taiwan BMI classification system. In the present study, the prevalence rates of diabetes and hypertension increased significantly with the BMI category; this finding is highly consistent with the intended meaning of the BMI classification system. However, the risks of other comorbidities such as asthma, hepatitis, peptic ulcer disease, and renal insufficiency were not correlated with the BMI category.
Obesity is a growing problem in Taiwan and is known to increase the risk of developing colon cancer[1-3]. It has also been reported that obese patients have a higher risk of surgical complications[11-13]. Previous studies have produced controversial results regarding the relationship between obesity and anastomotic leakage[14,15]. Sorensen et al[14] reported that obesity is not a risk factor for anastomotic leakage in colonic resection, while Biondo et al[15] reported that obesity is an independent risk factor, but is only associated with emergent procedures of the left colon. Miransky et al[16] reported that obesity and a contaminated surgical procedure independently predicted surgical-site infection in colorectal procedures. Riou et al[17] reported that obesity was a significant independent risk factor for wound dehiscence. Obesity has also been reported to be a risk factor for the postoperative occurrence of pulmonary complications[18,19]. The postoperative morbidity rate and anastomostic leakage rate did not differ significantly with the BMI category in our study. However, one of the limitations of this study is that we did not examine whether the occurrence rate of the other type of complications differed with the BMI category.
In the present study, the risk of postoperative mortality was highest in underweight patients. This finding is similar to that of Hickman et al[20] Although obese patients have higher rates of comorbidities with cardiovascular disease and diabetes, the postoperative morbidity and mortality rates were comparable with the normal-weight patients. Conversely, the underweight patients had a lower rate of comorbidities but a higher rate of postoperative mortality than did the other patients. However, a higher proportion of underweight patients in this study were hypoalbuminemic and anemic. The observed higher postoperative mortality rate may be at least partially attributed to the associated disease conditions.
Whether obese colon cancer patients have a worse long-term outcome than other patients remains a matter of controversy. Sinicrope et al[7] reported that underweight patients had a significantly worse OS (P = 0.0258), and that BMI ≥ 35 kg/m2 patients exhibited a trend toward a worse DFS (P = 0.0725) and OS (P = 0.0805) compared with normal-weight patients, but there was no significant difference. When they analyzed the data according to patient gender, males with BMI ≥ 35 kg/m2 exhibited a reduced OS, and females with obesity (BMI = 30-34 kg/m2) had a reduced OS when compared with their normal-weight counterparts. BMI category was significantly associated with both DFS and OS in multivariate analysis in their study. Meyerhardt et al[6] reported that neither BMI nor weight change was significantly associated with colon cancer patient survival indicators, including the OS, DFS, and recurrence-free survival, even for underweight patients. Dignam et al[5] reported that OS and DFS were significantly worse for underweight patients (BMI < 18.5 kg/m2) and very obese patients (BMI ≥ 35 kg/m2) than for normal-weight patients. Very obese patients had a greater risk of cancer recurrence or secondary primary tumors. In the present study, we found that BMI by itself was not a significant factor of CSS in colon cancer, but OS and DFS did tend to be worse for underweight patients than for the other patients. We found no differential effect of gender on either BMI or obesity. Compared with other patients, underweight patients had a worse OS but a similar CSS. This implies that many underweight patients died from noncancer events. It would have been reasonable to conclude that underlying comorbidities caused the higher mortality risk among the underweight patients, but in the present study this group actually had the smallest number of comorbidities. Further research should be conducted to establish the mechanisms responsible for the observed higher mortality risk in underweight patients. Furthermore, previous studies have shown that highly obese patients (BMI ≥ 35 kg/m2) or males may have a worse long-term outcome than normal-weight patients, but the obese cohort of the present study was not large enough to allow analysis of the difference.
In this study, tumor location was found to be correlated with BMI, such that the proportion of patients with right colon cancer increased as the BMI category decreased. Whether patients with a lower BMI tend to have right-side colon cancer is not well known or studied. However, since the lumen is larger for the right than the left colon, symptoms related to the tumor such as small-caliber or bloody stool need more time to be sensed by patients with right colon cancer, resulting in a longer period of nutrition depletion and body weight loss. Minoo et al[21] reported that proximally located tumors are significantly larger than those found in the distal colon. We have shown previously that the prevalence of malnutrition (hypoalbuminemia) is higher for right colon tumors than for left colon tumors[22]. Moreover, body weight loss is more common in right colon cancer than in left colon cancer (48.8% and 33.5%, respectively; P < 0.001). BMI is reduced in patients with body weight loss, and more patients with right colon tumors have body weight loss resulting in a lower BMI, which may partly explain the greater number of left-side tumors in the groups with a higher BMI.
The findings of this study suggest that a low BMI is a marker of weight loss, blood loss, nutrition depletion, and more-advanced disease, all of which are associated with a worse DFS and OS[22-24]. This could be the reason why the low-BMI group had a lower DFS and OS.
This study was subject to some limitations. It lacked data regarding changes in body weight before and after surgery, measurement of central obesity, physical activity, and diet changes after surgery, and involved a smaller sample than did previous studies. However, the study’s cohort came from a single medical institution with a standard collection of patients’ data, and so there was no selection bias as might be expected in a clinical trial. The results of this study pertain to patients from Taiwan and hence may not be generalizable to other populations.
For the population of Taiwan, which represents both Chinese people and Asians in general, BMI does not appear to be a significant factor of colon-CSS, but underweight patients appear to have a higher postoperative mortality and worse OS, and are less likely to experience DFS than those in the other BMI categories. The obese patients had a higher wound complication rate, but exhibited a similar survival rate when compared to the normal-weight patients.
COMMENTS
Background
The prevalence of obesity and the incidence of colon cancer continue to increase in Taiwan as in other developed countries. Studies that have investigated the influence of body mass index (BMI) on the outcomes of colon cancer patients have produced inconsistent findings. These previous reports were mostly from Western countries, and lacked data regarding Asian people. The authors therefore investigated the role of BMI in general colon cancer patients of Taiwan, in terms of the clinical characteristics and short- and long-term outcomes.
Research frontiers
BMI is a simple index that is used to classify individuals as being underweight, overweight, or obese. The cut-off points of BMI for classifications vary in different countries. The World Health Organization BMI classifications were used to evaluate the long-term outcome of colon cancer survivors and were compared with those determined using the Taiwan BMI classification system.
Innovations and breakthroughs
The findings of the present study demonstrate that a low BMI could be a marker of weight loss, blood loss, nutrition depletion, and more-advanced disease. For the population of Taiwan, which represents both Chinese people and other Asians in general, BMI does not appear to significantly affect the colon-cancer-specific survival, although underweight patients do appear to have a higher postoperative mortality and worse overall survival, and are less likely to experience disease-free survival than patients in the other BMI categories.
Applications
BMI is a simple index that can be used to evaluate the likelihood of colon cancer patients developing weight loss, blood loss, nutrition depletion, and more-advanced disease.
Terminology
BMI was calculated as the weight in kilograms divided by the height in meters squared. The classification of BMI was established to evaluate health risks such as type 2 diabetes and cardiovascular disease.
Peer review
This study investigated the effect of body mass index on the characteristics and overall outcome of colon cancer in Taiwan. This manuscript addresses a highly relevant subject and could be important for the cancer field.
Footnotes
Peer reviewer: Fausto Catena, MD, PhD, Department of General, Emergency and Transplant Surgery, St Orsola- Malpighi University Hospital, Via Massarenti 9, 40139 Bologna, Italy
S- Editor Cheng JX L- Editor A E- Editor Zhang DN
References
- 1.Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjønneland A, Halkjaer J, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2006;98:920–931. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 2.Rapp K, Schroeder J, Klenk J, Stoehr S, Ulmer H, Concin H, Diem G, Oberaigner W, Weiland SK. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer. 2005;93:1062–1067. doi: 10.1038/sj.bjc.6602819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr. 2007;86:556–565. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 4.Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Benson AB, Macdonald JS, Fuchs CS. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–495. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 5.Dignam JJ, Polite BN, Yothers G, Raich P, Colangelo L, O’Connell MJ, Wolmark N. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98:1647–1654. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 6.Meyerhardt JA, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Nelson H, Whittom R, Hantel A, Thomas J, Fuchs CS. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol. 2008;26:4109–4115. doi: 10.1200/JCO.2007.15.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinicrope FA, Foster NR, Sargent DJ, O’Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010;16:1884–1893. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Available from: http: //food.doh.gov.tw/FoodNew/health/1824/1824_102.aspx.
- 9.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 10.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 11.DeMaria EJ, Carmody BJ. Perioperative management of special populations: obesity. Surg Clin North Am. 2005;85:1283–1289, xii. doi: 10.1016/j.suc.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Benoist S, Panis Y, Alves A, Valleur P. Impact of obesity on surgical outcomes after colorectal resection. Am J Surg. 2000;179:275–281. doi: 10.1016/s0002-9610(00)00337-8. [DOI] [PubMed] [Google Scholar]
- 13.Gendall KA, Raniga S, Kennedy R, Frizelle FA. The impact of obesity on outcome after major colorectal surgery. Dis Colon Rectum. 2007;50:2223–2237. doi: 10.1007/s10350-007-9051-0. [DOI] [PubMed] [Google Scholar]
- 14.Sørensen LT, Jørgensen T, Kirkeby LT, Skovdal J, Vennits B, Wille-Jørgensen P. Smoking and alcohol abuse are major risk factors for anastomotic leakage in colorectal surgery. Br J Surg. 1999;86:927–931. doi: 10.1046/j.1365-2168.1999.01165.x. [DOI] [PubMed] [Google Scholar]
- 15.Biondo S, Parés D, Kreisler E, Ragué JM, Fraccalvieri D, Ruiz AG, Jaurrieta E. Anastomotic dehiscence after resection and primary anastomosis in left-sided colonic emergencies. Dis Colon Rectum. 2005;48:2272–2280. doi: 10.1007/s10350-005-0159-9. [DOI] [PubMed] [Google Scholar]
- 16.Miransky J, Ruo L, Nicoletta S, Eagan J, Sepkowitz K, Margetson N, Thaler H, Cohen AM, Guillem JG. Impact of a surgeon-trained observer on accuracy of colorectal surgical site infection rates. Dis Colon Rectum. 2001;44:1100–1105. doi: 10.1007/BF02234629. [DOI] [PubMed] [Google Scholar]
- 17.Riou JP, Cohen JR, Johnson H. Factors influencing wound dehiscence. Am J Surg. 1992;163:324–330. doi: 10.1016/0002-9610(92)90014-i. [DOI] [PubMed] [Google Scholar]
- 18.Brooks-Brunn JA. Predictors of postoperative pulmonary complications following abdominal surgery. Chest. 1997;111:564–571. doi: 10.1378/chest.111.3.564. [DOI] [PubMed] [Google Scholar]
- 19.Eichenberger A, Proietti S, Wicky S, Frascarolo P, Suter M, Spahn DR, Magnusson L. Morbid obesity and postoperative pulmonary atelectasis: an underestimated problem. Anesth Analg. 2002;95:1788–1792. doi: 10.1097/00000539-200212000-00060. [DOI] [PubMed] [Google Scholar]
- 20.Hickman DM, Miller RA, Rombeau JL, Twomey PL, Frey CF. Serum albumin and body weight as predictors of postoperative course in colorectal cancer. JPEN J Parenter Enteral Nutr. 1980;4:314–316. doi: 10.1177/014860718000400315. [DOI] [PubMed] [Google Scholar]
- 21.Minoo P, Zlobec I, Peterson M, Terracciano L, Lugli A. Characterization of rectal, proximal and distal colon cancers based on clinicopathological, molecular and protein profiles. Int J Oncol. 2010;37:707–718. doi: 10.3892/ijo_00000720. [DOI] [PubMed] [Google Scholar]
- 22.Lai CC, You JF, Yeh CY, Chen JS, Tang R, Wang JY, Chin CC. Low preoperative serum albumin in colon cancer: a risk factor for poor outcome. Int J Colorectal Dis. 2011;26:473–481. doi: 10.1007/s00384-010-1113-4. [DOI] [PubMed] [Google Scholar]
- 23.Diculescu M, Iacob R, Iacob S, Croitoru A, Becheanu G, Popeneciu V. The importance of histopathological and clinical variables in predicting the evolution of colon cancer. Rom J Gastroenterol. 2002;11:183–189. [PubMed] [Google Scholar]
- 24.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116 Suppl 7A:11S–26S. doi: 10.1016/j.amjmed.2003.12.008. [DOI] [PubMed] [Google Scholar]


