Abstract
AIM: To investigate the association between metabolic syndrome (MetS) and the development of gallstone disease (GSD).
METHODS: A cross-sectional study was conducted in 7570 subjects (4978 men aged 45.0 ± 8.8 years, and 2592 women aged 45.3 ± 9.5 years) enrolled from the physical check-up center of the hospital. The subjects included 918 patients with gallstones (653 men and 265 women) and 6652 healthy controls (4325 men and 2327 women) without gallstones. Body mass index (BMI), waist circumference, blood pressure, fasting plasma glucose (FPG) and serum lipids and lipoproteins levels were measured. Colorimetric method was used to measure cholesterol, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C). Dextrose oxidizing enzyme method was used to measure FPG. Subjects were asked to complete a questionnaire that enquired about the information on demographic data, age, gender, histories of diabetes mellitus, hypertension, and chronic liver disease and so on. Metabolic syndrome was diagnosed according to the Adult Treatment Panel III (ATP III) criteria. Gallstones were defined by the presence of strong intraluminal echoes that were gravity-dependent or attenuated ultrasound transmission.
RESULTS: Among the 7570 subjects, the prevalence of the gallstone disease was 12.1% (13.1% in men and 10.2% in women). BMI, waist circumference, systolic blood pressure, diastolic blood pressure, fasting blood glucose and serum triglyceride (TG) in cases group were higher than in controls, while serum high-density lipid was lower than in controls. There were significant differences in the waist circumference, blood pressure, FPG and TG between cases and controls. In an age-adjusted logistic regression model, metabolic syndrome was associated with gallstone disease. The age-adjusted odds ratio of MetS for GSD in men was 1.29 [95% confidence interval (CI), 1.09-1.52; P = 0.0030], and 1.68 (95% CI, 1.26-2.25; P = 0.0004) in women; the overall age-adjusted odds ratio of MetS for GSD was 1.42 (95% CI, 1.23-1.64; P < 0.0001). The men with more metabolic disorders had a higher prevalence of gallstone disease, the trend had statistical significance (P < 0.0001). The presence of 5 components of the MetS increased the risk of gallstone disease by 3.4 times (P < 0.0001). The prevalence of GSD in women who had 5 components of MetS was 5 times higher than in those without MetS component. The more the components of MetS, the higher the prevalence of GSD (P < 0.0001). The presence of 5 components of the MetS increased the risk of gallstone disease by 4.0 times.
CONCLUSION: GSD appears to be strongly associated with MetS, and the more the components of MetS, the higher the prevalence of GSD.
Keywords: Gallstone disease, Obesity, Hypertension, Dyslipidemia, Metabolic syndrome
INTRODUCTION
China is one of the fastest developing countries. Rapid economic development and industrialization have brought about changes in traditional diets and increasingly sedentary lifestyles. Metabolic syndrome (MetS) is defined as a cluster of multiple cardiovascular risk factors, including central obesity, elevated fasting plasma glucose, high blood pressure, lower high-density lipid (HDL), and higher serum triglyceride (TG) levels. The prevalence of MetS has been increasing gradually in China. In 1992, the overall prevalence of MetS in China was 13.3% (12.7% in men and 14.2% in women)[1]. By 2000, the prevalence of MetS had elevated to 15.1%; 13.6% in men and 16.6% in women[2]. In 2009, a population-based cross-sectional survey in China showed that the crude and age-standardized prevalence of MetS was 31.5% and 30.5%, respectively[3]. Numerous studies have indicated that MetS is closely associated with some common diseases, such as diabetes, hypertension, cardiovascular diseases, cancer, and gallstone disease. Consequently, the increasing prevalence of MetS may potentially associate with the increased prevalence of these diseases. Studies about the association between gallstone disease and MetS suggested that MetS is a risk factor for gallstone disease (GSD)[4], and some studies concluded that GSD might be a component of MetS[4,5] although it needs to be validated by more evidences.
GSD represents a significant burden for health care worldwide[6] and is one of the most common disorders among the patients admitted to the emergency rooms with abdominal discomfort, epigastric pain, nausea, vomiting, loss of appetite, etc[7]. Ethnicity and family traits are recognized as contributing factors[8]. GSD is known to affect 60%-70% of native Americans and a proportionately smaller number of individuals of mixed hispanic/native American origin[9]. The incidence of GSD is at least 10% among white adults in Western countries[10], but it is lower in African Americans and East Asians[9]. GSD is also on the rise and becoming a major health problem in China[11,12]; according to the reported estimates that the prevalence of GSD increased from 4.3% in 1989 to as high as 10.7% in 1995[12]. Risk factors associated with cholelithiasis include female gender, age, obesity, diabetes, hyperlipidemia, rapid appetite loss, hepatitis C, cirrhosis, and high caloric intake[9,13,14]. The association between GSD and MetS has been a focus of some recent studies. To the best of our knowledge, the prevalence of the disease and the association between the development of GSD and MetS are not fully elucidated. Moreover, there is currently only minimal data regarding the relationship between GSD and MetS in apparently healthy Asian people. This study aimed to establish if there is an association between the presence of MetS and the development of GSD. MetS is known to be strongly associated with lifestyle, and if MetS is proved to be related to gallstone, we may reduce the prevalence of gallstones through lifestyle interventions.
MATERIALS AND METHODS
Data resource and data collection
We conducted a cross-sectional study in 7570 subjects enrolled from the Physical Check-Up Center of the Sir Run Run Shaw Hospital. Among them, there were 4978 men, aged 45.0 ± 8.8 years, and 2592 women, aged 45.3 ± 9.5 years. The study protocol was approved by the Ethics Committee of the hospital. All the subjects signed the informed consent. And 918 (653 men and 265 women) subjects were found to have gallstones. The gallstone cases and controls consisted of a series of consecutive asymptomatic subjects. Exclusion criteria included histories of cholecystectomy, pancreatitis, sequela of clonorchis sinensis infection, gallbladder polyps, silt, dimly disease or gallbladder wall thickening, chronic kidney disease, pregnancy, and major gastrointestinal surgeries. Blood samples were collected via venipuncture from the study participants after they had fasted overnight for laboratory tests. Fasting plasma glucose (FPG), TG, total cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) concentrations were measured using the AEROSET analyze system (ADDOTT, America). Colorimetric method was used to measure cholesterol, HDL-C and LDL-C. Dextrose oxidizing enzyme method was used to measure FPG. Ultrasonographic examinations were also done.
Questionnaire
Subjects were asked to complete a questionnaire that enquired for information on demographic data, age, gender, marital status, address, telephone, histories of diabetes mellitus, hypertension, chronic liver disease, hyperlipidemia, systemic diseases, gastrointestinal surgery (vagotomy gastrectomy for peptic ulcer, ileal resection for inflammatory bowel disease, or any other disease or cause), gravidity, and the use of oral contraceptives, any other medications, and family history.
Physical examination
Body weight of the subjects, dressed in light clothing and without shoes, was measured to the nearest 0.10 kg. Height was measured to the nearest 0.5 cm. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared (kg/m2). Waist circumference (at the nearest 0.1 cm) was measured at the midpoint between the lower border of the rib cage and the iliac crest. Three blood pressure readings were obtained at 1-min intervals, and the second and third systolic and diastolic pressure readings were averaged and used in the analyses.
Diagnostic criteria
MetS was diagnosed using the Adult Treatment Panel III (ATP III) criteria. According to the ATP III criteria, MetS was defined as the presence of any three of the following five traits: (1) Abdominal obesity, defined as a waist circumference in men ≥ 90 cm and in women ≥ 80 cm; (2) Serum triglycerides ≥ 150 mg/dL (1.7 mmol/L) or medicinal treatment for elevated TG; (3) Serum HDL cholesterol < 40 mg/dL (1.03 mmol/L) in men and < 50 mg/dL (1.29 mmol/L) in women or medication for low HDL-C; (4) Blood pressure ≥ 130/85 mmHg or medication for high blood pressure; and (5) Fasting plasma glucose (FPG) ≥ 100 mg/dL (5.6 mmol/L) or medication for elevated blood glucose[15].
The diagnosis of GSD was established on the basis of the results of abdominal ultrasound (US) using a 3.5-MHz transducer. US was conducted by an experienced radiologist, who was unaware of the objectives of the study and blinded to laboratory values. Gallstones were defined by the presence of strong intraluminal echoes that were gravity-dependent or that attenuated ultrasound transmission (acoustic shadowing)[16].
Statistical analysis
All statistical analyses were conducted using SPSS version 13.0 (SPSS Inc, Chicago, Ill). The results were expressed as the mean ± SD. Binary variables were summarized by N and percentage. Student t test was applied to compare the differences between cases and controls for all continuous variables. χ2 test was used to compare the differences between cases and controls for all category variables. Age-adjusted logistic regression model was used to test the association between MetS and human gallstones.
RESULTS
Of the 7570 examined subjects, the prevalence of the GSD was 12.1% (13.1% in men and 10.2% in women). The results of univariate analysis of various factors and its relationship with GSD are shown in Table 1. In comparison with subjects without GSD, those with GSD were significantly older and had a higher waist circumference (WC), BMI, systolic blood pressure, diastolic blood pressure, fasting blood glucose and TG. Moreover, subjects with GSD had a significantly lower HDL cholesterol level than those without GSD. The incidence of larger waist circumference, higher blood pressure, increased FPG and TG was obviously higher in cases group than in the controls.
Table 1.
Demographic and clinical characteristics of the study subjects (mean ± SD) n (%)
| Variable | Cases (n=918) | Controls (n=6652) | P values |
| Age (yr) | 48.5 ± 9.1 | 44.7 ± 9.0 | < 0.0001 |
| Height (cm) | 165.6 ± 7.9 | 165.6 ± 7.7 | 0.2791 |
| Weight (kg) | 72.6 ± 11.3 | 69.4 ± 12.2 | < 0.0001 |
| Body mass index (kg/m2) | 26.3 ± 3.0 | 25.2 ± 3.4 | < 0.0001 |
| Waist circumference (cm) | 91.6 ± 9.4 | 87.8 ± 10.7 | < 0.0001 |
| Systolic blood pressure (mmHg) | 123.6 ± 14.3 | 119.8 ± 14.5 | < 0.0001 |
| Diastolic blood pressure (mmHg) | 74.1 ± 9.8 | 72.2 ± 10.4 | < 0.0001 |
| Fasting blood glucose (mmol/L) | 5.39 ± 1.37 | 5.11 ± 1.04 | < 0.0001 |
| High density lipid (mg/L) | 44.6 ± 10.9 | 46.0 ± 0.58 | 0.0004 |
| Triglyceride (mg/L) | 201.1 ± 183.3 | 183.7 ± 182.7 | 0.0069 |
| Male | 653 (71.1) | 4325 (65.0) | 0.0003 |
| Larger waist circumference | 673 (73.3) | 3918 (58.9) | < 0.0001 |
| Higher blood pressure | 401 (43.7) | 2072 (31.2) | < 0.0001 |
| Higher FPG | 146 (15.9) | 600 (9.0) | < 0.0001 |
| Lower high-density lipid | 407 (44.3) | 2842 (42.8) | 0.3551 |
| Higher triglyceride | 473 (51.5) | 3044 (45.8) | 0.0010 |
Student t test was applied to compare the differences between cases and controls for all continuous variables. Larger waist circumference denotes waist circumference ≥ 80 cm for females or ≥ 90 cm for males; higher blood pressure denotes systolic blood pressure ≥ 130 or diastolic blood pressure ≥ 85 mmHg or drug treatment; raised fasting plasma glucose (FPG) denotes FPG ≥ 5.6 mmol/L or drug treatment; raised triglyceride denotes triglyceride ≥ 1.70 mmol/L or drug treatment; lower HDL-C denotes HDL < 1.29 mmol/L for females or < 1.03 mmol/L for males or drug treatment. χ2 test was used to compare the differences between cases and controls for all category variables. HDL: High blood pressure; HDL-C: High-density lipoprotein cholesterol.
In age-adjusted logistic regression analyses (Table 2), MetS was significantly associated with the risk of GSD irrespective of the sex of the subjects. The age-adjusted odds ratio of MetS for GSD was 1.29 [95% Confidence interval (CI), 1.09-1.52; P = 0.0030] in men, and 1.68 (95% CI, 1.26-2.25; P = 0.0004) in women; and the overall age-adjusted odds ratio of MetS for GSD was 1.42 (95% CI, 1.23-1.64; P < 0.0001).
Table 2.
Association of metabolic syndrome with human gallstone n (%)
| MetS status |
Gallstone status |
OR (95% CI) | P values | |
| Cases | Controls | |||
| Male | ||||
| Non-MetS | 343 (11.6) | 2600 (88.4) | 0.0030 | |
| MetS | 310 (15.2) | 1725 (84.8) | 1.29 (1.09-1.52) | |
| Female | ||||
| Non-MetS | 169 (8.4) | 1852 (91.6) | 0.0004 | |
| MetS | 96 (16.8) | 475 (82.2) | 1.68 (1.26-2.25) | |
| Total | ||||
| Non-MetS | 512 (10.3) | 4452 (89.7) | < 0.0001 | |
| MetS | 406 (15.6) | 2200 (84.4) | 1.42 (1.23-1.64) | |
Age-adjusted logistic regression model was used to test the associations between metabolic syndrome (MetS) and human gallstone. OR: Odds ratio; CI: Confidence interval.
We also analyzed the association between the prevalence of GSD and the number of MetS components. Figure 1 shows that the more the components of MetS, the higher the prevalence of GSD in men, and the trend was significant (P < 0.0001). The presence of 5 components of the MetS increased the risk of gallstone disease by 3.4 times (P < 0.0001). We found the same trend in the women. The prevalence of GSD in women who had 5 components of MetS, was 5 times that of those without MetS component. The overall prevalence of GSD, and the trend was also significant (P < 0.0001).
Figure 1.
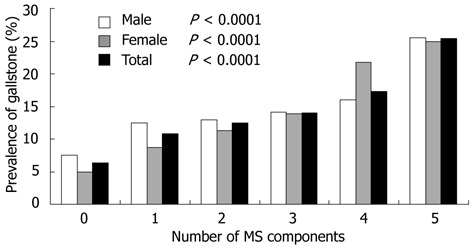
Trend test of the prevalence of gallstone disease and the number of metabolic syndrome components in males, females and total study subjects respectively. Trend test of the prevalence of gallstone disease and the number of metabolic syndrome components in males, in females and in total study subjects. MS: Metabolic syndrome.
DISCUSSION
In China, the study about the prevalence of GSD is rare, and the available studies are not sufficient in sample size or lack of appropriate statistical methods. We designed the cross-sectional study with a large sample of Chinese population. We found that the prevalence of the GSD was 12.1% (13.1% in men and 10.2% in women), which was slightly higher than the figure presented in a previous hospital-based study conducted at the West China Hospital, Sichuan University , which was reported to be 10.7% (9.9% in men and 11.6% in women)[17]. A population-based screening study conducted in Taiwan in 2006 reported that the overall prevalence of GSD was 5.0% (4.6% in men, 5.4% in women), without significant gender differences[18]. The apparently higher prevalence rate in our study may contribute to the Westernized lifestyle of our subjects who were of middle-to-high income class.
According to most of the previous epidemiologic studies, women have a higher prevalence of GSD than men in the Western world, and estrogen is considered to be an obvious factor for the gender difference[19]. However, our findings showed that the prevalence of GSD was higher in men than in women. Actually, gender as a risk factor for cholelithiasis still remains controversial. While the majority of studies conducted in the West have concluded that women are more likely to develop cholelithiasis[20,21] than men, studies among Asian patients have failed to identify a gender-related difference[22,23]. In fact, Liu et al[24] found a higher incidence of cholelithiasis in men than in women below 50 years of age, but a higher incidence in women than in men in age groups above 50 years. And Hung et al[25] indicated that menopause is a risk factor for cholelithiasis in women. Moreover, female predominance is less evident in Asia where the pigmented stone diseases are more common[26]. So the male predominance with GSD in our study may be attributed to the fact that our subjects were of Asian ethnicity and most of them were aged below 50 years, meanwhile most of our female subjects were premenopausal women.
Older age is another significant risk factor for GSD[27,28], so we used age-adjusted logistic regression model to test the associations between MetS and human gallstone. It has been reported that the presence of MetS as an insulin resistance phenotype was associated with an increased prevalence of gallstones[29]. In our age-adjusted logistic regression analysis, MetS was associated with an increased risk of GSD.
Obesity is a major cornerstone of MetS, in our study, the presence of a high waist circumference was common in patients with GSD. A population-based follow-up study on GSD in Kinmen also showed that greater waist circumference was associated with the development of GSD among type 2 diabetics[30]. Cojocaru et al[29] found that waist circumference and BMI were significantly associated with a higher risk of cholesterol gallstone. Obesity is a major risk factor for developing GSD because it can increase hepatic secretion of cholesterol[31].
Although dyslipidemia is very common in MetS, no conclusive evidence links dyslipidemia and GSD. A Korean study demonstrated that HDL cholesterol level was significantly lower in subjects with GSD; however, they did not find any component of dyslipidemia related to MetS that could be correlated with GSD formation[19]. A cross-sectional study in a check-up unit in a university hospital in Mexico City described the influence of low HDL cholesterol on developing GSD (OR = 2.32)[4]. In our study, subjects with GSD had lower HDL cholesterol and higher TG, but there was only difference in the incidence of higher TG between cases and controls. The relationship between HDL and GSD remains unclear. In most patients with higher TG, an association with overweight and insulin resistance is often observed based on the supersaturated (cholesterol) bile and diminished gallbladder motility, both contributing to gallstone formation[14]. Phase separation of cholesterol crystals from supersaturated bile is considered the key event in cholesterol gallstone formation. It is a basal framework of the interactions between the sterol, bile salts and phospholipids in aqueous solutions. Biliary bile acid and phospholipids are important to solubilize cholesterol[32]. Phospholipid transfer protein (PLTP) transfers lipids from low-density lipoproteins to high-density lipoproteins. It was found that an inhibitory effect of haptoglobin over PLTP activity in hyperlipidemic plasma may contribute to the regulation of reverse cholesterol transport[33]. Huang et al[34] revealed a hitherto unrecognized role of protein kinase Cβ (PKCβ) in the fine tuning diet-induced cholesterol and bile acid homeostasis, thus identifying PKCβ as a major physiological regulator of both triglyceride and cholesterol homeostasis. Moreover, polymorphisms in the gene encoding are also found to increase the gallstone risk. Such as the cholesterol transporter ABCG5-G8 and phospholipid floppase ABCB4[32].
Considering the obvious association between gallstone disease and MetS in this study, the fact that higher blood pressure was associated with MetS and GSD appears reasonable. Systolic blood pressure and diastolic blood pressure were higher in patients with GSD as compared with the controls. A study in Taiwan documented that cholelithiasis in Asian obese patients is significantly associated with increased diastolic blood pressure[35]. Blood pressure ≥ 130/85 mmHg was significantly associated with a higher risk of cholesterol gallstone[36]. The mechanism why higher blood pressure increased the risk of GSD still remains unclear. Some scholars considered that this association could be explained by the action of insulin in hypertension. To validate the mechanism, we will further study the relationship between hypertension and GSD. We have designed a study to investigate the association between BP and GSD, as well as the impact of medication for hypertension on GSD.
Previous studies indicated that diabetes mellitus was a risk factor for GSD[37-39]. GSD appeared strongly associated with fasting glycemia[29]. We noted that there was a positive correlation between prevalence of gallstone with higher FPG. The possible mechanisms for this association may be as follows: hyperglycemia inhibits bile secretion from the liver and disturbs gallbladder contraction[40]; hyperglycemia may affect gallbladder motility[41]; or some factors modifying the crystal nucleation and mucous secretion in bile[42].
Finally, GSD appears to be strongly associated with MetS. The result is consistent with the hypothesis that insulin resistance plays an important role in the pathogenesis of GSD. Animal experiments demonstrated that mice with isolated hepatic insulin resistance created by liver-specific disruption of the insulin receptor [Liver insulin receptor knockout (LIRKO) mice] are markedly predisposed towards cholesterol gallstone formation. After only one week on a lithogenic diet, 36% of LIRKO mice developed gallstones and 100% had gallstones by 12 wk[43]. Chang et al[16], showed that insulin resistance was positively associated with gallstones in non-diabetic Korean men, and this occurred regardless of obesity. Taking into account this association, some authors raised the question whether administration of lipid-lowering drug is a therapeutic option for GSD? Ezetimibe was shown to have a beneficial effect against cholelithiasis in both animal and humans[44], it is, therefore, possible to suggest that a clinical trial designed to investigate the potential efficiency of ezetimibe for reducing biliary cholesterol saturation and insulin resistance in populations with a predisposition to cholelithiasis should be now warranted.
In conclusion, GSD is common in China, and the present study shows an obvious association between MetS and GSD, and the more the metabolic components of MetS, the higher the prevalence of the GSD. But the mechanism for the association remains unclear, further research is needed to clarify how BP influences the formation of GSD, whether medication for dyslipidemia benefits the GSD patients, and whether we can reduce the prevalence of GSD through lifestyle interventions.
COMMENTS
Background
China is one of the fastest developing countries. The rapid economic growth and industrialization has brought about changes in traditional diets and lifestyles. The prevalence of metabolic syndrome (MetS) is increasing. But there have been few studies about the prevalence of gallstone disease (GSD), and the association between the development of GSD and MetS is not fully elucidated.
Research frontiers
The authors designed a cross-sectional study with a large sample of Chinese subjects to evaluate the association between MetS and the development of GSD. They concluded that GSD is strongly associated with MetS.
Innovations and breakthroughs
This study demonstrated a strong association between GSD and MetS, but the prevalence of GSD was higher in men than in women. This finding is not consistent with the results from most previous epidemiologic studies. The reason for the discrepancy may be that the subjects in this study are of Asian ethnicity and most of the subjects are below 50 years of age, meanwhile most of the female subjects are premenopausal women.
Applications
There is only minimal data regarding the relationship between GSD and MetS in apparently healthy Asian population. The findings of this study will make it possible to reduce the prevalence of gallstones with appropriate administration of medication and/or lifestyle interventions.
Peer review
This article is well written. This study demonstrated a strong association between GSD and MetS. The presentation of results is logic and the discussion is comprehensive.
Footnotes
Peer reviewers: Vasiliy I Reshetnyak, MD, PhD, Professor, Scientist Secretary of the Scientific Research Institute of General Reanimatology, 25-2, Petrovka str., 107031 Moscow, Russia; Wen Xie, MD, PhD, Assistant Professor, Center for Pharmacogenetics, University of Pittsburgh School of Pharmacy, 656 Salk Hall, 3501 Terrace Street, Pittsburgh, PA 15261, United States
S- Editor Lv S L- Editor A E- Editor Zhang DN
References
- 1.Further Study of Risk Factors for Stroke and Coronary Heart Disease Cooperation Group. The prevalence of metabolic syndrome in a 11 provinces cohort in China. Zhonghua Yufang Yixue Zazhi. 2002;36:298–300. [PubMed] [Google Scholar]
- 2.Gu D, Gupta A, Muntner P, Hu S, Duan X, Chen J, Reynolds RF, Whelton PK, He J. Prevalence of cardiovascular disease risk factor clustering among the adult population of China: results from the International Collaborative Study of Cardiovascular Disease in Asia (InterAsia) Circulation. 2005;112:658–665. doi: 10.1161/CIRCULATIONAHA.104.515072. [DOI] [PubMed] [Google Scholar]
- 3.Zuo H, Shi Z, Hu X, Wu M, Guo Z, Hussain A. Prevalence of metabolic syndrome and factors associated with its components in Chinese adults. Metabolism. 2009;58:1102–1108. doi: 10.1016/j.metabol.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Méndez-Sánchez N, Chavez-Tapia NC, Motola-Kuba D, Sanchez-Lara K, Ponciano-Rodríguez G, Baptista H, Ramos MH, Uribe M. Metabolic syndrome as a risk factor for gallstone disease. World J Gastroenterol. 2005;11:1653–1657. doi: 10.3748/wjg.v11.i11.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nervi F, Miquel JF, Alvarez M, Ferreccio C, García-Zattera MJ, González R, Pérez-Ayuso RM, Rigotti A, Villarroel L. Gallbladder disease is associated with insulin resistance in a high risk Hispanic population. J Hepatol. 2006;45:299–305. doi: 10.1016/j.jhep.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 6.Bodmer M, Brauchli YB, Krähenbühl S, Jick SS, Meier CR. Statin use and risk of gallstone disease followed by cholecystectomy. JAMA. 2009;302:2001–2007. doi: 10.1001/jama.2009.1601. [DOI] [PubMed] [Google Scholar]
- 7.Marschall HU, Einarsson C. Gallstone disease. J Intern Med. 2007;261:529–542. doi: 10.1111/j.1365-2796.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- 8.Wittenburg H, Lammert F. Genetic predisposition to gallbladder stones. Semin Liver Dis. 2007;27:109–121. doi: 10.1055/s-2006-960174. [DOI] [PubMed] [Google Scholar]
- 9.Shaffer EA. Gallstone disease: Epidemiology of gallbladder stone disease. Best Pract Res Clin Gastroenterol. 2006;20:981–996. doi: 10.1016/j.bpg.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep. 2005;7:132–140. doi: 10.1007/s11894-005-0051-8. [DOI] [PubMed] [Google Scholar]
- 11.Huang J, Chang CH, Wang JL, Kuo HK, Lin JW, Shau WY, Lee PH. Nationwide epidemiological study of severe gallstone disease in Taiwan. BMC Gastroenterol. 2009;9:63. doi: 10.1186/1471-230X-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu CM, Tung TH, Liu JH, Lee WL, Chou P. A community-based epidemiologic study on gallstone disease among type 2 diabetics in Kinmen, Taiwan. Dig Dis. 2004;22:87–91. doi: 10.1159/000078740. [DOI] [PubMed] [Google Scholar]
- 13.Acalovschi M, Buzas C, Radu C, Grigorescu M. Hepatitis C virus infection is a risk factor for gallstone disease: a prospective hospital-based study of patients with chronic viral C hepatitis. J Viral Hepat. 2009;16:860–866. doi: 10.1111/j.1365-2893.2009.01141.x. [DOI] [PubMed] [Google Scholar]
- 14.Smelt AH. Triglycerides and gallstone formation. Clin Chim Acta. 2010;411:1625–1631. doi: 10.1016/j.cca.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Heng D, Ma S, Lee JJ, Tai BC, Mak KH, Hughes K, Chew SK, Chia KS, Tan CE, Tai ES. Modification of the NCEP ATP III definitions of the metabolic syndrome for use in Asians identifies individuals at risk of ischemic heart disease. Atherosclerosis. 2006;186:367–373. doi: 10.1016/j.atherosclerosis.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Chang Y, Sung E, Ryu S, Park YW, Jang YM, Park M. Insulin resistance is associated with gallstones even in non-obese, non-diabetic Korean men. J Korean Med Sci. 2008;23:644–650. doi: 10.3346/jkms.2008.23.4.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun H, Tang H, Jiang S, Zeng L, Chen EQ, Zhou TY, Wang YJ. Gender and metabolic differences of gallstone diseases. World J Gastroenterol. 2009;15:1886–1891. doi: 10.3748/wjg.15.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CH, Huang MH, Yang JC, Nien CK, Etheredge GD, Yang CC, Yeh YH, Wu HS, Chou DA, Yueh SK. Prevalence and risk factors of gallstone disease in an adult population of Taiwan: an epidemiological survey. J Gastroenterol Hepatol. 2006;21:1737–1743. doi: 10.1111/j.1440-1746.2006.04381.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim SS, Lee JG, Kim DW, Kim BH, Jeon YK, Kim MR, Huh JE, Mok JY, Kim SJ, Kim YK, et al. Insulin resistance as a risk factor for gallbladder stone formation in Korean postmenopausal women. Korean J Intern Med. 2011;26:285–293. doi: 10.3904/kjim.2011.26.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tazuma S. Gallstone disease: Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic) Best Pract Res Clin Gastroenterol. 2006;20:1075–1083. doi: 10.1016/j.bpg.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Völzke H, Baumeister SE, Alte D, Hoffmann W, Schwahn C, Simon P, John U, Lerch MM. Independent risk factors for gallstone formation in a region with high cholelithiasis prevalence. Digestion. 2005;71:97–105. doi: 10.1159/000084525. [DOI] [PubMed] [Google Scholar]
- 22.Lai SW, Muo CH, Liao KF, Sung FC, Chen PC. Risk of acute pancreatitis in type 2 diabetes and risk reduction on anti-diabetic drugs: a population-based cohort study in Taiwan. Am J Gastroenterol. 2011;106:1697–1704. doi: 10.1038/ajg.2011.155. [DOI] [PubMed] [Google Scholar]
- 23.Novacek G. Gender and gallstone disease. Wien Med Wochenschr. 2006;156:527–533. doi: 10.1007/s10354-006-0346-x. [DOI] [PubMed] [Google Scholar]
- 24.Liu CM, Tung TH, Chou P, Chen VT, Hsu CT, Chien WS, Lin YT, Lu HF, Shih HC, Liu JH. Clinical correlation of gallstone disease in a Chinese population in Taiwan: experience at Cheng Hsin General Hospital. World J Gastroenterol. 2006;12:1281–1286. doi: 10.3748/wjg.v12.i8.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung SC, Liao KF, Lai SW, Li CI, Chen WC. Risk factors associated with symptomatic cholelithiasis in Taiwan: a population-based study. BMC Gastroenterol. 2011;11:111. doi: 10.1186/1471-230X-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stinton LM, Myers RP, Shaffer EA. Epidemiology of gallstones. Gastroenterol Clin North Am. 2010;39:157–169, vii. doi: 10.1016/j.gtc.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Liew PL, Lee WJ, Wang W, Lee YC, Chen WY, Fang CL, Huang MT. Fatty liver disease: predictors of nonalcoholic steatohepatitis and gallbladder disease in morbid obesity. Obes Surg. 2008;18:847–853. doi: 10.1007/s11695-007-9355-0. [DOI] [PubMed] [Google Scholar]
- 28.Festi D, Dormi A, Capodicasa S, Staniscia T, Attili AF, Loria P, Pazzi P, Mazzella G, Sama C, Roda E, et al. Incidence of gallstone disease in Italy: results from a multicenter, population-based Italian study (the MICOL project) World J Gastroenterol. 2008;14:5282–5289. doi: 10.3748/wjg.14.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cojocaru C, Pandele GI. [Metabolic profile of patients with cholesterol gallstone disease.] Rev Med Chir Soc Med Nat Iasi. 2010;114:677–682. [PubMed] [Google Scholar]
- 30.Tung TH, Ho HM, Shih HC, Chou P, Liu JH, Chen VT, Chan DC, Liu CM. A population-based follow-up study on gallstone disease among type 2 diabetics in Kinmen, Taiwan. World J Gastroenterol. 2006;12:4536–4540. doi: 10.3748/wjg.v12.i28.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CH, Huang MH, Yang JC, Nien CK, Yang CC, Yeh YH, Yueh SK. Prevalence and risk factors of nonalcoholic fatty liver disease in an adult population of taiwan: metabolic significance of nonalcoholic fatty liver disease in nonobese adults. J Clin Gastroenterol. 2006;40:745–752. doi: 10.1097/00004836-200609000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Van Erpecum KJ. Pathogenesis of cholesterol and pigment gallstones: an update. Clin Res Hepatol Gastroenterol. 2011;35:281–287. doi: 10.1016/j.clinre.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Henderson RJ, Wasan KM, Leon CG. Haptoglobin inhibits phospholipid transfer protein activity in hyperlipidemic human plasma. Lipids Health Dis. 2009;8:27. doi: 10.1186/1476-511X-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang W, Bansode RR, Xie Y, Rowland L, Mehta M, Davidson NO, Mehta KD. Disruption of the murine protein kinase Cbeta gene promotes gallstone formation and alters biliary lipid and hepatic cholesterol metabolism. J Biol Chem. 2011;286:22795–22805. doi: 10.1074/jbc.M111.250282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liew PL, Wang W, Lee YC, Huang MT, Lin YC, Lee WJ. Gallbladder disease among obese patients in Taiwan. Obes Surg. 2007;17:383–390. doi: 10.1007/s11695-007-9068-4. [DOI] [PubMed] [Google Scholar]
- 36.Misciagna G, Guerra V, Di Leo A, Correale M, Trevisan M. Insulin and gall stones: a population case control study in southern Italy. Gut. 2000;47:144–147. doi: 10.1136/gut.47.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakeeb A, Comuzzie AG, Al-Azzawi H, Sonnenberg GE, Kissebah AH, Pitt HA. Insulin resistance causes human gallbladder dysmotility. J Gastrointest Surg. 2006;10:940–948; discussion 948-949. doi: 10.1016/j.gassur.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Shebl FM, Andreotti G, Meyer TE, Gao YT, Rashid A, Yu K, Shen MC, Wang BS, Han TQ, Zhang BH, et al. Metabolic syndrome and insulin resistance in relation to biliary tract cancer and stone risks: a population-based study in Shanghai, China. Br J Cancer. 2011;105:1424–1429. doi: 10.1038/bjc.2011.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lioudaki E, Ganotakis ES, Mikhailidis DP. Lipid lowering drugs and gallstones: a therapeutic option? Curr Pharm Des. 2011;17:3622–3631. doi: 10.2174/138161211798220909. [DOI] [PubMed] [Google Scholar]
- 40.Chen CY, Lu CL, Huang YS, Tam TN, Chao Y, Chang FY, Lee SD. Age is one of the risk factors in developing gallstone disease in Taiwan. Age Ageing. 1998;27:437–441. doi: 10.1093/ageing/27.4.437. [DOI] [PubMed] [Google Scholar]
- 41.Misciagna G, Leoci C, Guerra V, Chiloiro M, Elba S, Petruzzi J, Mossa A, Noviello MR, Coviello A, Minutolo MC, et al. Epidemiology of cholelithiasis in southern Italy. Part II: Risk factors. Eur J Gastroenterol Hepatol. 1996;8:585–593. doi: 10.1097/00042737-199606000-00017. [DOI] [PubMed] [Google Scholar]
- 42.Kim JM, Lee HL, Moon W, Koh DH, Lee OY, Yoon BC, Choi HS, Hahm JS, Lee MH, Lee DH, et al. [Association between insulin, insulin resistance, and gallstone disease in Korean general population.] Korean J Gastroenterol. 2007;50:183–187. [PubMed] [Google Scholar]
- 43.Zhao YD, Springall DR, Hamid Q, Yacoub MH, Levene M, Polak JM. Localization and characterization of endothelin-1 binding sites in the transplanted human lung. J Cardiovasc Pharmacol. 1995;26 Suppl 3:S336–S340. [PubMed] [Google Scholar]
- 44.Wang HH, Portincasa P, Mendez-Sanchez N, Uribe M, Wang DQ. Effect of ezetimibe on the prevention and dissolution of cholesterol gallstones. Gastroenterology. 2008;134:2101–2110. doi: 10.1053/j.gastro.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


