Abstract
As the nitrogenous precursor of nitric oxide, L-arginine regulates multiple metabolic pathways involved in the metabolism of fatty acids, glucose, amino acids, and proteins through cell signaling and gene expression. Specifically, arginine stimulates lipolysis and the expression of key genes responsible for activation of fatty acid oxidation to CO2 and water. The underlying mechanisms involve increases in the expression of peroxisome proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1 alpha), mitochondrial biogenesis, and the growth of brown adipose tissue growth. Furthermore, arginine regulates adipocyte-muscle crosstalk and energy partitioning via the secretion of cytokines and hormones. In addition, arginine enhances AMP-activated protein kinase (AMPK) expression and activity, thereby modulating lipid metabolism and energy balance toward the loss of triacylglycerols. Growing evidence shows that dietary supplementation with arginine effectively reduces white adipose tissue in Zucker diabetic fatty rats, diet-induced obese rats, growing-finishing pigs, and obese patients with type II diabetes. Thus, arginine can be used to prevent and treat adiposity and the associated metabolic syndrome.
Keywords: Arginine, Lipid metabolism, Energy partitioning, Pigs, Nutrition, Amino acids, Review
2. INTRODUCTION
Obesity or overweight in humans is a growing epidemic worldwide and excessive accumulation of white adipose tissue also occurs in livestock species at market weight (1–3). White adipocytes are the major site for the storage of excess energy in the form of triacylglycerols. When dietary intake of energy is insufficient relative to its needs by the body, white adipose tissue undergoes lipolysis to provide skeletal muscle and other organs with non-esterified fatty acids. However, when dietary intake of energy exceeds whole-body energy expenditure, fat is accumulated in white adipose tissue, leading to obesity and other health problems (4–5). Identifying new means to reduce excess fat accretion and maintain lean body mass is important for both human health and animal production (6–8). Accordingly, many researchers have focused on the regulation of lipid metabolism and energy partitioning to prevent and treat obesity and its associated disorders.
Emerging evidence from animal studies indicates an important role of arginine in regulating the metabolism of energy substrates (9). Arginine is synthesized from citrulline in virtually all cell types (3). In most mammals, including humans, pigs, and rats, citrulline is derived from glutamine, glutamate, and proline via pyrroline-5-carboxylate as the common intermediate (7, 10–11). Although it was traditionally considered that arginine was not required to maintain a positive nitrogen balance in adult humans (12), this nutrient has now been recognized to be nutritionally and physiologically essential for the maintenance of cardiovascular health and reproductive functions in both males and females (13–14). Notably, dietary supplementation with arginine enhances protein deposition in skeletal muscle and intramuscular lipid concentrations, while reducing body fat mass in growing-finishing pigs (15). These studies suggest that arginine regulates lipid and protein metabolism in a tissue-specific manner. The major objectives of the article are: 1) To highlight recent advances in arginine metabolism as related to energy homeostasis; and 2) To provide insights into novel strategies of treating and preventing obesity as well as the associated metabolic syndrome.
3. EFFECTS OF ARGININE ON LIPID METABOLISM AND ENERGY PARTITIONING
3.1. Regulatory role of arginine in fat deposition
Accumulation of white adipose tissue in the body is determined by the balance between lipogenesis and lipolysis. Reduced accretion of the body fat may result from increased lipolysis, diminished synthesis of fatty acids, or both processes. Extensive studies in vitro have revealed that arginine stimulates lipolysis in adipocytes and promotes oxidation of long-chain fatty acids in insulin-sensitive tissues (1, 8, 16–17). For example, Jobgen (18) treated 3T3-L1 preadipocytes with different extracellular concentrations of L-arginine (50 – 400 μM) and found that the rates of glucose and oleic acid oxidation were 45% and 40% greater, respectively, in the presence of arginine than in its absence. Similarly, in cultured human adipocytes, increasing extracellular concentrations of arginine from 0.4 to 2 mM increased the oxidation of 1 mM palmitate and 5 mM glucose by 32% and 51%, respectively (9). Interestingly, the reported effects of arginine on lipogenesis in white adipocytes have not been consistent. For example, in differentiated 3T3-L1 preadipocytes, the incorporation of oleic acid into lipids was increased by 190% in the presence of 400 μM arginine than in its absence (18), and high levels of arginine enhanced the expression of peroxisome proliferator-activated receptor (PPAR) gamma (a key regulator of adipogenesis) in preadipocytes and their differentiation (19). In contrast, arginine decreased the incorporation of palmitate and glucose into triglycerides in human adipocytes by 35% and 39%, respectively (9).
Thus, the lipogenic responses of adipocytes to arginine may critically depend on cell type and the stage of cell differentiation.
Studies with obese rats have demonstrated that oral administration of arginine effectively reduces carcass white fat and enhances whole-body insulin sensitivity (3, 20). After 10 week of treatment, Zucker diabetic fatty rats receiving arginine supplementation (1.25% in drinking water) had 25% less epididymal fat and 45% less abdominal fat, compared with the control group (Table 1). Similar results have been reported for diet-induced obese rats (Table 2). Excitingly, long-term oral administration of arginine can decrease fat mass in adult obese humans with type II diabetes (21). Consistent with the reduction of white-fat mass, arginine treatment decreased circulating levels of glucose and nonesterified fatty acids and increased the oxidation of glucose and octanoate in abdominal and epididymal adipose tissues of obese rats (20). Moreover, arginine increased the expression of carnitine palmitoyltransferase 1 (CPT1), PCG-1alpha, and malonyl-CoA decarboxylase (MCD) in liver and depressed the expression of fatty acid synthase (FAS) and stearoyl coenzyme-A desaturase-1 (SCD1) (18). These results indicate a potent effect of arginine on inhibiting hepatic fatty acid synthesis from glucose.
Table 1.
Dietary supplementation with arginine reduces white fat mass in Zucker diabetic fatty rats
| Treatment | Alanine | Arginine | Alanine | Arginine |
|---|---|---|---|---|
| Tissue | Absolute weight (g) | Absolute weight (g) | Proportional weight (g tissue/kg body wt) | Proportional weight (g tissue/kg body wt) |
| Epididymal fat | 9.06 ± 0.66 | 6.82 ± 0.53* | 22.0 ± 1.40 | 20.1 ± 1.10 |
| Abdominal fat | 15.9 ± 0.96 | 8.83 ± 0.55* | 39.0 ± 1.45 | 25.7 ± 1.05* |
Values are means ± SEM, n = 6.
Different from alanine-treated rats, P < 0.01. At 9 weeks of age, male Zucker diabetic fatty rats were assigned randomly to receive drinking water containing either 1.51% L-arginine-HCl or 2.55% L-alanine (isonitrogenous control) (n = 6 per treatment). Epididymal fat and abdominal fat were collected and weighed at the end of a 10-week period of arginine supplementation. Adapted from Fu et al. (20).
Table 2.
Dietary supplementation with L-arginine reduces white adipose tissues and increases brown adipose tissue in diet-induced obese male Sprague-Dawley rats
| Retroperitoneal fat depots (g) | Epididymal fat depots (g) | Mesenteric fat depots (g) | Inguinal fat depots (g) | Brown adipose tissue (g) | ||
|---|---|---|---|---|---|---|
| Low-fat | Control | 7.41 ± 0.69 b | 7.45 ± 0.63 c | 3.16 ± 0.35 b | 6.78 ± 0.46 c | 0.57 ± 0.02 b |
| Low-fat | Arginine | 4.51 ± 0.33 c | 5.89 ± 0.33 d | 1.75 ± 0.16 c | 4.54 ± 0.27 d | 0.73 ± 0.03 a |
| High-fat | Control | 11.2 ± 1.34 a | 11.0 ± 0.86 a | 4.57 ± 0.63 a | 13.3 ± 1.18 a | 0.62 ± 0.04 b |
| High-fat | Arginine | 7.44 ± 0.46 b | 8.79 ± 0.60 b | 2.84 ± 0.18 b | 8.83 ± 0.76 b | 0.81 ± 0.04 a |
Data are mean ± SEM, n = 8. Means with different letters (a–d) within a column differ (P < 0.01). At 4 weeks of age, male Sprague-Dawley rats were fed a low-fat or high-fat diet for 15 weeks. Thereafter, rats in the low-fat and the high-fat group received drinking water either 1.51% L-arginine-HCl or 2.55% L-alanine (isonitrogenous control) for 12 weeks. Energy intake did not differ among the four groups of rats during the entire experimental period. Brown adipose tissue was obtained from the interscapular region. Data are adapted from Jobgen et al. (40).
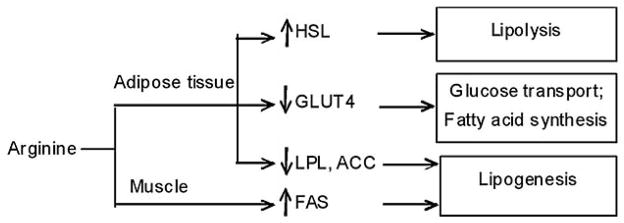
In growing-finishing pigs, we found that dietary supplementation with 1% arginine reduced fat accretion and promoted protein deposition in the whole body (15). Furthermore, the arginine treatment beneficially increased intramuscular fat content and the percentage of skeletal muscle in the carcass, while decreasing carcass fat content, compared with the control group (Table 3). Results of nuclear magnetic resonance (NMR)-based metabolomic analysis showed that concentrations of nitrogenous and lipid signaling molecules in serum were altered in response to the arginine treatment, in keeping with enhanced protein accretion and reduced fat deposition in the body (22). We also discovered that arginine differentially regulates expression of fat-metabolic genes in skeletal muscle and adipose tissue (Figure 1), favoring lipogenesis in skeletal muscle but lipolysis in white adipose tissue. Specifically, arginine down-regulates expression of lipogenic genes, such as lipoprotein lipase (LPL), acetyl-CoA carboxylas (ACC) alpha but up-regulates expression of genes for lipolysis in white adipose tissue, including hormone-sensitive lipase (HSL). In skeletal muscle of arginine-treated pigs, LPL activity and the ratio of FAS mRNA to HSL mRNA were enhanced, leading to an increase in intramuscular fat content (23). In addition, decreased expression of GLUT4 in adipose tissue of arginine-treated pigs is sufficient to impair glucose transport and fatty acid synthesis, suggesting that energy substrates are preferentially partitioned to skeletal muscle rather than white adipose tissue (23).
Table 3.
Dietary arginine supplementation reduces fat accretion but increases intramuscular fat content in growing-finishing pigs
| Alanine | Arginine | |
|---|---|---|
| Percentage of total skeletal muscle in the carcass (%) | 57.9 ± 0.61 | 61.1 ± 0.93 * |
| Percentage of total fat in the carcass (%) | 23.1 ± 0.35 | 20.5 ± 0.60 ** |
| Content of protein in longissimus dorsi muscle (g/100 g) | 18.9 ± 0.18 | 19.8 ± 0.21 ** |
| Content of lipids in longissimus dorsi muscle (g/100 g) | 1.81 ± 0.17 | 3.08 ± 0.48 * |
Values are means ± SEM, n = 8. Different from alanine-treated growing-finishing pigs,
P < 0.05 and
P < 0.01. Twenty-four 110-day-old barrows were assigned randomly to receive corn- and soybean meal-based diet supplementing with 1.0% L-arginine or 2.05% L-alanine (isonitrogenous control). After a 60-day period of arginine supplementation, total skeletal muscle and fat depots in the carcass were weighed. Longissimus dorsi muscle was analyzed for protein and lipids. Adapted from Tan et al. (15).
Figure 1.
Dietary supplementation with 1% arginine differentially regulates expression of lipid-metabolic genes in porcine adipose tissue and skeletal muscle. HSL, hormone sensitive lipase; GLUT4, glucose transporter 4; LPL, lipoprotein lipase; ACC, acetyl CoA carboxylas; FAS, fatty acid synthase. The symbol “↑” denotes an up-regulation of arginine on gene expression. The symbol “↓” denotes a down-regulation of arginine on gene expression.
3.2. Role of the arginine-NO pathway in lipid metabolism
Arginine is converted to nitric oxide (NO) by NO synthase (NOS) in almost all mammalian cells (27, 28). Increasing extracellular levels of arginine from 0.1 to 5 mM dose-dependently increases NO synthesis in diverse cell types, including skeletal muscle and adipose tissue (29–31). NO may function as an important autocrine regulator, thereby controlling both lipogenesis and lipolysis (24–26). Feeding an NOS inhibitor (L-Nω-nitroarginine) to rats increased total body fat, concomitant with a reduction in serum nitrate (27). Notably, this effect of the NOS inhibitor could be reversed by the addition of 4% arginine to the diet, providing the earliest evidence that NO may reduce adiposity in rats. Similarly, Nisoli et al. (28) found that mice with the knockout of endothelial NOS had higher body-fat weight than wild-type mice with normal expression of the NOS protein although food intake was similar between the two groups of animals. Results from our studies indicate that an increase in NO availability within physiological ranges through arginine supplementation markedly increased lipolysis as well as the oxidation of fatty acids and glucose in rat adipose tissue, contributing to a marked reduction in body fat mass (20). In addition to NO, arginine may regulate the mammalian target of rapamycin (mTOR) cell signaling (32), thereby increasing protein synthesis and inhibiting protein synthesis.
3.3. Arginine and mitochondrial biogenesis
Mitochondria play a key role in modulating adipocyte lipid metabolism and adipogenesis (33–34).
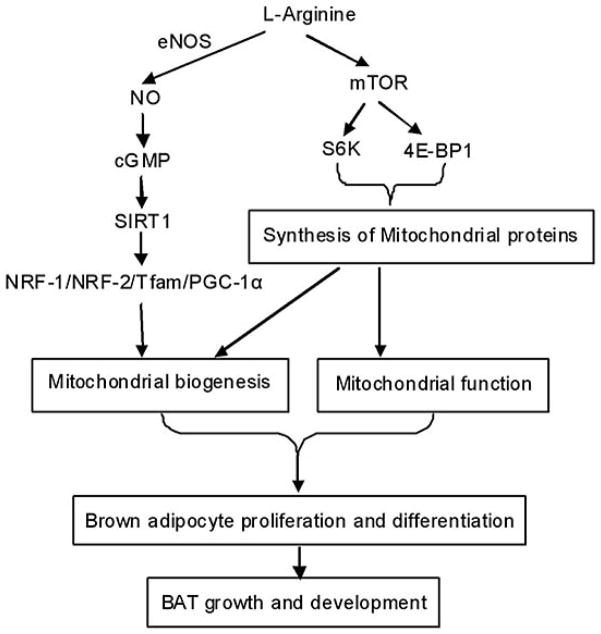
PGC-1alpha is a key nuclear receptor co-activator that can induce mitochondrial biogenesis (35). Thus, PGC-1alpha is crucial for the modulation of mitochondrial number and mass, fatty acid oxidation, and thermogenesis (35–36). An increase in PGC-1alpha expression can promote oxidation of long-chain fatty acids in adipocytes (37–38). Additionally, over-expression of this gene in mouse skeletal muscle increases mitochondrial abundance, resulting in increased energy expenditure and reduced body weight (38). Recent studies have provided convincing evidence that arginine can enhance mitochondrial biogenesis (39), brown adipose tissue development (40), and whole-body energy metabolism (9, 20) (Figure 2). These metabolic actions of arginine are associated with increased expression of PGC-1alpha (20). Consistent with the finding that NO is a modulator of mitochondrial biogenesis in a PGC-1alpha-dependent mechanism (41), dietary supplementation with arginine can increase the number and mass of mitochondria in adult rats (42).
Figure 2.
Possible mechanisms for arginine to enhance mitochondrial biogenesis and growth of brown adipose tissue in animals. NO, nitric oxide; eNOS, endothelial NO synthase; cGMP, cyclic guanosine monophosphate; SIRT1, Silent mating type information regulator 2 homolog 1; NRF-1/2, nuclear respiratory factor 1/2; Tfam, mitochondrial transcription factor A; PGC, peroxisome proliferator-activated receptor-gamma coactivator; mTOR, mammalian target of rapamycin; 4E-BP1, eukaryotic initiation factor 4E-binding protein-1; S6, ribosomal protein S6 kinase.
3.4. Endocrine effects of arginine
Elevated plasma levels of arginine have been found to correlate with alterations in the secretion of various cytokines (e.g., interleukin-6, interleukin-1beta, interferon-gamma, and tumor necrosis factor-alpha) and hormones (e.g., leptin, adiponectin, insulin, and growth hormone) (1). These cytokines and hormones, in turn, may influence insulin sensitivity, glucose homeostasis, and lipid metabolism. Interestingly, some mechanisms responsible for the effects of arginine include a direct cholinergic effect, membrane depolarization, calcium influx (43–44), and NO signaling (45–46).
It has been reported that pharmacological levels of arginine stimulate insulin and glucagon release in pigs (47–48) and healthy humans (49–51), as well as diabetic (52–53) and obese subjects (54). In our studies, we found that acute infusion of arginine increased plasma concentrations of insulin and glucagon at infusion time of 30, 60 and 90 min (unpublished data). In response to starvation signals, secretion of insulin by pancreatic beta-cells is reduced but secretion of glucagon by pancreatic alpha-cells is increased in an attempt to promote gluconeogenesis in the liver and kidneys. Interestingly, secretions of both insulin and glucagon are enhanced by arginine or a protein meal (55). These two hormones act on different tissues via cAMP-dependent signaling pathways, resulting in an overall decrease in circulating levels of glucose in diabetic animals (1).
Hormones derived from white adipose tissue may play a key role in the adipocyte-muscle cross-talk and, therefore, energy partitioning (56). For example, leptin and adiponectin may alter lipid partitioning in skeletal muscle by increasing fat oxidation and decreasing fatty acid incorporation into triacylglycerols (57–59). In growing-finishing pigs, a decrease in serum leptin concentration was associated with a reduction in body fat mass (15, 23). Similarly, Stingl et al. (53) reported that arginine infusion transiently decreased plasma concentrations of leptin both in insulin-deficient and hyperinsulinemic diabetic patients. Circulating levels of leptin are highly correlated with adipose tissue mass (60) and may not be altered after a meal (61), short-term exposure to insulin and glucagon (62–63), or acute administration of arginine (our unpublished data).
3.5. Role of the AMPK signaling pathway in lipid metabolism
AMPK is a key modulator of lipid metabolism and energy balance (64–65). AMPK is activated by a rise in the intracellular AMP/ATP ratio within the cell. AMPK activation stimulates ATP-generating metabolic pathways while inhibiting anabolic reactions (65). Thus, AMPK signaling is crucial for the regulation of fatty acid oxidation in liver and skeletal muscle, as well as mitochondrial biogenesis, glucose uptake and the control of insulin sensitivity in skeletal muscle (1). Exceedingly high levels of amino acids have been considered to inhibit AMPK activity in pancreatic beta-cells (66–67) and Caco 2 cells (68). In contrast, there are reports that physiological concentrations of arginine increased AMPK expression and activity in the rat liver at transcriptional, translational, and post-translational levels (18). These different results may be due to the tissue-specific regulation of AMPK activity (69) and energy metabolism by arginine in a concentration-dependent manner. For example, in mammals, arginine is mainly metabolized in the liver and dietary arginine supplementation enhanced hepatic expression of AMPK (indicated by increases in both mRNA and protein levels of AMPK) as well as hepatic AMPK phosphorylation in obese rats (18, 40). Multiple mechanisms may be responsible for the effect of arginine on activating AMPK and, therefore, regulating lipid metabolism and energy partitioning. AMPK phosphorylation inhibits ACC activity, thereby reducing the flux of energy substrates to anabolic pathways (64, 71). Specifically, activated AMPK not only inhibits fatty acid synthesis but also enhances fatty acid oxidation by reducing the levels of malonyl-CoA, the product of ACC, which is a potent allosteric inhibitor of CPT1, the enzyme that transport long-chain fatty acyl-CoA into mitochondria for β-oxidation (64, 71–72). AMPK phosphorylation also activates malonyl-CoA decarboxylase, the enzyme responsible for the degradation of malonyl-CoA (73). Furthermore, AMPK stimulates GLUT-4 translocation and glucose transport in skeletal muscle, thereby increasing whole-body utilization of glucose (74–75). In addition, AMPK inhibits lipolysis via promoting dephosphorylation of HSL and blocking protein kinase A (PKA)-induced HSL activation (76–77).
4. USE OF ARGININE IN TREATMENT OF OBESITY AND DIABETES
Obesity in humans is a major public health crisis worldwide (78) and probably the most important factor in the development of Type 2 diabetes (also known as an “insulin-resistant” disease). Additionally, the incidence of obesity in companion animals is increasing worldwide. Abdominal obesity is directly related to insulin resistance, leading to metabolic syndrome including hypertension as well as alterations in lipid profiles and blood glucose levels (79). Patients with the metabolic syndrome have a defect of NO bioavailability (80). Additionally, serum levels of NO metabolites were associated with other components or risk factors of the metabolic syndrome (81). Gruber et al. (82) suggested that reduced availability of NO, arginine and citrulline in juvenile obesity may contribute to the development of atherogenesis. As noted in the preceding sections, physiological levels of NO can ameliorate all of the adverse features of the metabolic syndrome in animal models of obesity (20). Therefore, arginine supplementation to enhance NO synthesis may provide an effective strategy to treat and prevent adiposity and associated metabolic syndrome.
As noted above, physiological levels of arginine modulate expression of key genes responsible for fatty acid and glucose oxidation, thereby decreasing white fat in the whole body (9, 83). These changes are associated with: a) reductions in circulating levels of glucose, homocysteine, and asymmetric dimethylarginine (risk factors for the metabolic syndrome); and b) improvement of endothelium-dependent relaxation (an indicator of cardiovascular function) in both type I and type II models of diabetes mellitus (20–21). These findings are timely and highly relevant to the development of sound therapeutic strategies to prevent and treat obesity in humans and companion animals.
Results from both animal and human studies indicate that short- and long-term oral administration of L-arginine can improve insulin sensitivity, endothelial function, and anti-oxidative capacities in type-2 diabetes mellitus (21, 84–85). In addition, intravenous infusion of L-arginine (0.52 mg·kg−1·min−1) enhances insulin sensitivity in obese patients, non-insulin-dependent diabetes mellitus (NIDDM) patients, and healthy subjects, and also restores defective insulin-mediated vasodilatation in obesity and NIDDM (85). Furthermore, a chronic administration of L-arginine reduces glucose levels in plasma, hepatic glucose production, and insulin resistance in type-2 diabetic patients (84). Finally, prolonged oral administration of L-arginine can increase insulin sensitivity, ameliorate abnormalities of glucose metabolism, improve endothelial function, reduces oxidative stress and white-fat mass, attenuate production of inflammatory cytokines, and spare skeletal muscle during a period of hypocaloric diet in obese type-2 diabetic patients (21).
5. SUMMARY AND PERSPECTIVE
Emerging evidence shows that L-arginine plays an important role in regulating lipid metabolism and energy partitioning in animals and humans. Results of biochemical and molecular studies with young animals indicate that arginine enhances the partitioning of dietary and metabolic energy from adipose tissue storage to skeletal muscle growth. In adults, arginine acts to maintain the mass and function of skeletal muscle in the face of decreased intake of dietary energy. Thus, dietary supplementation with arginine is beneficial in: a) reducing obesity in humans and companion animals; and b) enhancing economic returns in livestock production by increasing lean muscle growth and reducing fat accretion in the carcass. Although a number of biochemical and molecular mechanisms have been proposed to explain roles for arginine in metabolism of energy substrates, direct experimental evidence is needed to support these propositions. Additionally, it is important to look at the whole picture of arginine metabolism in the body, ranging from arginine degradation (86, 87) and synthesis (88, 89) in the small intestine to tissue-specific expression of arginase isoforms (90, 91), and to whole-body protein turnover (92, 93). Moreover, studies should be conducted to determine whether or not arginine supplementation can increase energy expenditure in animals and humans. New knowledge about arginine biochemistry and physiology is expected to aid in designing a novel, safe and effective way to prevent and treat obesity as well as metabolic syndrome in humans. The future holds great promise for the use of L-arginine to improve health in humans and animals and to enhance the efficiency of animal production worldwide.
Acknowledgments
This research was supported by grants from NSFC (31001016; 31110103909), Special Foundation of President of The Chinese Academy of Sciences, National 863 Project (2008AA10Z316), National Basic Research Project (2009CB118800), the CAS/SAFEA International Partnership Program for Creative Research Teams, the Thousand-People-Talent program at China Agricultural University, and Texas AgriLife Research project (No. 8200). Carmen D. Tekwe was supported by a postdoctoral training grant from the National Cancer Institute (R25T - 090301).
Abbreviations
- PGC-1
peroxisome proliferator-activated receptor-gamma coactivator-1
- AMPK
AMP-activated protein kinase
- NO
nitric oxide
- NMR
nuclear magnetic resonance
- NOS
NO synthase
- mTOR
mammalian target of rapamycin
- LPL
lipoprotein lipase
- ACC
acetyl CoA carboxylase
- FAS
fatty acid synthase
- HSL
hormone-sensitive lipase
- CPT1
carnitine palmitoyltransferase 1
- MCD
malonyl-CoA decarboxylase
- UCP
uncoupling protein
- PPAR
peroxisome proliferator-activated receptor
- GLUT
glucose transporter
- BCAT
branched-chain amino acid aminotransferase
- PKA
protein kinase A
References
- 1.Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G. Regulatory role for the arginine nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem. 2006;17:571–588. doi: 10.1016/j.jnutbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Mersmann HJ, Smith SB. Development of white adipose tissue lipid metabolism. In: Burrin DG, Mersmann HJ, editors. Biology of metabolism in growing animals. Elsevier; Oxford: 2005. [Google Scholar]
- 3.Wu G, Bazer FW, Davis TA, Kim SW, Li P, Rhoads JM, Satterfield MC, Smith SB, Spencer TE, Yin Y. Arginine metabolism and nutrition in growth, health and disease. Amino Acids. 2009;37:153–168. doi: 10.1007/s00726-008-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medina-Gomez G, Vidal-Puig A. Gateway to the metabolic syndrome. Nat Med. 2005;11:602–603. doi: 10.1038/nm0605-602. [DOI] [PubMed] [Google Scholar]
- 5.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 6.Hwang SY, Taylor CG, Zahradka P, Bankovic-Calic N, Ogborn MR, Aukema HM. Dietary soy protein reduces early renal disease progression and alters prostanoid production in obese fa/fa Zucker rats. J Nutr Biochem. 2008;19:255–262. doi: 10.1016/j.jnutbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37:1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 8.Wu G, Meininger CJ. Nitric oxide and vascular insulin resistance. BioFactors. 2009;35:21–27. doi: 10.1002/biof.3. [DOI] [PubMed] [Google Scholar]
- 9.McKnight JR, Satterfield MC, Jobgen WS, Smith SB, Spencer TE, Meininger CJ, McNeal CJ, Wu G. Beneficial effects of L-arginine on reducing obesity: Potential mechanisms and important implications for human health. Amino Acids. 2010;39:349–357. doi: 10.1007/s00726-010-0598-z. [DOI] [PubMed] [Google Scholar]
- 10.Geng MM, Li TJ, Kong XF, Song XY, Chu WY, Huang RL, Yin YL, Wu G. Reduced expression of intestinal N-acetylglutamate synthase in suckling piglets: a novel molecular mechanism for arginine as a nutritionally essential amino acid for neonates. Amino Acids. 2011;40:1513–1522. doi: 10.1007/s00726-010-0761-6. [DOI] [PubMed] [Google Scholar]
- 11.Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li P, Li XL, McKnight JR, Satterfield MC, Spencer TE. Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids. 2011;40:1053–1063. doi: 10.1007/s00726-010-0715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li XL, Rezaei R, Li P, Wu G. Composition of amino acids in feed ingredients for animal diets. Amino Acids. 2011;40:1159–1168. doi: 10.1007/s00726-010-0740-y. [DOI] [PubMed] [Google Scholar]
- 13.Wu X, Ruan Z, Gao YL, Yin YL, Zhou XH, Wang L, Geng MM, Hou YQ, Wu G. Dietary supplementation with L-arginine or N-carbamylglutamate enhances intestinal growth and heat shock protein-70 expression in weanling pigs fed a corn- and soybean meal-based diet. Amino Acids. 2010;39:831–839. doi: 10.1007/s00726-010-0538-y. [DOI] [PubMed] [Google Scholar]
- 14.Kim JY, Burghardt RC, Wu G, Johnson GA, Spencer TE, Bazer FW. Select nutrients in the ovine uterine lumen: VII. Effects of arginine, leucine, glutamine, and glucose on trophectodem cell signaling, proliferation, and migration. Biol Reprod. 2011;84:62–69. doi: 10.1095/biolreprod.110.085738. [DOI] [PubMed] [Google Scholar]
- 15.Tan BE, Yin YL, Liu ZQ, Li XG, Xu HJ, Kong XF, Huang RL, Tang WJ, Shinzato I, Smith SB, Wu GY. Dietary L-arginine supplementation increases muscle gain and reduces body fat mass in growing finishing pigs. Amino Acids. 2009;37:169–175. doi: 10.1007/s00726-008-0148-0. [DOI] [PubMed] [Google Scholar]
- 16.Trujillo ME, Sullivan S, Harten I, Schneider SH, Greenberg AS, Fried SK. Interleukin-6 Regulates Human Adipose Tissue Lipid Metabolism and Leptin Production in vitro. JCEM. 2004;89:5577–5582. doi: 10.1210/jc.2004-0603. [DOI] [PubMed] [Google Scholar]
- 17.Wu G, Lee MJ, Fried SK. The arginine-NO pathway modulates lipolysis in adipose tissues of obese human subjects. FASAB J. 2007;21:A1052. [Google Scholar]
- 18.Jobgen WJ. PhD Dissertation. Texas A&M University; 2007. Dietary L-arginine Supplementation Reduces Fat Mass in Diet-Induced Obese Rats. [Google Scholar]
- 19.Yan HY, Aziz E, Shillabeer G, Wong A, Shanghavi D, Kermouni A, Abdel-Hafez M, Lau DC. Nitric oxide promotes differentiation of rat white preadipocytes in culture. J Lipid Res. 2002;43:2123–2129. doi: 10.1194/jlr.m200305-jlr200. [DOI] [PubMed] [Google Scholar]
- 20.Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu GY. Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr. 2005;135:714–21. doi: 10.1093/jn/135.4.714. [DOI] [PubMed] [Google Scholar]
- 21.Lucotti P, Setola E, Monti LD, Galluccio E, Costa S, Sandoli EP, Fermo I, Rabaiotti G, Gatti R, Piatti P. Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2006;291(5):E906–E912. doi: 10.1152/ajpendo.00002.2006. [DOI] [PubMed] [Google Scholar]
- 22.He QH, Kong XF, Wu GY, Ren PP, Tang HR, Hao FH, Huang RL, Li TJ, Tan BE, Li P, Tang ZR, Yin YL, Wu YN. Metabolomic analysis of the response of growing pigs to dietary L-arginine supplementation. Amino Acids. 2009;3(7):199–208. doi: 10.1007/s00726-008-0192-9. [DOI] [PubMed] [Google Scholar]
- 23.Tan BE, Liu ZQ, Tang WJ, Xu HJ, Kong XF, Li XG, Yao K, Gu WT, Shinzato I, Smith SB, Wu GY. Dietary arginine supplementation differentially regulates expression of fat-metabolic genes in porcine adipose tissue and skeletal muscle. J Nutr Biochem. 2011;22:441–445. doi: 10.1016/j.jnutbio.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Andersson K, Gaudiot N, Ribiere C, Elizalde M, Giudicelli Y, Arner PA. nitric oxide-mediated mechanism regulates lipolysis in human adipose tissue in vivo. Br J Pharmacol. 1999;126:1639–45. doi: 10.1038/sj.bjp.0702430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frühbeck G, Gómez-Ambrosi J. Modulation of the leptin-induced white adipose tissue lipolysis by nitric oxide. Cell Signal. 2001;13:827–833. doi: 10.1016/s0898-6568(01)00211-x. [DOI] [PubMed] [Google Scholar]
- 26.Gaudiot N, Jaubert A-M, Charbonnier E, Sabourault D, Lacasa D, Giudicelli Y, Ribière C. Modulation of white adipose tissue lipolysis by nitric oxide. J Biol Chem. 1998;273:13475–81. doi: 10.1074/jbc.273.22.13475. [DOI] [PubMed] [Google Scholar]
- 27.Khedara A, Goto T, Morishima M, Kayashita J, Kato N. Elevated body fat in rats by the dietary nitric oxide synthase inhibitor, L-N omega nitroarginine. Biosci Biotechnol Biochem. 1999;63:698–702. doi: 10.1271/bbb.63.698. [DOI] [PubMed] [Google Scholar]
- 28.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 29.Kohli R, Meininger CJ, Haynes TE, Yan W, Self JT, Wu G. Dietary L-arginine supplementation enhances endothelial nitric oxide synthesis in streptozotocin-induced diabetic rats. J Nutr. 2004;134:600–608. doi: 10.1093/jn/134.3.600. [DOI] [PubMed] [Google Scholar]
- 30.Morris SM., Jr Recent advances in arginine metabolism. Curr Opin Clin Nutr Metab Care. 2004;7:45–51. doi: 10.1097/00075197-200401000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336:1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan BE, Yin YL, Kong XF, Li P, Li XL, Gao HJ, Li XG, Huang RL, Wu GY. L-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids. 2010;38:1227–1235. doi: 10.1007/s00726-009-0334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrière A, Carmona MC, Fernandez Y, Rigoulet M, Wenger RH, Pénicaud L, Casteilla L. Mitochondrial reactive oxygen species control the transcription factor CHOP-10/GADD153 and adipocyte differentiation: a mechanism for hypoxia-dependent effect. J Biol Chem. 2004;279:40462–40469. doi: 10.1074/jbc.M407258200. [DOI] [PubMed] [Google Scholar]
- 34.Vankoningsloo S, Piens M, Lecocq C, Gilson A, De Pauw A, Renard P, Demazy C, Houbion A, Raes M, Arnould T. Mitochondrial dys-function induces triglyceride accumulation in 3T3-L1 cells: role of fatty acid beta-oxidation and glucose. J Lipid Res. 2005;46:1133–1149. doi: 10.1194/jlr.M400464-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Bastin J, Aubey F, Rötig A, Munnich A, Djouadi F. Activation of per-oxisome proliferator-activated receptor pathway stimulates the mitochondrial respiratory chain and can correct deficiencies in patients’ cells lacking its components. J Clin Endocrinol Metab. 2008;93:1433–1441. doi: 10.1210/jc.2007-1701. [DOI] [PubMed] [Google Scholar]
- 36.Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 37.Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 38.Miura S, Tomitsuka E, Kamei Y, Yamazaki T, Kai Y, Tamura M, Kita K, Nishino I, Ezaki O. Overexpression of peroxisome proliferator-activated receptor gamma co-activator-1alpha leads to muscle atrophy with depletion of ATP. Am J Pathol. 2006;169:1129–1139. doi: 10.2353/ajpath.2006.060034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satterfield MC, Wu G. Growth and development of brown adipose tissue: significance and nutritional regulation. Front Biosci. 2011;16:1589–1608. doi: 10.2741/3807. [DOI] [PubMed] [Google Scholar]
- 40.Jobgen WJ, Meininger CJ, Jobgen SC, Li P, Lee MJ, Smith SB, Spencer TE, Fried SK, Wu G. Dietary L-arginine supplementation reduces white-fat gain and enhances skeletal muscle and brown fat masses in diet-induced obese rats. J Nutr. 2009;139:230–237. doi: 10.3945/jn.108.096362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nisoli E, Cozzi V, Carruba MO. Amino Acids and Mitochondrial Biogenesis. Am J Cardiol. 2008;101:E22–E25. doi: 10.1016/j.amjcard.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 42.Petrovicć V, Korać A, Buzadžć B, Vasilijvić A, Janković A, Mićunović K, Korać B. Nitric oxide regulates mitochondrial re-modelling in interscapular brown adipose tissue: ultrastructural and morphometric-stereologic studies. J Microscopy. 2008;232(3):542–548. doi: 10.1111/j.1365-2818.2008.02132.x. [DOI] [PubMed] [Google Scholar]
- 43.Charles S, Tamagawa T, Henquin JC. A single mechanism for the stimulation of insulin release and Rb efflux from rat islets by cationic amino acids. Biochemical J. 1982;208:301–308. doi: 10.1042/bj2080301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henquin JC. The biophysical events involved in the stimulation of insulin release by arginine. In: De Deyn PP, Marescau B, Stalon V, Qureshi IA, editors. Guanidino Compounds in Biology and Medicine. John Libbey and Company Ltd; London: 1992. [Google Scholar]
- 45.Bouwens L, Kloppel G. Cytochemical localisation of NADPH-diaphorase in the four types of pancreatic islet cell. Histochemistry. 1994;101:209–214. doi: 10.1007/BF00269546. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt HH, Warner TD, Ishii K, Sheng H, Murad F. Insulin secretion from pancreatic beta-cells caused by L-arginine derived nitrogen oxides. Science. 1992;255:721–723. doi: 10.1126/science.1371193. [DOI] [PubMed] [Google Scholar]
- 47.Kim SW, Wu G. Dietary arginine supplementation enhances the growth of milk-fed young pigs. J Nutr. 2004;134:625–630. doi: 10.1093/jn/134.3.625. [DOI] [PubMed] [Google Scholar]
- 48.Yao K, Yin YL, Chu WY, Liu ZQ, Deng D, Li TJ, Huang RL, Zhang JS, Tan BE, Wang WC, Wu GY. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J Nutr. 2008;138:867–872. doi: 10.1093/jn/138.5.867. [DOI] [PubMed] [Google Scholar]
- 49.Eaton RP, Schade DS. Effect of clofibrate on arginine-stimulated glucagon and insulin secretion in man. Metabolism. 1974;23:445–454. doi: 10.1016/0026-0495(74)90092-4. [DOI] [PubMed] [Google Scholar]
- 50.Floyd JC, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966;45:1487–1502. doi: 10.1172/JCI105456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palmer JP, Walter RM, Ensinck JW. Arginine-stimulated acute phase of insulin and glucagon secretion. I. In normal man. Diabetes. 1975;24:735–740. doi: 10.2337/diab.24.8.735. [DOI] [PubMed] [Google Scholar]
- 52.Palmer JP, Bensen JW, Walter RM. Arginine-stimulated acute phase of insulin and glucagon secretion in diabetic subjects. J Clin Invest. 1976;58:565–570. doi: 10.1172/JCI108502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stingl H, Raffesberg W, Nowotny P, Waldhäusl W, Roden M. Reduction of plasma leptin concentrations by arginine but not lipid infusion in humans. Obes Res. 2002;10:1111–1119. doi: 10.1038/oby.2002.151. [DOI] [PubMed] [Google Scholar]
- 54.Copinschi G, Wegienka LC, Hane S, Forsham PH. Effect of arginine on serum levels of insulin and growth hormone in obese subjects. Metabolism. 1967;16:485–491. doi: 10.1016/0026-0495(67)90076-5. [DOI] [PubMed] [Google Scholar]
- 55.Muller WA, Faloona GR, Unger RH. The influence of the antecedent diet upon glucagon and insulin secretion. N Engl J Med. 1971;285(26):1450–1454. doi: 10.1056/NEJM197112232852603. [DOI] [PubMed] [Google Scholar]
- 56.Sun X, Zemel MB. Leucine and Calcium Regulate Fat Metabolism and Energy Partitioning in Murine Adipocytes and Muscle Cells. Lipids. 2007;42:297–305. doi: 10.1007/s11745-007-3029-5. [DOI] [PubMed] [Google Scholar]
- 57.Dyck DJ, Heigenhauser GJ, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol (Oxf) 2006;186:5–16. doi: 10.1111/j.1748-1716.2005.01502.x. [DOI] [PubMed] [Google Scholar]
- 58.Muoio DM, Dohm GL, Fiedorek FT, Jr, Tapscott EB, Coleman RA, Dohn GL. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes. 1997;46(8):1360–1363. doi: 10.2337/diab.46.8.1360. [DOI] [PubMed] [Google Scholar]
- 59.Steinberg GR, Parolin ML, Heigenhauser GJ, Dyck DJ. Leptin increases FA oxidation in lean but not obese human skeletal muscle: evidence of peripheral leptin resistance. Am J Physiol Endocrinol Metab. 2002;283:E187–E192. doi: 10.1152/ajpendo.00542.2001. [DOI] [PubMed] [Google Scholar]
- 60.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 61.Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1:1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- 62.Kauter K, Ball M, Kearney P, Tellam R, McFarlane JR. Adrenaline, insulin and glucagon do not have acute effects on plasma leptin levels in sheep: development and characterisation of an ovine leptin ELISA. J Endocrinol. 2000;166:127–135. doi: 10.1677/joe.0.1660127. [DOI] [PubMed] [Google Scholar]
- 63.Pratley RE, Nicolson M, Bogardus C, Ravussin E. Effects of acute hyperinsulinemia on plasma leptin concentrations in insulin-sensitive and insulin-resistant Pima Indians. J Clin Endocrinol Metab. 1996;81:4418–4421. doi: 10.1210/jcem.81.12.8954052. [DOI] [PubMed] [Google Scholar]
- 64.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. J Biol Chem. 2007;282:10341–51. doi: 10.1074/jbc.M610631200. [DOI] [PubMed] [Google Scholar]
- 67.Leclerc I, Sun G, Morris C, Fernandez-Millan E, Nyirenda M, Rutter GA. AMP-activated protein kinase regulates glucagon secretion from mouse pancreatic alpha cells. Diabetologia. 2011;54:125–134. doi: 10.1007/s00125-010-1929-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chotechuang N, Chaumontet Catherine, Azzout-Marniche Dalila, Gaudichon Claire, Gausserès Nicolas, Vinoy Sophie, Tomé Daniel. AMPK phosphorylation is decreased in response to amino acids and glucose in Caco-2 intestinal cells. The FASEB Journal. 2007;21:839.7. [Google Scholar]
- 69.Hou YQ, Wang L, Ding BY, Liu YL, Zhu HL, Liu J, Li YT, Wu X, Yin YL, Wu G. Dietary alpha-ketoglutarate supplementation ameliorates intestinal injury in lipopolysaccharide-challenged piglets. Amino Acids. 2010;39:555–564. doi: 10.1007/s00726-010-0473-y. [DOI] [PubMed] [Google Scholar]
- 70.Hou YQ, Wang L, Ding BY, Liu YL, Zhu HL, Liu J, Li YT, Kang P, Yin YL, Wu G. Alpha-ketoglutarate and intestinal function. Front Biosci. 2011;16:1186–1196. doi: 10.2741/3783. [DOI] [PubMed] [Google Scholar]
- 71.López M, Lelliott CJ, Vidal-Puig A. Hypothalamic fatty acid metabolism: a housekeeping pathway that regulates food intake. Bioessays. 2007;29:248–261. doi: 10.1002/bies.20539. [DOI] [PubMed] [Google Scholar]
- 72.Munday MR. Regulation of mammalian acetyl-CoA carboxylase. Biochem Soc Trans. 2002;30:1059–1064. doi: 10.1042/bst0301059. [DOI] [PubMed] [Google Scholar]
- 73.Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002;277:32571–7. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- 74.Li J, Hu X, Selvakumar P, Russell RR, III, Cushman SW, Holman GD, Young LH. Role of the nitric oxide pathway in AMPK-mediated glucose uptake and GLUT4 translocation in heart muscle. Am J Physiol Endocrinol Metab. 2004;287:E834–E841. doi: 10.1152/ajpendo.00234.2004. [DOI] [PubMed] [Google Scholar]
- 75.Russell RR, III, Bergeron R, Shulman GI, Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol. 1999;277:H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]
- 76.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 77.Dagon Y, Avraham Y, Berry EM. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2 in adipocytes. Biochem Biophys Res Commun. 2006;340:43–47. doi: 10.1016/j.bbrc.2005.11.159. [DOI] [PubMed] [Google Scholar]
- 78.Hill JO, Peters JC, Catenacci VA, Wyatt HR. International strategies to address obesity. Obesity Rev. 2008;9:S41–S47. doi: 10.1111/j.1467-789X.2007.00437.x. [DOI] [PubMed] [Google Scholar]
- 79.Cersosimo E, DeFronzo RA. Insulin resistance and endothelial dysfunction: the road map to cardiovascular diseases. Diab Metab Res Rev. 2006;22:423–436. doi: 10.1002/dmrr.634. [DOI] [PubMed] [Google Scholar]
- 80.Piatti PM, Monti LD, Zavaroni I, Valsecchi G, Van Phan C, Costa S, Conti M, Sandoli EP, Solerte B, Pozza G, Pontiroli AE, Reaven G. Alterations in nitric oxide/cyclic-GMP pathway in nondiabetic siblings of patients with type 2 diabetes. J Clin Endocrinol Metab. 2000;85:2416–2422. doi: 10.1210/jcem.85.7.6667. [DOI] [PubMed] [Google Scholar]
- 81.Ghasemi A, Zahediasl S, Azizi F. Nitric oxide and clustering of metabolic syndrome components in pediatrics. Eur J Epidemiol. 2010;25:45–53. doi: 10.1007/s10654-009-9382-3. [DOI] [PubMed] [Google Scholar]
- 82.Gruber HJ, Mayer C, Mangge H, Fauler G, Grandits N, Wilders-Truschnig M. Obesity reduces the bioavailability of nitric oxide in juveniles. Int J Obes Lond. 2008;32:826–831. doi: 10.1038/sj.ijo.0803795. [DOI] [PubMed] [Google Scholar]
- 83.Jobgen W, Fu WJ, Gao H, Li P, Meininger CJ, Smith SB, Spencer TE, Wu G. High fat feeding and dietary L-arginine supplementation differentially regulate gene expression in rat white adipose tissue. Amino Acids. 2009;37:187–198. doi: 10.1007/s00726-009-0246-7. [DOI] [PubMed] [Google Scholar]
- 84.Piatti PM, Monti LD, Valsecchi G, Magni F, Setola E, Marchesi F, Galli-Kienle M, Pozza G, Alberti KG. Long-term oral L-arginine administration improves peripheral and hepatic insulin sensitivity in type 2 diabetic patients. Diabetes Care. 2001;24(5):875–880. doi: 10.2337/diacare.24.5.875. [DOI] [PubMed] [Google Scholar]
- 85.Wascher TC, Graier WF, Dittrich P, Hussain MA, Bahadori B, Wallner S, Toplak H. Effects of low-dose Larginine on insulin-mediated vasodilatation and insulin sensitivity. Eur J Clin Invest. 1997;27:690–695. doi: 10.1046/j.1365-2362.1997.1730718.x. [DOI] [PubMed] [Google Scholar]
- 86.Wu G, Knabe DA, Flynn NE, Yan W, Flynn SP. Arginine degradation in developing porcine enterocytes. Am J Physiol Gastrointest Liver Physiol. 1996;271:G913–G919. doi: 10.1152/ajpgi.1996.271.5.G913. [DOI] [PubMed] [Google Scholar]
- 87.Wu G. An important role for pentose cycle in the synthesis of citrulline and proline from glutamine in porcine enterocytes. Arch Biochem Biophys. 1996;336:224–230. doi: 10.1006/abbi.1996.0552. [DOI] [PubMed] [Google Scholar]
- 88.Flynn NE, Wu G. An important role for endogenous synthesis of arginine in maintaining arginine homeostasis in neonatal pigs. Am J Physiol Regul Integr Comp Physiol. 1996;271:R1149–R1155. doi: 10.1152/ajpregu.1996.271.5.R1149. [DOI] [PubMed] [Google Scholar]
- 89.Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li P, Li XL, McKnight JR, Satterfield MC, Spencer TE. Proline and hydroxyproline metabolism. implications for animal and human nutrition. Amino Acids. 2011;40:1053–1063. doi: 10.1007/s00726-010-0715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morris SM., Jr Recent advances in arginine metabolism. roles and regulation of the arginases. Br J Pharmacol. 2009;157:922–930. doi: 10.1111/j.1476-5381.2009.00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang JJ, Chen LX, Li P, Li XL, Zhou HJ, Wang FL, Li DF, Yin YL, Wu G. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. J Nutr. 2008;138:1025–1032. doi: 10.1093/jn/138.6.1025. [DOI] [PubMed] [Google Scholar]
- 92.Marliss EB, Chevalier S, Gougeon R, Morais JA, Lamarche M, Adegoke OAJ, Wu G. Elevations of plasma methylarginines in obesity and ageing are related to insulin sensitivity and rates of protein turnover. Diabetologia. 2006;49:351–359. doi: 10.1007/s00125-005-0066-6. [DOI] [PubMed] [Google Scholar]
- 93.Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Li XL, Satterfield MC, Spencer TE. Impacts of amino acid nutrition on pregnancy outcome in pigs: mechanisms and implications for swine production. J Anim Sci. 2010;88:E195–E204. doi: 10.2527/jas.2009-2446. [DOI] [PubMed] [Google Scholar]