Abstract
Objective
In sub-Saharan Africa, many patients initiate antiretroviral therapy (ART) at CD4+ cell counts much lower than those recommended in national guidelines. We examined program-level and contextual-level factors associated with low median CD4+ cell count at ART initiation in populations initiating ART.
Design
Multilevel analysis of aggregate and program-level service delivery data.
Methods
We examined data on 1690 cohorts of patients initiating ART during 2004–2008 in eight sub-Saharan African countries. Cohorts with median CD4+ less than 111 cells/μl (the lowest quartile) were classified as having low median CD4+ cell count at ART initiation. Cohort information was combined with time-updated program-level data and subnational contextual-level data, and analyzed using multilevel models.
Results
The 1690 cohorts had median CD4+ cell count of 136 cells/μl and included 121 504 patients initiating ART at 267 clinics. Program-level factors associated with low cohort median CD4+ cell count included urban setting [adjusted odds ratio (AOR) 2.1; 95% confidence interval (CI) 1.3–3.3], lower provider-to-patient ratio (AOR 2.2; 95% CI 1.3–4.0), no PMTCT program (AOR 3.6; 95% CI 1.0–12.8), outreach services for ART patients only vs. both pre-ART and ART patients (AOR 2.4; 95% CI 1.5–3.9), fewer vs. more adherence support services (AOR 1.6; 95% CI 1.0–2.5), and smaller cohort size (AOR 2.5; 95% CI 1.4–4.5). Contextual-level factors associated with low cohort median CD4+ cell count included initiating ART in areas where a lower proportion of the population heard of AIDS, tested for HIV recently, and a higher proportion believed ‘limiting themselves to one HIV-uninfected sexual partner reduces HIV risk’.
Conclusion
Determinants of CD4+cell count at ART initiation in populations initiating ART operate at multiple levels. Structural interventions targeting points upstream from ART initiation along the continuum from infection to diagnosis to care engagement are needed.
Keywords: CD4, HIV/AIDS, HIV scale-up, implementation science, late antiretroviral therapy initiation, multilevel, operations research, PEPFAR
Introduction
Efforts to scale-up access to HIV care and treatment have been successful at initiating large numbers of patients on antiretroviral therapy (ART). In sub-Saharan Africa, the estimated number of persons on ART increased from 100 000 persons in 2003 to 3.9 million by the end of 2009, reaching an estimated 37% of those in need of ART [1]. Despite these successes, there remain persistent challenges to optimizing the success of HIV care and treatment scale-up in resource-limited settings. Among the most important challenges are very high rates of late ART initiation (i.e., in the advanced stages of HIV disease) [2,3], which is associated with early mortality after initiation of ART [4,5]. A three-fold to four-fold higher mortality rate has been reported in the first year after ART initiation in resource-limited compared with resource-rich settings [6], and high mortality rates have also been noted among persons enrolled in care in the period prior to ART initiation among those who are eligible for ART [2]. In addition to diminishing the potential successes of ART scale-up, late ART initiation also adds significant burden and costs to care [7] and missed opportunities for secondary HIV prevention due to late diagnosis [1,8–10].
With few exceptions, national guidelines in the sub-Saharan African region during 2004–2009 specified ART eligibility for patients with CD4+ cell counts less than 200 cells/μl [11]. However, most patients initiate ART at substantially lower CD4+cell counts [2,4,6,12–27], often with advanced clinical stages [28]. Further, there is substantial variability in the CD4+ cell count at ART initiation across clinics and settings. For example, in an analysis from the ART-LINC collaboration, which included 19 967 patients from 27 centers in sub-Saharan Africa, Latin America, and Asia, the median CD4+ cell count at ART initiation was 114 cells/μl (range across centers 61–181) among patients in sub-Saharan Africa [17]. WHO recently issued new HIV care and treatment guidelines for resource-limited settings recommending initiation of ART at CD4+cell counts substantially higher than most current national guidelines [29].
The reasons for late ART initiation are complex and multidimensional, and likely include factors at the contextual level (e.g., urban/rural, testing coverage, levels of stigma, and HIV knowledge), program level (e.g., clinic patient load, clinic burden of sick patients, staffing level, clinic policies, and practices such as provision of peer/adherence support), and individual level (e.g., sociodemographic, health beliefs, depression, social support, and substance use). The objective of this analysis was to identify factors at the program and contextual levels that are associated with low median CD4+ cell count at ART initiation in cohorts from several countries in sub-Saharan Africa.
Methods
We used quarterly aggregate monitoring and evaluation (M&E) indicator data from HIV care clinics in eight sub-Saharan African countries (Ethiopia, Kenya, Lesotho, Mozambique, Nigeria, Rwanda, South Africa, and Tanzania) that received support from ICAP at Columbia University (www.columbia-icap.org) via the US Government’s President’s Emergency Plan for AIDS Relief (www.pepfar.gov) initiative. The use of data for this study was approved as nonhuman subjects research by the US Centers for Disease Control and Prevention (CDC) and the Institutional Review Board of Columbia University Medical Center.
Aggregate patient data
Aggregate data included PEPFAR program M&E indicators that were routinely collected for M&E purposes [30]. Such aggregate data on patients initiating ART were manually tallied from paper-based systems (e.g., from pre-ART/ART registers and national forms) that were maintained by site staff. Data were reported per site per quarter on cohorts of patients initiating ART in a given 3-month period. Information on each cohort included the number of ART patients aged 6 years and above (cohort size), the number with a CD4+ cell count at ART initiation, and the median CD4+ cell count at ART initiation, which served as the outcome variable in this analysis.
Program-level data
Data on program-level and site-level characteristics were gathered via routinely conducted, structured site surveys completed through in-person queries of site staff. Site surveys were conducted in June 2007, December 2007, and July 2008. Site and program characteristics included the following: geographic setting (urban/rural), type of facility (primary, secondary, tertiary), availability of voluntary counseling and testing (VCT), prevention of mother-to-child transmission (PMTCT), and tuberculosis (TB) treatment services (onsite, offsite, not available at facility), availability of CD4+ cell count testing (onsite or offsite), most common entry point into care at the site (VCT, PMTCT or labor and delivery, TB program, inpatient ward, outpatient clinic), inquiry about HIV status of family members of index patients and referral for testing, availability of supportive services such as outreach for patients who miss visits (none, outreach for ART patients only, or for both pre-ART and ART patients), presence of a peer educator program, and availability of adherence support services [number of different types adherence support services available (0–3, 4, 5, 6–12)]. Other cohort-level and program-level characteristics included the cumulative number of patients enrolled in care, the ratio of cumulatively enrolled to the number of patients cumulatively enrolled in care, and the clinical provider-to-patient ratio (e.g., physicians, clinical officers, and nurses), with cumulatively enrolled patients in the denominator.
The definition of urban and rural was determined locally using the categorizations of the Central Statistics Office or other national authority that determines the official category of a location. Broadly speaking, urban clinics were those in settings that were officially designated to be a city, with city administration and political bodies, or were located in big and small towns, peri-urban areas, growth points, and mining communities. Clinics in rural are as were those located in a village, subsistence farming areas, as well as large-scale and small-scale commercial farming areas.
Contextual-level characteristics
Country-specific, population-based household interview data from Demographic and Health Surveys (DHS) [31] conducted during 2003–2005 were used to construct subnational (regional) contextual variables on population-level factors hypothesized to be associated with late ART initiation (e.g., HIV prevalence and AIDS knowledge). HIV care and treatment sites included in this analysis were distributed across 31 subnational regions of the cited eight countries, with DHS data available for all 31 regions. The most recently available DHS data for each country were used: Ethiopia (2005), Kenya (2003), Lesotho (2004), Mozambique (2003), Nigeria (2003), Rwanda (2005), South Africa (2003), and Tanzania (2004). The most recently available population-based HIV prevalence estimates for each region were used from DHS (Ethiopia, Kenya, Lesotho, Rwanda, and Tanzania) [32] or other population-based prevalence survey (South Africa, 2003 [33]). Antenatal clinic HIV prevalence estimates published UNAIDS were used for Mozambique (2003) [34] and Nigeria (2003) [35].
Contextual variables were constructed at the subnational regional level (n = 31 regions) and included measures of socioeconomic status (percentage of households with electricity, piped water), HIV prevalence among persons aged 15–49 years, AIDS knowledge (proportion of respondents who have heard of AIDS and proportion who have comprehensive knowledge about AIDS, according to a standard DHS scale), population-level stigma (proportion of respondents with accepting attitudes toward persons living with AIDS), knowledge regarding the risk of HIV transmission (proportion who know that condoms can prevent HIV, percentage reporting that limiting themselves to one uninfected sexual partner limits risk of HIV transmission), and HIV testing coverage (proportion tested for HIV and received their results in the last 12 months).
Study sample and inclusion criteria
A total of 287 sites in 31 regions of the eight countries had patients who initiated ART during the study period (May 2004 to July 2008) were included in this analysis. Of these, 11 did not report data on cohorts of patients initiating ART, and additional nine sites did not report program-level data from the site survey available. The final sample in this analysis included 267 sites, where a total of 1690 3-month cohorts initiated ART during the study period, representing 121 504 patients aged 6 years and older. Cohort information from these 267 sites was combined with time-updated program-level data from site surveys according to the date during which cohort initiated ART and with contextual-level information from the DHS surveys.
Low median CD4+ cell count at antiretroviral therapy initiation (outcome)
Three-month cohorts with median CD4+ cell counts in the lowest quartile (<111 cells/μl) were classified as having low median CD4+ cell counts at ART initiation. Among all 1690 3-month cohorts, the median proportion of patients in the cohort who had a CD4+ count test result at ART initiation was 93% [interquartile range (IQR) 78–100]. We repeated our analysis excluding cohorts in which less than 75% of patients initiating ART had a baseline CD4+ cell count, and the results were not altered. Analyses presented here include all 1690 cohorts.
Statistical methods
Univariate/bivariate analyses
We used generalized linear mixed models to examine factors associated with low CD4+ cell count at ART initiation among 3-month cohorts of patients who started ART. As the site surveys were repeated at different time points, program-level variables were treated as time-dependent according to the quarter in which a given cohort initiated ART. Bivariate associations between hypothesized predictors and low cohort median CD4+ cell counts were examined using chi-squared P values and crude odds ratios, accounting for within site clustering effects.
Multivariate analyses
We used generalized linear mixed models to examine the program-level and contextual-level factors that were independently associated with low cohort median CD4+ cell count at ART initiation. After checking for linearity in the log odds, contextual-level factors were modeled continuously. All variables that were associated with the outcome at α = 0.20 in bivariate analysis were examined in multivariate models that controlled for calendar time of ART initiation. A backward stepwise method was used, with final models including all factors significant at α = 0.05. We used SAS Version 9.2 (Cary, North Carolina, USA), PROC GLIMMX for all statistical analyses.
Results
Sample description
The 267 sites reported cohort data on 1690 3-month cohorts, representing 121 504 patients who initiated ART during the study period (Table 1). Sixty percent of the sites were in urban settings, representing 67% of the cohorts and 85% of the patients initiating ART. Ninety-five percent of the sites were in either secondary (55%) or primary (40%) care facilities. Nearly 65% of patients initiated ART in the upper quartile of cohort size (i.e., 25% of cohorts accounted for 65% of patients). Eighty-six percent of patients and 85% of cohorts initiated ART in 2007 or later.
Table 1.
Number of cohorts and patients older than 6 years initiating antiretroviral at 267 sites in eight sub-Saharan African countries, May 2004 to July 2008.
| Number of sites [n (%)] | Total number of cohorts [n (%)] | Total number of patients initiating ART [n (%)] | |
|---|---|---|---|
| Total | 267 (100) | 1690 (100) | 121 504 (100) |
| Setting | |||
| Urban | 159 (60) | 1109 (66) | 10 5138 (87) |
| Rural | 108 (40) | 581 (34) | 16 366 (13) |
| Type of facility | |||
| Primary | 112 (42) | 585 (35) | 24 924 (21) |
| Secondary | 144 (54) | 988 (58) | 83 082 (68) |
| Tertiary | 11 (4) | 117 (7) | 13 495 (11) |
| Cohort size | |||
| 1–19 (median: 11) | 423 (25) | 4530 (4) | |
| 20–42 (median: 28) | 425 (25) | 12 479 (10) | |
| 43–88 (median: 64) | 406 (24) | 26 015 (21) | |
| 89–928 (median: 149) | 436 (26) | 78 480 (65) | |
| Calendar time of starting ART services | |||
| 2004-2 | 7 (3)a | 11 (1) | 292 (0) |
| 2005-1 | 10 (4) | 21 (1) | 1394 (1) |
| 2005-2 | 29 (11) | 69 (4) | 5478 (5) |
| 2006-1 | 34 (13) | 115 (7) | 8601 (7) |
| 2006-2 | 67 (25) | 240 (14) | 18 141 (15) |
| 2007-1 | 49 (18) | 343 (20) | 25 290 (21) |
| 2007-2 | 32 (12) | 410 (24) | 28 799 (24) |
| 2008-1 | 39 (15) | 481 (28) | 33 309 (27) |
| Country | |||
| Ethiopia | 39 (15) | 336 (20) | 26 514 (22) |
| Kenya | 41 (15) | 210 (12) | 10 191 (8) |
| Lesotho | 21 (6) | 82 (5) | 8842 (7) |
| Mozambique | 38 (14) | 274 (16) | 29 941 (25) |
| Nigeria | 16 (6) | 70 (4) | 10 492 (9) |
| Rwanda | 42 (16) | 323 (19) | 11 854 (10) |
| South Africa | 36 (13) | 234 (14) | 14 764 (12) |
| Tanzania | 34 (13) | 161 (10) | 8906 (7) |
Sites categorized according to the time when the first cohort initiated antiretroviral therapy (ART) at the site.
Median CD4+ cell count across cohorts at antiretroviral initiation
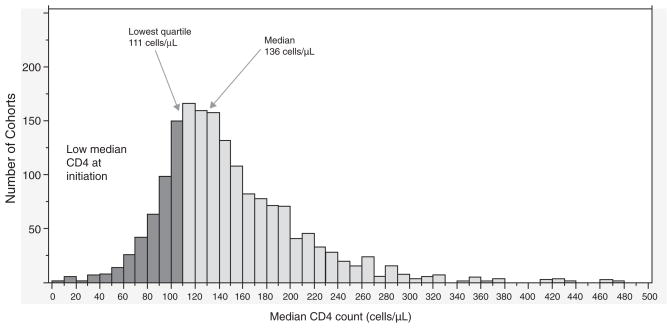
Figure 1 shows the distribution of the median CD4+ cell count at ART initiation for the 1690 cohorts of patients initiating ART. The median CD4+ cell count across the 1690 cohorts initiating ART was 136 cells/μl and a lower quartile of median was 111 cells/μl. The majority of cohorts had median CD4+ cell counts less than 200 cells/μl, with 25% of the 121 504 patients initiating ART in cohorts with median CD4+ cell counts in the lowest quartile of 111 cells/μl or less.
Fig. 1.
Distribution of cohort median CD4+ cell count at antiretroviral initiation (n =1690 cohorts).
Program-level factors
In crude analyses (Table 2), program-level factors associated with greater odds of low cohort median CD4+ cell count at ART initiation included urban vs. rural sites [odds ratio (OR) 1.8; 95% confidence interval (CI) 1.2–2.6], unaffiliated vs. affiliated with a PMTCT program (OR 4.4; 95% CI 1.6–12.4) or a VCT service (OR 2.9; 95% CI 1.1–7.5), onsite CD4+ testing (OR 1.7; 95% CI 1.2–2.3), being in the lowest vs. highest quartile of provider-to-patient ratio (OR 1.7; 95% CI 1.1–2.6), no availability of outreach program for patients who miss clinic visits (OR 1.7; 95% CI 1.2–2.4) or availability of outreach targeting ART patients only (OR 2.0; 95% CI 1.3–3.2) vs. availability of outreach targeting both pre-ART and ART patients, and availability of 0–3 adherence support programs vs. six or more (OR 2.4; 95% CI 1.6–3.7). Program-level factors significantly associated with a lower odds of low cohort median CD4+ cell count at ART initiation included offsite VCT (OR 0.27; 95% CI 0.15–0.47) and offsite TB program (OR 0.46; 95% CI 0.27–0.78).
Table 2.
Crude odds ratio for program-level factors associated with low median CD4 cell counts among cohorts of patients initiating antiretroviral therapy.
| Total number of cohorts [n (%)] | Median cohort CD4 cell count <111 cells/μl [n (%)] | Median cohort CD4 cell count ≥111 cells/μl [n (%)] | Odds ratio (95% CI) | |
|---|---|---|---|---|
| 1690 (100) | 422 (25) | 1268 (75) | ||
| Site characteristics | ||||
| Setting | ||||
| Rural | 581 (34) | 107 (25) | 474 (37) | 1 |
| Urban | 1109 (66) | 315 (75) | 794 (63) | 1.8 (1.2–2.6) |
| Type of site | ||||
| Primary | 585 (35) | 121 (29) | 464 (37) | 1 |
| Secondary | 988 (58) | 276 (65) | 712 (56) | 1.5 (1.0–2.2) |
| Tertiary | 117 (7) | 25 (6) | 92 (7) | 1.0 (0.46–2.4) |
| PMTCT program | ||||
| Onsite | 1504 (89) | 383 (91) | 1121 (89) | 1 |
| Offsite | 169 (10) | 30 (7) | 139 (11) | 0.63 (0.35–1.1) |
| Unaffiliated with a PMTCT program | 15 (1) | 9 (2) | 6 (0.5) | 4.4 (1.6–12.4) |
| VCT service | ||||
| Onsite | 1619 (96) | 413 (98) | 1206 (95) | 1 |
| Offsite | 60 (4) | 5 (1) | 55 (4) | 0.27 (0.15–0.47) |
| Unaffiliated with a VCT program | 6 (0.4) | 3 (1) | 3 (1) | 2.9 (1.1–7.5) |
| TB treatment program | ||||
| Onsite | 1400 (83) | 379 (90) | 1021 (81) | 1 |
| Offsite | 166 (10) | 24 (6) | 142 (11) | 0.46 (0.27– 0.78) |
| Unaffiliated with a TB program | 17 (1) | 8 (2) | 9 (1) | 2.4 (0.37– 15.7) |
| Not reported | 107 (6) | 11 (3) | 96 (8) | 0.31 (0.12–0.80) |
| CD4 testing | ||||
| Offsite | 924 (55) | 190 (45) | 734 (58) | 1 |
| Onsite | 762 (45) | 229 (55) | 533 (42) | 1.7 (1.2–2.3) |
| Program characteristics | ||||
| Query patients about family members’ HIV status | ||||
| No | 621 (37) | 167 (40) | 454 (36) | 1 |
| Yes | 1069 (63) | 255 (60) | 814 (64) | 0.85 (0.64–1.1) |
| Testing or referring to VCT | ||||
| No | 575 (34) | 155 (37) | 420 (33) | 1 |
| Yes | 1115 (66) | 267 (63) | 848 (67) | 0.85 (0.64–1.1) |
| Outreach for missed visits | ||||
| Not present | 642 (38) | 184 (44) | 458 (36) | 1.7 (1.2–2.4) |
| Pre-ART and ART | 770 (46) | 148 (35) | 622 (49) | 1 |
| ART patients only | 264 (16) | 86 (21) | 178 (14) | 2.0 (1.3–3.2) |
| Peer education access | ||||
| No | 623 (37) | 147 (35) | 476 (38) | 1 |
| Yes | 1054 (63) | 272 (65) | 782 (62) | 1.1 (0.82–1.6) |
| No. of adherence support programs | ||||
| 0–3 | 548 (32) | 194 (46) | 354 (28) | 2.4 (1.6–3.7) |
| 4–5 | 691 (41) | 144 (34) | 547 (43) | 1.2 (0.74–1.8) |
| ≥6 | 451 (27) | 84 (20) | 367 (29) | 1 |
| Program burden | ||||
| Cohort size | ||||
| ≤19 | 423 (25) | 104 (25) | 319 (25) | 1.0 (0.67–1.7) |
| 20–42 | 425 (25) | 93 (22) | 332 (26) | 0.91 (0.58–1.4) |
| 43–88 | 406 (24) | 122 (29) | 284 (22) | 1.4 (0.86–2.2) |
| ≥89 | 436 (26) | 103 (24) | 333 (26) | 1 |
| Cumulative number patients in care | ||||
| <100 | 239 (14) | 61 (15) | 178 (44) | 0.96 (0.59– 1.6) |
| 100–199 | 303 (18) | 80 (19) | 223 (18) | 1 |
| 200–499 | 463 (27) | 103 (25) | 360 (28) | 0.80 (0.54–1.2) |
| >500 | 682 (40) | 176 (42) | 506 (40) | 0.97 (0.65–1.5) |
| Ratio of ART patients to total care population | ||||
| ≤35 | 410 (25) | 110 (26) | 300 (24) | 1 |
| 36–45 | 431 (26) | 95 (23) | 336 (27) | 0.77 (0.50–1.2) |
| 46–55 | 432 (26) | 109 (26) | 323 (26) | 0.92 (0.60– 1.4) |
| ≥56 | 399 (24) | 105 (25) | 294 (23) | 0.97 (0.66–1.4) |
| Number of providers per 1000 patients in HIV care | ||||
| <4 | 433 (26) | 125 (30) | 308 (24) | 1.7 (1.1–2.6) |
| 4–9 | 436 (26) | 117 (28) | 319 (25) | 1.5 (0.98–2.3) |
| 9.1–18 | 389 (23) | 95 (23) | 294 (23) | 1.3 (0.87–2.0) |
| >18 | 432 (26) | 85 (20) | 347 (27) | 1 |
ART, antiretroviral therapy; CI, confidence interval; PMTCT, prevention of mother-to-child transmission; TB, tuberculosis; VCT, voluntary counseling and testing.
Contextual-level factors
Table 3 shows contextual-level factors for the 31 subnational regions according to the tertiles of the distribution for each variable as of 2003–2005. In univariate analyses, the proportion of households in regions with electricity and piped water were significantly associated with lower median cohort CD4+ cell count at ART initiation (Table 3). Cohorts initiating ART in regions with HIV prevalence rates of 19% or more were less likely to have low median CD4+ cell counts (OR 0.53; 95% CI 0.32–0.90). A higher proportion of persons in the region who believed that ‘limiting themselves to one HIV-uninfected sexual partner reduces HIV risk’ was significantly associated with low cohort median CD4+ cell count at ART initiation (ORtertile 2 vs. 1 2.1; 95% CI 1.2–3.5; ORtertile 3 vs. 1 3.9; 95% CI 2.3–6.8).
Table 3.
Crude odds ratio for contextual-level factors associated with low median CD4 cell counts among cohorts of patients initiating antiretroviral therapy.
| Total sub-regions [n (%)] | Total cohorts [n (%)] | Median CD4 cell count <111 cells/μl [n (%)] | Median CD4 cell count ≥111 cells/μl [n (%)] | Odds ratio (95% CI) | P value | |
|---|---|---|---|---|---|---|
| 31 (100) | 1690 (100) | 422 (25) | 1268 (75) | |||
| Percentage of households with electricity | ||||||
| Tertile 1 (1.7–5.8) | 10 (32) | 362 (21) | 73 (17) | 289 (23) | 1 | |
| Tertile2 (7.0–15.3) | 11 (35) | 822 (49) | 188 (45) | 634 (50) | 1.2 (0.76–1.8) | 0.03 |
| Tertile 3 (15.5–59.2) | 10 (32) | 506 (30) | 161 (38) | 345 (27) | 1.9 (1.1–3.0) | |
| Percentage of households with piped water | ||||||
| Tertile 1 (2.3–8.1) | 10 (32) | 277 (16) | 46 (11) | 231 (18) | 1 | |
| Tertile 2 (8.7–14.5) | 11 (35) | 495 (29) | 145 (34) | 350 (28) | 2.1 (1.2–3.6) | 0.03 |
| Tertile 3 (15.1–31.7) | 10 (32) | 918 (54) | 231 (55) | 687 (54) | 1.7 (0.98–2.9) | |
| HIV prevalence | ||||||
| Tertile 1 (0.70–4.90) | 10 (32) | 820 (49) | 233 (55) | 587 (46) | 1 | |
| Tertile 2 (5.10–12.0) | 11 (35) | 601 (36) | 142 (34) | 459 (36) | 0.78 (0.53–1.1) | 0.05 |
| Tertile 3 (19.0–39.1) | 10 (32) | 269 (16) | 47 (11) | 222 (18) | 0.53 (0.32–0.90) | |
| Percentage who have heard of AIDS | ||||||
| Tertile 1 (53.9–91.7) | 10 (32) | 427 (25) | 109 (26) | 318 (25) | 1 | |
| Tertile 2 (93.3–98.7) | 11 (35) | 467 (28) | 125 (30) | 342 (27) | 1.1 (0.71–1.6) | 0.7 |
| Tertile 3 (98.76–99.97) | 10 (32) | 796 (47) | 188 (45) | 608 (48) | 0.90 (0.58–1.4) | |
| Percentage with comprehensive knowledge about AIDS | ||||||
| Tertile 1 (5.1–18.2) | 10 (32) | 493 (29) | 116 (27) | 377 (30) | 1 | |
| Tertile 2 (19.8–35.5) | 11 (35) | 432 (26) | 125 (30) | 307 (24) | 1.3 (0.89–2.0) | 0.27 |
| Tertile 3 (35.9–64.1) | 10 (32) | 765 (45) | 181 (43) | 584 (46) | 1.0 (0.66–1.6) | |
| Percentage men and women accepting (stigma) | ||||||
| Tertile 1 (3.0–10.0) | 10 (32) | 529 (31) | 118 (28) | 411 (32) | 1 | |
| Tertile 2 (12.0–25.5) | 11 (35) | 535 (32) | 149 (35) | 386 (30) | 1.3 (0.91–2.0) | 0.34 |
| Tertile 3 (28.0–59.0) | 10 (32) | 626 (37) | 155 (37) | 471 (37) | 1.2 (0.74–1.8) | |
| Percentage know condom use prevents HIV transmission | ||||||
| Tertile 1 (12–55) | 10 (32) | 529 (31) | 118 (28) | 411 (32) | 1 | 0.14 |
| Tertile 2 (60–75) | 11 (35) | 777 (46) | 222 (53) | 555 (44) | 1.4 (0.95–2.1) | |
| Tertile 3 (76–89) | 10 (32) | 384 (23) | 82 (19) | 302 (24) | 0.95 (0.58–1.5) | |
| Percentage know limiting to one sexual partner prevents HIV risk | ||||||
| Tertile 1 (27–69) | 10 (32) | 341 (20) | 43 (10) | 298 (24) | 1 | |
| Tertile 2 (69.1–81) | 11 (35) | 835 (49) | 193 (46) | 642 (51) | 2.1 (1.2–3.5) | <0.01 |
| Tertile 3 (82–94) | 10 (32) | 514 (30) | 186 (44) | 328 (26) | 3.9 (2.3–6.8) | |
| Percentage know condom use and limit to one sexual partner prevents HIV transmission | ||||||
| Tertile 1 (10–50) | 10 (32) | 529 (31) | 118 (28) | 411 (32) | 1 | |
| Tertile 2 (54–68) | 11 (35) | 836 (49) | 226 (54) | 610 (48) | 1.3 (0.88–1.9) | 0.43 |
| Tertile 3 (69–83) | 10 (32) | 325 (19) | 78 (18) | 247 (19) | 1.1 (0.68–1.8) | |
| Percentage tested for HIV | ||||||
| Tertile 1 (0.50–7.3) | 10 (32) | 617 (37) | 139 (33) | 478 (38) | 1 | |
| Tertile 2 (8.3–13.1) | 11 (35) | 178 (11) | 55 (13) | 123 (10) | 1.5 (0.95–2.5) | 0.21 |
| Tertile 3 (13.9–43.5) | 10 (32) | 895 (53) | 228 (54) | 667 (53) | 1.2 (0.81–1.7) | |
ART, antiretroviral therapy; CI, confidence interval.
Multivariate models
We ran three multivariate models (Table 4). The first model included only program-level factors, the second only contextual-level factors, the third combined both program-level and contextual-level factors that were significant in the prior two models. Each model controlled for calendar time of ART initiation. In model 1, urban vs. rural setting [adjusted odds ratio (AOR) 2.1; 95% CI 1.3–3.3], being unaffiliated with a PMTCT program (AOR 4.1; 95% CI 1.1–15.1), availability of outreach services targeted toward ART patients only vs. both pre-ART and ART patients (AOR 2.1; 95% CI 1.3–3.5), availability of fewer vs. more adherence support services (AOR 2.0; 95% CI 1.3–3.2), lowest vs. highest quartile of provider-to-patient ratio (AOR 2.3; 95% CI 1.3–4.0), and smaller cohort size (AOR 2.6; 95% CI 1.4–4.7) were independently associated with a greater odds of low cohort median CD4+ cell count at ART initiation. Offsite vs. onsite CD4+ testing (AOR 0.56; 95% CI 0.36–0.88) and offsite vs. onsite PMTCT services (AOR 0.49; 95% CI 0.25–0.98) were independently associated with a lower risk of low cohort median CD4+ cell count at ART initiation. In model 2, which examined only contextual-level variables, cohorts of persons initiating ART in areas where a higher proportion of individuals who indicate that they had heard of AIDS had a lower odds of low cohort median CD4+ cell count (AOR 0.93 per unit increase; 95% CI 0.87–0.99) as did cohorts initiating ART in areas where a higher proportion of the population had been tested for HIV and received results (AOR 0.95 per unit increase; 95% CI 0.90–0.99). However, cohorts initiating ART in regions where a higher proportion of the population believed that ‘limiting themselves to one HIV-uninfected sexual partner reduces HIV risk’ had a higher odds of an increased low cohort median CD4+ cell count (AOR 1.06 per unit increase; 95% CI 1.02–1.10). Finally, in model 3, which combined program-level and contextual-level factors, all of the factors that were independently associated with low cohort median CD4+ cell count in models 1 and 2 remained significant, with the exception of onsite vs. offsite PMTCT services and offsite vs. onsite CD4+ testing. Independent of program and contextual factors, the odds of low cohort median CD4+ cell count at ART initiation decreased with calendar time in dose–response fashion.
Table 4.
Multivariate models of program and contextual-level factors and low CD4 cell count among cohorts initiating antiretroviral therapy.
| Model 1 [AOR (95% CI)], site/program level | Model 2 [AOR (95% CI)], contextual level | Model 3 [AOR (95% CI)], site/program + contextual level | |
|---|---|---|---|
| Program-level factors | |||
| Setting | |||
| Rural | 1 | 1 | |
| Urban | 1.8 (1.1–2.9) | 2.1 (1.3–3.3) | |
| Availability of CD4 test | |||
| Onsite | 1 | ||
| Offsite | 0.56 (0.36–0.88) | ||
| Availability of PMTCT | |||
| Onsite | 1 | 1 | |
| Offsite | 0.49 (0.25–0.98) | 0.73 (0.37–1.4) | |
| Unaffiliated with a PMTCT program | 4.1 (1.1–15.1) | 3.6 (1.0–12.8) | |
| Access to outreach services | |||
| Not present | 1.1 (0.74–1.7) | 0.88 (0.58–1.3) | |
| Pre-ART and ART | 1 | 1 | |
| ART patients only | 2.1 (1.3–3.5) | 2.4 (1.5–3.9) | |
| No. of adherence support programs | |||
| 0–3 | 2.0 (1.3–3.2) | 1.6 (1.0–2.5) | |
| 4–5 | 0.96 (0.60–1.5) | 0.94 (0.6–1.5) | |
| ≥6 | 1 | 1 | |
| No. of provider per 1000 patients | |||
| <4 | 1.9 (1.1–3.5) | 2.3 (1.3–4.0) | |
| 4–9 | 1.7 (1.0–2.8) | 1.8 (1.1–3.0) | |
| 9.1–18 | 1.5 (0.99–2.4) | 1.6 (1.0–2.5) | |
| >18 | 1 | 1 | |
| Cohort size | |||
| ≤19 | 2.6 (1.4–4.7) | 2.5 (1.4–4.5) | |
| 20–42 | 1.69 (0.97–2.9) | 1.7 (0.98–2.8) | |
| 43–88 | 1.52 (0.97–2.4) | 1.5 (0.96–2.3) | |
| ≥89 | 1 | 1 | |
| Contextual-level factors | |||
| Percentage have heard of AIDS | 0.92 (0.86–0.98) | 0.88 (0.83–0.93) | |
| Percentage ever tested HIV and got results | 0.94 (0.90–0.99) | 0.95 (0.93–0.98) | |
| Percentage know limiting one sexual partner reduces HIV risk | 1.06 (1.03–1.11) | 1.09 (1.06–1.11) | |
| Calendar time of starting ART | |||
| 2004-2 | 3.9 (0.85–17.4) | 3.0 (0.72–12.3) | 5.6 (1.2–25.5) |
| 2005-1 | 4.8 (1.6–14.6) | 4.6 (1.6–13.5) | 6.8 (2.2–21.0) |
| 2005-2 | 1.0 (0.50–2.3) | 1.2 (0.58–2.6) | 1.1 (0.5–2.5) |
| 2006-1 | 1 | 1 | 1 |
| 2006-2 | 0.77 (0.43–1.4) | 0.75 (0.43–1.3) | 0.74 (0.40–1.3) |
| 2007-1 | 0.60 (0.34–1.0) | 0.53 (0.31–0.92) | 0.54 (0.31–0.96) |
| 2007-2 | 0.50 (0.28–0.89) | 0.44 (0.26–0.76) | 0.43 (0.24–0.77) |
| 2008-1 | 0.35 (0.20–0.62) | 0.34 (0.20–0.58) | 0.29 (0.16–0.52) |
AOR, adjusted odds ratio; ART, antiretroviral therapy; CI, confidence interval; PMTCT, prevention of mother-to-child transmission.
Discussion
The findings from this analysis of CD4+ cell count at ART initiation among cohorts included 121 504 patients from 267 public sector HIV care sites from eight sub Saharan African countries and demonstrated that a number of program-level factors were significantly associated with low median cohort CD4+ cell counts at ART initiation. Program-level associations were independent of site characteristics (e.g., urban/rural, primary/secondary/tertiary), program burden (e.g., cohort size and provider-to-patient ratio), and context (e.g., AIDS awareness, testing coverage). The observed low median CD4+ cell counts at ART initiation (i.e., with 25% of the cohorts in our sample having a median CD4+ cell count of <111 cells/μl) are consistent with other reports from sub-Saharan Africa and further highlight the substantial amount of variability CD4+ cell count at ART initiation across diverse sites and contexts. A number of program-level and contextual-level factors were found to explain some of this variability and may represent important and potentially modifiable determinants of low CD4+ cell count in populations of persons initiating ART.
The very high rates of late ART initiation in the region [2,3] may in turn be driving early mortality after initiation of ART [4,5], and feasible upstream clinic and program-level interventions continue to be desperately needed. Our findings demonstrate a clear need to further investigate the role of upstream precursors and determinants of late ART initiation (e.g., late diagnosis, late engagement into care, and delayed ART initiation after eligibility determination) when designing HIV treatment programs. Specifically, we observed factors that may be related to the timeliness of diagnosis after infection (PMTCT affiliation, AIDS knowledge, and testing coverage), the willingness to engage into care given an HIV diagnosis (AIDS knowledge), and the timeliness of ART initiation given enrollment into care (availability of outreach, adherence support services provider-to-patient ratio). The latter finding may reflect better retention and ability to conduct clinical and immunologic monitoring to determine eligibility for ART.
With regard to the contextual-level factors examined, our analyses suggest that greater awareness of AIDS and higher testing coverage in the region are associated with lower risk of low CD4+ cell count at ART initiation. These findings are encouraging to programs aimed at increasing awareness and knowledge of serostatus. However, the finding regarding awareness of HIV risk as measured by the proportion of persons in the population who believed that ‘limiting themselves to one HIV-uninfected sexual partner reduces HIV risk’ is unexpected and more difficult to interpret. It may reflect ‘reverse causality’ if, for example, greater efforts aimed at HIV prevention education are occurring in areas where HIV morbidity and mortality is highest, or where the epidemic is more mature. It could also reflect a false sense of security among some as yet undiagnosed HIV-infected individuals who perhaps do not fully appreciate their risk (which in turn could result in higher rates of delayed testing and diagnosis). Interestingly, we did not observe a similar association with knowledge around the need to use condoms to reduce HIV risk.
The risk of low median CD4+ cell counts among cohorts of patients initiating ART declined with calendar time, possibly reflecting the effect of increasing HIV testing uptake among individuals in the community and ART availability [1]. In sub-Saharan Africa, HIV testing uptake is variable [36–46], but generally, low throughout the region (e.g., 22% [1]). That lower cohort size was associated with a higher likelihood of low cohort median CD4+ cell count could reflect the startup of programs with newer sites initially focusing on initiation of ART among the sickest patients. As testing and ART coverage increase, the risk of late ART initiation would be expected to decrease. However, even in areas with relatively higher ART availability and coverage (e.g., the USA), there remain high rates of late diagnosis followed by delayed enrollment into care and consequently initiation of ART at lower CD4+ cell counts. Approximately 26% of persons diagnosed with HIV in the USA [47] and in New York City [48] (the epicenter for HIV in the USA) have concomitant diagnosis of AIDS at the time of HIV diagnosis, and delayed access to HIV care after HIV diagnosis is common [49–51]. For example, a recent study in New York City showed that nearly 20% of newly diagnosed individuals had not yet enrolled in HIV care 1 year later [52] and this figure may be as high as 30–40% nationwide [49,53,54]. Thus, although there are encouraging reports of CD4+ cell count at ART initiation increasing in the sub-Saharan African region [26], without substantial expansion in HIV testing, increased efforts at engagement in care, and close clinical and immunological monitoring for ART eligibility, the challenge of late ART initiation may well persist.
The median CD4+ cell count among cohorts of patients initiating ART was, on average, lower at urban sites compared with rural sites (131 vs. 155 cells/μl), and cohorts at urban sites were more than twice as likely to have low median CD4+ cell count than those at rural sites (AOR 2.1; 95% CI 1.3–3.3). The reasons for this are not immediately clear and further investigation is needed. A few other studies in the region (from sites in Ghana and South Africa) have reported similar findings [55,56]. There are plausible explanations as to why cohorts of patients initiating ART at urban clinics might be on an average sicker than those at rural clinics in some instances. Rural HIV epidemics are generally less mature in most areas than their urban counterparts [32,57], and there may be many more patients with very low CD4+ cell count in urban than in rural areas. Additionally, similar to the spread of the HIV epidemic itself, HIV care and treatment scale-up in many sub-Saharan African countries started in large centers in urban areas before expanding to secondary and primary care sites in more rural areas [58]. Therefore, another possible explanation might be that many of the sickest patients in rural areas actually sought care and initiated ART in urban areas before HIV care clinics nearer to their homes became available. Moreover, sicker patients in rural areas may be more likely to go to a clinic in an urban area even if there is a clinic nearer to their home, perhaps because they believe the care to be better or because they are concerned about stigma in their community. Finally, HIV prevalence is generally lower in rural than in urban areas [32] and the patient load, even relative to staffing, may be substantially lower in rural clinics. This in turn could allow the sickest pool of patients to be treated more quickly in rural than in urban areas. Although we attempted to control for staffing ratios in a time-updated fashion, and these were found to be important correlates of low median CD4+ cell count at ART initiation, staffing ratios are likely more complex than we were able to capture on our program survey, and ultimately control for in our analysis. Conversely, there are also plausible reasons why some cohorts of rural patients initiating ART might have lower median CD4+ cell counts compared with urban patients, due, for example, to slower rollout of HIV care services compared with urban settings, lower HIV testing rates, and greater barriers associated with access such as longer distance to clinic, with costs and logistics of transport that may comparatively be more prohibitive [59].
Strengths of our study included the use of routinely collected aggregate data used for monitoring and evaluation purposes, which allowed for the use of data for cohorts that included more than 120 000 patients starting ART in several sub-Saharan African countries. The use of data from a diversity of sites, including rural and small sites and sites without electronic data systems, enhances the generalizability of our findings. The large number of clinics and contexts enabled us to examine a wide array of relevant program-level and contextual-level factors and control for confounding at each level. Our findings were robust to a number of sensitivity analyses, including the exclusion of individual countries (to examine the extent to which associations may be driven by sites in individual country), smaller cohorts (where median CD4+ cell counts may be more variable and less informative), and cohorts that initiated ART in 2004–2006 (to assess the influence of maturation of scale-up effect).
However, our study also has limitations requiring that the results be interpreted with caution. We examined the role of program and contextual factors that were observed, rather than randomly assigned (i.e., experimentally), and as such observed associations may be prone to confounding. Although we attempted to control for confounding by adjusting for measured factors hypothesized to be important, the associations we observed could be still be a result of uncontrolled confounding by other factors at the program and contextual levels. Finally, although a standard operating procedure on indicator definitions and limited quality assurance procedures help ensure consistency of reported indicators across sites and settings, data quality in the service delivery context is often limited and could have influenced our results (e.g., outcome misclassification). Further, the site surveys could have resulted in exposure misclassification. However, each misclassification is likely nondifferential with regard to one another, making it harder to observe true associations when they exist. In addition, site surveys elicited information on availability of specific services and not their coverage or utilization by patients at these particular sites. Further, although we used the most recent DHS data as source of our contextual-level data, these surveys were conducted from 2003 to 2005 and thus prior to the period during which most of our cohorts initiated ART (2007 and 2008). Time-updated contextual-level information may have given different results. Finally, although our analysis included many rural sites (n = 108), the proportion of patients from rural sites was small (15%) and may further limit generalizability. However, given the strength of the associations, future investigations should explore these areas further.
In conclusion, the associations observed in our analyses of factors associated with low CD4+ cell count at ART initiation may represent potentially influential and modifiable determinants. Although the decreasing odds of low median CD4+ cell count at ART initiation with calendar time is encouraging, a substantial proportion of cohorts continue to initiate ART with low median CD4+ cell counts (e.g., 50% below 136 cells/μl). Our findings suggest specific approaches that may motivate earlier ART initiation in populations of patients (e.g., development of interventions such as active tracing and tracking of patients in care who miss visits in order to ensure early identification of ART eligibility) and a need for more focused operational research on this issue.
Acknowledgments
We would like to thank and acknowledge staff from the International Center for AIDS Care and Treatment Programs (ICAP), including Deborah Horowitz, Caroline Korves, Suzue Saito, and Matthew Lamb from ICAP Headquarters in New York, USA; Senior Monitoring and Evaluation Advisors in the ICAP country offices, including: Tsigereda Gadisa (ICAP Ethiopia), Muhsin Sherrif, (ICAP Kenya), Melanie Manyasha (ICAP Lesotho), Maria Fernanda Alvim (ICAP Mozambique), Frank Oransaye (ICAP Nigeria), Veronicah Mugisha (ICAP Rwanda), Harriet Nuwagaba-Biribonwoha (Uganda), Molly Strachan (ICAP Tanzania), Kanchan Reed (ICAP South Africa), and Hermann Brou (ICAP Cote d’Ivoire); ICAP Clinical Officers in each country who contributed to data collection; and ICAP Country Directors for overall support and guidance; Staff from the Center for International Earth Science Information Network (CIESIN), Columbia University (Tricia Chai-Onn and Mark Becker) for geographical information system-related assistance. We would also like to acknowledge the Ministries of Health (MOH) from Ethiopia, Kenya, Lesotho, Mozambique, Nigeria, Rwanda, South Africa, and Tanzania whose HIV care and treatment programs we endeavor to support through this work.
The activities described in this paper were funded through the President’s Emergency Plan for AIDS Relief (PEPFAR, www.pepfar.gov) through the US Centers for Disease Control and Prevention (CDC; www.cdc.gov), by a grant from the Doris Duke Charitable Foundation’s Operations Research on AIDS Care and Treatment in Africa (ORACTA; www.ddcf.org), and by a grant from the US National Institutes of Health (NIH; www.nih.-gov), National Institute of Mental Health (NIMH grant 1R01MH089831-01A1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
D.N., Y.W., D.H., B.E., and W.E.S. designed the experiments/the study. Y.W. and D.N. analyzed the data. D.H., W.E.S., D.N., and B.E. were responsible for the data contributed by participating cohorts. D.N., Y.W., and W.E.S. wrote the first draft of the paper. Y.W., D.H., B.E., and W.E.S. contributed to the writing of the paper.
D.N., Y.W., D.H., B.E., and W.E.S. have no competing interests.
References
- 1.WHO. Towards Universal Access: scaling up priority HIV/AIDS interventions in the health sector: progress report 2010. Geneva: WHO; 2010. [Google Scholar]
- 2.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 3.Colebunders R, Ronald A, Katabira E, Sande M. Rolling out antiretrovirals in Africa: there are still challenges ahead. Clin Infect Dis. 2005;41:386–389. doi: 10.1086/431490. [DOI] [PubMed] [Google Scholar]
- 4.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinkhof MW, Boulle A, Weigel R, Messou E, Mathers C, Orrell C, et al. Mortality of HIV-infected patients starting antiretro-viral therapy in sub-Saharan Africa: comparison with HIV-unrelated mortality. PLoS Med. 2009;6:e1000066. doi: 10.1371/journal.pmed.1000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 7.Krentz HB, Auld MC, Gill MJ. The high cost of medical care for patients who present late (CD4 <200 cells/μl) with HIV infection. HIV Med. 2004;5:93–98. doi: 10.1111/j.1468-1293.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- 8.Bunnell R, Mermin J, De Cock KM. HIV prevention for a threatened continent: implementing positive prevention in Africa. JAMA. 2006;296:855–858. doi: 10.1001/jama.296.7.855. [DOI] [PubMed] [Google Scholar]
- 9.Cohen MS, Hellmann N, Levy JA, DeCock K, Lange J. The spread, treatment, and prevention of HIV-1: evolution of a global pandemic. J Clin Invest. 2008;118:1244–1254. doi: 10.1172/JCI34706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, Clark JE. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. quiz CE11–CE14. [PubMed] [Google Scholar]
- 11.Egger M. Outcomes of ART in Resource-limited and Industrialized Countries [paper 62]. Conference on Retroviruses and Opportunistic Infections (CROI); Denver, CO, USA. 2007. [Google Scholar]
- 12.Bussmann H, Wester CW, Ndwapi N, Grundmann N, Gaolathe T, Puvimanasinghe J, et al. Five-year outcomes of initial patients treated in Botswana’s National Antiretroviral Treatment Program. AIDS. 2008;22:2303–2311. doi: 10.1097/QAD.0b013e3283129db0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–895. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 14.Djomand G, Roels T, Ellerbrock T, Hanson D, Diomande F, Monga B, et al. Virologic and immunologic outcomes and programmatic challenges of an antiretroviral treatment pilot project in Abidjan, Cote d’Ivoire. AIDS. 2003;17 (Suppl 3):S5–S15. doi: 10.1097/00002030-200317003-00002. [DOI] [PubMed] [Google Scholar]
- 15.Etard JF, Ndiaye I, Thierry-Mieg M, Gueye NF, Gueye PM, Laniece I, et al. Mortality and causes of death in adults receiving highly active antiretroviral therapy in Senegal: a 7-year cohort study. AIDS. 2006;20:1181–1189. doi: 10.1097/01.aids.0000226959.87471.01. [DOI] [PubMed] [Google Scholar]
- 16.Laurent C, Diakhate N, Gueye NF, Toure MA, Sow PS, Faye MA, et al. The Senegalese government’s highly active antiretroviral therapy initiative: an 18-month follow-up study. AIDS. 2002;16:1363–1370. doi: 10.1097/00002030-200207050-00008. [DOI] [PubMed] [Google Scholar]
- 17.Nash D, Katyal M, Brinkhof MW, Keiser O, May M, Hughes R, et al. Long-term immunologic response to antiretroviral therapy in low-income countries: a collaborative analysis of prospective studies. AIDS. 2008;22:2291–2302. doi: 10.1097/QAD.0b013e3283121ca9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toure S, Kouadio B, Seyler C, Traore M, Dakoury-Dogbo N, Duvignac J, et al. Rapid scaling-up of antiretroviral therapy in 10 000 adults in Cote d’Ivoire: 2-year outcomes and determinants. AIDS. 2008;22:873–882. doi: 10.1097/QAD.0b013e3282f768f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wools-Kaloustian K, Kimaiyo S, Musick B, Sidle J, Siika A, Nyandiko W, et al. The impact of the President’s Emergency Plan for AIDS Relief on expansion of HIV care services for adult patients in western Kenya. AIDS. 2009;23:195–201. doi: 10.1097/QAD.0b013e32831cc0e6. [DOI] [PubMed] [Google Scholar]
- 20.Lawn SD, Myer L, Harling G, Orrell C, Bekker LG, Wood R. Determinants of mortality and nondeath losses from an anti-retroviral treatment service in South Africa: implications for program evaluation. Clin Infect Dis. 2006;43:770–776. doi: 10.1086/507095. [DOI] [PubMed] [Google Scholar]
- 21.Kabugo C, Bahendeka S, Mwebaze R, Malamba S, Katuntu D, Downing R, et al. Long-term experience providing antiretro-viral drugs in a fee-for-service HIV clinic in Uganda: evidence of extended virologic and CD4+ cell count responses. J Acquir Immune Defic Syndr. 2005;38:578–583. doi: 10.1097/01.qai.0000134742.26338.2f. [DOI] [PubMed] [Google Scholar]
- 22.Laurent C, Ngom Gueye NF, Ndour CT, Gueye PM, Diouf M, Diakhate N, et al. Long-term benefits of highly active antire-troviral therapy in Senegalese HIV-1-infected adults. J Acquir Immune Defic Syndr. 2005;38:14–17. doi: 10.1097/00126334-200501010-00003. [DOI] [PubMed] [Google Scholar]
- 23.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 24.Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 25.Bekker LG, Myer L, Orrell C, Lawn S, Wood R. Rapid scale-up of a community-based HIV treatment service: programme performance over 3 consecutive years in Guguletu, South Africa. S Afr Med J. 2006;96:315–320. [PubMed] [Google Scholar]
- 26.Keiser O, Anastos K, Schechter M, Balestre E, Myer L, Boulle A, et al. Antiretroviral therapy in resource-limited settings 1996 to 2006: patient characteristics, treatment regimens and monitoring in sub-Saharan Africa, Asia and Latin America. Trop Med Int Health. 2008;13:870–879. doi: 10.1111/j.1365-3156.2008.02078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowrance DW, Ndamage F, Kayirangwa E, Ndagije F, Lo W, Hoover DR, et al. Adult clinical and immunologic outcomes of the national antiretroviral treatment program in Rwanda during 2004–2005. J Acquir Immune Defic Syndr. 2009;52:49–55. doi: 10.1097/QAI.0b013e3181b03316. [DOI] [PubMed] [Google Scholar]
- 28.Kigozi IM, Dobkin LM, Martin JN, Geng EH, Muyindike W, Emenyonu NI, et al. Late-Disease Stage at Presentation to an HIV Clinic in the Era of Free Antiretroviral Therapy in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2009;52:282–289. doi: 10.1097/QAI.0b013e3181ab6eab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. Rapid advice: antiretroviral therapy for HIV infection in adults and adolescents – November 2009. Geneva: World Health Organization; 2009. [Google Scholar]
- 30.Wikipedia. President’s Emergency Plan for AIDS Relief. [Google Scholar]
- 31.Measure DHS. [Accessed 11 May 2011];Demographic and Health Surveys. www.measurehds.com.
- 32.Macro_International. HIV prevalence estimates from the demographic and health surveys. Calverton, MD: Macro International Inc; 2008. [Google Scholar]
- 33.South African National Department of Health. [[Accessed 11 May 2011].];The National HIV and Syphilis Prevalence Survey – South Africa. 2008 http://data.unaids.org/pub/Report/2008/20080904_southafrica_anc_2008_en.pdf.
- 34.UNAIDS. Epidemiological Fact Sheet on HIV and AIDS, Mozambique: core data on epidemiology and response. Geneva: UNAIDS; 2008. [Google Scholar]
- 35.UNAIDS. Epidemiological Fact Sheet on HIV and AIDS, Nigeria: core data on epidemiology and response. Geneva: UNAIDS; 2008. [Google Scholar]
- 36.Kenya Demographic and Health Survey 2003. Calverton, MD, USA: Central Bureau of Statistics Ministry of Planning & National Development; 2003. [Google Scholar]
- 37.Uganda Demographic and Health survey 2006. Calverton, MD, USA: Uganda Bureau of Statistics (UBOS) and Macro International Inc; 2007. [Google Scholar]
- 38.Central Statistical Office, Harare, Zimbabwe. Zimbabwe Demographic and Health Survey 2005–2006. Calverton, MD, USA: Macro International Inc; 2007. [Google Scholar]
- 39.Central Statistical Office, Mbabane. Swaziland Demographic and Health Survey 2006–07. Calverton, MD, USA: Macro International; 2007. [Google Scholar]
- 40.Central Statistical Office (CSO), Ministry of Health (MOH), Tropical Diseases Research Centre (TDRC), University of Zambia, Macro International Inc. Zambia Demographic and Health Survey 2007. Calverton, MD, USA: CSO and Macro International Inc; 2009. [Google Scholar]
- 41.Department of Health, Medical Research Council, OrcMacro. South Africa demographic and health survey 2003. Pretoria: Department of Health; 2007. [Google Scholar]
- 42.Institut National de la Statistique du Rwanda (INSR), and ORC Macro. Rwanda Demographic and Health Survey 2005. Calverton, MD, USA: INSR and ORC Macro; 2006. [Google Scholar]
- 43.National Bureau of Statistics (NBS) [Tanzania], and ORC Macro. Tanzania demographic and health survey 2004–2005. Dar es Salaam, Tanzania: National Bureau of Statistics and ORC Macro; 2005. [Google Scholar]
- 44.National Population Commission (NPC) [Nigeria.], and ORC Macro. Nigeria demographic and health survey 2003. Calverton, MD: National Population Commission and ORC Macro; 2004. [Google Scholar]
- 45.National Statistical Office (NSO) [Malawi.], ORC Macro. Malawi demographic and health survey 2004. Calverton, MD: NSO and ORC Macro; 2005. [Google Scholar]
- 46.De Cock KM, Bunnell R, Mermin J. Unfinished business: expanding HIV testing in developing countries. N Engl J Med. 2006;354:440–442. doi: 10.1056/NEJMp058327. [DOI] [PubMed] [Google Scholar]
- 47.CDC. Diagnosis and reporting of HIV and AIDS in states with HIV/AIDS surveillance: United States, 1994–2000. MMWR. 2002;51:595–598. [PubMed] [Google Scholar]
- 48.Nash D, Ramaswamy C, Manning S. Implementation of named HIV reporting: New York City. MMWR Morb Mortal Wkly Rep. 2004;52:1248–1252. [PubMed] [Google Scholar]
- 49.Samet JH, Freedberg KA, Stein MD, Lewis R, Savetsky J, Sullivan L, et al. Trillion virion delay: time from testing positive for HIV to presentation for primary care. Arch Intern Med. 1998;158:734–740. doi: 10.1001/archinte.158.7.734. [DOI] [PubMed] [Google Scholar]
- 50.Samet JH, Freedberg KA, Savetsky JB, Sullivan LM, Stein MD. Understanding delay to medical care for HIV infection: the long-term nonpresenter. AIDS. 2001;15:77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- 51.Mugavero MJ, Lin HY, Allison JJ, Willig JH, Chang PW, Marler M, et al. Failure to establish HIV care: characterizing the ‘no show’ phenomenon. Clin Infect Dis. 2007;45:127–130. doi: 10.1086/518587. [DOI] [PubMed] [Google Scholar]
- 52.Torian LV, Wiewel EW, Liu KL, Sackoff JE, Frieden TR. Risk factors for delayed initiation of medical care after diagnosis of human immunodeficiency virus. Arch Intern Med. 2008;168:1181–1187. doi: 10.1001/archinte.168.11.1181. [DOI] [PubMed] [Google Scholar]
- 53.Krawczyk CS, Funkhouser E, Kilby JM, Vermund SH. Delayed access to HIV diagnosis and care: special concerns for the Southern United States. AIDS Care. 2006;18 (Suppl 1):S35–S44. doi: 10.1080/09540120600839280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Krawczyk CS, Funkhouser E, Kilby JM, Kaslow RA, Bey AK, Vermund SH. Factors associated with delayed initiation of HIV medical care among infected persons attending a southern HIV/AIDS clinic. South Med J. 2006;99:472–481. doi: 10.1097/01.smj.0000215639.59563.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torpey K, Lartey M, Amenyah R, Addo NA, Obeng-Baah J, Rahman Y, et al. Initiating antiretroviral treatment in a resource-constrained setting: does clinical staging effectively identify patients in need? Int J STD AIDS. 2009;20:395–398. doi: 10.1258/ijsa.2008.008333. [DOI] [PubMed] [Google Scholar]
- 56.Martinson N, Heyer A, Steyn J, Struthers H, McIntyre J, Gray G, Pronyk P. Does WHO clinical stage reliably predict who should receive ARV treatment? [abstract WeFo0304]. The 3rd IAS Conference on HIV Pathogenesis and Treatment; Rio de Janeiro. 2005. [Google Scholar]
- 57.Mertens TE, Low-Beer D. HIV and AIDS: where is the epidemic going? Bull World Health Org. 1996;74:121–129. [PMC free article] [PubMed] [Google Scholar]
- 58.El-Sadr WM, Hoos D. The President’s Emergency Plan for AIDS Relief: is the emergency over? N Engl J Med. 2008;359:553–555. doi: 10.1056/NEJMp0803762. [DOI] [PubMed] [Google Scholar]
- 59.Geng EH, Nash D, Kambugu A, Zhang Y, Braitstein P, Christopoulos KA, et al. Retention in care among HIV-infected patients in resource-limited settings: emerging insights and new directions. Curr HIV/AIDS Rep. 2010;7:234–244. doi: 10.1007/s11904-010-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]