Abstract
The hippocampus is often considered to be an important site for stress and learning interactions; however, it has never been demonstrated whether these effects require the hippocampus. In the current study, hippocampal lesions prevented both enhancements of learning after stress in male rats and impairments of learning after stress in female rats without disrupting learning itself in either sex. Thus, the hippocampus is necessary for modifying learning in males and females after acute stressful experience.
Exposure to an acute stressful event can have long-lasting consequences for learning processes, and the effects of stress are presumed to be mediated by neuronal activity in the hippocampus1. This idea is based, in part, on the fact that hippocampal-dependent learning is susceptible to both stressful experience and direct infusions of stress hormones2,3. However, because many learning tasks depend on an intact hippocampus, it is unclear whether the hippocampus is required for the modulation of learning by stress or is simply involved in learning itself. Stressful experience can also modify learning that is not hippocampal dependent, such as delay eye-blink conditioning4. For example, delay conditioning in male rats is enhanced following an acute stressful event, whereas conditioning in females is impaired following the same stressor5,6. If the hippocampus is necessary for the modulation of learning by stress, then hippocampal lesions should prevent both the stress-induced facilitation of learning in males and the learning deficits following stress in females.
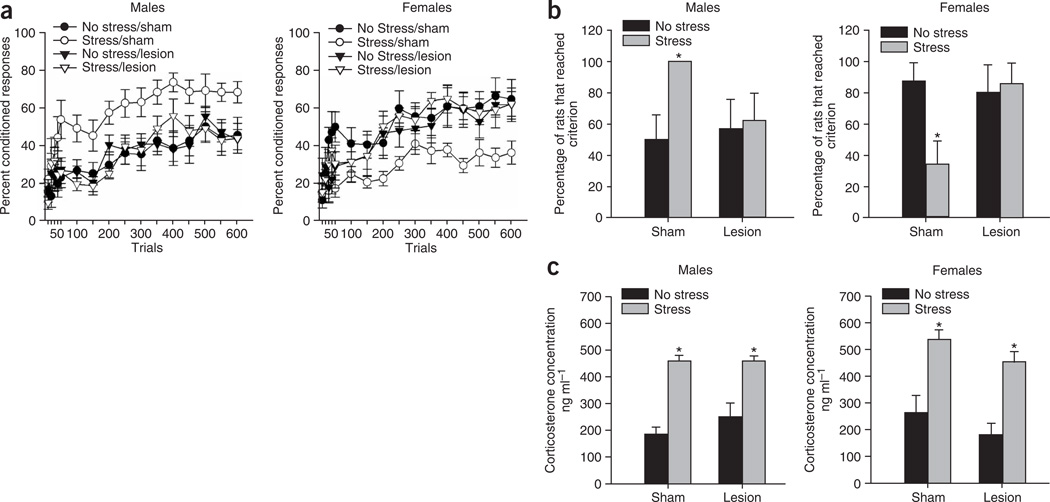
Adult (67–100 d) male and female Sprague-Dawley rats were given excitotoxic lesions of the complete hippocampus or were subjected to sham operations (Fig. 1), and were then fitted with head stages for classical eye-blink conditioning. After 1 week of recovery, half of the rats were exposed to a stressor of combined restraint stress and 30 periodic tail-shocks (1 s, 1 mA, 1 per min). Animals began training for delay eye-blink conditioning (150 trials per d for 4 d) 24 h later. A white-noise conditioned stimulus (850 ms) overlapped and coterminated with an eyelid shock, the unconditioned stimulus (100ms). To assess learning, we quantified conditioned responses, anticipatory eye blinks that occurred within 500 ms of the eyelid shock, using eyelid electromyography (Supplementary Methods online). An ANOVA revealed that the percentage of conditioned responses increased over trials for both males (F11,319 = 19.65, P < 0.05) and females (F11,286 = 19.85, P < 0.05) (Fig. 2a). Thus, both sexes learned to emit conditioned responses, with a trend for unstressed females to outperform unstressed males (F1,16 = 3.32, P = 0.09), an effect that has been reported in other studies6. An ANOVA revealed that exposure to the stressor interacted with the lesion to affect the percentage of conditioned responses that were emitted by both males and females (F1,29 = 5.38, P < 0.05 and F1,26 = 5.00, P < 0.05, respectively), but these conditions did not interact with blocks of trials (F11,319 = 0.61, P > 0.05 and F11,296 = 0.46, P > 0.05, respectively). Newman-Keuls post hoc tests indicated that sham-operated stressed males emitted more conditioned responses than did unstressed males (P < 0.05), whereas stressed males with hippocampal lesions did not emit more conditioned responses than did their unstressed lesioned counterparts (P > 0.05). Sham-operated females rats exposed to the stressor emitted fewer conditioned responses than did the unstressed females (P < 0.05), whereas females with hippocampal lesions did not emit fewer conditioned responses than did their unstressed lesioned counterparts (P > 0.05).
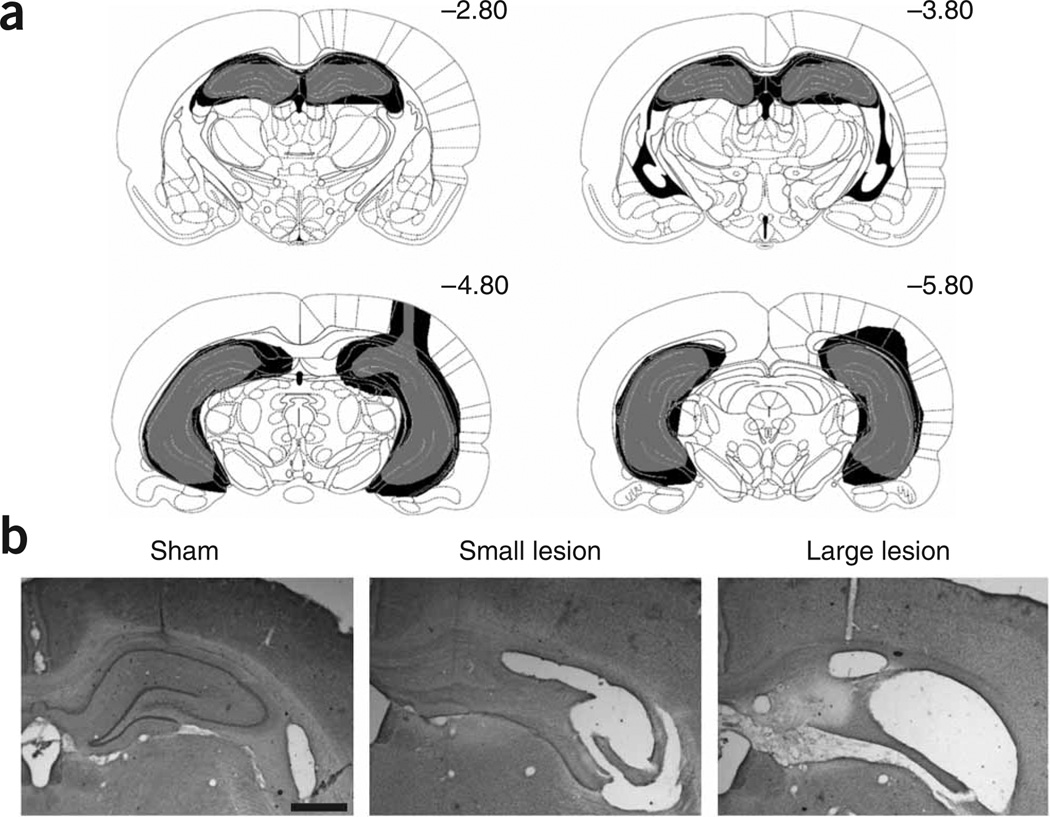
Figure 1.
Rats in the experimental group were given excitotoxic lesions of the complete hippocampus. (a) The largest lesion (in black) and smallest lesion (in gray) were selected from all animals included in the study, and are shown here according to a rat brain atlas15 (Supplementary Results online). (b) Representative examples of the smallest and largest lesion are shown. Scale bar, 1 mm. These experiments were conducted in accordance with procedures outlined by the Animal Care and Facilities Committee at Rutgers University.
Figure 2.
Hippocampal lesions prevent the modulation of learning by stress, without disrupting learning itself or corticosterone release. (a) Hippocampal lesions prevented the stress-induced enhancement of conditioning in males and the stress-induced impairment of conditioning in females. Data are represented as the mean ± s.e.m. percentage of conditioned responses over 600 training trials. (b) Stress increased the percentage of male rats that learned, whereas it decreased the percentage of female rats that learned. However, both effects were prevented by hippocampal lesions. The graphs show the percentage of the subjects that emitted at least 60% conditioned responses for a minimum of one trial block (± standard error of the proportion), and asterisks indicate significant differences from sham-operated counterparts (P ≤ 0.05). (c) Hippocampal lesions did not prevent the release of corticosterone after stressor exposure. Data are represented as the mean ± s.e.m. plasma levels of corticosterone (ng ml−1). Asterisks indicate increases in corticosterone levels relative to unstressed controls (P < 0.05).
Exposure to the stressor also affected the percentage of animals that learned, defined here as reaching a criterion of emitting at least 60% conditioned responses for a minimum of one block of training trials (50 trials per block; Fig. 2b). Only 50% of unstressed males reached this criterion compared with the 100% of sham-operated stressed males that did (χ2(1, n = 18) = 7.41, P < 0.05). However, a smaller percentage of stressed males with lesions reached criterion (62.5%) than their stressed sham-operated counterparts (χ2(1, n = 16) = 4.86, P < 0.05). In contrast, a large percentage of the unstressed females reached criterion (87.5%), whereas only 33% of the stressed females with sham-operations did (χ2(1, n = 17) = 3.68, P < 0.05). However, hippocampal lesions increased the percentage of stressed females that reached criterion (87.5%; χ2(1, n = 17) = 3.68, P < 0.05). Those that reached criteria significantly increased conditioned responding within the first 200 trials of training (F11,231 = 15.46, P < 0.05, and F11,220 = 22.3, P < 0.05 for males and females, respectively, with post hocs for both with P < 0.05). An analysis of all rats on the remaining trial blocks (250–600) revealed that the percentage of conditioned responses in the sham-operated males exposed to the stressor differed from those of the other male groups (F3,29 = 3.85, P < 0.05), and that the percentage of conditioned responses in stressed females with sham-operations differed from those of other female groups (F3,25 = 3.85, P < 0.05). Together, these results indicate that lesions of the hippocampus prevent both the enhanced conditioning that occurs in males after a stressful event and the decreased conditioning that occurs in females after the same stressor.
Although these findings suggest that the hippocampus is necessary for modulating learning after stress, an attenuation of the stress effect could occur if hippocampal lesions prevent the hypothalamic-pituitary adrenal axis response to stress. To assess this, we assayed plasma corticosterone concentrations 20 min after re-exposure to the stressor. Concentrations were elevated significantly in males and females after stressor exposure (F1,39 = 66.9, P < 0.001), regardless of whether or not the animals were lesioned (F1,39 = 0.60, P > 0.05; F1,39 = 0.26, P > 0.05; lesion alone and stress-lesion interaction, respectively) (Fig. 2c). Thus, hippocampal lesions did not reduce the stress-induced release of corticosterone. It has been reported that hippocampal lesions can disrupt negative feedback on the hypothalamus7 (See ref. 8.), resulting in a prolonged hypothalamic-pituitary adrenal axis response. It seems unlikely that a longer stress response would prevent the effects of stress. Also, previous studies have demonstrated that changes in corticosterone levels are not sufficient to establish the effects of stress on classical conditioning6,9. Moreover, in both sexes, corticosterone levels return to baseline by the time that training begins, 24 h later, and do not differ between groups during training10. Finally, exposure to the same amount of controllable stress does not modulate learning in males or females, even though corticosterone levels are similar to those of animals exposed to uncontrollable stress11.
Evidently, exposure to an acute stressful event modulates learning in males and females via changes in the hippocampus. This stressor also modulates the density of dendritic spines in the CA1 region of the hippocampus, increasing density in males and reducing density in females12. Because these anatomical changes mirror the sex difference in eye-blink conditioning, their presence (or absence in the case of females) may contribute to both the enhanced learning in males and the learning deficit in females that are observed after stress. Additionally, hippocampal cell excitability is increased following acute stress in males2. Such changes could in turn affect processing in the cerebellar eye-blink circuitry via polysynaptic connections with other critical structures, including the amygdala and bed nucleus of the stria terminalis13,14.
Our results here demonstrate that the hippocampus is necessary for the modulation of delay eye-blink conditioning by stressful experience. Hippocampal lesions did not disrupt conditioning itself nor reduce the release of corticosterone, dissociating the role of the hippocampus in learning and in the stress response from its role in the modulation of learning by stress4,7. Thus, the hippocampus is an intermediary structure that links the consequences of a stressful experience with learning circuitry, even when the learning task itself does not require the hippocampus. These findings underscore the necessity for the hippocampus in mediating both positive and negative responses to stressful life events, perhaps including those associated with post-traumatic stress disorder, a seriousmental illness with long-lasting repercussions, many of which are cognitive.
Supplementary Material
ACKNOWLEDGMENTS
Special thanks to D.E. Waxler for comments on the manuscript and to D. Vargas and A. Tang for technical support. This work was supported by US National Institute of Mental Health (59970) and National Science Foundation (IOB-0444364) grants to T.J.S. and a National Institute of Mental Health (AG19957-06) grant to D.A.B.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
References
- 1.McEwen BS, Sapolsky RM. Curr. Opin. Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 2.Weiss C, Sametsky E, Sasse A, Spiess J, Disterhoft JF. Learn. Mem. 2005;12:138–143. doi: 10.1101/lm.89005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roozendaal B, Griffith QK, Buranday J, de Quervain DJ, McGaugh JL. Proc. Natl. Acad. Sci. USA. 2003;100:1328–1333. doi: 10.1073/pnas.0337480100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Behav. Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- 5.Shors TJ, Weiss C, Thompson RF. Science. 1992;257:537–539. doi: 10.1126/science.1636089. [DOI] [PubMed] [Google Scholar]
- 6.Wood GE, Shors TJ. Proc. Natl. Acad. Sci. USA. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sapolsky RM, Krey LC, McEwen BS. Proc. Natl. Acad. Sci. USA. 1984;81:6174–6177. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuvnes FA, Steffenach HA, Murison R, Moser MB, Moser EI. J. Neurosci. 2003;23:4345–4354. doi: 10.1523/JNEUROSCI.23-10-04345.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beylin AV, Shors TJ. Horm. Behav. 2003;43:124–131. doi: 10.1016/s0018-506x(02)00025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shors TJ. Neurobiol. Learn. Mem. 2001;75:10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- 11.Leuner B, Mendolia-Loffredo S, Shors TJ. Biol. Psychiatry. 2004;56:964–970. doi: 10.1016/j.biopsych.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shors TJ, Chua C, Falduto J. J. Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shors TJ, Mathew PR. Learn. Mem. 1998;5:220–230. [PMC free article] [PubMed] [Google Scholar]
- 14.Bangasser DA, Santollo J, Shors TJ. Behav. Neurosci. 2005;119:1459–1466. doi: 10.1037/0735-7044.119.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd edn. Orlando, Florida, USA: Academic Press; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.