Abstract
Dendritic spines in the hippocampus are sources of synaptic contact that may be involved in processes of learning and memory [Moser (1999) Cell. Mol. Life Sci., 55, 593–600]. These structures are sensitive to sex differences as females in proestrus possess a greater density than males and females in other stages of the estrous cycle [Woolley et al. (1990) J. Neurosci., 10, 4035–4039]. Moreover, exposure to an acute stressful event increases spine density in the male hippocampus but decreases spine density in the female hippocampus [Shors et al.. (2001) J. Neurosci., 21, 6292–6297]. Here we demonstrate that antagonism of N-methyl-d-aspartate (NMDA) receptors prevents the increase in spine density as females transition from diestrus 2 to proestrus, when estrogen levels are rising. Antagonism of NMDA receptors during exposure to the stressful event also prevented the changes in spine density in males and females, despite differences in the direction of these effects. Thus, the stress-induced increase in spine density was prevented in the male hippocampus as was the stress-induced decrease in spine density in the female hippocampus. NMDA receptor antagonism during exposure to the stressful event did not alter corticosterone levels or the corticosterone response to stress. These data suggest that both increases and decreases in spine density can be dependent on NMDA receptor activation.
Keywords: corticosterone, estrogen, glutamate, hippocampus, learning, synapse
Introduction
There are many sex differences in behaviour and presumably these differences are accompanied, and in some cases mediated, by sex differences in neuronal anatomy and synaptic plasticity. Reports of sex differences in anatomical structures are many, although most are localized to brain regions involved in sexual behaviour, such as the hypothalamus, amygdala or bed nucleus of the stria terminalis (Becker et al., 1992). With regard to limbic regions, and in particular those associated with learning and memory, reports of sex differences in anatomy are less numerous (Gould et al., 1990; Galea et al., 1997; Miranda et al., 1999). For neurons in the hippocampus, sex of the animal, hormonal milieu and exposure to stressful events are all known to affect the density of dendritic spines. Specifically, treatment with estrogen increases spine density in area CA1 of the hippocampus and cycling levels of estrogen during the estrous cycle correlate with spine density (Gould et al., 1990; Woolley et al., 1990). Moreover, females in proestrus have a greater density of spines in area CA1 than do males (Shors et al., 2001). In response to an acute stressful event of intermittent tailshocks, spine density is increased in males but decreased in females (Shors et al., 2001). Thus, a number of manipulations, both exogenous and endogenous can alter spine density and the effects can be either positive or negative depending on the sex of the animal.
The increase in spine density after acute treatment with estrogen is dependent on activation of the N-methyl-d-aspartate (NMDA) type of glutamate receptor (Woolley & McEwen, 1994). There are also numerous reports that exposure to acute stress activates NMDA receptors to induce changes in synaptic and neuronal plasticity (Monaghan & Cotman, 1985; Morris et al., 1986; Shors & Servatius, 1995; Kim et al., 1996; Shors et al., 1997; McKinney et al., 1999; Gazzaley et al., 2002). Moreover, a number of studies indicate that glutamate receptor-mediated events are critically involved in altering the number and structure of dendritic spines on pyramidal neurons in area CA1 of the hippocampus (Woolley, 1998; Kirov & Harris, 1999; Korkotian, 1999; Rose & Konnerth, 2001). Here we hypothesized that NMDA receptor activation was necessary for inducing the effects of acute stressful experience on spine density. Specifically, we tested whether the difference in spine density between males and females is dependent on NMDA receptor activation in females in the transition from diestrus (when estrogen levels are low) to proestrus (when estrogen levels are elevated). We also tested whether stress would increase spine density in males and decrease spine density in females if NMDA receptors were antagonized during the stressful event.
Materials and methods
Subjects
Adult Sprague-Dawley male and female rats (250–400 g; 2–3 months) were purchased from Zivic Miller (Zelienople, PA, USA) and maintained in the Department of Psychology at Rutgers University. Rats were housed individually, had unlimited access to laboratory chow and water, and maintained on a 12: 12 light: dark cycle. Experimental protocols were approved by the Animal Care and Facilities Committee Review at Rutgers University, which maintains assurance with the Office of Laboratory Animal Welfare.
Estrous cycle
Vaginal cytology was obtained through daily vaginal smears (10.00–11.00 h). Sterile cotton-tipped applicators were immersed in physiological saline and gently inserted into the vaginal tract to remove loose cells and rolled onto a slide (Everett, 1989). Cells were dried and fixed in 95% ethanol, rinsed in buffered distilled water, stained in slightly alkaline 1% aqueous filtered Toluidine blue, and rinsed in 70% and then 95% ethanol. Based on their vaginal cytology, rats were classified into four stages of estrus: proestrus was associated with light purple staining epithelial cells with dark nuclei; estrus with masses of dark blue staining cornified cells; diestrus 1 with darkly stained leucocytes and numerous epithelial cells; and diestrus 2 with similar morphology but reduced numbers of cells. Vaginal smears were sampled through at least two consecutive cycles and only animals with normal 4–5-day cycles were used in the study.
Stressor exposure
Vaginal smears were obtained on the day of injection and stressor exposure. Immediately thereafter, cells were stained and the stage of estrus was determined. Groups of males and females in diestrus 2 received an intraperitoneal injection of the competitive NMDA receptor antagonist (+)-3-(2-carboxypiperazin-4-yl) propyl-1-phosphoric acid (CPP; 10 mg/kg; Sigma) or saline vehicle. Females in diestrus 2 were chosen because we have previously shown that the decrease in spine density following stressor exposure occurs when females are stressed during this stage (Shors et al., 2001). One hour later, half of the rats in each group were restrained and exposed to 30, 1-s, 1-mA, 60-Hz shocks to the tail at a rate of 1/min. The other groups remained in their home cages as unstressed controls. The eight groups consisted of: unstressed males injected with saline (n = 7); stressed males injected with saline (n = 7); unstressed males injected with CPP (n = 7); stressed males injected with CPP (n = 8); unstressed females injected with saline during diestrus 2 and killed in proestrus (n = 5); stressed females injected with saline, stressed during diestrus 2 and killed in proestrus (n = 5); unstressed females injected with CPP during diestrus 2 and killed in proestrus (n = 5); and stressed females injected with CPP, stressed during diestrus 2 and killed in proestrus (n = 5).
Hormonal evaluations and radioimmunoasssay
Twenty-four hours later, animals were deeply anaesthetized with 75 mg/kg sodium pentobarbital. Cardiac blood was collected, mixed with 0.1 mL heparin and centrifuged at 3000 r.p.m. for 20 min. Plasma was extracted and frozen for later radioimmunoassay of corticosterone, estradiol and testosterone. To determine whether treatment with the NMDA receptor antagonist altered hormone levels, separate groups of rats were tested. The groups included: males injected with CPP and exposed to the stressor (n = 10) or injected with CPP and left in their home cage (n = 9); males injected with saline and exposed to the stressor (n = 5) or injected with saline and left in their home cage (n = 5); females injected with CPP during diestrus 2 and exposed to the stressor (n = 5) or injected with CPP and left unstressed (n = 5) and females injected with saline during diestrus 2 and exposed to the stressor (n = 5) or injected with saline and left in their home cage (n = 5). Within minutes of stressor cessation, stressed animals were deeply anaesthetized with sodium pentobarbital. In this experiment, trunk blood was collected from stressed and unstressed controls and treated as described above. Plasma concentrations were determined using solid-phase radioimmunoassay (Coat-A-Count, Diagnostic Products, Los Angeles, CA, USA).
Golgi impregnation
Rats were perfused transcardially with 120 mL of 4.0% paraformaldehyde in 0.1 m phosphate buffer and 1.5% picric acid (v/v). Brains were post-fixed and stored for 24 h in the same solution. Following post-fixation, a modified version of the single-section Golgi impregnation procedure was used to process brains (Gabbott & Somogyi, 1984; Woolley & Gould, 1994; Shors et al., 2001). Serial coronal sections (150 µm) were cut on an oscillating tissue sheer (Electron Microscopy Sciences, Fort Washington, PA, USA) in a bath of 3.0% potassium dichromate in distilled water. Sections were incubated at room temperature overnight in individual Petri dishes containing 3.0% potassium dichromate. The next day, sections were rinsed and mounted onto ungelatinized slides, a coverslip glued over the sections at the four corners and the slide assembly placed in a Coplin jar containing 1.5% silver nitrate in distilled water. After 72 h, slide assemblies were dismantled and sections removed from the slides. Sections were rinsed in distilled water, dehydrated with 95% and 100% ethanol, cleared in xylene and mounted onto ungelatinized glass slides. Slides were coverslipped with Permount and dried before quantitative analysis.
Analysis of spine density
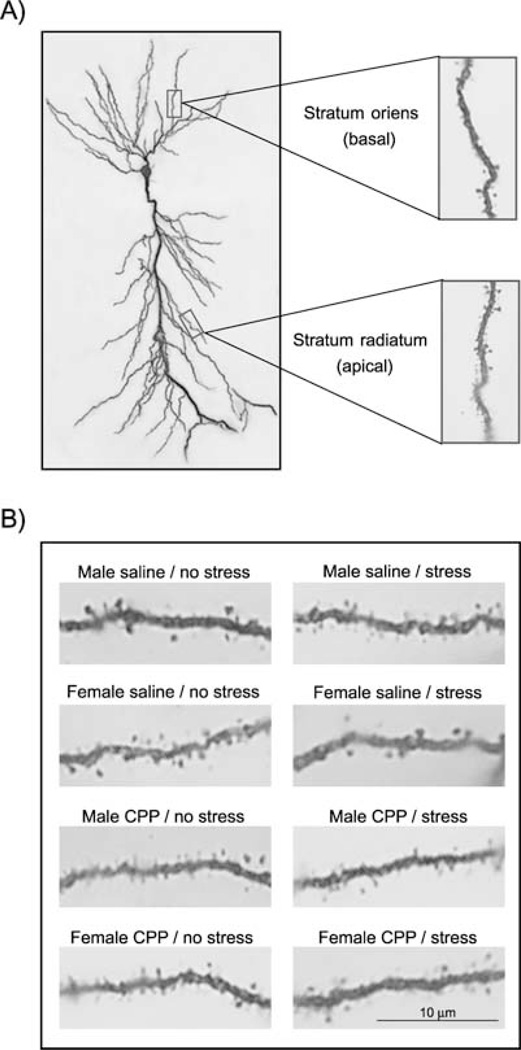
Counting of dendritic spines was conducted blind to experimental condition. All measurements were taken from pyramidal neurons in area CA1 of the dorsal hippocampal formation. Density was measured on both apical dendrites in the stratum radiatum and basal dendrites of the stratum oriens (Fig. 1A). Quantitative analysis was conducted on tissue stained dark with Golgi impregnation that was uniform throughout the section. To be included in the study, an animal had to possess six Golgi-impregnated pyramidal neurons discernible from nearby impregnated cells without breaks in staining along the dendrites. Measures were taken on secondary and tertiary branches and began at least 50 µm away from the soma for apical dendrites and 30 µm for basal dendrites. Five segments between 10 and 20 µm in length and in the same plane of focus were selected. In some cases, segments were from the same branch. Counting required focusing with the fine adjustment of the microscope (Nikon Eclipse E400, Nikon, Tokyo, Japan) using 1000 × and oil immersion. Only those spines that were distinct from the dendritic branch, but obviously connected to it, were counted. Using light microscopy, we were unable to count specific types of heads or exact length. However, we were conservative in our assessment and only counted those that were clearly visible along their entire length (approximately 0.5–2.0 µm).
Fig. 1.
Pyramidal neuron, and dendritic segments in area CA1 of the hippocampus. (A) Photomicrograph of a Golgi-impregnated cell illustrating basal spines in the stratum oriens and apical dendrites in the stratum radiatum. (B) Representative segment from an apical dendrite in area CA1 from each group is shown. Segments were stained with Golgi and magnified 1000 ×. Fewer spines are shown than measured because only one plane of focus is shown.
Spine density was calculated by dividing the number of spines on a segment by the length of the segment and was expressed as the number of spines per 10 µm of dendrite. Densities of spines on five segments of a cell were averaged for a cell mean, and the six cells from each animal were averaged for an animal mean. Spine density values using this method are underestimates because spines protruding either above or beneath the dendritic shaft are not accounted for (Woolley & Gould, 1994). To determine whether group differences in spine density were meaningful, analysis of variance (anova) was used with post hoc Neuman-Keuls for group comparisons.
Results
Sex differences in spine density
anova indicated an interaction between spine density on apical dendrites in area CA1 in groups injected with the NMDA receptor antagonist CPP vs. saline and in males vs. females (Fl,20 = 5.76; P (0.05; Figs 1B and 2). Post hoc analysis further indicated that spine density in the group of unstressed females that were injected with saline and killed during proestrus was greater than that obtained from unstressed males that were also injected with saline (P < 0.005). Group differences did not exist for those that were injected with the NMDA receptor antagonist (P > 0.05). Spine density in females that were injected with saline during diestrus 2 and killed in proestrus was elevated and different from all other groups (P-values < 0.05).
Fig. 2.
Sex differences and effects of stress on spine density in the hippocampus are NMDA receptor-dependent. Graph illustrates the mean (±SEM) density of apical dendritic spines on pyramidal cells in area CA1 of the hippocampus 24 h after exposure to an acute stressor of brief inescapable tailshock stimulation. Significant differences are noted with asterisks. Under unstressed conditions, proestrus females had a greater density of spines than males. Exposure to the stressor was associated with an increase in spine density in males and a decrease density in females. Treatment with the competitive NMDA receptor antagonist, CPP, prevented both the expression of sex differences and the opposite effects of stress on spine density.
A similar analysis on density along basal dendrites in area CA1 indicated that the effects were similar to those on apical dendrites but less pronounced. There was an interaction between spine density in males vs. females and in those treated with CPP vs. saline (F1,19 = 6.10; P < 0.05). Spine density in females that were injected with saline during diestrus 2 and killed during proestrus was greater than males that were injected with saline (P = 0.05). Spine density in females that were injected with CPP during diestrus 2 and killed during proestrus was not different from that in males injected with CPP (P > 0.05).
Stress effects on spine density
There was a three-way interaction between the density of spines on apical dendrites in area CA1 of males vs. females, those that were exposed to the stressor or left in their home cage and those that were injected with CPP or with saline [F1,41 = 18.72; P < 0.0001] (Figs 1B and 2). The density of spines in the hippocampus of males that were injected with saline and exposed to the stressor was greater than in the hippocampus of males that were also injected with saline but not exposed to the stressor (P < 0.05). The density of spines in the hippocampus of females that were injected with saline during diestrus 2, exposed to the stressor and killed in proestrus was less than that in the hippocampus of females that were also injected with saline but not exposed to the stressor (P < 0.05). In contrast to these effects of stressor exposure on spine density, there were no group differences in the hippocampus of groups that were injected with the NMDA receptor antagonist CPP and exposed to the stressor or not (females, P > 0.05; males, P > 0.05). Spine density was not different in the hippocampus of unstressed groups injected with CPP vs. those injected with saline (unstressed females injected with CPP vs. saline, P > 0.05; unstressed males injected with CPP vs. saline, P > 0.05).
As reported previously (Shors et al., 2001), the effects of stressor exposure on spine density in the stratum oriens (basal dendrites) were less evident than the effects on spine density in the stratum radiatum (the apical dendrites). Although there was a three-way interaction between stress and sex and treatment on these spines (F1,37 = 6.42; P < 0.05), post hoc analysis indicated that densities between groups were not different (P > 0.05). Exposure to CPP was not associated with changes in spine density on the basal dendrites (P > 0.05).
Hormonal responses to stress in males vs. females
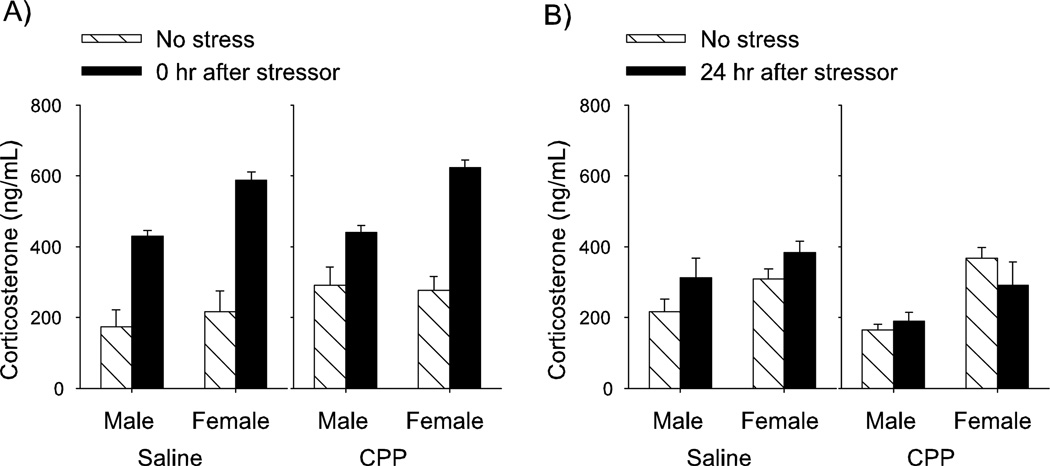
Concentrations of corticosterone, estradiol and testosterone from plasma collected at the time of perfusion (24 h after stressor cessation) were compared with those collected within minutes of stressor cessation. With sex (male vs. female), stress (yes or no), time (minutes or 24 h) and treatment (CPP or saline) as independent variables and corticosterone as the dependent variable, there was a three-way interaction between sex, stress and time after stressor cessation (F1,81 = 7.76; P < 0.01; Fig. 3). As expected, the concentration of corticosterone obtained from males and females that were stressed and killed immediately after stressor cessation was greater than that obtained from the unstressed controls (P-values < 0.001). Concentrations were not elevated in those that were exposed to the stressor and killed 24 h later (P-values > 0.05). Importantly, there was no main effect of treatment with the NMDA receptor antagonist on corticosterone (P > 0.05), nor was it involved in an interaction with the other independent variables. The analyses indicate that treatment with the NMDA receptor antagonist CPP during the stressful event did not prevent an hypofhalamic-pituitary-adrenal (HPA) stress response from occurring, as measured by a release of corticosterone into the blood during exposure to the stressful event of intermittent tailshocks. As reported previously (Viau & Meaney, 1991; Shors et al., 2001), the concentration of corticosterone was elevated in females compared with the concentration in males (P < 0.00001).
Fig. 3.
Effects of sex and stressor exposure on corticosterone. Blood concentrations of corticosterone in males and females under stressed and unstressed conditions. Samples were collected either immediately after the stressor (0 h) or 24 h later. Exposure to the stressful event increased endogenous levels of corticosterone immediately after stressor exposure, even in groups treated with the NMDA receptor antagonist (CPP). Twenty-four hours later, group levels had returned to baseline.
The concentration of estradiol in plasma obtained from males vs. females was different and interacted with concentrations obtained from groups killed either within minutes or 24 h after stressor exposure (F1,73 = 17.56; P < 0.0001). This was expected because two groups of females were in diestrus 2 at the time of blood collection and the other two were in proestrus, when estrogen concentrations are elevated. Estradiol was elevated in the plasma obtained from females in proestrus (with a mean of 38.26 pg/mL) and higher than any other group (P-values < 0.001) including females in diestrus 2 (13.65 pg/mL). Overall, the concentration of estradiol was greater in females than males (F1,73 = 43.84; P < 0.000001). Treatment with the NMDA receptor antagonist CPP did not alter the concentration of estradiol in males or females (F1,73 = 2.72; P > 0.05).
The concentration of testosterone was greater in males than females (F1,73 = 147.66; P < 0.000001) and negligible in females irrespective of stressor exposure or treatment with the antagonist (<5.00 ng/dL). Treatment with the NMDA receptor antagonist did not alter the concentration of testosterone in males (Fl,38 = 1.14; P = 0.29).
Discussion
The primary aim of the present experiments was to determine whether NMDA receptor activation was necessary for increasing spine density in the male hippocampus and decreasing spine density in the female hippocampus after exposure to an acute stressful event. To test this, groups of male and female rats were injected with a competitive NMDA receptor antagonist or a saline vehicle before exposure to the stressful event of intermittent tailshocks and restraint (30 shocks; 1 mA, 1 s, 1/min). Twenty-four hours later, their brains were prepared for Golgi impregnation and assessment of spine density. The effects of the stressor on spine density on apical dendrites in area CA1 in both males and females were prevented by administration of the antagonist before stressor exposure (Figs 1 and 2). These data indicate that the presence of the antagonist during the stressful event prevented the increase in spine density in males as well as the decrease in females, implicating NMDA receptor activation in these stress-induced phenomena. As noted in the Introduction, there are sex differences in spine density and thus the effects of stress must be interpreted in their context. Under unstressed conditions, females in proestrus possess more spines on dendrites of CA1 than do males (Shors et al., 2001). This effect was replicated in the present experiment and can be observed by comparing the density of spines in unstressed females injected with saline to that of unstressed males injected with saline (Fig. 2). In the presence of the NMDA receptor antagonist, however, spine density was not increased in females that were killed during proestrus. Therefore, the absence of a stress effect on spine density in females treated with the antagonist could reflect the absence of a proestrus-induced increase under unstressed conditions. With respect to the males, there was no effect of the antagonist on spine density in the unstressed groups. In males, then, it appears that NMDA receptor activation during exposure to the stressful event is involved in the subsequent increase in spine density.
Sex hormones can have remarkable effects on the presence and density of dendritic spines, especially those measured in area CA1 of the hippocampal. Gould et al. (1990) found that treatment with estradiol increased spine density on apical and basal dendrites of pyramidal cells in area CA1 in ovariectomized female rats. Woolley, Gould and McEwen went on to report that females possessed more spines during proestrus than other stages of estrus (Woolley et al., 1990; Woolley & McEwen, 1992), a result that we have also observed (Shors et al., 2001). The fluctuation across the estrous cycle included an impressive 30% change between diestrus and proestrus, and coincided with a significant increase in estradiol levels in the blood. They hypothesized that the fluctuation in spine density in response to changing levels of estrogen across the estrous cycle was mediated, or at least modulated, by NMDA receptor activation. In a series of experiments, they found that NMDA receptor antagonism prevented the increase in spine density in response to estrogen replacement in ovariectomized females (Woolley & McEwen, 1994). They also found that treatment with estradiol increased the number of NMDA binding sites and NR1 subunits in area CA1 (Gazzaley et al., 1996; Woolley et al., 1997) as well as synaptic input mediated via NMDA receptors (Woolley et al., 1997). In culture, estradiol increases the presence of spines and this effect is preventable by treatment with NMDA receptor antagonists (Murphy & Segal, 1996). Here we have extended these findings by showing that the increased observation of spines in females during proestrus relative to males is similarly prevented by NMDA receptor antagonism. Importantly, treatment with the antagonist did not prevent the increase in estradiol levels as the females transitioned from diestrus into proestrus. Also, females did transition from diestrus into proestrus as determined by the types of cells extracted from the vaginal tract. Overall then, treatment with the antagonist prevents estrogen from increasing the density of spines but does not prevent or alter the onset of proestrus itself.
For the most part, hormone levels at the time of perfusion were as expected and were not particularly informative about mechanisms that underlie these effects of stress on spine density and their prevention by NMDA receptor antagonism. There was an increase in corticosterone within minutes of stressor cessation in both males and females treated with either saline or the antagonist that returned to baseline within 24 h (Fig. 3). Treatment with the NMDA antagonist did not reduce corticosterone levels in response to the stressful event in either males or females. These data indicate that prevention of the stress effect by NMDA receptor antagonism is not because of a reduction in the stress response itself, as measured by changes in the HPA response to the stressor. In terms of other hormones, treatment with the NMDA receptor antagonist did not alter testosterone levels in the blood of males or females. As testosterone was present in all groups, these data do not allow us to eliminate the presence of testosterone as important to these effects of stress on spine density. In fact, others have recently found that testosterone might be important to the maintenance of synaptic structure. Using electron microscopy, Leranth et al. (2003) reported that castration in adulthood reduced the density of spine synapses on apical dendrites in area CA1 by nearly 50%. Interestingly, density was restored to normal levels with testosterone but not estrogen replacement.
It has long been proposed that dendritic spines are involved in processes of learning and memory (Moser, 1999; Leuner & Shors, 2003). Minimally, they represent anatomical substrates for enhancing synaptic connections between neurons. It is perhaps not coincidental that exposure to the acute stress used in the present studies has similarly opposite effects on associative memory formation in males vs. females (Wood & Shors, 1998; Wood et al., 2001). Twenty-four hours after exposure to the stressor used here, males emit many more learned responses during classical eyeblink conditioning whereas females emit fewer. Also, females in proestrus acquire the learned response faster when trained in proestrus than in other stages of estrus, and faster than males (Shors et al., 1998). Thus, sex differences in spine density and the effects of stress in males vs. females correlate with the ability to acquire this associative response. Based on these correlations, we have proposed that sex differences and stress-induced changes in spine density provide anatomical structures for learning (Shors, 2002; Shors & Miesegeas, 2002; Leuner et al, 2003). More specifically, we have proposed that an increased presence of spines during proestrus or after stress in males enhances their ability to form associations when opportunities for new learning arise (Leuner & Shors, 2003). In contrast, a decrease in their presence during estrus, diestrus and after stress in females reduces the ability to acquire associations under similar learning situations.
If the modulation of spine density by experience is related to subsequent learning ability, one would assume that learning itself would affect spine density. We recently tested this possibility using Golgi impregnation of dendritic spines in animals that had been trained on the hippocampal-dependent task of trace eyeblink conditioning and the hippocampal-independent task of delay conditioning (Leuner et al., 2003). Exposure to both training paradigms increased the observation of dendritic spines 24 h after they learned the response. Interestingly, the effects of learning itself on spine density were localized to the basal, and not apical, dendrites on the pyramidal neurons in area CA1. Moser et al. (1994) also reported learning-induced effects on the basal but not apical dendrites of CA1; the effects of stress were primarily localized to the apical dendrites. These studies highlight what could be an anatomical difference between the modulation of learning by stress and sex differences vs. that of learning itself. To further complicate the issue, sex differences and the effects of estradiol are evident on both apical and basal dendrites and preventable at both locations by antagonism of NMDA receptors (Woolley & McEwen, 1994). Together these data suggest that exposure to stressful experiences, hormones and learning situations influence the density of dendritic spines on pyramidal neurons within a cell region of the hippocampal formation, i.e. area CA1. These effects are localized to different regions within the dendritic tree and are modulated in different directions depending on the stimulus. As a group, however, they are all sensitive to NMDA receptor antagonism.
Acknowledgements
This work was supported by the National Institutes of Mental Health (MH59970), National Science Foundation (IBN0217403), National Alliance for Research on Schizophrenia and Depression (NARSAD) to T.J.S. and a NIMH (MH3568) predoctoral fellowship to B.L.
Abbreviations
- anova
analysis of variance
- CPP
(+)-3-(2-carboxypiperazin-4-yl)propyl-1-phosphoric acid
- HPA
hypothalamic–pituitary–adrenal
- NMDA
N-methyl-d-aspartate
References
- Becker JB, Breedlove SM, Crews D. Behavioral Endocrinology. Cambridge, MA: MIT Press; 1992. [Google Scholar]
- Everett BJ. Neurobiology of Reproduction in the Female Rat. Monographs on Endocrinology. Berlin: Springer-Verlag; 1989. [PubMed] [Google Scholar]
- Gabbott PL, Somogyi J. The ‘single’ section Golgi-impregnation procedure: methodological description. J. Neurosci. Meth. 1984;11:221–230. doi: 10.1016/0165-0270(84)90084-0. [DOI] [PubMed] [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Gazzaley AJ, Kay S, Benson DL. Dendritic spine plasticity in hippocampus. Neuroscience. 2002;111:853–862. doi: 10.1016/s0306-4522(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Gazzaley AJ, Weiland NG, McEwen BS, Morrison JH. Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J. Neurosci. 1996;16:6830–6838. doi: 10.1523/JNEUROSCI.16-21-06830.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Foy MR, Thompson RF. Behavioral stress modifies hippocampal plasticity through N-methyl-D-aspartate receptor activation. Proc. Natl. Acad. Sci. USA. 1996;93:4750–4753. doi: 10.1073/pnas.93.10.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov SA, Harris KM. Dendrites are more spiny on mature hippocampal neurons when synapses are inactivated. Nat. Neurosci. 1999;2:878–883. doi: 10.1038/13178. [DOI] [PubMed] [Google Scholar]
- Korkotian ESM. Bidirectional regulation of dendritic spine dimensions by glutamate receptors. Neuroreport. 1999;10:2875–2877. doi: 10.1097/00001756-199909090-00032. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gondal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J. Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J. Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Shors TJ. New spines, new memories. Mol. Neurobiol. 2003 doi: 10.1385/MN:29:2:117. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat. Neurosci. 1999;2:44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- Miranda P, Williams CL, Einstein G. Granule cells in aging rats are sexually dimorphic in their response to estradiol. J. Neurosci. 1999;19:3316–3325. doi: 10.1523/JNEUROSCI.19-09-03316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan DT, Cotman CW. Distribution of N-methyl-d-aspartate-sensitive L-[3H]glutamate-binding in rat brain. J. Neurosci. 1985;5:2909–2919. doi: 10.1523/JNEUROSCI.05-11-02909.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RGM, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-d-aspartate receptor antagonist, AP5. Nature. 1986;19:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Moser E. Making more synapses? A way to store information? Cell. Mol. Life Sci. 1999;55:593–600. doi: 10.1007/s000180050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests formation of new synapses. Proc. Natl. Acad. Sci. USA. 1994;91:12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Segal M. Regulation of dendritic spine density in cultured rat hippocampal neurons by steroid hormones. J. Neurosci. 1996;16:4059–4068. doi: 10.1523/JNEUROSCI.16-13-04059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Konnerth A. NMDA receptor-mediated Na+ signals in spines and dendrites. J. Neurosci. 2001;21:4207–4214. doi: 10.1523/JNEUROSCI.21-12-04207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ. Opposite effects of stressful experience on memory formation in males versus females. Dialogues Clin. Neurosci. 2002;4:39–47. doi: 10.31887/DCNS.2002.4.2/tshors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Chua C, Falduto J. Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J. Neurosci. 2001;21:6292–6297. doi: 10.1523/JNEUROSCI.21-16-06292.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Elkabes S, Selcher JC, Black IB. Stress persistently increases NMDA receptor-mediated binding of [3H]PDBu (a marker for protein kinase C) in the amygdala, and re-exposure to the stressful context reactivates the increase. Brain Res. 1997;750:293–300. doi: 10.1016/s0006-8993(96)01369-8. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Paczynski M, Mathew PR, Pickett J. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9:419–423. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegeas G. Testosterone in utero and at birth determine how a stressful experience will affect memory formation in adulthood. Proc. Natl Acad. Sci. USA. 2002;99:13955–13960. doi: 10.1073/pnas.202199999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Servatius RJ. Stress-induced sensitization and facilitated learning require NMDA receptor activation. Neuroreport. 1995;6:677–680. doi: 10.1097/00001756-199503000-00023. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav. Neurosci. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males but impairs conditioning in females through activational influences of ovarian hormones. Proc. Natl. Acad. Sci. USA. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm. Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- Woolley C, Gould E. Steroid action on neural structure. Meth. Neurosci. 1994;22:383–301. [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuations in dendritic spine density on adult hippocampal pyramidal neurons. J. Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J. Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-d-aspartate receptor-dependent mechanism. J. Neurosci. 1994;14:7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley C, Weiland NG, McEwen BS. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor mediated synaptic input: correlation with dendritic spine density. J. Neurosci. 1997;17:1848–1859. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]