Abstract
The ability to detect and monitor bladder cancer in noninvasively obtained urine samples is a major goal. While a number of protein biomarkers have been identified and commercially developed, none have greatly improved the accuracy of sample evaluation over invasive cystoscopy. The ongoing development of high-throughput proteomic profiling technologies will facilitate the identification of molecular signatures that are associated with bladder disease. The appropriate use of these approaches has the potential to provide efficient biomarkers for the early detection and monitoring of recurrent bladder cancer. Identification of disease-associated proteins will also advance our knowledge of tumor biology, which, in turn, will enable development of targeted therapeutics aimed at reducing morbidity from bladder cancer. In this article, we focus on the accumulating proteomic signatures of urine in health and disease, and discuss expected future developments in this field of research.
Keywords: biomarker, bladder cancer detection, molecular signature, oncoproteomics, urinalysis
Cancer of the urinary bladder is among the five most common malignancies worldwide [1]. Transitional cell carcinomas (TCCs) are the most common urothelial tumors in Western countries and constitute approximately 95% of all cases [2]. Early detection remains one of the most urgent issues in bladder cancer research. New urinary bladder cancer cases for 2009 are estimated to be 70,980, with estimated deaths at 14,330 [101]. When detected early, the 5-year survival rate is approximately 94%; therefore, timely intervention increases patient survival rate dramatically. Urothelial tumors can be classified into two groups based on histopathology and clinical behavior. At presentation, more than 80% of bladder tumors are non-muscle-invasive papillary tumors (pTa or pT1). The remaining 20% of tumors show muscle invasion at the time of diagnosis and have a much less favorable prognosis. While radical surgery is required for invasive bladder tumors, superficial lesions are treated more conservatively by transurethral resection. However, more than 70% of patients with Ta/T1 lesions confined to the mucosa have recurrence during the first 2 years. If left untreated, these initially superficial lesions can progress to being muscle invasive [3]. The gold standard for initial clinical diagnosis and staging of bladder cancer involves cystoscopic examination of the bladder. Cystoscopy is an unpleasant, invasive procedure that involves anesthetizing the patient and resection of biopsies for histopathological diagnosis and staging. The recurrence phenomenon of superficial bladder tumors is what makes bladder cancer one of the most prevalent cancers worldwide. Patients with superficial tumors are under continued surveillance by routine cystoscopy examinations of the bladder for the early detection of new tumor developments. Once bladder tumors are identified and removed, patients will routinely get surveillance cystoscopy every 3 months for 2 years, then every 6 months for 2 years, then yearly thereafter. Consequently, the development of noninvasive urinalysis assays using reliable diagnostic markers would be of tremendous benefit to both patients and healthcare systems.
Urinalysis for bladder cancer detection
Voided urine cytology (VUC) remains the method of choice for the noninvasive detection of bladder cancer. This method microscopically examines the morphology of the cells of the bladder lining, which are collected from the urine. The method is subjective and is open to considerable interobserver variation and, thus, accuracy is a problem, especially for low-grade and low-stage tumors [4,5]. Furthermore, results are not available rapidly, it is prone to interobserver variation and it is relatively expensive. Understandably, a good deal of research has focused on identifying potential urine tumor markers with higher accuracy than urine cytology. Promising commercially developed diagnostic protein markers for the urinary detection of bladder cancer include NMP-22 and BTA, and others with potential have been reported in the literature (Table 1). Unfortunately, these tests suffer from high false-positive rates and, thus, there is no protein test available to date that can replace urine cystoscopy or cytology [6–9]. The inadequate power of single markers must partly explain this. The concept that the presence or absence of one molecular marker will aid diagnostic or prognostic evaluation has not proved to be the case. To identify informative molecular signatures of bladder cancer in urine requires high-throughput proteomic profiling and sophisticated bioinformatics tools for complex data analysis and pattern recognition. Evolving proteomic techniques, such as TOF mass spectrometry (MS), SDS/ PAGE with MALDI-MS, and liquid-phase 2D separation techniques, greatly facilitate the detailed and systematic isolation, identification and characterization of proteins in complex biological samples. Some cancers are more amenable than others to early diagnosis by biochemical testing. Urine as a biological sample source has several advantages relative to other biological fluids. It is accessible in copious amounts through natural micturition and, thus, enables repeat or temporal sampling. Unlike blood, urinary proteins and peptides are relatively stable and require only routine processing prior to analyzing or archiving [10,11]. A disadvantage of urine sampling is the wide range of variability in protein concentrations between individual samples but this can be overcome by normalization to urinary creatinine or to peptides that are present in the urine irrespective of age, gender and health status [12]. In order to identify biomarkers of disease it is advantageous to have some idea of the normal proteome in the sample source. Consequently, much effort has been focused on defining a normal/healthy urinary proteome. Normal human urine contains up to 150 mg/24 h of protein. This protein originates both from the ultrafiltration of plasma and from natural turnover and secretion from the urinary tract itself, represented largely by the urothelium lining the bladder.
Table 1.
Protein-based urine tests for bladder cancer detection.
| Test | Molecule detected | Detection | Ref. |
|---|---|---|---|
| Bladder tumor antigen | Complement factor H-related 1 | ELISA | [9] |
| Urinary bladder cancer test | Fragments of cytokeratins 8 and 18 | ELISA | [8,60] |
| CYFRA21.1 | Fragments of cytokeratin 19 | ELISA | [61] |
| ImmunoCyt | Mucin-like antigen and high-molecular-weight glycosylated form of carcinoembryonic antigen | Fluorescent monoclonal antibodies | [62,63] |
| NMP22 | Member of the nuclear matrix family of proteins | ELISA | [64–67] |
| Hyaluronic acid and hyaluronidase | Hyaluronic acid and its degrading enzyme hyaluronidase | ELISA-like test | [68] |
| Soluble Fas | Soluble forms of Fas (TNF receptor superfamily, member 6) generated by alternative splicing | ELISA | [69,70] |
| BLCA-4 | Member of the nuclear matrix family of proteins | Indirect immunoassay or ELISA | [71,72] |
Urinary proteome
The establishment of a normal urinary proteome has evolved hand-in-hand with the improvement of proteomic techniques, and every new study that includes healthy controls contributes extra information. Early studies used 2D PAGE techniques [13–16], high-perfomance liquid chromatography (HPLC)-ESI-mass spectrometry (MS) [17] and LC-MS/MS [16] to identify over 100 protein components from unfractionated normal urine. In 2003, Wittke et al. used capillary electrophoresis coupled with ESI-TOF MS to obtain a profile of the peptides present in the urine of healthy subjects [18]. A ‘normal urine polypeptide pattern’ consisting of 247 polypeptides was obtained, but no protein identification was performed; hence, it is unclear how many intact proteins the detected peptides would collapse into. In 2004, a 2D PAGE survey identified 1400 distinct spots, of which, over 400 peptides and 150 unique proteins were identified [19]. As techniques become more sensitive, so the number of detected urinary proteins has increased. Using acetone precipitation and a combination of three separation approaches, a total of 226 urinary proteins were detected and the majority were identified [20]. Employing a bead-based concentration and differential elution technique, Castagna et al. identified 383 gene products in human urine, substantially adding to the catalog of the urinary proteome at that time [21]. Confirmation of this level of complexity was achieved using the application of a variety of different sample preparation methods coupled with nano-HPLC-ESI-MS/MS followed by peptide fragmentation pattern. Tyan et al. demonstrated that healthy-subject urine samples contained a total of 2283 peptides, corresponding to 311 unique proteins [22]. The most recent analyses of the urinary proteome have suggested that there are over 1000 proteins and as many as 5000 reasonably abundant peptides detectable in urine using highly sensitive techniques [23]. Combining 1D PAGE and reverse-phase LC coupled to (Orbitrap) MS, Adachi et al. estimated the number of identified urinary proteins to be 1500 [24]. Compared with the estimated 5000–10,000 proteins detectable in the serum, and potentially the majority of the human proteome found in solid tissues, urine is a less complex biological sample in which to identify specific disease-associated protein biomarkers. These studies focus primarily on the soluble proteins, but there is another source of protein found in the urine – those present in exosomes. Normal human urine contains large numbers of exosomes, which are 40- to 100-nm vesicles that originate from the renal epithelia facing the urinary space. Exosomes can be isolated from urine by either high-speed centrifugation or ultrafiltration, and studies on the protein content have been performed [25,26], primarily for biomarker discovery in renal disease.
The construction of biological-sample proteome databases greatly enables the standardization of data and the accurate comparison of results from multi-institutional healthy and patient cohorts. A number of databases have been created in order to integrate human protein data from a diversity of tissues and fluids, and multiple technological platforms. The Human Proteome Organization (HUPO) was founded in 2001 to organize data from multiple laboratories and to facilitate scientific collaborations. In 2007, HUPO initiated the Human Kidney and Urine Proteome Project (HKUPP) in order to better understand kidney function and disease, but also to establish standard protocols and guidelines for the proteome analysis of urine [27]. A European initiative (EuroKUP) to address the standardization of proteomic urinalysis is also under way [28], but guidelines and dataset access from HKUPP and EuroKUP are awaited. A large-scale effort to map body fluid proteomes is included in the Max-Planck Unified (MAPU) proteome database. MAPU contains several body-fluid proteomes, including plasma, urine and cerebrospinal fluid, mapped at the institute using MS techniques [29]. By employing high-resolution MS and stringent validation criteria, false-positive identification rates in MAPU are lower than 1:1000. Thus, MAPU datasets can serve as a reference for biomarker discovery in body fluids [29]. MAPU contains the peptides identifying each protein, measured masses, scores and intensities, and is freely available (Table 2). The recently created Sys-BodyFluid database [30] is an expansion of MAPU data through the curation of related literature and further annotation, and provides a comprehensive reference database for body fluid and clinical proteomics research (Table 2).
Table 2.
Proteome database resources that include urinary data.
| Database | URL | Ref. |
|---|---|---|
| Human Proteome Organization |
www.hupo.org www.hupo.org/research/urine |
[102] |
| Human Kidney and Urine Proteome Project | www.hkupp.org | [27] |
| European Kidney and Urine Proteomics | www.eurokup.org | [28] |
| Max-Planck Unified Proteome Database |
www.mapuproteome.com http://141.61.102.16/urine |
[29] |
| Human Protein Atlas | www.proteinatlas.org | [73] |
| Sys-BodyFluid | www.biosino.org/bodyfluid | [30] |
| International Protein Index | www.ebi.ac.uk/IPI | [74] |
Proteomic profiling of urine for bladder cancer biomarker discovery
The 2DE of proteins has been the conventional method for biomarker assessment in urological proteomics [31,32], and investigators have performed a systematic evaluation of sample preparation methods for such analyses [33]. A group of nuclear matrix proteins, termed BLCA, were identified using 2D gel electrophoresis (2DGE) in tissue [34], of which, BLCA1 and 4 have been followed up extensively [35] in urinary analysis. Rasmussen et al. used 2D PAGE and MS protein identification to identify 124 polypeptides in the urine of patients with squamous cell carcinoma (SCC) of the bladder [36]. Psoriasin, a major protein in human keratinocytes, was only observed in the urine from SCC patients. In another a study using 2DGE, Irmak et al. identified two proteins, orosomucoid (ORM) and zinc α-glycoprotein (ZAG), which were increased in urine samples of tumor-bearing patients in comparison with samples from a few healthy volunteers [37]. ZAG, also known as MAC16, is a protein that causes cachexia in mice and has previously been isolated from urine of patients with cancer cachexia, so this may be a marker for a subset of advanced cancer where severe alteration of lipid metabolism and weight loss are evident. The urinary proteome was explored by Pinero et al. using 2D-DIGE coupled with MS and database interrogation. 2D-DIGE analysis yielded 12 clearly differentially expressed spots, and identified regenerating protein-1 (Reg-1) and keratin 10 as being associated with bladder cancer. Reg-1 is proposed to act as an inhibitor of apoptosis leading to Reg-1-activated proliferative activity. Reg-1 expression was validated in biopsy material and was found to be associated with tumor progression and clinical outcome. An immunoassay to detect Reg-1 in urine was then used to survey 32 patients with and 48 without bladder cancer. The Reg-1 assay enabled the discrimination of patients with bladder cancer and controls [38]. Saito et al. used a 2D PAGE approach, but focused specifically on proteins of the extracellular matrix and matrix metalloproteinases (MMP) in urine [39]. Samples were treated with gelatin-affinity beads and analysis was performed on the enriched proteins by 2D PAGE. MMP-2, MMP-9, fibronectin (FN) and associated fragments were present in cancer patients but not in healthy individuals [39]. Investigators have also applied gel-free methodologies to urine analysis. SELDI-TOF MS technology has been employed to study urine samples from patients [40,41]. Vlahou et al. compared the proteomic profile of urine samples from healthy controls and with transitional cell carcinoma of the bladder [42]. Multiple protein changes were reproducibly detected in the cancer group, including five potential novel biomarkers and several protein clusters. One of the biomarkers, α-defensin, was subsequently shown to be present in bladder tumor cells. The combination of the biomarkers and protein clusters significantly improved the accuracy of patient classification. In a separate cross-validation study by the same authors [42], α-defensin monitoring was used to detect bladder cancer with better sensitivity and specificity than commercial bladder tumor antigen stat and the urinary bladder cancer tests. A promising application of evolving technology to urinary proteomic profiling is capillary electrophoresis (CE) MS. In CE, analytes are separated with high resolution based on their migration through a liquid-filled capillary column when subjected to an electric field. Using online coupling of CE to an ESI-TOF MS, it is possible to perform analysis of up to 6000 polypeptides in short order [43]. In urinary proteome analysis, the high thermodynamic stability of urinary peptides ensures that CE separation at low pH, which is required for CE-MS coupling, is feasible [44]. Theodorescu et al. used this technology to identify urinary biomarkers for bladder cancer in a training set composed of 46 patients with urothelial carcinoma and 33 healthy volunteers [11]. These were further refined using CE MS spectra of another cohort of urine samples from healthy volunteers and patients with malignant and nonmalignant genitourinary diseases. Using this two-step approach, a diagnostic biomarker signature of 22 urinary peptides was established. In a validation study, this signature enabled the correct classification of all urothelial carcinoma patients in a testset containing 31 urothelial carcinoma patients and 138 nonmalignant genitourinary disease patients [11]. A prominent polypeptide from the diagnostic pattern for urothelial carcinoma was identified as fibrinopeptide A, a known biomarker of ovarian cancer and gastric cancer.
Urinary glycoproteomics
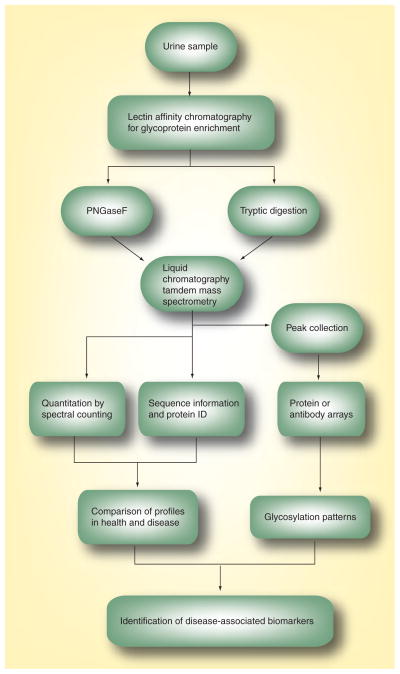
An efficient strategy to profile complex biological samples is to extract or enrich samples for subproteomes, for example, membrane proteins, glycoproteins or phosphoproteins. The reduction of complexity and the concomitant removal of interfering macro-abundant proteins enables a more accurate analysis of a subset of the total proteome. In our own ongoing work, we have focused on the analysis of these proteome subsets in cancer cell lines and clinical samples using novel strategies [45–48]. We have recently investigated the feasibility of profiling a glycoprotein component of the naturally micturated urinary proteome, and applied optimized analyses to compare the profile of a panel of urine samples obtained from patients with bladder cancer and nonmalignant bladder conditions. We utilized the ability of an immobilized α-mannose-binding lectin – Con A – to enrich N-linked glycoproteins from human urine. The enriched glycoproteins were then digested with trypsin and analyzed with nano-LC-MS/MS (Figure 1). A total of 186 distinct N-linked glycoproteins were identified with high confidence by multiple analysis of as little as 10 ml of naturally micturated urine. The majority of identified proteins were either secreted or membranous proteins, and a subset of proteins was identified that was commonly excreted in urine from bladder cancer patients. The combinatorial approach of Con A-affinity chromatography and nano-LC-MS/MS provides high sensitivity and, with relatively moderate labor demands, can greatly facilitate the identification of potential biomarkers of bladder cancer from non-invasively obtained human urine. The most discriminatory single protein was α-1B-glycoprotein (A1BG-human), a glycoprotein that has recently been implicated as a serum biomarker for other cancers [49,50]. This protein was detected in all Con A-captured fractions of bladder cancer patients’ samples, but was not found to occur in nontumor-bearing patients urine in this study. These studies are ongoing, and will follow a translational path of investigation that will aim to achieve application of the findings in the clinical arena. Once the discovery phase is complete, we will monitor the expression of the best candidate markers in a validation phase. This will include a larger cohort of patients with a typical presentation profile of a broad range of urological conditions. It is optimal to conduct the validation phase with a technique that is likely to have utility in the implementation phase, such as a laboratory or bedside assay format. The validation phase will determine the optimal biomarker panel through determination of sensitivity, specificity and overall assay accuracy. Unfortunately, many studies do not include enough patients in the discovery or validation phases and so, many promising biomarkers either remain to be validated or subsequently ultimately fail to show clinical utility.
Figure 1. Strategy for the analysis of the urinary glycoproteome.
Redrawn with permission from [45].
The majority of reported proteomic urine profile studies have used large amounts of sample material. Hundreds of milliliters of urine are typically used for gel-based analysis, and the pooling of samples from different individuals are typically observed in the published gel analysis of normal urine proteome studies in order to increase the amount of proteins [51]. By pooling samples, one is likely to lose the individual information of the intrinsic components present in urine from a single patient at a given time. Thus, a reliable and accurate profiling technique employing a small amount of urine is essential for investigating urinary proteomes and for marker identification in minimal samples. In order to improve efficiency and to make the glycoprotein-enrichment strategy applicable to minimal sample material, we have recently developed a nano-scale chelating Con A monolithic capillary prepared using glycidyl methacrylate-co-ethylene dimethacrylate as polymeric support [52]. Con A was immobilized on Cu(II)-charged iminodiacetic acid (IDA) regenerable sor-bents by forming a IDA:Cu(II):Con A sandwich affinity structure that has high column capacity, as well as stability. When compared with conventional Con A lectin chromatography, the monolithic capillary enabled the more reproducible detection of over double the number of unique N-glycoproteins in human urine samples. Utility for analysis of minimal biological samples was confirmed by the successful elucidation of glycoprotein profiles in mouse urine samples at the microliter scale [52]. We are currently employing the improved efficiency of the nanoscale monolithic capillary in large-scale urinary proteomic studies of bladder cancer, where available materials are often limited. Recent studies, such as those described earlier, are already highlighting the advances in proteomics technologies towards high mass accuracy and resolution. Novel MS-based methodologies, new bioinformatics tools and innovative strategies will lead to a comprehensive understanding of the urinary proteome in health and disease. As long as ongoing findings are collated in established and curated publicly available databases, the future for the discovery and validation of urinary biomarkers of multiple diseases is encouraging.
Expert commentary
Only proteomic profiling enables the evaluation of global changes in gene expression that result from both transcriptional and post-transcriptional processing of mRNA and translation and post-translation modifications. Although genomics may be more amenable to comprehensive surveys at this point, since phenotypic changes can only manifest themselves through altered protein expression, and it is clearly preferable to profile this component whenever possible. Moreover, the ability to identify protein factors involved in the progression of disease will most readily lead to the development of biomarkers that have clinical utility.
Optimal diagnostic and/or prognostic assays will likely comprise panels of biomarkers. Single markers may not provide high specificity and sensitivity but when the markers are analyzed in concert, they may prove to have clinical utility for clinical assessment. Such multimarker panels could enable the early detection of bladder cancer through screening of high-risk populations, define molecular characteristics with staging [53], and facilitate noninvasive surveillance regimens for the important monitoring of disease recurrence and prognosis [54], which will aid treatment decisions by possibly tailoring strategies to individual patients [55,56]. It is worth noting that cystoscopy by itself is not 100% sensitive, although it is considered the gold standard, so even protein biomarkers that can confirm or improve cystoscopic evaluations have clinical value. It may be fair to say that the majority of disease-associated proteomic (and genomic) profiling studies to date have attempted to develop genetic marker-based prognostic systems that might replace the existing clinical criteria, rather than incorporating the valuable clinical information contained in established clinical markers. A more promising strategy may be to combine both clinical- and genetic-marker information that may be complementary. To address this, we have recently developed computational algorithms that can efficiently parse high-dimensional data with associated clinical information. Through the combination of both molecular and clinical markers, the application of these algorithms to breast and prostate cancer-profile data has identified ‘hybrid signatures’ that perform significantly better than the molecular signature, or clinical criteria alone in the prediction of disease recurrence [57–59]. Ongoing computational developments that enable interstudy comparison and the incorporation of distinct forms of data, including proteomic, genomic and clinical data, will provide platforms for the refinement of cancer-related molecular signatures and lead to more accurate prognostic systems, which may facilitate personalized patient evaluation and treatment decisions. These integrated analyses also have the potential to highlight pivotal genes and pathways that are likely part of the biological driving force of metastasis.
Five-year view
Despite the high complexity of components in urine, the urinary proteome is highly amenable to clinical research owing to the broad availability of the samples, the noninvasive nature of collection and the possibility of repeat sampling. A survey of the research literature reveals the promise of proteomics in unraveling the molecular complexities associated with bladder cancer. If the continued rapid evolution of high-throughput proteomic technologies approaches the coverage currently achieved in the genomics field, proteomics will no doubt be at the core of studies into the pathology of human disease. The application of evolving bioinformatics and integrative systems biology will optimize the use of the data that are accumulating in publicly available databases, through initiatives by HUPO and others. The combination of data from multiple sources offers the best chance for identifying biomarkers for early detection, diagnosis and prognosis, and to reveal the most promising therapeutic targets.
Key issues.
The proteomic profiling of body fluids will identify biomarkers with potential clinical utility in noninvasive assays. Bladder cancer detection using urine sampling is an excellent example.
The reduced complexity achieved by the analysis of subclasses of the urinary proteome (glycoproteome, phoshoproteome) may accelerate the comprehensive profiling of the urinary proteome in health and disease.
The continued evolution of proteomic methods and concomitant reduction in costs will enable the accumulation of large amounts of data associated with human disease. The integration of proteomic data with multiple sources of global profiles will provide better information for the modeling of disease. Publicly available, curated databases are essential for this effort.
It is expected that compound molecular signatures, rather than single biomarkers, will achieve the required sensitivity and specificity for bladder cancer detection. Multi-institutional initiatives to validate these multimarker panels will be needed to take proteomic profile data to clinical utility.
Proteomics technology development is also required to facilitate the transition of biomarkers to clinical laboratory tests. Protein and antibody array strategies that enable simultaneous analysis of multiple markers have great promise in this context.
Footnotes
For reprint orders, please contact reprints@expert-reviews.com
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Steve Goodison, Email: goodison.steve@gmail.com, MD Anderson Cancer Center – Orlando, Cancer Research Institute, 6900 Lake, Nona Boulevard, Orlando, FL 32827, USA, Tel.: +1 904 633 0978, Fax: +1 904 633 0979.
Charles J Rosser, Email: charles.rosser@ufl.edu, MD Anderson Cancer Center – Orlando, Cancer Research Institute, 6900 Lake, Nona Boulevard, Orlando, FL 32827, USA.
Virginia Urquidi, Email: vurquidi@gmail.com, MD Anderson Cancer Center – Orlando, Cancer Research Institute, 6900 Lake, Nona Boulevard, Orlando, FL 32827, USA.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Pisani P, Parkin DM, Bray F, Ferlay J. Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer. 1999;83(1):18–29. doi: 10.1002/(sici)1097-0215(19990924)83:1<18::aid-ijc5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 2.Aben KK, Kiemeney LA. Epidemiology of bladder cancer. Eur Urol. 1999;36(6):660–672. doi: 10.1159/000020069. [DOI] [PubMed] [Google Scholar]
- 3.Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, et al. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol. 2000;164(3 Pt 1):680–684. doi: 10.1016/s0022-5347(05)67280-1. [DOI] [PubMed] [Google Scholar]
- 4.Rife CC, Farrow GM, Utz DC. Urine cytology of transitional cell neoplasms. Urol Clin North Am. 1979;6(3):599–612. [PubMed] [Google Scholar]
- 5.Cajulis RS, Haines GK, 3rd, Frias-Hidvegi D, McVary K, Bacus JW. Cytology, flow cytometry, image analysis, and interphase cytogenetics by fluorescence in situ hybridization in the diagnosis of transitional cell carcinoma in bladder washes: a comparative study. Diagn Cytopathol. 13(3):214–223. doi: 10.1002/dc.2840130307. discussion 224 (1995) [DOI] [PubMed] [Google Scholar]
- 6.Hautmann S, Toma M, Lorenzo Gomez MF, et al. Immunocyt and the HA-HAase urine tests for the detection of bladder cancer: a side-by-side comparison. Eur Urol. 2004;46(4):466–471. doi: 10.1016/j.eururo.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Giannopoulos A, Manousakas T, Mitropoulos D, et al. Comparative evaluation of the BTAstat test, NMP22, and voided urine cytology in the detection of primary and recurrent bladder tumors. Urology. 2000;55(6):871–875. doi: 10.1016/s0090-4295(00)00489-1. [DOI] [PubMed] [Google Scholar]
- 8.Babjuk M, Soukup V, Pesl M, et al. Urinary cytology and quantitative BTA and UBC tests in surveillance of patients with pTapT1 bladder urothelial carcinoma. Urology. 2008;71(4):718–722. doi: 10.1016/j.urology.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 9.Schroeder GL, Lorenzo-Gomez MF, Hautmann SH, et al. A side by side comparison of cytology and biomarkers for bladder cancer detection. J Urol. 2004;172(3):1123–1126. doi: 10.1097/01.ju.0000134347.14643.ab. [DOI] [PubMed] [Google Scholar]
- 10.Schaub S, Wilkins J, Weiler T, et al. Urine protein profiling with surface-enhanced laser-desorption/ionization time-of-flight mass spectrometry. Kidney Int. 2004;65(1):323–332. doi: 10.1111/j.1523-1755.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- 11••.Theodorescu D, Wittke S, Ross MM, et al. Discovery and validation of new protein biomarkers for urothelial cancer: a prospective analysis. Lancet Oncol. 2006;7(3):230–240. doi: 10.1016/S1470-2045(06)70584-8. Describes a proteomic approach from biomarker discovery to internal validation and to validation in an independent sample set. [DOI] [PubMed] [Google Scholar]
- 12.Schiffer E, Mischak H, Novak J. High resolution proteome/peptidome analysis of body fluids by capillary electrophoresis coupled with MS. Proteomics. 2006;6(20):5615–5627. doi: 10.1002/pmic.200600230. [DOI] [PubMed] [Google Scholar]
- 13.Oh J, Pyo JH, Jo EH, et al. Establishment of a near-standard two-dimensional human urine proteomic map. Proteomics. 2004;4(11):3485–3497. doi: 10.1002/pmic.200401018. [DOI] [PubMed] [Google Scholar]
- 14.Bueler MR, Wiederkehr F, Vonderschmitt DJ. Electrophoretic, chromatographic and immunological studies of human urinary proteins. Electrophoresis. 1995;16(1):124–134. doi: 10.1002/elps.1150160122. [DOI] [PubMed] [Google Scholar]
- 15.Marshall T, Williams K. Two-dimensional electrophoresis of human urinary proteins following concentration by dye precipitation. Electrophoresis. 1996;17(7):1265–1272. doi: 10.1002/elps.1150170716. [DOI] [PubMed] [Google Scholar]
- 16.Thongboonkerd V, McLeish KR, Arthur JM, Klein JB. Proteomic analysis of normal human urinary proteins isolated by acetone precipitation or ultracentrifugation. Kidney Int. 2002;62(4):1461–1469. doi: 10.1111/j.1523-1755.2002.kid565.x. [DOI] [PubMed] [Google Scholar]
- 17.Heine G, Raida M, Forssmann WG. Mapping of peptides and protein fragments in human urine using liquid chromatography-mass spectrometry. J Chromatogr A. 1997;776(1):117–124. doi: 10.1016/s0021-9673(97)00440-8. [DOI] [PubMed] [Google Scholar]
- 18.Wittke S, Fliser D, Haubitz M, et al. Determination of peptides and proteins in human urine with capillary electrophoresis-mass spectrometry, a suitable tool for the establishment of new diagnostic markers. J Chromatogr A. 2003;1013(1–2):173–181. doi: 10.1016/s0021-9673(03)00713-1. [DOI] [PubMed] [Google Scholar]
- 19.Pieper R, Gatlin CL, McGrath AM, et al. Characterization of the human urinary proteome: a method for high-resolution display of urinary proteins on two-dimensional electrophoresis gels with a yield of nearly 1400 distinct protein spots. Proteomics. 2004;4(4):1159–1174. doi: 10.1002/pmic.200300661. [DOI] [PubMed] [Google Scholar]
- 20.Sun W, Li F, Wu S, et al. Human urine proteome analysis by three separation approaches. Proteomics. 2005;5(18):4994–5001. doi: 10.1002/pmic.200401334. [DOI] [PubMed] [Google Scholar]
- 21.Castagna A, Cecconi D, Sennels L, et al. Exploring the hidden human urinary proteome via ligand library beads. J Proteome Res. 2005;4(6):1917–1930. doi: 10.1021/pr050153r. [DOI] [PubMed] [Google Scholar]
- 22.Tyan YC, Guo HR, Liu CY, Liao PC. Proteomic profiling of human urinary proteome using nano-high performance liquid chromatography/electrospray ionization tandem mass spectrometry. Anal Chim Acta. 2006;579(2):158–176. doi: 10.1016/j.aca.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 23.Coon JJ, Zurbig P, Dakna M, et al. CE-MS analysis of the human urinary proteome for biomarker discovery and disease diagnostics. Proteomics Clin Appl. 2008;2:964–973. doi: 10.1002/prca.200800024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adachi J, Kumar C, Zhang Y, Olsen JV, Mann M. The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome Biol. 2006;7(9):R80. doi: 10.1186/gb-2006-7-9-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pisitkun T, Johnstone R, Knepper MA. Discovery of urinary biomarkers. Mol Cell Proteomics. 2006;5(10):1760–1771. doi: 10.1074/mcp.R600004-MCP200. [DOI] [PubMed] [Google Scholar]
- 26.Gonzales PA, Pisitkun T, Hoffert JDT, et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol. 2009;20(2):363–379. doi: 10.1681/ASN.2008040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Yamamoto T, Langham RG, Ronco P, Knepper MA, Thongboonkerd V. Proteomics; Towards standard protocols and guidelines for urine proteomics: a report on the Human Kidney and Urine Proteome Project (HKUPP) symposium and workshop; 6 October 2007; Seoul, Korea. 1 November 2007; San Francisco, CA, USA. 2008. pp. 2156–2159. Describes a Human Proteome Organization initiative for the study of the urine proteome. These initiatives are essential for the standardization of data across studies. [DOI] [PubMed] [Google Scholar]
- 28•.Vlahou A, Schanstra J, Frokiaer J, et al. Establishment of a European Network for Urine and Kidney Proteomics. J Proteomics. 2008;71(4):490–492. doi: 10.1016/j.jprot.2008.06.009. Describes a European initiative for the study of the urine proteome. These initiatives are essential for the standardization of data across studies. [DOI] [PubMed] [Google Scholar]
- 29•.Zhang Y, Zhang Y, Adachi J, et al. MAPU: Max-Planck Unified database of organellar, cellular, tissue and body fluid proteomes. Nucleic Acids Res. 2007;35(Database issue):D771–D779. doi: 10.1093/nar/gkl784. Database that is an extension of the Max-Planck Unified database and is one of few to focus on body fluids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li SJ, Peng M, Li H, et al. Sys-BodyFluid: a systematical database for human body fluid proteome research. Nucleic Acids Res. 2009;37(Database issue):D907–D912. doi: 10.1093/nar/gkn849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tantipaiboonwong P, Sinchaikul S, Sriyam S, Phutrakul S, Chen ST. Different techniques for urinary protein analysis of normal and lung cancer patients. Proteomics. 2005;5(4):1140–1149. doi: 10.1002/pmic.200401143. [DOI] [PubMed] [Google Scholar]
- 32.Nabi G, N’Dow J, Hasan TS, Booth IR, Cash P. Proteomic analysis of urine in patients with intestinal segments transposed into the urinary tract. Proteomics. 2005;5(6):1729–1733. doi: 10.1002/pmic.200401125. [DOI] [PubMed] [Google Scholar]
- 33.Thongboonkerd V, Chutipongtanate S, Kanlaya R. Systematic evaluation of sample preparation methods for gel-based human urinary proteomics: quantity, quality, and variability. J Proteome Res. 2006;5(1):183–191. doi: 10.1021/pr0502525. [DOI] [PubMed] [Google Scholar]
- 34.Getzenberg RH, Konety BR, Oeler TA, et al. Bladder cancer-associated nuclear matrix proteins. Cancer Res. 1996;56(7):1690–1694. [PubMed] [Google Scholar]
- 35.Myers-Irvin JM, Landsittel D, Getzenberg RH. Use of the novel marker BLCA-1 for the detection of bladder cancer. J Urol. 2005;174(1):64–68. doi: 10.1097/01.ju.0000162022.36772.a4. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen HH, Orntoft TF, Wolf H, Celis JE. Towards a comprehensive database of proteins from the urine of patients with bladder cancer. J Urol. 1996;155(6):2113–2119. [PubMed] [Google Scholar]
- 37.Irmak S, Tilki D, Heukeshoven J, et al. Stage-dependent increase of orosomucoid and zinc-α2-glycoprotein in urinary bladder cancer. Proteomics. 2005;5(16):4296–4304. doi: 10.1002/pmic.200402005. [DOI] [PubMed] [Google Scholar]
- 38.Orenes-Pinero E, Corton M, Gonzalez-Peramato P, et al. Searching urinary tumor markers for bladder cancer using a two-dimensional differential gel electrophoresis (2D-DIGE) approach. J Proteome Res. 2007;6(11):4440–4448. doi: 10.1021/pr070368w. [DOI] [PubMed] [Google Scholar]
- 39.Saito M, Kimoto M, Araki T, et al. Proteome analysis of gelatin-bound urinary proteins from patients with bladder cancers. Eur Urol. 2005;48(5):865–871. doi: 10.1016/j.eururo.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 40.Vlahou A, Schellhammer PF, Mendrinos S, et al. Development of a novel proteomic approach for the detection of transitional cell carcinoma of the bladder in urine. Am J Pathol. 2001;158(4):1491–1502. doi: 10.1016/S0002-9440(10)64100-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mueller J, von Eggeling F, Driesch D, et al. ProteinChip technology reveals distinctive protein expression profiles in the urine of bladder cancer patients. Eur Urol. 2005;47(6):885–893. doi: 10.1016/j.eururo.2005.02.016. discussion 893–884. [DOI] [PubMed] [Google Scholar]
- 42.Holterman DA, Diaz JI, Blackmore PF, et al. Overexpression of α-defensin is associated with bladder cancer invasiveness. Urol Oncol. 2006;24(2):97–108. doi: 10.1016/j.urolonc.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Kolch W, Neususs C, Pelzing M, Mischak H. Capillary electrophoresis-mass spectrometry as a powerful tool in clinical diagnosis and biomarker discovery. Mass Spectrom Rev. 2005;24(6):959–977. doi: 10.1002/mas.20051. [DOI] [PubMed] [Google Scholar]
- 44.Ye B, Skates S, Mok SC, et al. Proteomic-based discovery and characterization of glycosylated eosinophil-derived neurotoxin and COOH-terminal osteopontin fragments for ovarian cancer in urine. Clin Cancer Res. 2006;12(2):432–441. doi: 10.1158/1078-0432.CCR-05-0461. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Ao X, Vuong H, et al. Membrane glycoproteins associated with breast tumor cell progression identified by a lectin affinity approach. J Proteome Res. 2008;7(10):4313–4325. doi: 10.1021/pr8002547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patwa TH, Wang Y, Miller FR, et al. A novel phosphoprotein analysis scheme for assessing changes in premalignant and malignant breast cell lines using 2D liquid separations, protein microarrays and tandem mass spectrometry. Proteomics Clin Appl. 2008;3(1):51–66. doi: 10.1002/prca.200800097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kreunin P, Yoo C, Urquidi V, Lubman DM, Goodison S. Differential expression of ribosomal proteins in a human metastasis model identified by coupling 2-D liquid chromatography and mass spectrometry. Cancer Genomics Proteomics. 2007;4(5):329–339. [PMC free article] [PubMed] [Google Scholar]
- 48••.Kreunin P, Zhao J, Rosser C, et al. Bladder cancer associated glycoprotein signatures revealed by urinary proteomic profiling. J Proteome Res. 2007;6(7):2631–2639. doi: 10.1021/pr0700807. Describes the enrichment and identification of the urinary glycoproteome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian M, Cui YZ, Song GH, et al. Proteomic analysis identifies MMP-9, DJ-1 and A1BG as overexpressed proteins in pancreatic juice from pancreatic ductal adenocarcinoma patients. BMC Cancer. 2008;8:241. doi: 10.1186/1471-2407-8-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Zhao J, Patwa TH, Qiu W, et al. Glycoprotein microarrays with multi-lectin detection: unique lectin binding patterns as a tool for classifying normal, chronic pancreatitis and pancreatic cancer sera. J Proteome Res. 2007;6(5):1864–1874. doi: 10.1021/pr070062p. Describes the analysis of the subproteome of glycoproteins and how the seperated proteins can be applied to microarrays for the analysis of both protein levels and glycosylation pattern. [DOI] [PubMed] [Google Scholar]
- 51.Wang L, Li F, Sun W, et al. Concanavalin A-captured glycoproteins in healthy human urine. Mol Cell Proteomics. 2006;5(3):560–562. doi: 10.1074/mcp.D500013-MCP200. [DOI] [PubMed] [Google Scholar]
- 52•.Feng S, Yang N, Pennathur S, Goodison S, Lubman DM. Enrichment of glycoproteins using nanoscale chelating concanavalin A monolithic capillary chromatography. Anal Chem. 2009;81(10):3776–3783. doi: 10.1021/ac900085k. Describes the development of improved glycoprotein enrichment chromatography. This will enable more efficient glycoprotein analyses in minimal sample material. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schiffer E, Vlahou A, Petrolekas, et al. Prediction of muscle-invasive bladder cancer using urinary proteomics. Clin Cancer Res. 2009;15(15):4935–4943. doi: 10.1158/1078-0432.CCR-09-0226. [DOI] [PubMed] [Google Scholar]
- 54.Habuchi T, Marberger M, Droller, et al. Prognostic markers for bladder cancer: International Consensus Panel on bladder tumor markers. Urology. 2005;66(6 Suppl 1):64–74. doi: 10.1016/j.urology.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 55.Lokeshwar VB, Habuchi T, Grossman HB, et al. Bladder tumor markers beyond cytology: international Consensus Panel on bladder tumor markers. Urology. 2005;66(6 Suppl 1):55–63. doi: 10.1016/j.urology.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 56.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374(9685):239–249. doi: 10.1016/S0140-6736(09)60491-8. [DOI] [PubMed] [Google Scholar]
- 57.Sun Y, Goodison S, Li J, Liu L, Farmerie W. Improved breast cancer prognosis through the combination of clinical and genetic markers. Bioinformatics. 2007;23(1):30–37. doi: 10.1093/bioinformatics/btl543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Y, Urquidi V, Goodison S. Derivation of molecular signatures for breast cancer recurrence prediction using a two-way validation approach. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0365-6. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Y, Goodison S. Optimizing molecular signatures for predicting prostate cancer recurrence. Prostate. 2009;69(10):1119–1127. doi: 10.1002/pros.20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hijazi A, Devonec M, Bouvier R, Revillard JP. Flow cytometry study of cytokeratin 18 expression according to tumor grade and deoxyribonucleic acid content in human bladder tumors. J Urol. 1989;141(3):522–526. doi: 10.1016/s0022-5347(17)40878-0. [DOI] [PubMed] [Google Scholar]
- 61.Fernandez-Gomez J, Rodriguez-Martinez JJ, Barmadah SE, et al. Urinary CYFRA 21.1 is not a useful marker for the detection of recurrences in the follow-up of superficial bladder cancer. Eur Urol. 2007;51(5):1267–1274. doi: 10.1016/j.eururo.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 62.Olsson H, Zackrisson B. ImmunoCyt a useful method in the follow-up protocol for patients with urinary bladder carcinoma. Scand J Urol Nephrol. 2001;35(4):280–282. doi: 10.1080/003655901750425846. [DOI] [PubMed] [Google Scholar]
- 63.Mian C, Pycha A, Wiener H, et al. Immunocyt: a new tool for detecting transitional cell cancer of the urinary tract. J Urol. 1999;161(5):1486–1489. doi: 10.1016/s0022-5347(05)68934-3. [DOI] [PubMed] [Google Scholar]
- 64.Miyanaga N, Akaza H, Tsukamoto S, et al. Usefulness of urinary NMP22 to detect tumor recurrence of superficial bladder cancer after transurethral resection. Int J Clin Oncol. 2003;8(6):369–373. doi: 10.1007/s10147-003-0357-1. [DOI] [PubMed] [Google Scholar]
- 65.Shariat SF, Bolenz C, Godoy G, et al. Predictive value of combined immunohistochemical markers in patients with pT1 urothelial carcinoma at radical cystectomy. J Urol. 2009;182(1):78–84. doi: 10.1016/j.juro.2009.02.125. discussion 84. [DOI] [PubMed] [Google Scholar]
- 66•.Grossman HB, Soloway M, Messing E, et al. Surveillance for recurrent bladder cancer using a point-of-care proteomic assay. JAMA. 2006;295(3):299–305. doi: 10.1001/jama.295.3.299. Discusses the utility of bedside, point-of-care urinalysis. This is a major translational goal of proteomics biomarker discovery. [DOI] [PubMed] [Google Scholar]
- 67.van Rhijn BW, van der Poel HG, van der Kwast TH. Urine markers for bladder cancer surveillance: a systematic review. Eur Urol. 2005;47(6):736–748. doi: 10.1016/j.eururo.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 68.Lokeshwar VB. Are there molecular signatures for predicting bladder cancer prognosis? J Urol. 2006;176(6 Pt 1):2347–2348. doi: 10.1016/j.juro.2006.08.142. [DOI] [PubMed] [Google Scholar]
- 69.Svatek RS, Herman MP, Lotan Y, et al. Soluble Fas – a promising novel urinary marker for the detection of recurrent superficial bladder cancer. Cancer. 2006;106(8):1701–1707. doi: 10.1002/cncr.21795. [DOI] [PubMed] [Google Scholar]
- 70.Cascino I, Fiucci G, Papoff G, Ruberti G. Three functional soluble forms of the human apoptosis-inducing Fas molecule are produced by alternative splicing. J Immunol. 1995;154(6):2706–2713. [PubMed] [Google Scholar]
- 71.Van Le TS, Miller R, Barder T, et al. Highly specific urine-based marker of bladder cancer. Urology. 2005;66(6):1256–1260. doi: 10.1016/j.urology.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 72.Konety BR, Nguyen TS, Brenes G, et al. Clinical usefulness of the novel marker BLCA-4 for the detection of bladder cancer. J Urol. 2000;164(3 Pt 1):634–639. doi: 10.1097/00005392-200009010-00004. [DOI] [PubMed] [Google Scholar]
- 73.Uhlen M, Bjorling E, Agaton C, et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics. 2005;4(12):1920–1932. doi: 10.1074/mcp.M500279-MCP200. [DOI] [PubMed] [Google Scholar]
- 74.Kersey PJ, Duarte J, Williams A, et al. The International Protein Index: an integrated database for proteomics experiments. Proteomics. 2004;4(7):1985–1988. doi: 10.1002/pmic.200300721. [DOI] [PubMed] [Google Scholar]
Websites
- 101.National Cancer Institute, US National Institutes of Health. Bladder Cancer Statistics. 2009 www.cancer.gov/cancertopics/types/bladder.
- 102.HUPO. 7th World Congress; 16–20 August 2008; Amsterdam, The Netherlands . 2008. www.hupo2008.nl/ [Google Scholar]