Abstract
Objective
Design and evaluation of the dietary intake monitoring application (DIMA) to assist varying-literacy patients receiving hemodialysis to adhere to their prescribed dietary regimen.
Methods
An iterative, user-centered design process informed by Bandura's social cognitive theory was employed to design DIMA—a mobile application that utilizes touch-screen, visual interfaces; barcode scanning; and voice recording to assist varying-literacy patients receiving hemodialysis to self-monitor their diet. A pilot field study was conducted where 18 patients receiving hemodialysis were recruited face-to-face from two dialysis facilities to use DIMA for 6 weeks. Subjects recorded their dietary intake using DIMA and met with research assistants three times each week. All interactions with DIMA were logged. Subjects' interdialytic weight gain was recorded throughout the study. At the end of the study, two face-to-face questionnaires were administered to assess usability and context of use.
Results
Subjects were able to use DIMA successfully—12 subjects used DIMA as much or more at the end of the study as they did at the beginning and reported that DIMA helped them change their diet. Subjects had difficulty using the barcode scanner. Viewing past meals was the most used of the reflection mechanisms in DIMA.
Conclusion
Results suggest that while many design features were useful, some could be improved. In particular, future versions of DIMA will be on a smartphone using a camera for barcode scanning, integrate feedback and past meal reflection into the normal flow of the application, and support visual cues when selecting food items.
Keywords: Design, evaluation, electronic monitoring, diet, fluid, hemodialysis, chronically ill, field study, HCI, pervasive healthcare, mobile computing, user-centered design, literacy
Background and significance
Patients receiving hemodialysis have little to no residual kidney function making fluid and electrolyte management key elements of their medical treatment. These patients typically undergo dialysis treatment three times a week and are prescribed a diet that restricts sodium, potassium, phosphorus, and fluid intake.1 Effective dietary self-management helps prevent poor health outcomes. Inadequate self-management may lead to increased blood pressure,2 reduced heart,3 physical,4 and cognitive function,5 reduced bone health,6 and increased mortality.7 8 Unfortunately, the target population often lacks the computational, literacy, and memory skills necessary to track their fluid and nutrient intake.9 10 Indeed, as many as 80% of patients do not restrict their fluid intake11 and 67% do not sufficiently limit their nutrients.12
Electronic dietary self-monitoring can provide patients with just-in-time13 information that can influence their dietary choices at critical decision-making moments. There are currently no dietary monitoring applications that meet the literacy and disease-specific requirements of patients receiving hemodialysis. Most dietary monitoring applications target individuals attempting to lose weight, and utilize text-based input,14–17 which is inappropriate for varying-literacy populations. Alternative methods of data input include scanning receipts and barcodes,13 18–20 both mechanisms which require significant technical sophistication. Taking pictures of food is an easy input mechanism—either as a delayed nutrition analysis tool21 or a reflective prompt.22 Although taking pictures is ideal for low-literacy populations, it is not conducive to real-time feedback.
In applications for patients with chronic disease, researchers have primarily explored how to help people with diabetes monitor and reflect on their dietary intake. A popular trend is to pair images of food with contextual23 or personal health data24 25 to support reflection. However, Arsand and colleagues found subjects were not interested in taking pictures of their food.24
There have been two studies that explored off-the-shelf nutrition monitoring PDA applications to assist patients receiving hemodialysis self-monitor diet.26 27 Dowell and Welch focused their results on the technology form factor,26 whereas Sevick and colleagues measured improved dietary compliance.27 Although both of these studies were encouraging, the small sample size (n<5) and required high literacy, numeracy, and technology knowledge made it unsuitable for the target population.
In this paper, we first describe the design of an offline mobile dietary intake monitoring application (DIMA) that was created by an interdisciplinary team. The team utilized an iterative design process where the needs and abilities of the target patient population were integrated into the final design. A significant challenge was the varying literacy and numeracy in the target population, requiring a non-textual and non-numerical design. We describe the DIMA application and the rationalization for our design choices.
We then provide a user evaluation of DIMA from a 6-week pilot field study with 18 patients receiving hemodialysis. Another paper examines the clinical outcomes of subjects enrolled in the pilot study (unpublished data), whereas this paper focuses on usage and user perceptions. Specifically, our research questions were:
RQ1: Will subjects use DIMA over the 6-week period to monitor their diet?
RQ2: Which features of DIMA are most useful to help subjects monitor their diet?
Methods
We designed DIMA through an iterative, user-centered design process. Our design was informed by Bandura's social cognitive theory28 where we aimed to improve health outcomes by designing a self-monitoring application that provided subjects with the ability to observe, recognize, and change their behavior. We were careful to incorporate foods, beverages, and supplements that were relevant to this particular disease and population.
DIMA application design
DIMA (figure 1) runs on an iPAQ hx2495b PDA (Hewlett-Packard, Palo Alto, California, USA) running Windows Mobile 5.0. We chose a PDA because it is a low-cost, mobile device with a large touch screen. A mobile device provides patients with the ability to record foods and drinks as they consume them within or outside of their homes. DIMA utilizes a Socket SDIO In-Hand Scan Card (SDSC Series 3E; Socket Mobile, Newark, California, USA) barcode scanner for easy food item input, and a touch screen to input those items without barcodes. Computer scientists on the Indiana University Bloomington research team designed and developed DIMA with feedback from the Indiana University Indianapolis health professional research team. Overall, the DIMA application underwent four revisions, three β-tests, and a comprehensive review by health professionals before being deployed for evaluation.
Figure 1.
The dietary intake monitoring application (DIMA) is a mobile, electronic food diary.
Navigation
In a previous study,29 we tested three typical navigation structures with the target population. We found that they make the least mistakes with linear navigation where users traverse down one path and make a single choice on each screen. Once a task is complete, the user is brought back to the beginning or home screen. Consequently, DIMA uses a linear navigation with home buttons on every screen.
Build a meal
We found patients receiving hemodialysis typically think of their daily nutrition in terms of prepared meals (eg, ‘I ate lasagna’), instead of how nutrition monitoring applications typically work—logging individual ingredients that make up a meal (eg, ‘I ate pasta, tomato sauce, and cheese’).30 The build-a-meal metaphor emphasizes this conceptualization of an entire meal. The meal concept is immediately evident on the first DIMA screen (figure 2A) where users can review past meals they have entered by clicking the multiple place settings, or enter a new meal by clicking the single place setting. When the single place setting is clicked, users go to the build-a-meal screen (figure 2B).
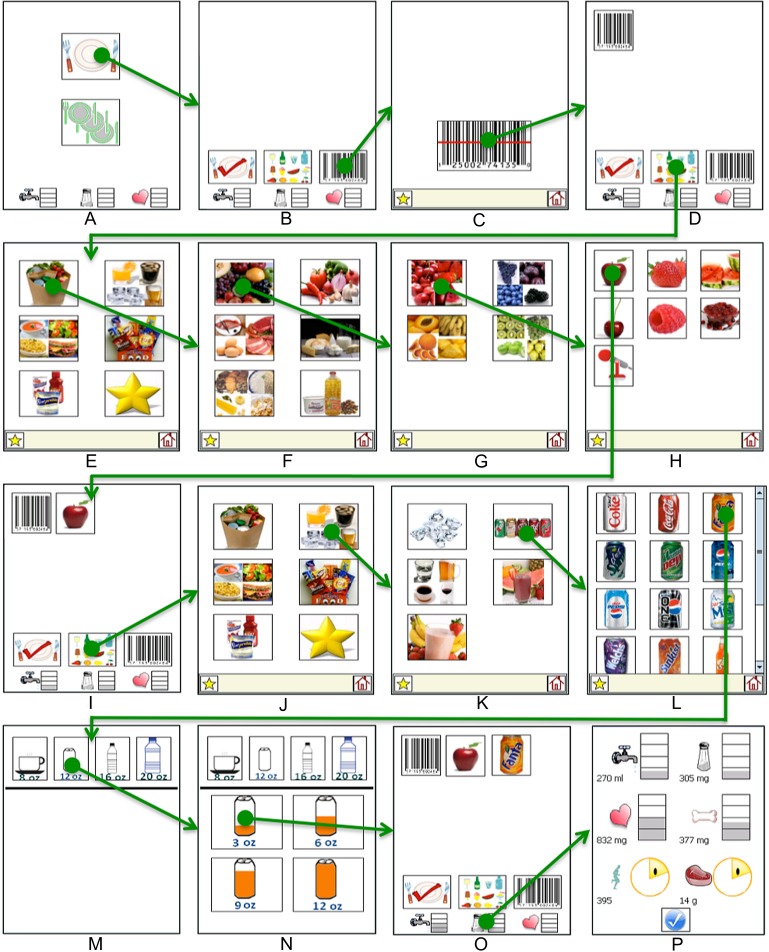
Figure 2.
Application flow to add three items to a meal and then look at detailed feedback: (A) first screen; (B) build-a-meal screen; (C) empty scanning screen; (D) build-a-meal screen with barcode; (E) food category screen for icon selection; counter clockwise from top left: food groups, drinks and ice, snack foods, favorites, supplements, and prepared foods; (F) food group screen; (G) fruits screen; (H) search by color screen for red fruits—voice recording is at the bottom; (I) build-a-meal with apple added; (J) food category screen; (K) drinks and ice screen; (L) soda screen; (M) drink container screen; (N) portion of container consumed screen; (O) build-a-meal with soda added; and (P) detailed feedback screen.
To add a food item from the build-a-meal screen, the user must select either scanning (right) or icon input (middle). To delete a food item, the user taps on the food icon they wish to delete on the build-a-meal screen and confirms the deletion on a subsequent screen.
Barcode scanning
Previous studies showed that this population was capable of scanning barcodes during dialysis sessions and at home.31 Once the user selects entering a food item with scanning, they simply press the single button on the scanning screen and aim the scanner at the barcode (figure 2C). Once a subject successfully scans a barcode, DIMA automatically returns to the build-a-meal screen, and a picture of a barcode is included in the current meal (figure 2D).
Icon interface
When designing the icon interface, there must be a balance between how subjects think about food they consume and information presentation on a small screen. Too many food items on a single screen make it difficult to find the food, so food must be grouped into categories (figure 2E). The USDA food groups are an obvious categorization because dietitians use them when educating patients. In addition, our prior work found patients often think about foods that they should not eat (eg, many snacks) and prepared meals.30 Other important categories are ice and supplements, as patients receiving hemodialysis use ice as a self-care strategy to limit fluid intake and consume liquid supplements to prevent malnutrition. Finally, patients often eat the same foods regularly. Thus, DIMA has a favorites screen to access frequently consumed foods.
Portion sizes
While it may be too overwhelming for a non-technical population to select portion sizes for all foods, DIMA includes a portion interface for fluids because it is critical information to collect and display to patients receiving hemodialysis. As soon as a drink, ice, or supplement is selected, a portion screen appears, prompting the user to select the size of the container (figure 2M) and then the amount they drank (figure 2N). The two-part portion size interface allows subjects to select volumes from 2 to 20 oz.
Voice recording
DIMA includes a mechanism to voice record a food item if users are unable to scan the barcode or find the icon. Voice recorded items are not processed in real time, and thus their nutrient values are not included in the real-time feedback. We previously found that subjects preferred voice recording food items over barcode scanning31; however, they had difficulty recording items that could be parsed in real time.32 Thus for DIMA, the voice recording option is only available after the user either tries but is unsuccessful in scanning a barcode, or navigates the icon screens in an attempt to find a food item that is not present (figure 2H). This design decision forced subjects to attempt to enter food another way and receive real-time feedback before they tried voice recording.
Feedback
Patients receiving hemodialysis must balance their diet by limiting fluid, sodium, potassium, and phosphorus, while ensuring they consume enough calories and protein. Providing real-time feedback is essential to empower patients to make good dietary choices in everyday life. DIMA feedback is tailored to individual patients based on their prescribed diet. DIMA could not include feedback for all six items on every screen because of the limited screen size. Instead, DIMA has a feedback bar at the bottom of the build-a-meal screen that provides the user with the ability to see their consumption levels for the most critical nutrients at a glance—fluid, sodium, and potassium. If users tap on the feedback bar, they are taken to a screen to view all of the monitored nutrients (figure 2P).
The feedback icons for nutrients that should be limited use a fill-up metaphor, where they start empty and gradually fill up as nutrients are consumed, turning red when near their limit.30 In contrast, the two nutrients that patients need to consume a minimum of each day have smiley face feedback icons that are gradually filled in. Patients know they have reached their daily goal when the smiley face is completed.
Study design
The Indiana University Institutional Review Board approved a randomized controlled pilot study of patients receiving hemodialysis, and this paper only reports findings from the intervention (DIMA) group. Subjects were recruited in-person from two dialysis facilities. Subjects were at least 18 years old, alert and oriented, prepared their own meals, on hemodialysis for at least 3 months, and self-reported difficulty with at least one component of their diet. Literacy, numeracy, and technology knowledge were known to be varied in the recruitment sites, and thus were not considered for individual subject recruitment.
Our previous studies testing specific design features in isolation had a clear ‘wow factor’, where subjects actively used the technology for 1–2 weeks, but then usage dropped off dramatically once the technology was no longer novel and exciting.31 A major goal of this study was to see if subjects would use DIMA longer if they received real-time dietary feedback. We were also interested in the specific design considerations discussed in the ‘DIMA application design’ section above. We logged every interface click and constructed rich usage interaction patterns for each subject. Unfortunately, subject 17 lost his PDA during the study, and so we only have a partially complete interaction log for that subject.
Setup
Research assistants (RAs) were trained to use DIMA. The RAs met and trained subjects to use the PDA and DIMA on average for three dialysis sessions. After subjects passed a competency assessment test for DIMA and PDA usage, they entered the 6-week self-monitoring phase.
During self-monitoring, subjects took their PDAs home to record their dietary intake. They met with an RA each subsequent dialysis session. RAs charged the PDAs, downloaded DIMA food logs and saved voice recordings to help the research team identify foods that were not included in DIMA. At the end of the study, RAs verbally administered two usability questionnaires to assess subjects' perceptions of DIMA. Subjects received a total of $25 for completing each interview. Compensation was not linked to DIMA usage.
Subjects
Twenty-four subjects were recruited to use DIMA from two inner-city dialysis units that have varying literacy rates.10 29 Five subjects dropped out after baseline interviews: one subject did not want the research team to access his/her medical records, two subjects had difficulty seeing or using DIMA, one subject had a stroke and one subject became pregnant. Of the remaining 19, one subject withdrew during the intervention delivery because of extended illness. Two additional subjects withdrew post-intervention and prior to completing the final instruments (one had a kidney transplant and one started home dialysis); however, they had completed the usability questionnaires reported here, so we include them in the current analysis. The subject attrition rate for this study was 25%, less than the average attrition rates in our previous work.31 32
In this paper, we report on 18 subjects. Thirteen subjects were female and all were African Americans. On average, subjects were aged 53 years (SD 15.1). Subjects had been on hemodialysis from 3 months to 9 years. The main causes of renal disease were hypertension and type II adult onset diabetes. Each subject had at least one other comorbidity.
Many low-literacy patients refuse to participate in any study that requires reading (including taking a literacy test), because they lose standing with their peers. Further, patients are reluctant to participate in studies outside of the dialysis unit because of the time commitment required by hemodialysis. Thus, we used education levels as a proxy for literacy. In the USA, 76% of adults without a high school diploma and 44% of those with only a high school diploma have basic or below basic literacy skills.33 Four subjects had less than a high school diploma and 14 subjects had only a high school diploma, which would indicate approximately 50% of subjects had basic or below basic literacy levels. This corresponds to a previous study where 60% of the patients in this unit had basic or below basic literacy levels as measured by the Rapid Estimate of Adult Literacy in Medicine test (REALM).34 35
Results
To answer our two research questions, we used four sets of metrics:
Monitoring frequency computed through recorded meal logs
Use of individual features within DIMA using detailed application usage logs
A 27-item post-intervention usability questionnaire developed by Susan Rawl and colleagues, modified for DIMA
A 33-item post-intervention questionnaire measuring the usability of specific features and context of use, developed specifically for the DIMA pilot.
Sample questions from the post-intervention questionnaires are included in box 1. Here, we highlight the most relevant results as we discuss each research question.
Box 1. Sample questions from two post-intervention questionnaires.
Sample questions from the 25-item modified Rawl usability questionnaire (5-point Likert scale)
The PDA was easy to use.
I enjoyed using the PDA.
The feedback pictures on the PDA were helpful.
The PDA made me think about how to change my diet and fluid intake.
Sample questions from a 33-item context-of-use and usability questionnaire
When you use the PDA, do you find the feedback clear and understandable?
When you use the PDA, do you use it at home?
When you use the PDA, do you use it when you eat away from home?
When you use the PDA, do you take it when you leave the house?
When you use the PDA, do you think that the feedback applies to you?
When you use the PDA, do you change what you eat and drink based on the feedback?
When you use the PDA, do you ever use it in front of friends?
When you use the PDA, do you ever use it in front of strangers?
When you use the PDA, do you ever use it in a restaurant?
When you use the PDA, do you ever use it at home?
When using the PDA, was it easy or hard for you to use the scanner?
When using the PDA, was it easy or hard for you to select the icons on the screen?
When using the PDA, was it easy or hard for you to use the voice recorder?
When using the PDA, was it easy or hard for you to find a food on the screen that you have found before?
When using the PDA, was it easy or hard for you to find a food on the screen for the first time?
Did you find it easy or hard to find the food you are looking for with the icons?
Did you find it easy or hard to find the feedback about your water and diet intake?
Did you find it easy or hard to see the total meal you have entered?
Did you find it easy or hard to understand the information on the screen?
RQ1: Will subjects use DIMA over the 6-week period to monitor their diet?
Seventeen subjects felt the PDA was easy to use and enjoyed using it. All subjects used DIMA in their homes; however, six did not use DIMA while they were out or around strangers.
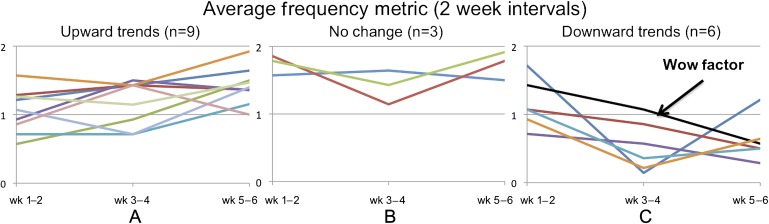
We defined subjects' monitoring frequency in terms of how many times they input food items during a day. A high rating (2) indicates a subject entered food items at different times during the day and thus could make the most of the real-time feedback for decision making. A moderate rating (1) was for when subjects entered items only once a day, and thus could reflect on their meal choices but were less likely to benefit from real-time feedback throughout the day. A low rating (0) was for when subjects did not enter any food items in a day. We highlighted the frequency trends in figure 3 by plotting the 2-week average frequencies for all subjects. Figure 3A shows subjects who increased their frequency by more than 10% (n=9), figure 3C shows subjects who decreased their frequency by more than 10% (n=6), and figure 3B shows the subjects (n=3) who stayed within 10% of their monitoring frequency from the beginning to the end of the study.
Figure 3.
Two-week frequency averages by subjects with (A) upward trends, (B) no change, and (C) downwards trends.
In previous studies, we found subjects typically undergo a wow factor phase where they use an application a lot in the beginning of the study and then decrease participation because they lose interest over time. An example of this is shown in figure 3C, where the line points to a subject who had a monitoring score of 1.43 in the first 2 weeks of the study, but had dropped off significantly by the last 2 weeks (monitoring score=0.57). While there were six subjects who demonstrated the wow factor to varying degrees, the majority of subjects (n=12) had little change in monitoring frequency or actually increased frequency by the end of the study. Thus, we conclude that DIMA was useful for the majority of subjects to continue using the application for the entire 6 weeks when compared to our previous study where subjects did not receive feedback and did not regularly participate after 2 weeks.31
Despite this continued usage, subjects did not record everything they consumed. Over the course of the study, the average interdialytic weight gain of subjects was 0.84 kg, which equates to approximately 840 ml of fluid consumption. We found that the average fluid intake recorded with DIMA per day for each subject was 471 ml—56% of what they actually consumed.
RQ2: Which features of DIMA are most useful to help subjects monitor their diet?
Application logs showed that with the exception of barcode scanning, subjects were able to use most features within DIMA. The icon interface was used an average of 445 times per subject, whereas scanning was used an average of 42 times per subject. Subjects could not successfully scan an item 87.9% of the time they attempted to do so. Only four subjects successfully scanned an item on their own. Of the 3622 items entered by subjects with the icon interface, 43% could reasonably be expected to have had barcodes.
Fourteen subjects thought it was easy to find foods using the icon interface as opposed to nine subjects reporting the scanner as an easy input mechanism. Although 10 subjects thought finding a food item was difficult the first time they attempted to find the item via the icon interface, 15 subjects reported that it was easy to find the food the next time they tried to find the item.
A total of 71 voice recordings were made by six subjects during the study—12 recordings had multiple food items. Of the total 95 items recorded, 18 could have been scanned and 39 entered with icons.
The disease-specific interface components were used by subjects. The most frequently used was ice, which was selected a total of 140 times by 15 subjects. In addition, seven subjects recorded supplements over the course of the study for a total of 53 times.
Reflection on diet
DIMA provides two ways for subjects to reflect on their dietary intake—viewing intake levels and reviewing past meal recordings. Subjects viewed their past meals (average=93) more than their full feedback levels (average=70). Subjects could always view the three most important feedback levels on the first screen and build-a-meal screen, making it difficult to fully evaluate feedback through the usage logs.
Reflection: All subjects who viewed past meals started a new meal soon afterwards. Thirteen subjects started or ended their day by reflecting on their past meals for 11.61 days during the 42-day study. Subjects reflected more on past meals during the first 2 weeks of the study (43.11% during this time).
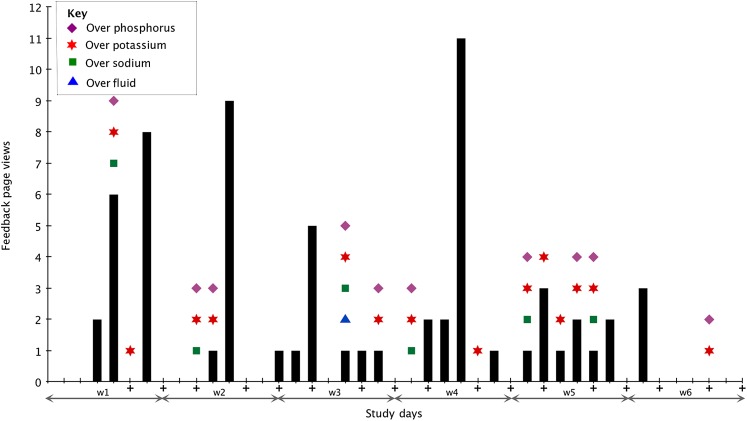
Feedback use: All subjects agreed that the feedback icons were helpful in monitoring their dietary intake, but one subject noted that the feedback was not understandable. Nine subjects rarely went over limits and thus sporadically looked at the feedback page. The other nine subjects viewed the feedback page at least twice a week throughout the study. We expected subjects to look at their feedback levels more after they went over their limits and then not exceed the limits as much, as shown in figure 4 during week 2; indeed three subjects were over their limits until they began accessing the feedback page at the end of the study. Six subjects continued to go over their limits even after looking at the feedback page.
Figure 4.
Overview of subject 7's feedback page views and days over limits. The + under the number indicates meetings with research assistants.
To further examine subject usage of specific interface components, we split subjects' 2-week average frequency metric into three groups: high (>1.3), medium (≥0.6, ≤1.3), and low (<0.6). Table 1 summarizes the results from one-way ANOVA testing for frequency of using the different input mechanisms (Icon, Scan, and Voice) and reflection mechanisms (PastMeals and Feedback). Frequency of using the feedback page differed significantly (F(2,45)=4.31, p=0.019). Post-hoc Tukey–Kramer tests showed that the high group used feedback significantly more than the other two groups at the 0.05 level of significance. There was no statistically significant difference between the medium and low groups. Overall, frequency of using the PastMeals page missed the significant cut-off (F(2,45)=3.01, p=0.059), but Tukey–Kramer post hoc comparisons indicate that the high group used PastMeals significantly more than the low group.
Table 1.
One-way ANOVA results for interface component by three DIMA usage levels based on 2-week average frequency: L<0.6, 0.6≤M≤1.3, 1.3<H
| Interface component | F score | p Value | Tukey–Kramer (0.05), mean difference (honesty significant difference) | ||
| H–M (HSD) | H–L (HSD) | M–L (HSD) | |||
| Icon | F(2,45)=10.85 | 0.00014* | 4.72 (4.62)* | 10.83 (5.73)* | 6.10 (5.87)* |
| Voice Record | F(2,45)=1.70 | 0.20 | |||
| Scan | F(2,45)=1.26 | 0.29 | |||
| Feedback | F(2,45)=4.31 | 0.019* | 1.22 (1.22)* | 1.51 (1.51)* | 0.29 (1.55) |
| PastMeals | F(2,45)=3.01 | 0.059 | 0.43 (1.37) | 1.72 (1.70)* | 1.23 (1.74) |
Significant results.
H, high; L, low; M, medium.
Icon use differed significantly across all three groups (F(2,45)=10.85, p=0.00014), and post hoc Tukey–Kramer tests showed significant differences between all pairs of groups at the 0.05 significance level. There were no statistically significant differences between groups for using scanning or voice recording input.
Discussion
One limitation of the study was that given the difficulty in obtaining actual consumption levels, we do not know how compliant subjects were in monitoring. Interdialytic weight gain values do tell us subjects only recorded 56% of the fluid they consumed, so we would expect similar percentages for non-fluid foods as well. Another limitation is that we used a proxy for literacy instead of measuring literacy directly. Our proxy is consistent with a prior study that measured literacy with this population,35 increasing our confidence that it is sufficient.
The majority of subjects could and did use DIMA to monitor their nutrient and fluid intake. Only six subjects significantly decreased their DIMA usage during the field trial—in comparison to our previous work where all subjects decreased their usage after the wow factor wore off. One of the major differences in this study is that subjects received personalized, real-time feedback based on what they input. All subjects felt the feedback was directly applicable to them and the majority reported that using DIMA caused them to change (n=11) or think about how to change (n=17) their diet. Subjects who used DIMA more viewed the feedback page significantly more as well. Other research also provided real-time feedback, but had decreased usage by the end of the study.17
Table 2 summarizes the key results that suggest changes to the DIMA design. When we started this project, low-cost, touch-screen smartphones were not available. The next iteration of DIMA will be on a smartphone platform. While smartphones have a similar affordance to a PDA, the phone functionality makes them more convenient. DIMA on a smartphone is likely to result in fewer subjects forgetting the device at home; however, the shorter battery life will require subjects to remember to charge the phone. We must carefully consider privacy, usability, and cost issues to determine if DIMA should synchronize with a remote personal or electronic health record. While such synchronization may be desired by healthcare workers, the necessary data plan is currently too costly for many subjects with low-socioeconomic status, and subjects may not want to share the information with providers. Indeed, many subjects over the course of this project have specifically indicated they were not always truthful to their providers about their eating habits, and would be reluctant to use a technology that shared such information.
Table 2.
Results that suggest a needed change to DIMA, along with associated suggested changes
| Result | Suggestion for design | Issues to consider |
| Some subjects did not take DIMA out of the home and/or did not use DIMA in front of strangers. | Make DIMA available on a smartphone. The phone features will encourage users to have DIMA with them at all times, and the phone is less likely to have a stigma associated with it when used in the presence of others. | Battery will drain faster, so may need to remind users to charge phone. Additional monthly cost of phone, and potentially a data plan. |
| Subjects only recorded on average 56% of fluid intake in DIMA. | Include a reminder feature during common eating times. With phone implementation, context-aware reminders can also be used, based on the location of the subject and past meal entry times. | Reminders can become irritating, resulting in users ignoring them. |
| Subjects failed most of the times they attempted scanning. | Use the camera feature in the phone to take a picture of the barcode, which is easier to use than the socket scanner used in this study. | |
| Most subjects did not scan when a barcode was available. | In addition to change in scanner technology, include a scanned item picture in the current meal, instead of a barcode picture. | Several thousand barcodes must be associated with a picture in the database. |
| Some subjects started a new meal immediately after viewing PastMeals. Some subjects often viewed PastMeals at the beginning or end of the day. PastMeals was used more often during the first 2 weeks. | Support easier viewing of past meals by altering the first page so it has one button to start a new meal at the top third of the screen, with the bottom of the screen containing a review of the most recent meals. Thus, past meals are always easily viewable before starting a new meal. | For evaluation, it will be difficult to measure if subjects view PastMeals, since they will no longer have to perform an action to access it. |
| Some subjects stayed within their limits only after starting to view feedback near the end of the study. | Incorporate viewing full feedback automatically: after saving the current meal, have DIMA go to the feedback page. User must acknowledge feedback to continue to the first page. | Again, it will be difficult to measure if subjects look at the full feedback, although in this case, we can detect how long they keep the feedback page open before accepting it and returning to the first page. |
| Some subjects continued to go over their limits after reviewing feedback. | Incorporate visual cues as subjects select food icons: foods that will keep consumption of critical nutrients within limits have a different background/border than foods that will exceed limits. |
The smartphone will allow us to integrate context-aware reminders to remind subjects to record meals when they are most likely to be eating (based on location and time). In addition, we will switch to an easier-to-use camera-based scanner and incorporate images of the items scanned on the build-a-meal page, not just a generic barcode icon.
Finally, we plan to enhance our feedback and reflection mechanisms by making them viewable by default: the first page will provide a view of the most recently consumed meals, the full feedback page will be shown every time a meal is saved, and visual cues will be integrated during food entry to highlight foods that will not place the subject over their daily limits. We are currently making these modifications before we plan a longer and larger field study.
Conclusions
This study provides insights and validates many of the design choices suggested by our prior user studies. Two-thirds of subjects continued to use the application and reflect on their diet over a 6-week period—bypassing the typical wow factor period. The usage logs and user feedback also suggest changes to the DIMA design.
Footnotes
Contributors: All of the authors contributed to the design of the study, the performance of the study, and the writing of the paper.
Funding: This research was supported by a grant made available by the National Institute of Biomedical Imaging and Bioengineering (R21 EB007083), a National Science Foundation research infrastructure grant (NSF RI Grant CNS-0202048 A004), and a T32 Postdoctoral Training Grant (NIH T32 NR007066).
Competing interests: None.
Ethics approval: This research was approved by the Institutional Review Board of Indiana University.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Denhaerynck K, Manhaeve D, Dobbels F, et al. Prevalence and consequences of nonadherence to hemodialysis regimens. Am J Crit Care 2007;16:222–35 [PubMed] [Google Scholar]
- 2.Inrig JK, Patel UD, Gillespie BS, et al. Relationship between interdialytic weight gain and blood pressure among prevalent hemodialysis patients. Am J Kidney Dis 2007;50:108–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunal AI, Karaca I, Aygen B, et al. Strict volume control and left ventricular hypertrophy in hypertensive patients on chronic haemodialysis: a cross-sectional study. J Int Med Res 2004;32:70–7 [DOI] [PubMed] [Google Scholar]
- 4.Christensen AJ, Smith TW, Turner CW, et al. Family support, physical impairment, and adherence in hemodialysis: An investigation of main and buffering effects. J Behav Med 1992;15:313–25 [DOI] [PubMed] [Google Scholar]
- 5.Dogukan A, Guler M, Yavuzkir MF, et al. The effect of strict volume control on cognitive functions in chronic hemodialysis patients. Ren Fail 2009;31:641–6 [DOI] [PubMed] [Google Scholar]
- 6.Martin KJ, Gonzalez EA. Prevention and control of phosphate retention/hyperphosphatemia in CKD-MBD: what is normal, when to start, and how to treat? Clin J Am Soc Nephrol 2011;6:440–6 [DOI] [PubMed] [Google Scholar]
- 7.Acchiardo SR, Moore LW, Burk L. Morbidity and mortality in hemodialysis patients. ASAIO Trans 1990;36:M148–51 [PubMed] [Google Scholar]
- 8.Port FK, Pisoni RL, Bragg-Gresham JL, et al. DOPPS estimates of patient life years attributable to modifiable hemodialysis practices in the United States. Blood Purif 2004;22:175–80 [DOI] [PubMed] [Google Scholar]
- 9.Evans JD, Wagner CD, Welch JL. Cognitive status in hemodialysis as a function of fluid adherence. Ren Fail 2004;26:575–81 [DOI] [PubMed] [Google Scholar]
- 10.Welch JL, Siek KA, Connelly KH, et al. Merging health literacy with computer technology: self-managing diet and fluid intake among adult hemodialysis patients. Patient Educ Couns 2010;79:192–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings KM, Becker MH, Kirscht JP, et al. Psychosocial factors affecting adherence to medical regiments in a group of hemodialysis patients. Med Care 1982;20:567–80 [DOI] [PubMed] [Google Scholar]
- 12.Brown J, Fitzpatrick R. Factors influencing compliance with dietary restrictions in dialysis patients. J Psychosom Res 1988;32:191–6 [DOI] [PubMed] [Google Scholar]
- 13.Intille SS, Kukla C, Farzanfar R, et al. Just-in-time technology to encourage incremental, dietary behavior change. AMIA Annu Symp Proc 2003:874. [PMC free article] [PubMed] [Google Scholar]
- 14.CalorieKing. http://www.calorieking.com
- 15.DietMatePro. http://www.dietmatepro.com
- 16.MyPyramidTracker. http://www.mypyramidtracker.gov
- 17.Tsai C, Lee G, Raab F, et al. Usability and feasibility of PmEB: a mobile phone application for monitoring real time caloric balance. Mobile Network Appl 2007;12:173–84 [Google Scholar]
- 18.DailyBurn. http://www.dailyburn.com/foodscanner
- 19.Mankoff J, Hsieh G, Hung HC, et al. Using low-cost sensing to support nutritional awareness. Proc. of 4th International Conf. on Ubiquitous Computing, Götebörg, Sweden. Springer, 2002:371–8 [Google Scholar]
- 20.QuickCalories. http://www.quickcalories.com
- 21.MyFoodPhone. http://www.mycanutrition.com
- 22.Frost J, Smith BK. Visualizing health: imagery in diabetes education. Proc Designing for User Experiences. 2003:1–14 [Google Scholar]
- 23.Aarhus R, Ballegaard S, Hansen T. The eDiary: bridging home and hospital through healthcare technology. In: Wagner I, Tellioglu H, Balka E, et al., eds. ECSCW 2009. London: Springer, 2009:63–83 [Google Scholar]
- 24.Arsand E, Tufano JT, Ralston JD, et al. Designing mobile dietary management support technologies for people with diabetes. J Telemed Telecare 2008;14:329–32 [DOI] [PubMed] [Google Scholar]
- 25.Smith BK, Frost J, Albayrak M, et al. Integrating glucometers and digital photography as experience capture tools to enhance patient understanding and communication of diabetes self-management practices. Personal and Ubiquitous Computing 2007;11:273–86 [Google Scholar]
- 26.Dowell SA, Welch JL. Use of electronic self-monitoring for food and fluid intake: a pilot study. Nephrol Nurs J 2006;33:271–7 [PubMed] [Google Scholar]
- 27.Sevick MA, Piraino B, Sereika S, et al. A preliminary study of PDA-based dietary self-monitoring in hemodialysis patients. J Ren Nutr 2005;15:304–11 [DOI] [PubMed] [Google Scholar]
- 28.Bandura A. Social Foundations of Thought and Action: a Social Cognitive Theory. Englewood Cliffs, NJ: Prentice Hall, 1986:617 [Google Scholar]
- 29.Chaudry BM, Connelly KH, Siek KA, et al. Mobile interface design for low-literacy populations. In Proceedings of the 2nd ACM SIGHIT International Health Informatics Symposium (IHI ′12), New York, NY, USA: ACM, 2012:91–100 doi:10.1145/2110363.2110377 [Google Scholar]
- 30.Siek KA, Connelly KH, Rogers Y. Pride and prejudice: learning how chronically ill people think about food. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. Montreal, Quebec, Canada: ACM, 2006:947–50 [Google Scholar]
- 31.Siek KA, Connelly KH, Rogers Y, et al. When do we eat? An evaluation of food items input into an electronic food monitoring application. Proceedings of the International Conference Pervasive Computing Technology for Healthcare. Innsbruck, Austria: IEEE, 2006 [Google Scholar]
- 32.Siek KA, Connelly KH, Chaudry B, et al. Evaluation of two mobile nutrition tracking applications for chronically ill populations with low literacy skills. In: Tan J, Olla O, eds. Mobile Health Solutions for Biomedical Applications. Hershey, PA: IGI Global, 2009:1–23 [Google Scholar]
- 33.Kutner M, Greenberg E, Jin Y, et al. The Health Literacy of America's Adults: Results From the 2003 National Assessment of Adult Literacy (NCES 2006–483). Washington, DC: U.S.Department of Education: National Center for Education Statistics, 2006 [Google Scholar]
- 34.Murphy PW, Davis TC, Long SW, et al. Rapid estimate of adult literacy in medicine: a quick reading test for patients. J Read 1993;37:124–30 [Google Scholar]
- 35.Chaudry B, Connelly KH, Siek KA, et al. The design of a mobile portion size estimation interface for a low literacy population. 5th International Conference on Pervasive Computing Technologies for Healthcare. Dublin, Ireland: IEEE, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]