Abstract
We hypothesized that chronic administration of the angiotensin-converting enzyme inhibitor, ramipril, to young adult male rats would prevent/ameliorate fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Eighty 12–14-week-old young adult male Fischer 344 rats received either: (1) sham irradiation, (2) 40 Gy of fractionated whole-brain irradiation delivered as two 5 Gy fractions/week for 4 weeks, (3) sham irradiation plus continuous administration of 15 mg/L of ramipril in the drinking water starting 3 days before irradiation, or (4) fractionated whole-brain irradiation plus ramipril. Cognitive function was assessed using a perirhinal cortex-dependent version of the novel object recognition task 26 weeks after irradiation. Microglial activation was determined in the perirhinal cortex and the dentate gyrus of the hippocampus 28 weeks after irradiation using the ED1 antibody. Neurogenesis was assessed in the granular cell layer and subgranular zones of the dentate gyrus using a doublecortin antibody. Fractionated whole-brain irradiation led to: (1) a significant impairment in perirhinal cortex-dependent cognitive function, (2) a significant increase in activated microglia in the dentate gyrus but not in the perirhinal cortex, and (3) a significant decrease in neurogenesis. Continuous administration of ramipril before, during, and after irradiation prevented the fractionated whole-brain irradiation-induced changes in perirhinal cortex-dependent cognitive function, as well as in microglial activation in the dentate gyrus. Thus, as hypothesized, continuous administration of the angiotensin-converting enzyme inhibitor, ramipril, can prevent the fractionated whole-brain irradiation-induced impairment in perirhinal cortex-dependent cognitive function.
INTRODUCTION
Up to 30% of the ~1.6 million new cancer patients diagnosed in the U.S. in 2011 will develop brain metastases (1, 2). Fractionated, partial or whole-brain irradiation is widely used for the treatment of brain metastases, and ~170,000 patients a year will receive cranial irradiation (3). Long-term survivors face the risk of developing radiation-induced late effects that can severely impact their quality of life, with chronic, progressive cognitive impairment observed in up to 50% of adult patients surviving ≥6 months after fractionated whole-brain irradiation (4). These patients exhibit progressive deficits in information processing speed, frontal lobe executive functions, memory, spatial relationships, visual motor processing, quantitative skills, and/or attention (5, 6). This cognitive impairment in memory is not solely hippocampal, but is associated with declines in spatial and non-spatial learning, as well as in verbal and figural memory, the latter reflecting perirhinal cortex-dependent pathways (7). Rodent studies have demonstrated significant reductions in both hippocampal-dependent (8, 9) and perirhinal cortex-dependent (10) cognitive function after single-dose or fractionated whole-brain irradiation.
Hippocampal dysfunction has been hypothesized to be a causal mechanism underlying some of these radiation-induced cognitive deficits (11, 12). Alterations in neurogenesis and the neurogenic microenvironment have been noted in the irradiated hippocampus, including changes in the neurovasculature and granular precursor cell populations in the dentate gyrus (13), and an elevation of the microglial inflammatory response (14). Microglia, the immune cells of the brain, can act as negative regulators of neurogenesis by producing proinflammatory cytokines that block neuronal differentiation and increase precursor cell death (14, 15). Prolonged microglial activation can also lead to a sustained inflammatory response that has been implicated in acute and chronic neurodegenerative diseases, as well as in late radiation-induced brain injury (16, 17).
Although the pathogenic mechanism(s) involved in radiation-induced cognitive impairment remain(s) unclear, recent studies aimed at blocking the renin-angiotensin system (18) have shown promise in preventing or mitigating radiation-induced late effects in the central nervous system. The angiotensin-converting enzyme (ACE) inhibitor, ramipril, has been shown to modulate radiation-induced optic neuropathy (19), and the angiotensin type 1 receptor antagonist (AT1RA), L-158,809, can prevent/ameliorate fractionated whole-brain irradiation-induced cognitive impairment (20). Angiotensin II (Ang II) is increasingly recognized as a potent inflammatory peptide (21, 22), and the ability of renin-angiotensin system blockade to modulate radiation-induced brain injury has been hypothesized to reflect, in part, inhibition of renin-angiotensin system-mediated neuroinflammation (18).
To date, no studies have evaluated the effects of continuous administration of ramipril on cognition after fractionated whole-brain irradiation. We used our well-characterized rat model of radiation-induced brain injury (10) to test the hypothesis that continuous administration of ramipril prevents radiation-induced cognitive impairment. In addition, given the hypothesized role of inflammation and its impact on neurogenesis in radiation-induced brain injury, we assessed the ability of ramipril to modulate microglial activation in the perirhinal cortex and the dentate gyrus, as well as in neurogenesis in the dentate gyrus. The data presented here demonstrate that continuous administration of ramipril prevented the radiation-induced impairment in perirhinal cortex-dependent cognitive function.
MATERIALS AND METHODS
Animals and Whole-Brain Irradiation Procedures
Eighty 10–12-week-old young adult male Fischer 344 (F344) rats were obtained from Harlan Laboratories, Inc. (Indianapolis, IN) and housed in pairs on a 12-h light/dark cycle with food and water ad libitum. All experiments and handling of animals were performed in strict accordance with the NIH Guide for Care and Use of Laboratory Animals as approved by the Wake Forest School of Medicine Institutional Animal Care and Use Committee.
After an acclimation period of 2 weeks, rats were randomized to 4 experimental groups (n = 20 rats/group); Group 1: sham irradiation, Group 2: fractionated whole-brain irradiation alone, Group 3: sham irradiation plus 15 mg/l of ramipril (King Pharmaceuticals, Cary, NC) in the drinking water, and Group 4: fractionated whole-brain irradiation plus 15 mg/l of ramipril in the drinking water. Rats received ramipril beginning 3 days before the start of fractionated whole-brain irradiation and continuously until the end of the experiment. All rats were weighed weekly to assess their overall health, and fresh drinking water with or without ramipril was supplied every other day. A 40 Gy total dose of fractionated whole-brain irradiation was delivered in 8 fractions of 5 Gy, twice per week, for 4 weeks as described previously (23). The biologically effective dose (24) calculated for this regimen was 106.7 Gy, assuming an α/β ratio of 3 Gy for late radiation-induced brain damage (25). The corresponding biologically effective dose for a conventional irradiation regimen for glioma patients (30 fractions of 2 Gy in 6 weeks) was 100.2 Gy. Thus, the biological effects of this prolonged fractionated whole-brain irradiation regimen are anticipated to be more relevant to the clinical situation than the biological effects observed after large single doses or large fractionated doses delivered over a short time period.
All irradiations were performed in a 267 TBq (7,214 Ci) self-shielded 137Cs irradiator using lead and Cerrobend® shielding devices to collimate the beam so that the eyes were shielded to prevent optic nerve damage and vision loss, and the whole brain, including the brain stem, was irradiated. The average dose rate to the midline of the brain was ~4 Gy/min: the eyes and body received about 15% and 1–3% of the brain dose, respectively. To ensure that each rat received the same midline brain dose, each lightly anesthetized rat (Ketamine [75 mg/kg]/xylazine [7 mg/kg]) had the twice-weekly dose delivered to alternate sides of the head on alternate days. Sham-irradiated rats were anesthetized twice weekly for 4 weeks.
Novel Object Recognition Task
Cognitive function was assessed in each animal 26 weeks after the completion of fractionated whole-brain irradiation using a perirhinal cortex-dependent version of the novel object recognition task (10, 26). The novel object recognition task is comprised of three phases: familiarization, delay and test. Before testing, each animal was habituated to the testing arena (a rectangular container: 24” × 18” × 20”) by allowing exploration of the empty arena for 5 min a day for 5 days. After habituation, the sessions that followed were divided into a sample phase and a test phase. During the sample phase, the rodent was allowed to explore and become familiar with 2 identical objects located within the testing arena for 3 min. Following the sample phase, a 1 min delay was introduced during which the animal was removed from the arena and returned to its home cage. A test phase followed in which one of the 2 objects from the sample phase was paired with a novel object. The subject was left in the arena for a total of 3 min during the test phase and its activity was recorded. The objects were secured to the arena floor with Velcro patches so that the subject could not displace them during exploration. Recognition memory was inferred from the preferential exploration (measured as time) of the novel object compared with that of the familiar object. The position (left or right) of the novel object in the test phase was balanced between sessions to avoid any spatial preference.
The basic measurement collected was the time the rat spent exploring an object, defined as placing his nose within ≤2 cm of the object and actively exploring it. The following parameters were obtained: E1, the total time spent exploring the identical objects A1 and A2 in the sample phase (3 min); E2, the total time spent exploring object A3 (identical to A1 and A2) and the novel object B1 in the test phase (3 min); and D1, the index of discrimination defined as the difference in time spent exploring objects A3 and B1 in the test phase (i.e., B1–A3). Rats with an E1 <10 s were judged to have failed to complete the task and were eliminated from the study (1 sham and 1 sham + ramipril). For the remaining rats, the discrimination ratio (D1/E2), a measure of the rat’s recognition memory, was calculated, and the average discrimination ratio for each treatment group was compared.
Tissue Processing and Blood Collection
Rats were weighed weekly during the study. Following completion of the cognitive function testing at 28 weeks post-fractionated whole-brain irradiation/sham-fractionated whole-brain irradiation, they were euthanized by decapitation (guillotine). Arterial blood was collected from decapitated rats into chilled Vacutainer blood collection tubes (Becton-Dickinson, Franklin Lakes, NJ). The extractions, assays and antibody characteristics were as previously described (27–29). The tubes contained a mixture of peptidase inhibitors, in a cocktail of protease inhibitors (0.12 mM pepstatin A, 0.44 mmol/L o-phenanthroline, 25 mmol/L EDTA, 1 mM 4-chloromercuribenzoic acid, and 3 µM acetyl-His-Pro-Phe-Val-statine-Leu-Phe, a specific rat renin inhibitor). Blood samples were centrifuged within 20 min of collection at 3000g for 20 min, plasma was removed and aliquots were stored at −80°C until radioimmunoassay of angiotensin (Ang) peptides. For plasma extraction, Sep-Pak columns (Waters, Milford, MA) were activated with 5-mL sequential washes of a mixture of ethanol/water/4% acetic acid (83: 13: 4), methanol, ultrapure water, and 4% acetic acid. The sample was applied to the column, washed with ultrapure water and acetone, and eluted with 2:1 mL and 1:1.5 mL washes of a mixture of ethanol/water/4% acetic acid. Samples were reconstituted in assay buffer and for Ang I and Ang-(1–7), a TRIS buffer with 0.1% bovine serum albumin was used. Samples were subjected to 3 different radioimmunoassays. Ang II was measured using a commercial kit (Nichols Institute radioimmunoassay, Alpco, Windham, NH), Ang-(1–7) was measured using an antibody characterized by us, and Ang I was assayed with a modified Peninsula assay (Peninsula Laboratories, San Carlos, CA). Samples were corrected for recoveries of trace radiolabeled angiotensins added to samples, which averaged 92%. The minimum detectable levels of the assays were 1 fM/mL, 0.8 fM/mL and 2.8 fM/mL for Ang I, Ang II, and Ang-(1–7), respectively, and the minimum detectable level of the assay was arbitrarily assigned for samples less than the detectable limit. Intra-assay and inter-assay coefficient of variation was 18% and 22% for Ang I, 12% and 22% for Ang II, and 8% and 20% for Ang-(1–7). Cross-reactivity for the angiotensin-(1–7) radioimmunoassay is 100% for Ang-(1–7) and Ang-(2–7), and less than 0.01% with Ang-(3–7), Ang II, Ang I, and their fragments; for the Ang II radioimmunoassay is 100% for Ang II, Ang III, Ang-(3–8), and Ang-(4–8), but less than 0.01% with Ang I and Ang-(1–7); and for the Ang I radioimmunoassay is 100% for Ang I, Ang-(2–10) and Ang-(3–10), but less that 0.01% with Ang II and Ang-(1–7).
The brains were removed rapidly and hemisected at the midline. The right hemisphere was flash frozen in liquid nitrogen, and the left hemisphere was immersion fixed in phosphate-buffered 4% paraformaldehyde for 24 h. The left hemispheres were then cryoprotected for 24 h in 10%, 20%, and 30% sucrose, frozen in embedding medium, cryosectioned at a thickness of 40-µm in the coronal plane and then placed in an antifreeze solution (1:1:2 ethylene glycol, glycerol and 0.1 M of phosphate-buffered saline).
Immunohistochemistry
Based on previously published studies, immunohistochemistry was performed on the left hemispheres of 4 brains selected from each experimental group (30). For each brain, every 12th section through the hippocampus dentate gyrus (bregma −1.8 to bregma −6.8) was systematically random sampled (the first section chosen was randomly selected from the first 12 sections of dentate gyrus for each animal and then one in every 12th section was systematically selected throughout the dentate gyrus). These same sections were also used to assess immunohistochemistry in the perirhinal cortex region. Sections were then washed in tris-buffered saline (TBS: pH 7.4) to remove the cryoprotectant. For immunohistochemical staining, endogenous peroxidase activity was reduced by 30 min incubation in 0.6% hydrogen peroxide in TBS. Sections were then incubated in blocking solution (5% normal serum and 0.2% Triton X-100 in TBS), and then overnight at 4°C in primary antibody diluted in blocking solution. Primary antibodies were: (1) mouse monoclonal anti-rat CD68 (ED1 clone), which labels activated macrophages/microglia (MCA341R, AbD Serotec, Raleigh, NC; 1:400); (2) rabbit polyclonal anti-ionized calcium-binding adaptor molecule (Iba1), which labels all macrophages/microglia (Wako, Richmond, VA; 0.083 µg/mL); and (3) goat polyclonal anti-rat doublecortin (Dcx+), which labels immature neurons [sc-8066 (C18), Santa Cruz Biotechnology, Inc., Santa Cruz, CA; 1 µg/mL; 1:200].
Primary antibodies were detected using biotinylated secondary antibodies [ED1: horse anti-mouse, Iba1: goat anti-rabbit, Dcx: horse anti-goat (Santa Cruz; 1:300)] and were visualized using peroxidase-conjugated avidin-biotin complex (ABC Elite kit) with nickel enhanced diaminobenzidine as substrate (Vector Laboratories, Burlingame, CA). Sections were counterstained with the nuclear binding dye 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma Aldrich, St. Louis, MO) to facilitate recognition of anatomical landmarks.
Stereological Analyses of Tissue Sections
Stereologic quantification is superior to traditional, single-section estimation techniques because the result is not biased by the shape, size, and orientation of the counting objects or by volumetric variation (31). All analyses were performed blindly by using coded sections. A modification of the optical disector method was used for quantification of immunolabeled cells in the dentate gyrus (granular cell layer and subgranular zone) and hilus (area included between both blades of dentate gyrus) (32). ED1+ cells in the dentate gyrus/hilus were then exhaustively counted, excluding cells in the top focal plane to avoid overestimation, using the Neurolucida system (Microbrightfield, Inc., Colchester, VT). The estimated total number of: (1) Iba1+ cells in the dentate gyrus/hilus and perirhinal cortex, (2) ED1+ cells in the perirhinal cortex, and (3) Dcx+ cells in the granular cell layer/subgranular zone, were estimated by the optical fractionator method using StereoInvestigator software (Microbrightfield, Inc.). The perirhinal cortex was determined for quantification purposes to be the area 100 µm on either side of the rhinal fissure extending to, but not including, the adjacent white matter (~bregma −2.0 to −4.4). The counting parameters for Iba1+ and ED1+ cells in the dentate gyrus/hilus and perirhinal cortex were: sampling grid size (175 × 175 µm), counting frame size (75 × 75 µm), disector height (12 µm), and guard-zone thickness (2 µm). For Dcx+ cells in the granular cell layer/subgranular zone the counting parameters were: sampling grid size (100 × 100 µm), counting frame size (100 × 100 µm), disector height (10 µm), and guard-zone thickness (2 µm). To estimate the precision of the stereological counts we determined the coefficient of error (CE) using the Gundersen-Jensen coefficient of error estimator estimates. The variance introduced by the stereological analysis should not account for more than 50% of the observed group variance (33), i.e., the ratio between CE2 and the observed variance of the group, CV2, should be less than 0.5. All estimates in the current study were less than 0.5 (34).
Statistical Analysis
Data are presented as the mean ± SEM and the CE for the mean of individual estimates of all stereological group data are also reported. A two-way analysis of variance (ANOVA) was used to detect a drug (ramipril) effect, a radiation effect, and any interaction (drug × radiation) effects. Bonferroni and Student-Newman-Keuls tests were used for the pairwise comparisons (controlling for multiple comparisons). All analyses were performed using Graphpad Prism (La Jolla, CA) and/or SAS (Cary, NC) software.
RESULTS
All animals appeared healthy during the study. Data analysis of the body weights determined 28 weeks after irradiation revealed a significant effect of both fractionated whole-brain irradiation (F(1.70) = 4.38, P = 0.040) and ramipril (F(1.70) = 11.69, P = 0.001), as well as a significant interaction effect (F(1.70) = 7.46, P = 0.008). Bonferroni posttest analysis showed no significant difference in body weight between the fractionated whole-brain irradiation, sham-irradiated, or fractionated whole-brain irradiation + ramipril animals. The mean body weights (± SEM) for these groups measured 28 weeks after irradiation were 485.7 ± 9.9 g, 490.3 ± 5.9 g, and 480.8 ± 6.8 g, respectively. However, the mean body weight in the sham-irradiated + ramipril rats of 446.5 ± 5.8 g was significantly lower (P < 0.05) compared with the other groups (see Supplementary Material S1; http://dx.doi.org/10.1667/RR2731.1.S1).
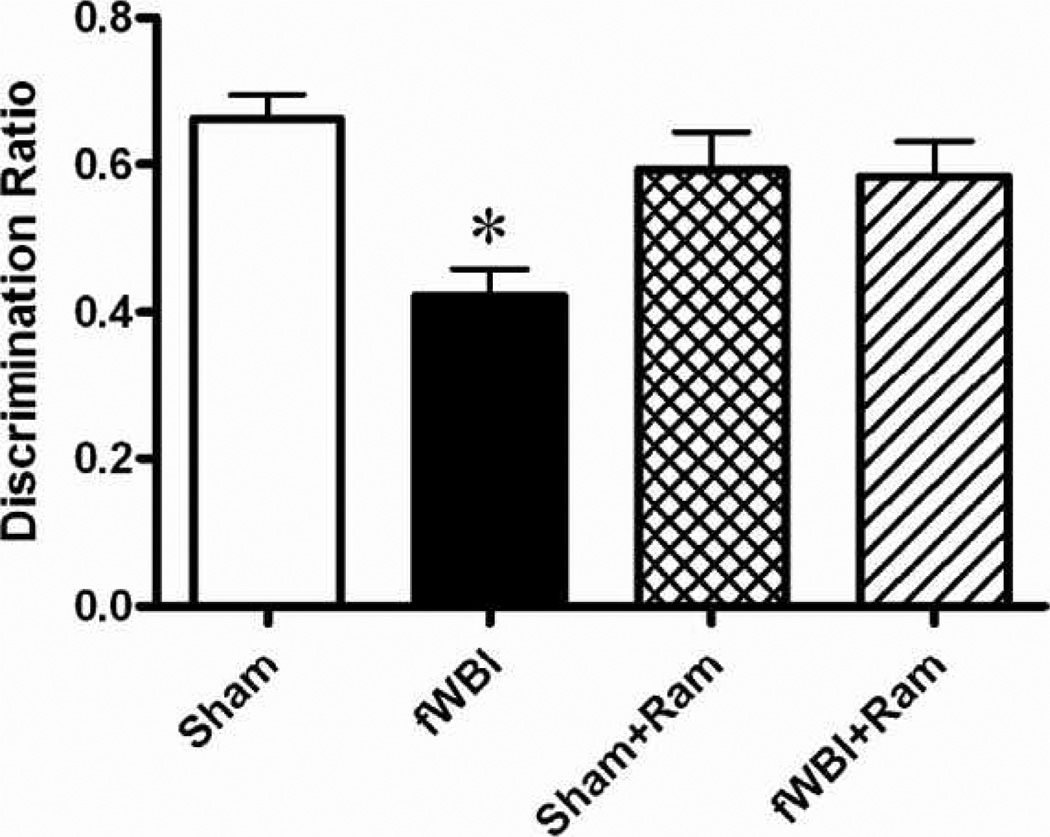
Perirhinal cortex-dependent cognitive function was determined 26 weeks after the completion of fractionated whole-brain irradiation. Data analysis using two-way ANOVA showed that there was no significant difference in E2 (the total time spent exploring both objects in the test phase) between any of the groups: radiation (F(1,70) = 0.76, P = 0.388); ramipril (F(1,70) = 0.17, P = 0.682); and interaction (F(1,70) = 0.06, P = 0.812). Values (seconds) were 23.00 ± 1.94 (sham irradiation), 21.78 ± 1.93 (fractionated whole-brain irradiation), 24.26 ± 2.14 (sham + ramipril), and 22.10 ± 1.70 (fractionated whole-brain irradiation + ramipril). All rats showed a preference for exploring the novel object during the test phase. However, the fractionated whole-brain irradiation rats spent significantly more time investigating the familiar object (A3) compared to the sham-irradiated group (interaction effect: F(1,70) = 4.351, P = 0.041; Bonferroni P < 0.05; no significant main effects of radiation (F(1,70) = 3.189, P = 0.079) or ramipril (F(1,70) = 1.083, P = 0.302), which indicates that they were impaired in discriminating the novel from the familiar object compared to the other groups (see Supplementary Material S2; http://dx.doi.org/10.1667/RR2731.1.S2). Analyzing the D1 (B1–A3) values revealed no effect of ramipril (F(1,70) = 2.02, P = 0.160) and no interaction between fractionated whole-brain irradiation and ramipril (F(1,70) = 2.02, P = 0.160). However, there was a significant radiation effect (F(1,70) = 7.08, P = 0.010), due to the fractionated whole-brain irradiation rats exploring the familiar object (A3) longer. The mean D1 value for fractionated whole-brain irradiation rats was 8.89 ± 1.02, which was significantly less than that observed in the other groups [14.79 ± 1.34 (sham), 14.79 ± 1.92 (sham + ramipril) and 13.0 ± 1.30 (fractionated whole-brain irradiation + ramipril)]. Analyzing the discrimination ratio data revealed a ramipril × fractionated whole-brain irradiation interaction (F(1.70) = 7.35, P = 0.008). Consistent with previously published results for this rat model (10, 20), fractionated whole-brain irradiation significantly reduced perirhinal cortex-dependent cognitive function (Fig. 1, Bonferroni, P < 0.05). Continuous administration of ramipril in the drinking water before, during, and after fractionated whole-brain irradiation prevented this reduction in cognitive function without any significant effect on the cognitive function of the sham-irradiated controls (Fig. 1; two-way ANOVA: fractionated whole-brain irradiation effect F(1.70) = 8.58, P = 0.005; ramipril effect F(1.70) = 1.20, P = 0.276; no significant difference between sham and sham + ramipril: Bonferroni, P > 0.05).
FIG. 1.
Administration of the angiotensin-converting enzyme-inhibitor, ramipril, to young adult male F344 rats before, during, and after fractionated whole-brain irradiation prevents radiation-induced perirhinal-cortex-dependent cognitive impairment measured by the perirhinal-cortex-dependent version of the novel object recognition task. Rats received either sham irradiation (Sham), 40 Gy of fractionated whole-brain irradiation (fWBI), sham irradiation + 15 mg/L of ramipril in the drinking water (Sham + Ram), or 40 Gy of fractionated whole-brain irradiation + ramipril (fWBI + Ram). Ramipril was continuously administered to the rats starting 3 days before the beginning of fractionated whole-brain irradiation. Cognitive function was assessed 26 weeks after completion of fractionated whole-brain irradiation. Data represent the mean ± SEM; n = 20/group; *P < 0.05.
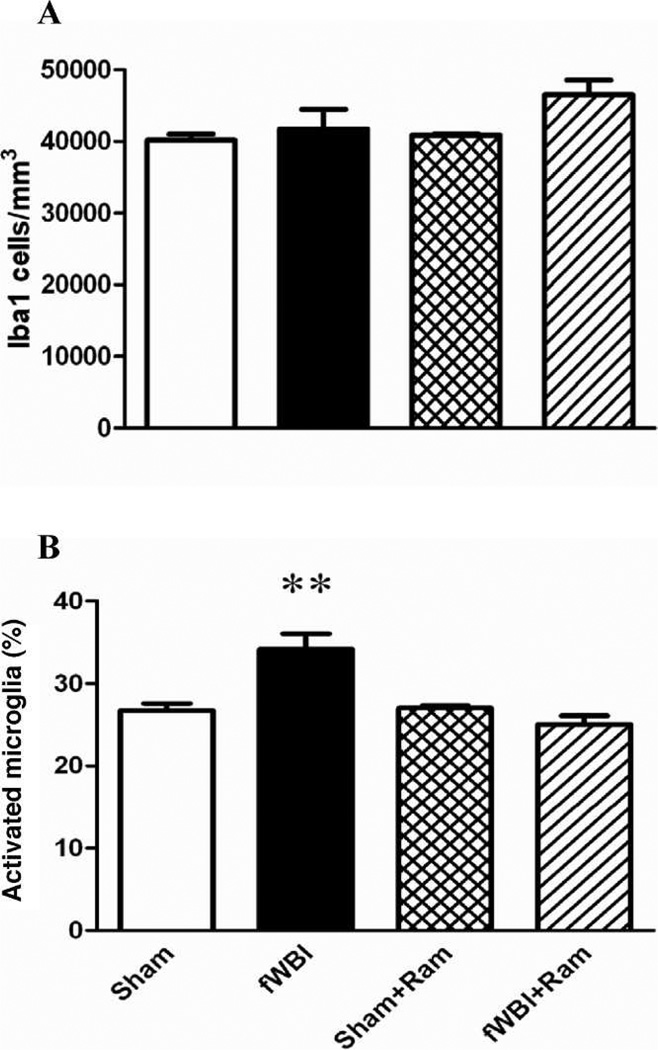
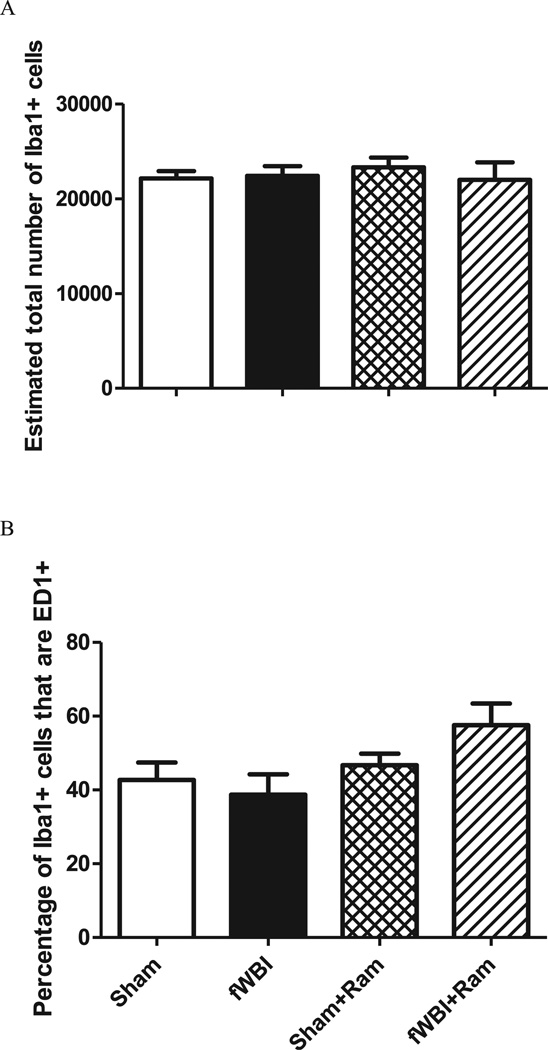
No significant change in the number of Iba1+ cells/mm3 (total microglia) was measured in the dentate gyrus in any of the groups 28 weeks after fractionated whole-brain irradiation (Fig. 2A; two-way ANOVA: fractionated whole-brain irradiation effect F(1.12) = 4.23, P = 0.062; ramipril effect F(1.12) = 2.48, P = 0.141; ramipril × fractionated whole-brain irradiation F(1.12) = 1.42, P = 0.257; coefficient of error: sham = 0.03, fractionated whole-brain irradiation = 0.03, sham + ramipril = 0.03, fractionated whole-brain irradiation + ramipril = 0.03). As expected (35), expressing the data as the percentage of total microglia that were activated revealed that, (1) fractionated whole-brain irradiation led to a significant increase in the percentage of activated microglia, and (2) this increase was prevented by continuous administration of ramipril (Fig. 2B: two-way ANOVA: fractionated whole-brain irradiation effect F(1.12) = 5.65, P = 0.035; ramipril effect F(1.12) = 14.68, P = 0.002; ramipril × fractionated whole-brain irradiation F(1.12) = 16.74, P = 0.002). In contrast, no significant difference in microglial activation or in the percentage of microglia that were activated in the perirhinal cortex, was measured between any of the treatment and sham-irradiation groups (Fig. 3; two-way ANOVA: fractionated whole-brain irradiation effect F(1.12) = 0.48, P = 0.500; ramipril effect F(1.12) = 5.32, P = 0.040; ramipril × fractionated whole-brain irradiation F(1.12) = 2.26, P = 0.159, Bonferroni group comparisons P > 0.05; coefficient of error: sham = 0.07, fractionated whole-brain irradiation = 0.07, sham + ramipril = 0.06, fractionated whole-brain irradiation + ramipril = 0.05). Additionally there was no significant difference in the total number of microglia (Iba1+) in the perirhinal cortex (two-way ANOVA: fractionated whole-brain irradiation F(1.12) = 0.18, P = 0.6814; ramipril effect F(1.12) = 0.9, P = 0.7656; ramipril × fractionated whole-brain irradiation F(1.12) = 0.43, P = 0.5243, coefficient of error = 0.07 for all groups).
FIG. 2.
Ramipril decreases radiation-induced microglial activation in the dentate gyrus of the hippocampus. The number of microglia/mm3 was unchanged after fractionated whole-brain irradiation (fWBI) (panel A). The percentage of activated microglia (panel B) increased after fractionated whole-brain irradiation. Administering ramipril prevented these increases. Rats received either sham irradiation (Sham), 40 Gy of fractionated whole-brain irradiation (fWBI), sham irradiation +15 mg/L of ramipril in the drinking water (Sham + Ram), or 40 Gy of fractionated whole-brain irradiation + ramipril (fWBI + Ram). Ramipril was continuously administered to the rats starting 3 days before the beginning of fractionated whole-brain irradiation. Data represent the mean ± SEM; n = 4 rats/group; **P < 0.01.
FIG. 3.
Fractionated whole-brain irradiation and/or ramipril treatment fails to alter total number of microglia (panel A) as well as microglial activation (panel B) in the perirhinal cortex. Rats received either sham irradiation (Sham), 40 Gy of fractionated whole-brain irradiation (fWBI), sham irradiation +15 mg/L of ramipril in the drinking water (Sham + Ram), or 40 Gy of fractionated whole-brain irradiation + ramipril (fWBI + Ram). Ramipril was continuously administered to the rats starting 3 days before the beginning of fractionated whole-brain irradiation. Data represent the mean ± SEM; n = 4 rats/group.
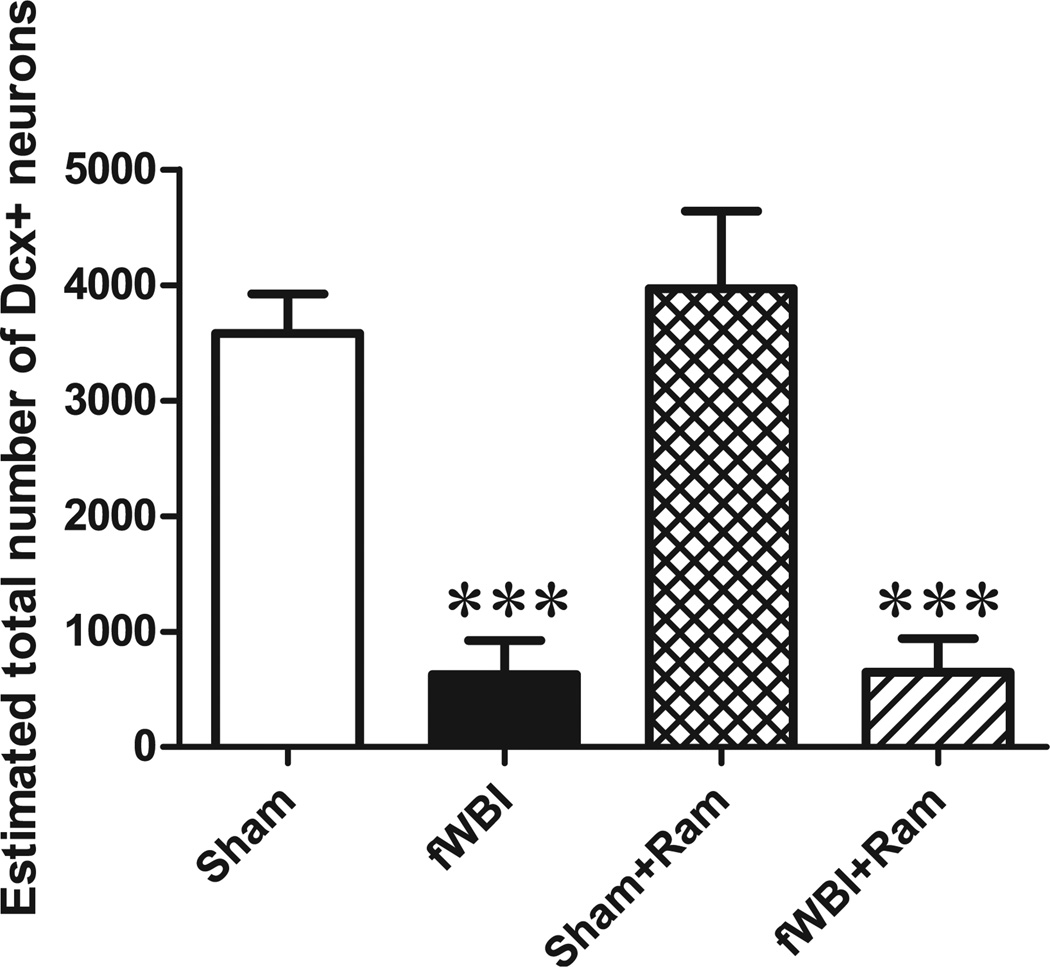
Immature neurons (DCX+ cells) were quantified within the granular cell layer/subgranular zone as an indirect marker of neurogenesis, since not all newborn neurons are incorporated into functional networks (36). There was an effect of fractionated whole-brain irradiation (F(1.12) = 53.33, P < 0.0001), but no effect of ramipril (F(1.12) = 0.23, P = 0.642) and no ramipril and fractionated whole-brain irradiation interaction (F(1.12) = 0.19, P = 0.674). A significant decrease in neurogenesis was measured in fractionated whole-brain irradiation rats compared to sham-irradiated rats. Continuous administration of ramipril did not prevent this fractionated whole-brain irradiation-induced decrease in neurogenesis (Fig. 4; coefficient of error: sham = 0.08, fractionated whole-brain irradiation = 0.32, sham + ramipril = 0.07, fractionated whole-brain irradiation + ramipril = 0.26).
FIG. 4.
Ramipril does not prevent the fractionated whole-brain irradiation-induced decrease in hippocampal neurogenesis. The marked reduction in the number of immature neurons (DCX+) in the dentate gyrus after fractionated whole-brain irradiation determined 28 weeks after fractionated whole-brain irradiation was not modulated by continuous administration of ramipril. Rats received either sham irradiation (Sham), 40 Gy of fractionated whole-brain irradiation (fWBI), sham irradiation +15 mg/L of ramipril in the drinking water (Sham + Ram), or 40 Gy of fractionated whole-brain irradiation + ramipril (fWBI + Ram). Ramipril was continuously administered to the rats starting 3 days before the beginning of fractionated whole-brain irradiation. Data represent the mean ± SEM; n = 4 rats/group; **P < 0.01, ***P < 0.001.
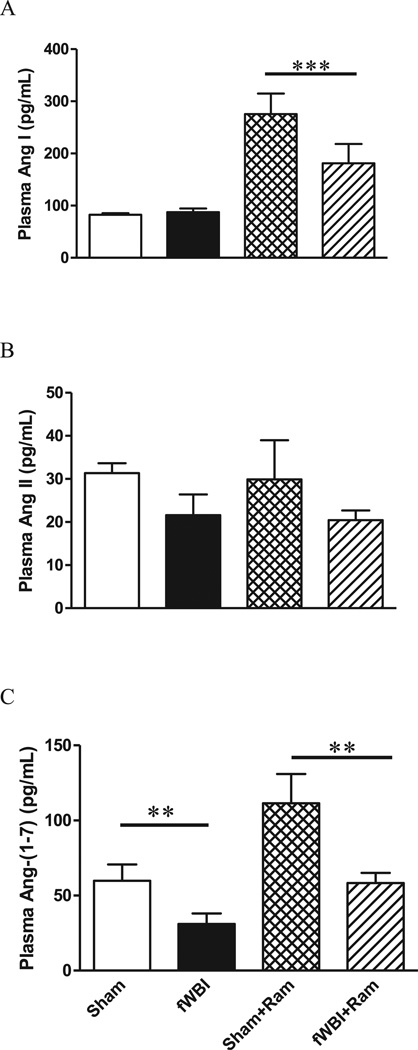
With respect to plasma Ang I levels, a two-way ANOVA showed no interaction between ramipril and fractionated whole-brain irradiation (F(1.20) = 3.29, P = 0.085). Also, no significant difference in the plasma level of Ang I was observed between fractionated whole-brain irradiation and sham-irradiated rats that did not receive ramipril (Fig. 5A; F(1.20) = 2.67, P = 0.118). However, continuous administration of ramipril significantly increased the plasma Ang I level in sham-irradiated rats and fractionated whole-brain irradiation rats (Fig. 5A; F(1.20) = 27.37, P = 0.0001). Two-way ANOVA showed no significant interaction between ramipril and fractionated whole-brain irradiation, and neither fractionated whole-brain irradiation or ramipril significantly altered plasma levels of Ang II (Fig. 5B; two-way ANOVA fractionated whole-brain irradiation F(1.20) = 3.21, P = 0.088; ramipril effect F(1.20) = 0.06, P = 0.811; ramipril × fractionated whole-brain irradiation F(1.20) = 0.00, P = 0.980). There was also no significant interaction between ramipril and fractionated whole-brain irradiation in terms of plasma Ang-(1–7) levels (Fig. 5C, F(1.20) = 1.01, P = 0.327). However, fractionated whole-brain irradiation produced a significant decrease in the plasma Ang-(1–7) level compared to the level in sham-irradiated rats (Fig. 5C, F(1.20) = 11.28, P = 0.003). Continuous administration of ramipril returned the plasma Ang-(1–7) level in the fractionated whole-brain irradiation rats to the level in sham-irradiated rats, but not to the level in the sham-irradiated rats receiving ramipril (Fig. 5C, F(1.20) = 10.48, P = 0.004).
FIG. 5.
Continuous administration of ramipril results in a significant increase in plasma Ang I and Ang-(1–7), but not Ang II. Panel A: Ang I levels; panel B: Ang II levels; panel C: Ang-(1–7) levels. Rats received either sham irradiation (Sham), 40 Gy of fractionated whole-brain irradiation (fWBI), sham irradiation +15 mg/L of ramipril in the drinking water (Sham + Ram), or 40 Gy of fractionated whole-brain irradiation + ramipril (fWBI + Ram). Ramipril was continuously administered to the rats starting 3 days before the beginning of fractionated whole-brain irradiation. Data represent mean ± SEM; n = 6 rats/group; * P < 0.05.
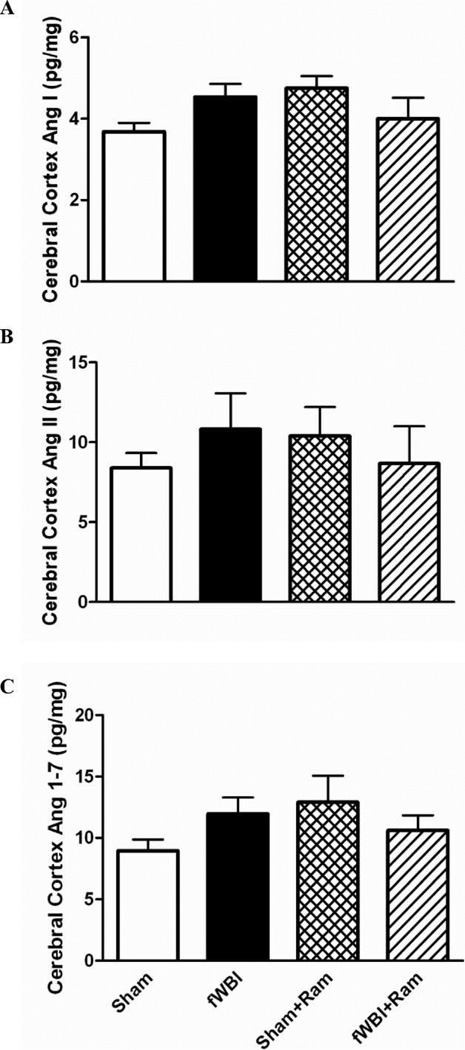
In contrast, no significant differences in Ang peptide levels were observed in the cerebral cortex of the rats from any of the groups [Fig. 6; two-way ANOVA: (Ang I: fractionated whole-brain irradiation F(1.18) = 0.02, P = 0.884; ramipril effect F(1.18) = 0.50, P = 0.487; ramipril × fractionated whole-brain irradiation F(1.18) = 4.66, P = 0.045), (Ang II: fractionated whole-brain irradiation F(1.18) = 0.03, P = 0.863; ramipril effect F(1.18) = 0.00, P = 0.969; ramipril × fractionated whole-brain irradiation F(1.18) = 1.11, P = 0.305), (Ang-(1–7): fractionated whole-brain irradiation F(1.18) = 0.05, P = 0.823; ramipril effect F(1.18) = 0.69, P = 0.418; ramipril × fractionated whole-brain irradiation F(1.18) = 2.90, P = 0.106].
FIG. 6.
Continuous administration of ramipril does not significantly alter the levels of angiotensin peptides in the cerebral cortex. Panel A: Ang I levels; panel B: Ang II levels; panel C: Ang-(1–7) levels. Rats received either sham irradiation (Sham), 40 Gy of fractionated whole-brain irradiation (fWBI), sham irradiation +15 mg/L of ramipril in the drinking water (Sham + Ram), or 40 Gy of fractionated whole-brain irradiation + ramipril (fWBI + Ram). Ramipril was continuously administered to the rats starting 3 days before the beginning of fractionated whole-brain irradiation. Cognitive function was assessed 26 weeks after completion of fractionated whole-brain irradiation. Data represent mean ± SEM; n = 6 rats/group.
DISCUSSION
In our model of radiation-induced brain injury in young adult male rats, continuous administration of the angiotensin-converting enzyme inhibitor, ramipril, before, during, and after fractionated whole-brain irradiation prevented the decline in perirhinal cortex-dependent cognitive function measured 26 weeks after fractionated whole-brain irradiation. Continuous administration of ramipril also prevented the radiation-induced increase in activated microglia in the dentate gyrus, but had no effect on the radiation-induced decrease in hippocampal neurogenesis. Moreover, ramipril treatment increased systemic levels of Ang 1, and prevented the fractionated whole-brain irradiation-induced decrease in plasma Ang-(1–7) levels, indicating effective angiotensin-converting enzyme inhibition. Thus, chronic administration of ramipril in the young adult male rat appears to prevent the fractionated whole-brain irradiation-induced impairment in perirhinal cortex-dependent cognitive function.
Chronic renin-angiotensin system blockade using the angiotensin-converting enzyme inhibitor, ramipril, was well tolerated, with no apparent morbidity evident in any of the experimental groups. Body weight was reduced by ~8.0% in the sham-irradiation + ramipril rats compared with the other groups. This ramipril-mediated reduction in body weight confirms previous reports showing that chronic administration of angiotensin-converting enzyme inhibitors results in weight loss in rats (37, 38). This reduction in weight gain seen with chronic renin-angiotensin system blockade does not appear to reflect changes in food intake, but rather inhibition of an age-related increase in body weight due to improvements in metabolic function (39) and/or a decrease in body adiposity (38).
Previous studies in the kidney and, to a lesser extent the lung have provided clear evidence that renin-angiotensin system blockade using either angiotensin-converting enzyme inhibitors or AT1RA can prevent/ameliorate radiation-induced late effects (40). Given the presence of a functioning brain renin-angiotensin system (41) and its importance in normal cognitive processing and potential treatment of dysfunctional memory disease states (42, 43), the use of renin-angiotensin system blockers in the treatment of radiation-induced brain injury, including cognitive impairment, appears logical (18). Indeed, a key role for the brain renin-angiotensin system in the development of dyscirculatory encephalopathy in Chernobyl cleanup workers has been recently proposed (44). Using a rat model of radiation-induced optic neuropathy, Kim et al. (19) were the first to demonstrate ramipril-mediated neuroprotection against late radiation-induced brain injury. Administering ramipril (1.5 mg/kg/day) to young adult male F344 rats starting 2 weeks after stereotactic irradiation (30 Gy) of the optic nerves and chiasm and, for 6 months after irradiation, mitigated the severity of functional and histologic optic nerve damage. Of interest, delaying the start of ramipril administration until 4 weeks after irradiation failed to prevent radiation-induced injury, which suggests that effective mitigation with ramipril necessitates early administration of the drug (45). More recent studies have shown that ramipril produced modest protection against whole-brain irradiation-induced decreases in neurogenesis, but did not modulate radiation-induced neuroinflammation, which was assessed in terms of microglial activation (46).
The current findings extend the above observations, and demonstrate for the first time that ramipril can prevent fractionated whole-brain irradiation-induced impairment in perirhinal cortex-dependent cognitive function (Fig. 1). It should be noted that the degree of cognitive impairment observed 26 weeks after fractionated whole-brain irradiation is subtle. However, we have previously reported that fractionated whole-brain irradiation leads to a chronic, progressive decline in cognitive function assessed using the novel object recognition task, such that by 52 weeks after fractionated whole-brain irradiation, the animals no longer exhibit a preference for either object (20). It remains unclear as to whether the prevention of fractionated whole-brain irradiation-induced impairment in perirhinal cortex-dependent cognitive function seen at 26 weeks reflects permanent protection or a delayed response that might appear at later times. Although this will require additional studies, previous findings using the AT1RA, L-158,809 suggest that renin-angiotensin system blockade prior to, during, and for only 5 weeks after fractionated whole-brain irradiation, was sufficient to prevent perirhinal cortex-dependent cognitive impairment determined 26 weeks after irradiation (20). These findings also confirm the ability of angiotensin-converting enzyme inhibitors to modulate memory and learning. In preclinical studies, angiotensin-converting enzyme inhibitors have been shown to improve learning and memory in aged rats (47), as well as enhance memory learned by fear or habituation (48). In contrast, intra-hippocampal infusion of Ang II in rats induces amnesia (49). Clinically, angiotensin-converting enzyme inhibitors improve cognitive function in essential hypertensive patients independent of their effects on blood pressure (50), and stabilize cognitive function in patients with mild cognitive impairment (51). However, the specific mechanism(s) involved in this angiotensin-converting enzyme inhibitor-mediated modulation of cognitive function remains unclear.
In contrast to the previous report (19), the current study indicated that ramipril treatment prevented the increased microglial activation measured in the dentate gyrus 28 weeks after fractionated whole-brain irradiation (Fig. 2). This may reflect differences in the timing of ramipril administration. In the previous study, ramipril dosing was started 24 h after whole-brain irradiation (19). Whereas, in the current study the drug was administered before, during, and after fractionated whole-brain irradiation. It may also reflect differences in the degree of neuroinflammation that occurs after single-dose and fractionated whole-brain irradiation. Our unpublished data indicate that a range of single whole-brain irradiation doses results in a greater increase in activated microglia in the dentate gyrus determined 2 months after irradiation compared with that observed in rats irradiated with a range of biologically equivalent fractionated whole-brain irradiation doses.
The mechanism by which angiotensin-converting enzyme inhibition leads to a decrease in radiation-induced microglial activation is not fully understood. Angiotensin II plays a major role in inflammation in several tissues, including the brain (21), through, in part, binding to AT1 receptors with resultant activation of NADPH oxidases and reactive oxygen species production (52). In vitro studies indicate that Ang II enhances the lipopolysaccharide (LPS)-induced activation of primary rat microglial cells, and that incubating the cells with an AT1RA prevents this activation, in part through suppressing NFκB and AP-1 activation (53, 54). Similarly, administration of the AT1RA, candesartan, which can cross the blood-brain barrier, reduced LPS-induced microglial activation and pro-inflammatory cytokines in the young adult male rat brain (53). Given the well-documented pro-inflammatory actions of Ang II, it is possible that the ramipril-mediated prevention of the fractionated whole-brain irradiation-induced increase in microglial activation in the dentate gyrus seen in the current study reflects inhibition of Ang II-mediated microglial activation. However, elucidating the precise mechanisms involved in the ramipril-mediated inhibition of radiation-induced neuroinflammation requires additional investigations.
A fractionated whole-brain irradiation-induced increase in microglial activation has also been reported in the perirhinal cortex (35). However, we did not detect any significant change in the number of activated microglia in the perirhinal cortex following fractionated whole-brain irradiation (Fig. 3). This might reflect differences in the rat strains. The current study used F344 rats while our previous study used F344 × BN rats. The lack of microglial activation in the perirhinal cortex of fractionated whole-brain irradiation rats suggests that the concomitant decrease in perirhinal cortex-dependent cognitive function observed 26 weeks after irradiation did not result from increased neuroinflammation. However, additional studies are needed to assess other aspects of the microglial phenotype, including production of anti-inflammatory mediators and/or trophic factors (55) that might provide a mechanistic link between ramipril and modulation of fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment.
Given that Ang II receptors can influence cell proliferation and neuronal differentiation (56), it is reasonable to hypothesize that renin-angiotensin system blockade might modulate the radiation-induced reduction in neurogenesis observed in the dentate gyrus. However, we did not observe any effect of ramipril on the fractionated whole-brain irradiation-induced reduction in neurogenesis, which confirms previous observations using this adult rat model of fractionated whole-brain irradiation (35). Although a previous study has noted that ramipril ameliorated a (10 Gy) radiation-induced reduction in neurogenesis in the rat brain, the effect was modest and was not observed after 15 Gy whole-brain irradiation (46).
Since ramipril can mitigate the severity of radiation-induced optic neuropathy (19, 45), prevention of the fractionated whole-brain irradiation-induced impairment in performance on the perirhinal cortex-dependent novel object recognition task reported here might reflect a drug effect on the optic apparatus. However, previous histologic studies have failed to demonstrate any gross evidence of optic nerve lesions following fractionated whole-brain irradiation in this rat model (10, 20). Moreover, no loss of visual acuity was observed in this study, which is consistent with previous reports in adult male rats exposed to total doses of 40 or 45 Gy of fractionated whole-brain irradiation (8, 57, 58). Thus, it seems unlikely that the ability of ramipril to protect perirhinal cortex-dependent cognitive function after fractionated whole-brain irradiation reflects modulation of optic nerve function.
We have previously hypothesized that renin-angiotensin system blockers act to prevent radiation-induced brain injury by inhibiting an ongoing interaction between radiation and the pro-inflammatory Ang II peptide (59). However, recognition of the growing complexity of the renin-angiotensin system, as well as the functional relevance of other Ang peptides, particularly Ang-(1–7) (60) point to a need to update this working model. Ang-(1–7) is a recently identified Ang peptide that plays important physiologic roles by binding to the Mas receptor and counterbalancing the actions of Ang II through its vasodilator, anti-proliferative, anti-fibrotic, and anti-inflammatory properties (61, 62).
Ang-(1–7) is formed from Ang I or Ang II by several endopeptidases and carboxypeptidases, including ACE-2 (60). During angiotensin-converting enzyme inhibition or Ang receptor blockade, plasma Ang-(1–7) levels and cardiac ACE-2 mRNA expression (28) increase, suggesting that the beneficial effects of renin-angiotensin system blockade may be due, in part, to a shift in the renin-angiotensin system from the ACE-Ang II-AT1R axis to the ACE-2-Ang-(1–7)-Mas receptor axis (63). Indeed, we observed a marked increase in systemic levels of Ang I and Ang-(1–7) in our rats treated with ramipril. Of interest, fractionated whole-brain irradiation resulted in a decrease in plasma Ang-(1–7) levels that was prevented in the fractionated whole-brain irradiation rats receiving ramipril. Although we did not detect any change in cerebral cortex Ang peptide levels, this may reflect regional differences in response. Analysis of renin-angiotensin system components in the dorsomedial medulla of male F344 rats treated with L-158,809 for 1 year revealed a significant increase in the mRNA of the enzymes ACE-2 and neprilysin, as well as the Mas receptor (64). Thus, continuous renin-angiotensin system blockade may activate enzymes and receptors that would shift the balance from Ang II to Ang-(1–7), and thereby counteract the pathogenic function of Ang II in the irradiated brain and enhance production of Ang-(1–7), which itself is implicated in enhancing learning and memory (65). Defining the putative role of Ang-(1–7) as a modulator of radiation-induced cognitive impairment is a focus of ongoing studies.
In summary, continuous administration of the angiotensin-converting enzyme inhibitor, ramipril, before, during, and after completion of fractionated whole-brain irradiation prevented the radiation-induced perirhinal cortex-dependent cognitive impairment measured by the novel object recognition task 26 weeks after irradiation. The pathogenic mechanism(s) involved remain(s) unclear. However, given these characteristics of angiotensin-converting enzyme inhibitors are, (1) well-tolerated drugs routinely prescribed for hypertension and cardiovascular disease (66), (2) selectively modulate radiation-induced normal tissue injury without altering tumor response (67), and (3) may enhance cancer therapies (68), they appear to be promising drugs for future clinical trials aimed at improving the survival and quality of life of brain tumor patients treated with radiotherapy.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH grants AG11370 (DRR), CA122318, CA113267 (MER), and HL51952 (DID). The authors would like to thank Dr. Ken Wheeler for helpful discussions, Liz Forbes and Dr. Kun Hua (Department of Neurobiology and Anatomy, WFSM) for providing technical assistance with the immunohistochemistry and microscopy, and Dr. Edward Leung of King Pharmaceuticals for kindly providing the ramipril.
REFERENCES
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer Statistics, 2011. Ca Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29:533–540. doi: 10.1016/s0305-7372(03)00105-1. [DOI] [PubMed] [Google Scholar]
- 3.Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–1304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 4.Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 5.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24:1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 6.Shaw EG, Rosdahl R, D’Agostino RB, Lovato J, Naughton MJ, Robbins ME, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24:1415–1420. doi: 10.1200/JCO.2005.03.3001. [DOI] [PubMed] [Google Scholar]
- 7.Murray EA, Bussey TJ, Saksida LM. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annu Rev Neurosci. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- 8.Brown WR, Blair RM, Moody DM, Robbins ME, Wheeler KT. Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: A potential rat model of vascular dementia. J Neurol Sci. 2007;257:67–71. doi: 10.1016/j.jns.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Raber R, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 10.Atwood T, Payne VS, Zhao W, Brown WR, Wheeler KT, Zhu J-M, et al. Quantitative magnetic resonance spectroscopy reveals a potential relationship between radiation-induced changes in rat brain metabolites and cognitive impairment. Radiat Res. 2007;168:574–581. doi: 10.1667/RR0735.1. [DOI] [PubMed] [Google Scholar]
- 11.Monje M. Cranial radiation therapy and damage to hippocampal neurogenesis. Devel Disabil Res Rev. 2008;14:238–242. doi: 10.1002/ddrr.26. [DOI] [PubMed] [Google Scholar]
- 12.Barani IJ, Benedict SH, Lin P-S. Neural stem cells: implications for the conventional radiotherapy of central nervous system malignancies. Int J Radiat Oncol Biol Phys. 2007;68:324–333. doi: 10.1016/j.ijrobp.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 13.Monje ML, Mizumatsu S, Fike JR, Palmer T. Irradiation induced neural precursor-cell dysfunction. Nat Med. 2002;8:955–961. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 14.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 15.Vallières L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astroctyic production of interleukin-6. J Neurosci. 2002;22:486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekdahl CT, Kokaia Z, Lindvall G. Brain inflammation and adult neurogenesis: the dual role of microglia. Neuroscience. 2009;158:1021–1029. doi: 10.1016/j.neuroscience.2008.06.052. [DOI] [PubMed] [Google Scholar]
- 17.Mildenberger M, Beach TG, McGeer EG, Ludgate CM. An animal model of prophylactic cranial irradiation: histologic effects at acute, early and delayed stages. Int J Radiat Oncol Biol Phys. 1990;18:1051–1060. doi: 10.1016/0360-3016(90)90440-u. [DOI] [PubMed] [Google Scholar]
- 18.Robbins ME, Zhao W, Garcia-Espinosa MA, Diz DI. Renin-Angiotensin system blockers and modulation of radiation-induced brain injury. Curr Drug Targets. 2010;11:1413–1422. doi: 10.2174/1389450111009011413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JH, Brown SL, Kolozsvary A, Jenrow KA, Ryu S, Rosenblum ML, et al. Modification of radiation injury by ramipril, inhibitor of Angiotensin-converting enzyme, on optic neuropathy in the rat. Radiat Res. 2004;161:137–142. doi: 10.1667/rr3124. [DOI] [PubMed] [Google Scholar]
- 20.Robbins ME, Payne V, Tommasi E, Diz DI, Hsu F-C, Brown WR, et al. The AT1 receptor antagonist, L-158,809, prevents or ameliorates fractionated whole-brain irradiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2009;73:499–505. doi: 10.1016/j.ijrobp.2008.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saavedra JM. Brain Angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol Neurobiol. 2005;25:485–511. doi: 10.1007/s10571-005-4011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benicky J, Sanchez-lemus E, Pavel J, Saavedra JM. Anti-inflammatory effects of angiotensin receptor blockers in the brain and periphery. Cell Mol Neurobiol. 2009;29:781–792. doi: 10.1007/s10571-009-9368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 2005;164:662–668. doi: 10.1667/rr3453.1. [DOI] [PubMed] [Google Scholar]
- 24.Fowler JF. Brief summary of radiobiological principles in fractionated radiotherapy. Semin Radiat Oncol. 1992;2:16–21. [Google Scholar]
- 25.van der Kogel AJ. The nervous system: Radiobiology and experimental pathology. In: Schrer E, Streffer C, Trott KR, editors. Radiopathlogy of organs and Tissues. Berlin: Springer-Verlag; 1991. pp. 191–212. [Google Scholar]
- 26.Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Senanayake PD, Moriguchi A, Kumagai H, Ganten, Ferrario CM, Brosnihan KB. Increased expression of angiotensin peptides in the brain of transgenic hypertensive rats. Peptides. 1994;15:919–926. doi: 10.1016/0196-9781(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 28.Iyer SN, Chappell MC, Averill DB, Diz DI, Ferrario CM. Vasodepressor actions of Angiotensin-(1–7) unmasked during combined treatment with linopril and losartan. Hypertension. 1998;31:669–705. doi: 10.1161/01.hyp.31.2.699. [DOI] [PubMed] [Google Scholar]
- 29.Allred A, Chappell MC, Ferrario CM, Diz DI. Differential actions of renal ischemic injury on the intrarenal Angiotensin system. Am J Physiol Renal Physiol. 2000;279:F636–F645. doi: 10.1152/ajprenal.2000.279.4.F636. [DOI] [PubMed] [Google Scholar]
- 30.Ramanan S, Kooshki M, Zhao W, Hsu F-C, Riddle DR, Robbins ME. The PPARα agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int J Radiat Oncol Biol Phys. 2009;75:870–877. doi: 10.1016/j.ijrobp.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.West MJ, Slomianka S, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionators. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 32.Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 33.Gundersen HJG, Jensen EBV, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology-reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- 34.Slomianka L, West MJ. Estimators of the precision of stereological estimates: an example based on the CA1 pyrimidal cell layer of rats. Neuroscience. 2005;136:757–767. doi: 10.1016/j.neuroscience.2005.06.086. [DOI] [PubMed] [Google Scholar]
- 35.Conner KR, Payne VS, Forbes ME, Robbins ME, Riddle DR. Effects of the AT1 receptor antagonist L-158,809 on microglia and neurogenesis after fractionated whole-brain irradiation. Radiat Res. 2010;173:49–61. doi: 10.1667/RR1821.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ehninger D, Kempermann G. Neurogenesis in the adult hippocampus. Cell Tissue Res. 2008;331:243–250. doi: 10.1007/s00441-007-0478-3. [DOI] [PubMed] [Google Scholar]
- 37.Campbell DJ, Duncan AM, Kladis A, Harrap SB. Converting enzyme inhibition and its withdrawal in spontaneously hypertensive rats. J Cardiovasc Pharamcol. 1995;26:426–436. doi: 10.1097/00005344-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 38.Weisinger HS, Begg DP, Egan GF, Jayasooriya AP, Lie F, Mathai ML, et al. Angiotensin converting enzyme inhibition from birth reduces body weight and body fat in Sprague-Dawley rats. Physiol Behav. 2008;93:820–825. doi: 10.1016/j.physbeh.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 39.Gilliam-Davis S, Payne VS, Kasper SO, Tommasi EN, Robbins ME, Diz DI. Long-term AT1 receptor blockade improves metabolic function and provides renoprotection in Fischer-344 rats. Am J Physiol Heart Circ Physiol. 2007;293:H1327–H1333. doi: 10.1152/ajpheart.00457.2007. [DOI] [PubMed] [Google Scholar]
- 40.Moulder JF, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Semin Radiat Oncol. 2007;17:141–148. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 41.von Bohlen und Halbach O, Albrecht A. The CNS renin-angiotensin system. Cell Tissue Res. 2006;326:599–616. doi: 10.1007/s00441-006-0190-8. [DOI] [PubMed] [Google Scholar]
- 42.Phillips MI, Menezes de Oliveira E. Brain renin angiotensin in disease. J Mol Med. 2008;86:715–722. doi: 10.1007/s00109-008-0331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wright JW, Harding JW. The brain RAS and Alzheimer’s disease. Exptl Neurol. 2010;223:326–333. doi: 10.1016/j.expneurol.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 44.Kehoe AD, Nikiforov AM, Alexanin SS, Neronov EG, Tikhomirova OV, Shun’kov VB, et al. Angiotensin-converting enzyme genotype and encephalopathy in Chernobyl cleanup workers. Eur J Neurol. 2009;16:95–100. doi: 10.1111/j.1468-1331.2008.02355.x. [DOI] [PubMed] [Google Scholar]
- 45.Ryu S, Kolozsvary A, Jenrow KA, Brown SL, Kim JH. Mitigation of radiation-induced optic neuropathy in rats by ACE inhibitor ramipril: importance of ramipril dose and treatment time. J Neurooncol. 2007;82:119–124. doi: 10.1007/s11060-006-9256-4. [DOI] [PubMed] [Google Scholar]
- 46.Jenrow KA, Brown SL, Liu J, Kolozsvary A, Lapanowski K, Kim JH. Ramipril mitigates radiation-induced impairment of neurogenesis in the rat dentate gyrus. Radiat Oncol. 2010;5:6. doi: 10.1186/1748-717X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basso N, Paglia N, Stella I, de Cavanagh EMV, Ferder L, del Rosario Lores Arnaiz M, et al. Protective effect of the inhibition of the renin-angiotensin system on aging. Regul Peptides. 2005;128:247–252. doi: 10.1016/j.regpep.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 48.Kerr DS, Bevilaqua LR, Bonini JS, Rossato JL, Kohler CA, Medina JH, et al. Angiotenisn II blocks memory consolidation through an AT2 receptor-dependent mechanism. Psychopharmacology. 2005;179:529–535. doi: 10.1007/s00213-004-2074-5. [DOI] [PubMed] [Google Scholar]
- 49.Bonini JS, Bevilaqua IR, Zinn CG, Kerr DS, Medina JH, Izquierso I, et al. Angiotensin II disrupts inhibitory avoidance memory retrieval. Horm Behav. 2006;50:308–313. doi: 10.1016/j.yhbeh.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 50.Amenta F, Di Tullio MA, Tomassoni D. The cholinergic approach for the treatment of vascular dementia: evidence from preclinical and clinical studies. Clin Exp Hypertens. 2002;24:697–713. doi: 10.1081/ceh-120015346. [DOI] [PubMed] [Google Scholar]
- 51.Kehoe PG, Wilcock GK. Is inhibition of the renin-angiotensin system a new treatment option for Alzheimer’s disease? Lancet Neurol. 2007;6:373–378. doi: 10.1016/S1474-4422(07)70077-7. [DOI] [PubMed] [Google Scholar]
- 52.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benicky J, Sanchez-lemus E, Honda M, Pang T, Orecna M, Wang J, Leng Y, Chiang D-M, Saavedra JM. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology. 2011;36:857–870. doi: 10.1038/npp.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyoshi M, Miyano K, Moriyama N, Taniguchi M, Watanabe T. Angiotensin type 1 receptor antagonist inhibits lipoploysaccharide-induced stimulation of rat microglial cells by suppressing nuclear factor κB and activator protein-1 activation. Eur J Neurosci. 2008;27:343–351. doi: 10.1111/j.1460-9568.2007.06014.x. [DOI] [PubMed] [Google Scholar]
- 55.Nakajima K, Kohsaka S. Microglia: neuroprotective and neurotrophic cells in the central nervous system. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:65–84. doi: 10.2174/1568006043481284. [DOI] [PubMed] [Google Scholar]
- 56.Gendron L, Payet MD, Gallo-Payet N. The angiotensin type 2 receptor of angiotensin II and neuronal differentiation: from observations to mechanisms. J Mol Endocrinol. 2003;31:359–372. doi: 10.1677/jme.0.0310359. [DOI] [PubMed] [Google Scholar]
- 57.Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE. Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat Res. 2006;166:892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- 58.Shi L, Olson J, D’Agostino R, Jr, Linville C, Nicolle M, Robbins ME, et al. Aging masks detection of radiation-induced brain injury. Brain Res. 2011;1385:307–316. doi: 10.1016/j.brainres.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robbins M, Diz DI. Pathogenic role of the renin-angiotensin system in modulating radiation-induced late effects. Int J Radiat Oncol Biol Phys. 2006;64:6–12. doi: 10.1016/j.ijrobp.2005.08.033. [DOI] [PubMed] [Google Scholar]
- 60.Varagic J, Trask AJ, Jessup JA, Chappell MC, Ferrario CM. New angiotensins. J Mol Med. 2008;86:663–671. doi: 10.1007/s00109-008-0340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chappell MC, Modrall JG, Diz DI, Ferrario CM. Novel aspects of the renal renin-Angiotensin system: Angiotensin-(1–7), ACE2 and blood pressure regulation. Contrib Nephrol. 2004;143:77–89. doi: 10.1159/000078713. [DOI] [PubMed] [Google Scholar]
- 62.Ratovich MJ, Grobe JL, Raizada MK. Angiotensin-(1–7) as an antihypertensive, antifibrotic target. Curr Hypertens Res. 2008;10:227–232. doi: 10.1007/s11906-008-0043-9. [DOI] [PubMed] [Google Scholar]
- 63.Xu P, Sriramula S, Lazartiques E. ACE2/ANG-(1–7)Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol. 2011;300:R804–R817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gilliam-Davis S, Gallagher PE, Payne V, Kasper SO, Tommasi EN, Westwood BN, et al. Long-term systemic angiotensin II type receptor blockade regulates mRNA expression of dorsomedial medulla renin-angiotensin system components. Physiol Genom. 2011;43:829–835. doi: 10.1152/physiolgenomics.00167.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hellner K, Walthrt T, Schubert M, Albrecht D. Angiotensin-(1–7) enhances LTP in the hippocampus through the G-protein-coupled receptor Mas. Mol Cell Neurosci. 2005;29:427–435. doi: 10.1016/j.mcn.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 66.Asselbergs FW, van Gilst WH. Angiotensin converting enzyme inhibition in cardiovascular risk populations: a practical approach to identify the patient who will benefit most. Curr Opin Cardiol. 2007;22:267–272. doi: 10.1097/HCO.0b013e3281a7ec81. [DOI] [PubMed] [Google Scholar]
- 67.Kohl RR, Kolozsvary A, Brown SL, Zhu G, Kim JH. Differential effect in tumor and normal tissue after treatment with ramipril, an angiotensin-converting enzyme inhibitor. Radiat Res. 2007;168:440–445. doi: 10.1667/RR0707.1. [DOI] [PubMed] [Google Scholar]
- 68.George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer. 2010;10:745–759. doi: 10.1038/nrc2945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.