Abstract
Acute stressful experience enhances subsequent learning in males and impairs learning in females. These sex differences emerge soon after puberty in adulthood. Whether these opposite effects of stress on learning extend into older age is unknown. To examine this, young adult (2–3 months) and middle aged (17–18 months) Fischer 344 rats of both sexes were exposed to an acute stress or of brief tail shocks and trained 24 h later with classical eyeblink conditioning using a trace paradigm. Whereas stressful experience enhanced conditioning in adult males and impaired conditioning in adult females, it had no effect whatsoever on conditioning in the aging animals of either sex. There was no effect of aging itself on acquisition of the conditioned response, suggesting that trace conditioning is not necessarily compromised during this period of life. Together, these data indicate that associative learning in the aging animal is resistant to both the negative and positive consequences of stressful experience.
Keywords: Trace conditioning, Sex differences, Glucocorticoids, Estrogen, Learning, Aging
1. Introduction
Stressful life experiences affect learning differently in males versus females. In a series of studies, it has been shown that exposure to an acute stressful event increases classical eyeblink conditioning in males, but exposure to the same stressful event impairs conditioning in females [16,21,22]. These effects emerge after puberty. Thus, learning in animals that are exposed to the stressor and trained prior to puberty is unaffected. During puberty, learning in both males and females is facilitated by exposure to the stressful event [4]. The effects of stress on learning also fluctuate across the lifespan of the adult, at least in females. For example, learning is unaffected by exposure to the stressor in postpartum females that are lactating or in virgins that are induced to behave maternally after exposure to another females offspring [7]. This suppression of the stress effect is in contrast to the impairment observed in females that are cycling. Overall, the data indicate that the effects of stress on associative learning emerge with sexual maturation and are evident when females are receptive to and capable of reproduction. In males, the data suggest the enhanced effect of stress on learning emerges with puberty and is maintained throughout adulthood. However, we do not know whether the effects of stress on learning occur in aged animals.
The effects of stress on learning are usually mediated or at least influenced by the presence of hormones. For example, the effect of stress on learning in females is most pronounced if females are stressed during diestrus and trained 1–2 days later during proestrus when the concentration of estrogen and progesterone in the blood is increased [16,21,22]. It has also been shown that either removal of the ovaries or treatment with the estrogen antagonist Tamoxifin blocks the detrimental effects of stress on conditioning. Together, these findings indicate that the detrimental effect of stress on learning in females is dependent on the presence of estrogen [21]. In males, the effect of stress is not dependent on the presence of reproductive hormones, i.e. testosterone [17], but rather is dependent on the presence of corticosterone. Interestingly enough, the effect of stress on learning in females is not dependent on the presence of corticosterone, despite the fact that female rats have elevated levels of glucocorticoids under both basal and stress conditions when compared to adult male rats. Thus, learning in male rats without an adrenal cortex is not enhanced after exposure to an acute stressor, whereas that in males with adrenals is enhanced; learning in females without their adrenals is reduced after exposure to the stressful event, just as it is in females that are intact [1,22]. In summary then, exposure to a stressful event has opposite effects on learning the classically conditioned eyeblink response in males versus females and these effects are mediated by different hormonal systems.
It is well established that hormones fluctuate with age. Aged male rats tend to experience elevated basal levels of glucocorticoids, lower levels of testosterone, as well as disrupted feedback of the hypothalamic–pituitary–adrenal axis [3,5,12,14,19]. By middle age (17 months) female rats also experience increased basal concentrations of corticosterone and a prolonged glucocorticoid response to ACTH [8]. Their estrogen levels begin to decline in middle age, with cessation of the estrous cycle by 12 months [10,18].
In the present studies, we examined the effects of acute stress on new learning in aged animals of both sexes. One concern that we had in attempting these studies is that learning itself would be impaired with age. Many cognitive abilities decline with age, including those assessed during classical eyeblink conditioning [2,20]. In fact, it is reported that performance begins to decline as early as middle age in both humans and rats [23], although the effect in rats may be limited to strains with a shorter lifespan [6,20]. We thereby chose to study middle-aged animals, because they would likely be able to perform the conditioned response and also because the females would have recently ceased to have an estrous cycle. Provided that the aged animals could learn the conditioned response, it was hypothesized that the effects of stress on that learning would be different in the middle aged animal than in the young adult.
2. Methods
2.1. Subjects
Adult (2–3 months) and middle aged (17–18 months) male and female Fischer 344 rats (n= 59) were obtained from the colony of the National Institute on Aging maintained by Harlan (Indianapolis, IN). For the study, groups consisted of young males (stress = 7; no stress = 5), middle-aged males (stress = 8; no stress = 7), young females (stress = 8; no stress = 8) and middle-aged females (stress= 8; no stress= 8). Rats were housed in wire rack cages for at least 1 week prior to testing, provided food and water ab libitum and maintained on a 12:12 h light/dark cycle with lights on at 8 a.m.
2.2. Surgery
Subjects were anesthetized with sodium pentobarbital (females 8 mg/kg; males 12mg/kg) and then maintained throughout surgery with isoflurane and oxygen. Head stages were attached to the skull using anchoring screws and dental acrylic. Four electrodes (insulated stainless steel wire 0.013 cm) were implanted in the upper eyelid (obicularis oculi) and the insulation was removed. All rats were provided at least 1 week of recovery.
2.3. Vaginal cytology
To assess stages of the estrous cycle, vaginal swabs were taken daily. Cotton Q-tips immersed in saline were inserted into the vaginal track. Cells were removed and placed onto slides which were stained with 1% toluidine blue. Stage of the estrous cycle was verified by visualization under 10× magnification. The stages of the cycle were characterized as follows—Estrus: large blue staining cornified cells, diestrus 1: small dark staining leukocytes with scattered epithelial cells, diestrus 2: similar appearance to diestrus 1 but with a lesser density of cells, proestrus: round clumped nucleated purple stained cells. Only young adult females that demonstrated two 4–5-day cycles with all stages of the estrous cycle were included in the study. All of the aged females presented with a persistent stage of diestrus throughout the experiment, i.e. they did not possess an estrous cycle.
2.4. Eyeblink conditioning and stressor exposure
One day prior to training, rats were acclimated to the conditioning environment, which consisted of 10 separate lit (7.5 wt bulb) conditioning chambers (22 cm × 26 cm × 25 cm) with metal and Plexiglas walls and a metal floor grid. The conditioning chambers were housed within sound attenuating chambers (51cm × 52 cm × 35 cm). After acclimation, half of the subjects were returned to their home cages. The others were taken into a different context, restrained in Plexiglas chambers and exposed to 30, 1 mA, 60 Hz, 1 s, shocks, delivered 1 per min to two electrodes on each side of the tail. The young adult females were exposed to the stressor during the diestrus 2 stage of their cycle and then trained 24 h later in proestrus. After stressor exposure all rats were returned to their home cage.
Twenty-four hours after stressor exposure, rats were returned to the conditioning chambers (9 a.m.–12 p.m.). First spontaneous blinks were recorded for 10 min (30 trials with no stimuli and a total of 15 s recording time). Then rats were exposed to 10 presentations of the white noise (84 dB, 250 ms, 25 ms rise/fall time) in order to assess age or sex differences in eyeblink responses to the conditioned stimulus (CS) prior to any training. Animals were then trained with 200 trials a day for 3 days of trace eyeblink conditioning. In this paradigm a white noise CS (84 dB, 250 ms, 25 ms rise/fall time) was separated from an eyelid stimulation unconditioned stimulus (US—0.70 mA, 60 Hz, AC, 100 ms) by a 500 ms trace interval. Trials were administered in sets of 10 in the following order: one CS alone trial, four paired trials, one US alone trial, four paired trials. Intertrial intervals were randomized at 25 ± 5 s.
Eyeblinks were detected using EMG activity as recorded from the obicularis oculi muscle. Eyelid electrodes were connected via a headstage to a differential amplifier with a 300–500Hz band pass filter and amplified by 10 K. EMG signals were digitized at 1 kHz with a 16 bit A/D card (Keithley-Metrabyte, Taunton, MA) and relayed for analysis via computers. Changes in the EMG were considered eyeblinks if they persisted for >3 ms and were >4 times the standard deviation of baseline responses recorded during a 250 ms stimulus-free period at the beginning of each trial. Eyeblinks were considered CRs if they occurred during the 500 ms trace interval.
2.5. Necropsy
One day after the end of training, animals were injected with a lethal dose of pentobarbital. Rats were necropsied for gross abnormalities. Any subjects with tumors were eliminated from the analysis.
3. Results
The data were analyzed using a four-way ANOVA with sex, age, stressor exposure as the independent measures and percentage of conditioned responses as the dependent repeated measure (6 blocks of 100 trials each). There was a three-way interaction between stressor exposure (stress versus no stress), age (young adult versus middle aged) and sex (male versus female) [F(1,51) = 5.06, p < 0.05]. Post hoc analysis with planned comparisons indicated that the stressed adult males emitted a greater percentage of CRs (59% ± 8) when averaged across six blocks of trials than the other groups (no stress/young adult male = 40% ± 15; stress/middle age males = 29% ± 15; no stress/middle aged males = 31% ± 13; stress/young adult females = 24% ± 6; no stress/young adult females = 38% ± 15; stress/middle aged females = 33% ± 13; no stress/middle aged females = 38% ± 12) (p < 0.001) (Fig. 1A). Stressed young adult females emitted fewer CRs averaged across six blocks, than both stressed and unstressed young males. In addition stressed young adult females produced fewer averaged CRs than unstressed females of both ages (p values < 0.05) (Fig. 1C). Conditioning in middle-aged males that had been exposed to the stressor was not different from conditioning in unstressed middle-aged males or middle-aged females of either condition (p values > 0.05) (Fig. 1B). Conditioning in stressed middle-aged females did not differ from unstressed females that were young or middle aged (p values > 0.05) (Fig. 1D).
Fig. 1.
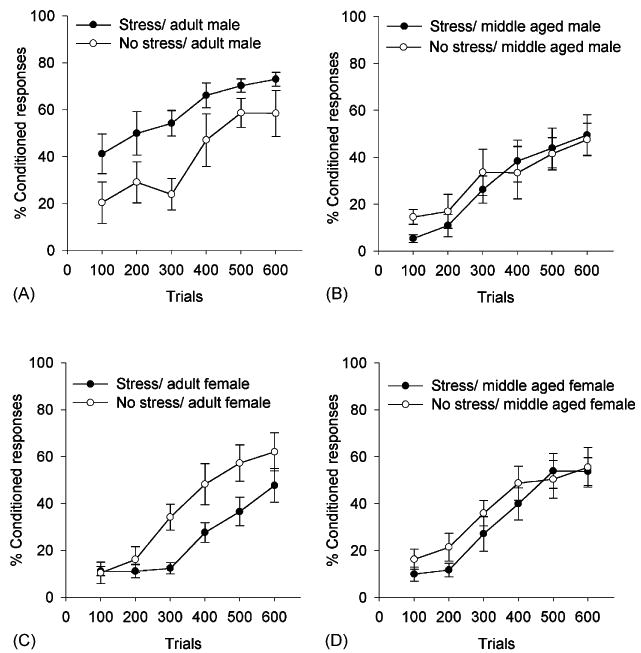
Effects of stress on trace eyeblink conditioning in young adult and middle-aged male and female rats. The graph depicts the mean (± S.E.M.) percentage of CRs averaged in six blocks (100 trials each) for a total of 600 trials of trace eyeblink conditioning for stressed and unstressed subjects. (A) Adult male rats were exposed to the stressful event and trained 24 h later. They emitted a greater percentage of CRs across the blocks of training trials. (B) In contrast, middle-aged males that were exposed to the same stressor did not express any change in the percentage of conditioned responses. (C) Adult females were exposed to the stressful event and trained 24 h later. As shown, they emitted a smaller percentage of conditioned responses when compared to females that were not exposed to the stressor. (D) Exposure to the stressful event did not alter the percentage of conditioned responses in middle-aged females.
As a further measure learning, we subjected the data to an arbitrary criterion. The criterion was determined to be 60%, which equals 54 blinks during the trace interval of a trial out of the 90 paired trials that occurred in a block [15]. Rats that did not achieve that criterion within six blocks of 100 trials each were awarded a score of 600. ANOVA on this measure indicated an interaction between stress or exposure (stress versus no stress) and sex (male versus female) [F(1,51) = 4.18, p < 0.05]; stressed males required fewer training trials to reach the criterion than did stressed females. There was also an interaction between age (young versus middle aged) and sex (male versus female) [F(1,51) = 4.18, p < 0.05]; the group of young adult males required fewer training trials to reach criterion than all other groups. The interactions between condition, sex and age were not below the 0.05 level of probability (p = 0.057), but the trend suggests that the young adult males that had been exposed to the stress or required fewer trials to reach criterion than all the other groups (Fig. 2).
Fig. 2.
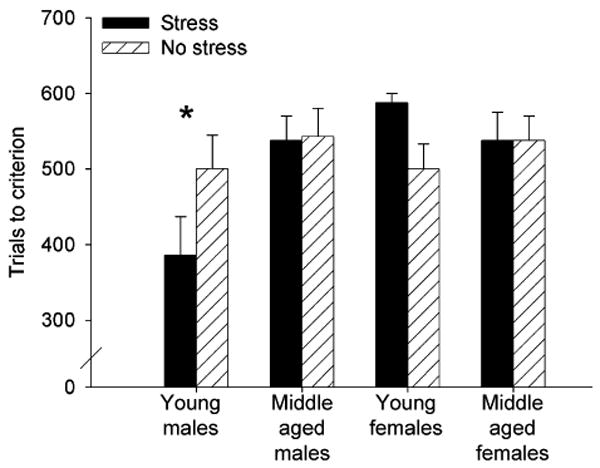
The graph depicts the mean (±S.E.M.) number of trials that were necessary to reach a criterion of 60% conditioned responses in 100 trials. As a group, the young adult males required fewer trials to reach criterion. This effect is attributed to the rapid learning in those that were exposed to the stressor before training.
A number of performance measures were used to assess nonspecific changes in performance because of age or sex differences. The number of spontaneous blinks prior to any training was examined using an ANOVA with three independent variables (sex × age × stressor exposure). Post hoc analysis indicated that young adults (M = 1.7/S.D. = 1.4) blinked fewer times than did the middle-aged animals (M = 2.6, S.D. = 1.7) over a 10 min session (15 s total recording time) [F(1,51) = 5.91, p < 0.05]. Eyeblink responses to the CS alone prior to any training were used to detect sensitized responses to the CS. The number of blinks in response to 10 stimulus presentations was analyzed. There was a significant effect of sex on the number of responses [F(1,51) = 6.86, p < 0.05]. Males blinked in response to one of the 10 auditory stimuli and females did not respond to any.
4. Discussion
Overall, we report here that trace conditioning in the middle-aged animal is resistant to the negative and positive consequences of stressful experience. As in previous studies, we found that exposure to the acute stressful event of brief periodic tailshocks induced opposite effects on trace eyeblink conditioning in young adult males versus females [16,21,22]. Exposure to the stressor enhanced learning in young males when compared to learning in unstressed males of the same age, but decreased learning in young females, when compared to the females that were not stressed. However, exposure to the stressful event had no effect on learning in middle age rats of either sex. Thus, contrary to what might be expected, exposure to an acute stressful event did not induce an exaggerated response in the middle-aged animal, regardless of sex. There were minor differences in the spontaneous and sensitized blink rates prior to training. Older subjects blinked once more than the young adults over the course of a 15 s recording period and males blinked once more than females to a white noise stimulus delivered prior to any training. However, neither of these measures was affected by exposure to the stressful event. Because learning itself was unaffected by age, these age differences in nonspecific responding are apparently inconsequential to the effects of stress on learning (or absence thereof).
Exactly how learning in the middle age animal becomes resistant to stress is unknown and cannot be determined from the data presented here. It seems likely that changes in hormonal systems are critical, especially those related to the HPA and HPG axes. It is reported that the HPA response to stress is prolonged in aged animals [3,5,14]. Interestingly, a similar response has been reported for pre-pubescent male rats [13]. Because learning in the juvenile is also unresponsive to stress [4], it could be that a prolonged HPA response to stress contributes to the absence of the stress effect on learning in both age groups. The corticosterone response to stress is likely involved in males, because it is necessary for the enhanced learning during young adulthood [1]. There are also numerous studies indicating that glucocorticoids increase with age and that the HPA response becomes deregulated [3,5,14]. According to the “glucocorticoid cascade hypothesis,” a persistent increase in basal levels of glucocorticoids leads to cellular damage, particularly in the hippocampus, a brain region necessary for this type of learning [14]. Based on this idea, it could be proposed that exposure to the stressful event does not enhance glucocorticoids levels enough from those occurring under unstressed conditions to induce the processes necessary to enhance learning in the aged male animal.
The detrimental effect of stress on conditioning in the cycling female is not dependent on the presence of glucocorticoids but is rather dependent on the presence of estrogen [21]. Since females cease to cycle and estrogen levels decrease substantially by 12 months of age [10,18] it is likely that low levels of estrogen contribute to, or are responsible for their inability to respond to the stressor. Consistent with this hypothesis, all of the middle-aged females were in a stage of persistent diestrus, a state associated with relatively low levels of estrogen.
As noted, learning itself was unaffected by age in this study. This was somewhat unexpected because others had reported deficits in eyeblink conditioning as early as 18 months, a stage associated with middle age [11,20]. However, not all studies find learning deficits in the middle aged. For example, the F1 hybrid rats learn at similar rates as young adults and better than the aged Fisher 344 rats. It has been suggested that differences in performance and rate of acquisition may be due to differences in lifespan; the F1 hybrids tend to live longer than the Fisher 344 rats [6]. This explanation does not necessarily apply to our data because we used Fisher 344 rats, which have a relatively short lifespan and reportedly age more rapidly. Rather, the discrepancy probably reflects differences in the testing procedures, some of which are more sensitive to subtle changes in learning abilities than others. Importantly, we did not train animals to a fixed criterion. Young adults did achieve a criterion of 60% conditioned responding (54 out of 90 trials), but this effect was primarily attributed to performance in the young stressed adult males which surpassed that in all other groups.
There was no deficit in learning in the unstressed middle-aged females when their performance was compared to young adult females, despite that they were in a persistent stage of diestrus and thus exposed to low levels of estrogen. We typically find that young adult females emit more conditioned eyeblink responses than young adult males. The enhanced responding in the female is especially evident if they are trained when they are in proestrus, when estrogen levels are high [16]. In the present study, we did not observe the sex difference in learning. A possible reason for this may be strain differences, as our previous studies were performed on Sprague-Dawley rats. It is interesting that the ability to learn this response was unaffected by age in the female, even though they were no longer cycling. To our knowledge, trace eyeblink conditioning in the aged female rat has not been examined before. There was a report that trace conditioning of a fear response is compromised in aged females. However, the females were 23–24 months of age, much older that those used here [11]. There are reports of performance deficits in middle age female rats trained to navigate a Morris water maze using spatial cues [9]. Apparently, learning deficits in females arose months before similar ones were observed in males.
Until recent times, postmenopausal life was relatively short-lived but as our life expectancy increases, women are spending a longer percentage of their lifespan in a post-menopausal state. These changes underscore the importance of describing the biology of menopause and post-menopause. Often coinciding with hormonal fluctuation are overt changes in behavior and affect. In the present study, there was no obvious learning deficit in the aging females, despite the cessation of estrus. There was however a marked resistance to stress; exposure to a stressful event that typically impairs learning in a cycling female had no effect on learning in the middle-aged female. This resistance to stress has also been observed during other stages of reproductive life, most notably those times associated with prepubescence and postpartum [4,7]. Taken together, these studies demonstrate that stressful experience can have different effects on learning during different phases of life, particularly those associated with the emergence and cessation of reproductive capacity.
Acknowledgments
This work was supported by the National Institute of Mental Health (59970) and National Institute on Aging to TJS.
References
- 1.Beylin AV, Shors TJ. Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Horm Behav. 2003;43(1):124–31. doi: 10.1016/s0018-506x(02)00025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finkbiner RG, Woodruff-Pak DS. Classical eyeblink conditioning in adulthood: effects of age and interstimulus interval on acquisition in the trace paradigm. Psychol Aging. 1991;6(1):109–17. doi: 10.1037//0882-7974.6.1.109. [DOI] [PubMed] [Google Scholar]
- 3.Hauger RL, Thrivikraman KV, Plotsky PM. Age-related alterations of hypothalamic–pituitary–adrenal axis function in male Fischer 344 rats. Endocrinology. 1994;134(3):1528–36. doi: 10.1210/endo.134.3.8119195. [DOI] [PubMed] [Google Scholar]
- 4.Hodes GE, Shors TJ. Distinctive stress effects on learning during puberty. Horm Behav. 2005;48(2):163–71. doi: 10.1016/j.yhbeh.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Issa AM, Rowe W, Gauthier S, Meaney MJ. Hypothalamic–pituitary– adrenal activity in aged, cognitively impaired and cognitively unimpaired rats. J Neurosci. 1990;10(10):3247–54. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knuttinen MG, Gamelli AE, Weiss C, Power JM, Disterhoft JF. Age-related effects on eyeblink conditioning in the F344 × BN F1 hybrid rat. Neurobiol Aging. 2001;22(1):1–8. doi: 10.1016/s0197-4580(00)00194-9. [DOI] [PubMed] [Google Scholar]
- 7.Leuner B, Shors TJ. Learning during motherhood: a resistance to stress. Horm Behav. 2006;50(1):38–51. doi: 10.1016/j.yhbeh.2006.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo MJ, Kau MM, Wang PS. Effect of aging on corticosterone secretion in diestrous rats. J Cell Biochem. 2006;97:351–8. doi: 10.1002/jcb.20576. [DOI] [PubMed] [Google Scholar]
- 9.Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci. 1999;19(18):8122–33. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matt DW, Sarver PL, Lu JK. Relation of parity and estrous cyclicity to the biology of pregnancy in aging female rats. Biol Reprod. 1987;37(2):421–30. doi: 10.1095/biolreprod37.2.421. [DOI] [PubMed] [Google Scholar]
- 11.McEchron MD, Cheng AY, Gilmartin MR. Trace fear conditioning is reduced in the aging rat. Neurobiol Learn Mem. 2004;82(2):71–6. doi: 10.1016/j.nlm.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Meites J. Changes in neuroendocrine control of anterior pituitary function during aging. Neuroendocrinology. 1982;34:151–6. doi: 10.1159/000123293. [DOI] [PubMed] [Google Scholar]
- 13.Romeo RD, Lee SJ, Chhua N, McPherson CR, McEwen BS. Testosterone cannot activate an adult-like stress response in prepubertal male rats. Neuroendocrinology. 2004;79(3):125–32. doi: 10.1159/000077270. [DOI] [PubMed] [Google Scholar]
- 14.Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7(3):284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 15.Shors TJ. Acute stress rapidly and persistently enhances memory formation in the male rat. Neurobiol Learn Mem. 2001;75(1):10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- 16.Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. Stages of estrus mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9(3):419–23. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- 17.Shors TJ, Miesegaes G. Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood. Proc Natl Acad Sci USA. 2002;99(21):13955–60. doi: 10.1073/pnas.202199999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vom Saal FS, Finch CE, Nelson JF. Natural history and mechanisms of reproductive aging in humans, laboratory rodents, and other selected vertebrates. In: Knobil E, Neill JD, editors. The physiology of reproduction. 2nd. Vol. 2. New York: Raven Press; 1994. pp. 1215–314. [Google Scholar]
- 19.Wang C, Leung A, Sinha Hikim AP. Reproductive aging in the male brown-norway rat: a model for the human. Endocrinology. 1993;133(6):2773–81. doi: 10.1210/endo.133.6.8243304. [DOI] [PubMed] [Google Scholar]
- 20.Weiss C, Thompson RF. The effects of age on eyeblink conditioning in the freely moving Fischer-344 rat. Neurobiol Aging. 1991;12(3):249–54. doi: 10.1016/0197-4580(91)90105-s. [DOI] [PubMed] [Google Scholar]
- 21.Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA. 1998;95(7):4066–71. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci. 2001;115(1):175–87. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- 23.Woodruff-Pak DS, Jaeger ME, Gorman C, Wesnes KA. Relationships among age, conditioned stimulus-unconditioned stimulus interval, and neuropsychological test performance. Neuropsychology. 1999;13(1):90–102. doi: 10.1037//0894-4105.13.1.90. [DOI] [PubMed] [Google Scholar]


