Abstract
OBJECTIVES
The exclusion of the left atrial appendage (LAA) has been used to reduce the risk of stroke associated with atrial fibrillation (AF). While LAA exclusion has been associated with a reduced risk of stroke, the effect on the electrical activity of the LAA (a potential source of AF) remains unknown. As such, we sought to demonstrate whether surgical epicardial clip occlusion leads to the electrical isolation of the LAA.
METHODS
From December 2010 until August 2011, 10 patients with paroxysmal AF underwent off-pump coronary artery bypass surgery with bilateral pulmonary vein isolation and an LAA clip occlusion with a new epicardial clip. Before and after the clip was placed, pacing manoeuvres were performed to assess the electrical exit and entry blocks from the LAA.
RESULTS
All clips were applied successfully. The mean procedure time for the clip application was 4 ± 1 min. No complications occurred related to clip application. Prior to the pericardial closure, 18 ± 3 min after the clip placement, the LAA stimulation and pacing manoeuvres demonstrated complete electrical isolation of the LAA in all cases.
CONCLUSIONS
Epicardial LAA clip occlusion leads to the acute electrical isolation of the LAA and may not only provide stroke prevention but also reduce the recurrence of AF.
Keywords: Atrial fibrillation, Left atrial appendage, Clip, Electrical isolation, Stroke, Occlusion, Maze, Ablation, Surgery
BACKGROUND
Minimally invasive surgical ablations of atrial fibrillation (AF) have emerged as a valuable option for patients with AF, and guidelines suggest that patients with AF who are undergoing cardiac surgery should receive AF therapy [1–3]. During these cardiac surgery procedures, some surgeons routinely remove the left atrial appendage (LAA) to reduce the risk of stroke associated with AF. Recently, a new clip (AtriClip, Atricure Inc., West Chester, OH, USA) has been shown to be safe, effective and durable in terms of isolating the LAA from the left atrium (LA) with regard to blood flow [4, 5], hence potentially decreasing the potential for stroke. However, recent data also suggest that the LAA may be a source of AF triggers [6]. It is unknown whether LAA clip occlusion also provides the electrical isolation of the LAA, which may lead to reduced AF burden. In the present study, we sought to determine whether LAA clip occlusion leads to acute electrical isolation of the LAA.
MATERIAL AND METHODS
From December 2010 until August 2011, we applied an LAA clip (AtriClip, Atricure Inc.) by an epicardial approach in 10 sequential patients undergoing off-pump coronary artery bypass (OPCAB) procedure at our institution, who also had paroxysmal AF and were in sinus rhythm at the time of surgery. None had a history of atrioventricular block. All patients underwent median sternotomy with an OPCAB procedure, followed by bilateral pulmonary vein isolation (PVI) with a bipolar radiofrequency clamp (Isolator Synergy Ablation Clamp, Atricure Inc.) and finally placement of an LAA clip (AtriClip, Atricure Inc.). Transoesophageal echocardiography (TEE) was used to rule out LAA thrombus at the beginning of the procedure and to assess the perfusion of the LAA after the clip application [4]. At the beginning of the procedure and just after the pericardial opening, pacing and sensing of the LA, LAA and right ventricle (RV) using a bipolar pacing pen (Max1, Atricure Inc.) and bipolar temporary pacing wires were performed (Fig. 1). The electrocardiographical results of the pacing manoeuvres were documented with the MicroPace ORLab Stimulator/EP Recorder system (Atricure Inc.) and the ICS 3000 (Biotronik, Berlin, Germany) to record and store both the surface and epicardial electrocardiograms. First right ventricular, then left atrial and finally LAA pacing were performed (Fig. 2). The RV was paced at 10.0 V/0.5 ms for 30 s to document the capture of the RV and to demonstrate excitation of the ventricle. Next, LA was paced at 10.0 V/0.5 ms for 30 s to assess conduction to the RV as well as block to the LAA (‘entry block’). Finally, the LAA was paced at 10.0 V/0.5 ms for 30 s to confirm block to the LA and the lack of acceleration to the RV (‘exit block’). All manoeuvres were documented in relation to the surface electrocardiogram (Figs 3 and 4).
Figure 1:
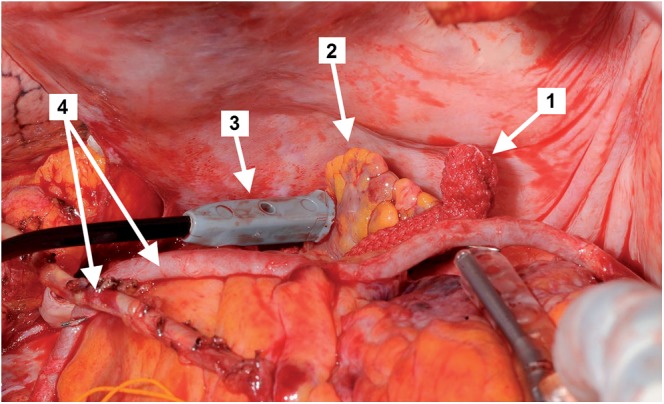
The intraoperative stimulation of the LAA after the LAA clip application (1: applied LAA clip; 2: LAA; 3: pacing pen; 4: bypass grafts).
Figure 2:
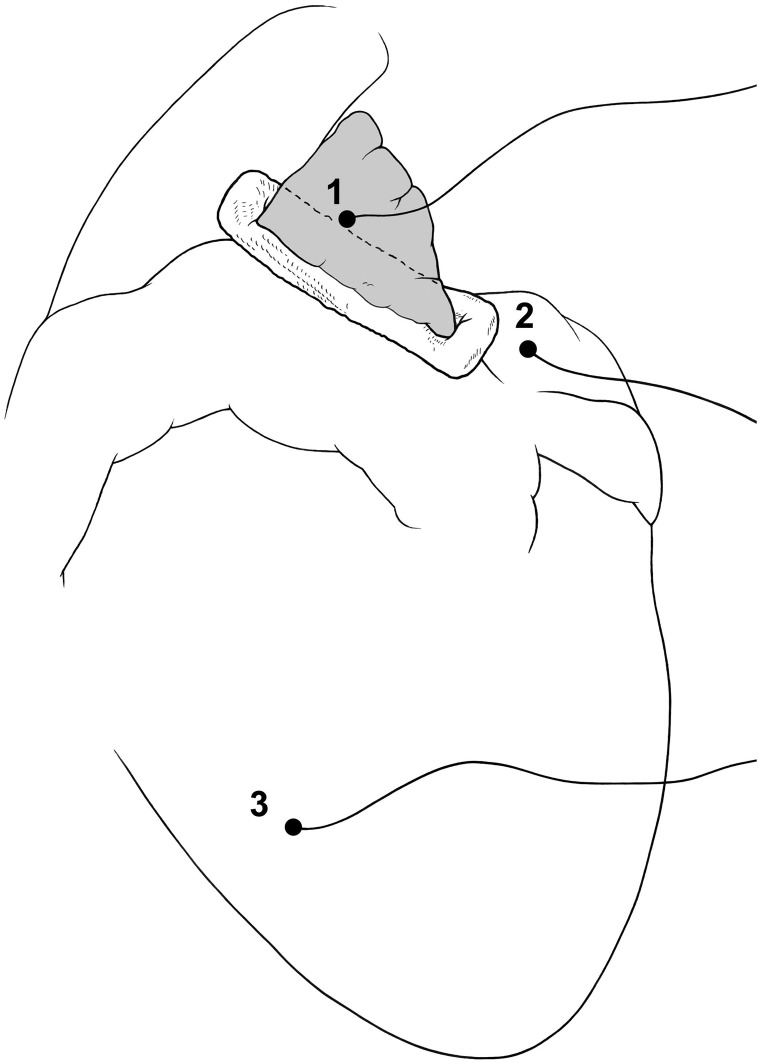
Pacing and sensing sites for the intraoperative testing of the electrical isolation of the LAA (1: LAA; 2: LA; 3: RV).
Figure 3:
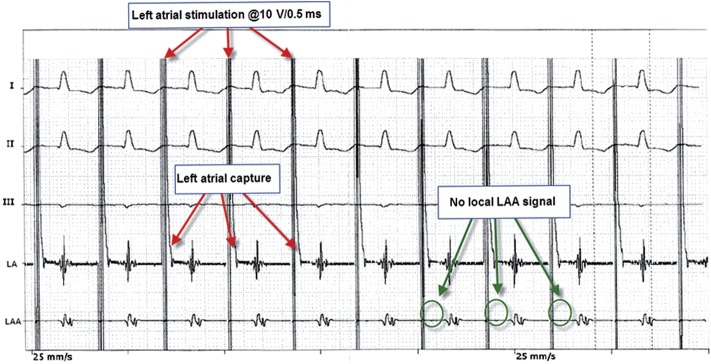
Surface and epicardial ECG during the stimulation of the LA demonstrating an entry block to the LAA.
Figure 4:
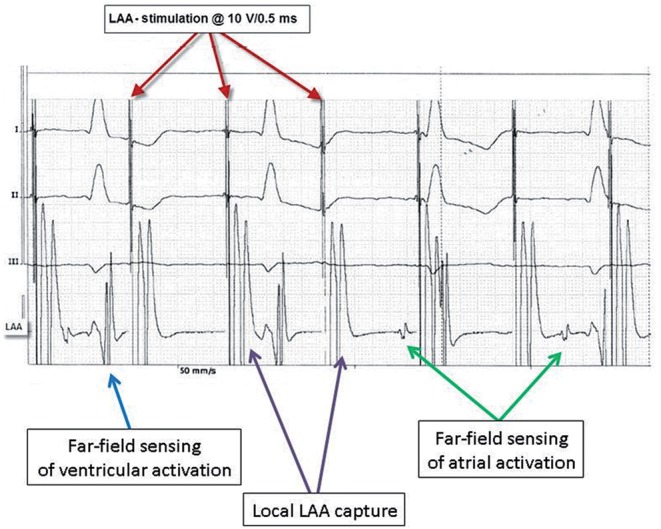
Surface and epicardial ECG during the stimulation of the LAA demonstrating an exit block from the LAA.
RESULTS
A total of 10 patients underwent an OPCAB with concomitant epicardial bilateral pulmonary vein isolation and the LAA clip application. The mean age of the patients was 73 ± 5 years, and eight patients (80%) were male. The mean left ventricular function was 58 ± 17% and the mean preoperative logistic EuroSCORE 12.1 ± 13.7%.
At the start of surgery, and as per surgical routine in the OPCAB setting, right atrial and RV pacing was possible in all patients. After coronary revascularization, epicardial PVI was performed in all patients. The entrance and the exit block were documented for all pulmonary veins separately. In all patients (100%), the LAA clip was applied successfully as documented by the complete lack of residual blood flow on TEE. The mean procedure time with the application of the LAA clip was 4 ± 1 min. During the procedure, no complications occurred with relation to the device application. Prior to the pericardial closure, all patients underwent pacing as outlined above. In all 10 patients, RV pacing at a speed above the intrinsic sinus rate resulted in the acceleration of the heart rate on the surface ECG, indicating the local capture of the RV. Similarly, 1:1 AV conduction and the subsequent heart rate acceleration were demonstrated after LA pacing. In contrast, no electrical acceleration of local LAA potentials was observed after LA pacing indicating an entry block from the LA to the LAA (Fig. 3). Conversely, in all patients, the stimulation of the LAA at a speed above the sinus rate showed no electrical response in the LA as documented by an epicardial electrocardiogram. Furthermore, no acceleration of the heart rate was observed indicating the dissociation of LAA stimulation and ventricular activation, hence confirming an exit block from the LAA. Importantly, the local capture of the LAA was clearly documented on the intracardiac electrocardiogram (Fig. 4).
DISCUSSION
The primary reason for occluding the LAA in patients with AF or patients receiving arrhythmia surgery is to reduce the stroke risk due to the clot formation in this structure [7]. However, recent reports suggest that the LAA could be a source of AF triggers in up to 30% of patients [6]. In our current study, we were able to demonstrate not only 100% successfully complete circulatory exclusions, but also acute electrical isolation of the LAA after application of the clip in all patients. As such, this procedure may not only reduce thromboembolic complications from AF, but may also lead to a reduction in the recurrence of AF.
Although other series using the same clip have been published, to the best of our knowledge, only one report addressing the electrical isolation of the LAA after the clip placement in a single patient with stand-alone thoracoscopic LAA occlusion has been published so far [8]. In our study, we report 10 such cases with consistent results of both the exit and the entry block from and into the LAA. Other devices used for occluding the LAA, including endocardial transcatheter devices such as Watchman (Atritech, Plymouth, MN, USA) and Amplatzer cardiac plug (AGA Medical, St Jude Medical Plymouth, MN, USA), may possibly prevent the LAA clots from reaching the LA; they have not been shown to electrically isolate the LAA. In fact, endocardial devices render any future left atrial and LAA ablations in particular impossible. As the incidence of surgical ablation of AF either as a stand-alone procedure or as an add-on to another procedure grows, the role of LAA exclusion may also grow. Using tools such as the LAA CLIP which also isolate the LAA electrically may improve the success rates of these procedures. It is possible that the additional isolation of the LAA may partly explain the success rate in surgical series vs catheter series [9].
Limitations
Although, in the current study, we did not assess an electrical block in the long term, in previous work, we [10] and others [11] have seen that LAA clipping leads to complete fibrosis of the LAA and development of a scar. Hence, reconnection (as it may be observed especially after catheter-based PVI) is unlikely and the persistence of electrical isolation is highly probable.
CONCLUSION
Epicardial LAA clip occlusion leads to the complete electrical isolation of the LAA, resulting in not merely the elimination of an important source of systemic thromboembolism but also the electrical exclusion of potential triggers for the recurrence of AF.
Conflict of interest: S. Mahapatra is a consultant for St Jude Medical (St Paul, MN, USA). S. P. Salzberg is a consultant for Atricure (Westchester, OH, USA).
REFERENCES
- 1.Mahapatra S, LaPar DJ, Kamath S, Payne J, Bilchick KC, Mangrum JM, et al. Initial experience of sequential surgical epicardial-catheter endocardial ablation for persistent and long-standing persistent atrial fibrillation with long-term follow-up. Ann Thorac Surg. 2011;91:1890–8. doi: 10.1016/j.athoracsur.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krul SP, Driessen AH, van Boven WJ, Linnenbank AC, Geuzebroek GS, Jackman WM, Wilde AA, de Bakker JM, de Groof JR. Thoracoscopic video-assisted pulmonary vein antrum isolation, ganglionated plexus ablation and periprocedural confirmation of ablation lesions. First results of a hybrid surgical-electrophysiological approach for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4:262–70. doi: 10.1161/CIRCEP.111.961862. [DOI] [PubMed] [Google Scholar]
- 3.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–61. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Salzberg SP, Plass A, Emmert MY, Desbiolles L, Alkadhi H, Grunenfelder J, et al. Left atrial appendage clip occlusion: early clinical results. J Thorac Cardiovasc Surg. 2010;139:1269–74. doi: 10.1016/j.jtcvs.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 5.Ailawadi G, Gerdisch MW, Harvey RL, Hooker RL, Damiano RJ, Jr, Salamon T, et al. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg. 2011;142:1002–9.e1. doi: 10.1016/j.jtcvs.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 6.Di Biase L, Burkhardt JD, Mohanty P, Sanchez J, Mohanty S, Horton R, et al. Left atrial appendage: an underrecognized trigger site of atrial fibrillation. Circulation. 2010;122:109–18. doi: 10.1161/CIRCULATIONAHA.109.928903. [DOI] [PubMed] [Google Scholar]
- 7.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–42. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 8.Benussi S, Mazzone P, Maccabelli G, Vergara P, Grimaldi A, Pozzoli A, et al. Thoracoscopic appendage exclusion with an atriclip device as a solo treatment for focal atrial tachycardia. Circulation. 2011;123:1575–8. doi: 10.1161/CIRCULATIONAHA.110.005652. [DOI] [PubMed] [Google Scholar]
- 9.Boersma LV, Castella M, van Boven W, Berruezo A, Yilmaz A, Nadal M, et al. Atrial fibrillation catheter ablation versus surgical ablation treatment (FAST): a 2-center randomized clinical trial. Circulation. 2012;125:23–30. doi: 10.1161/CIRCULATIONAHA.111.074047. [DOI] [PubMed] [Google Scholar]
- 10.Salzberg SP, Gillinov AM, Anyanwu A, Castillo J, Filsoufi F, Adams DH. Surgical left atrial appendage occlusion: evaluation of a novel device with magnetic resonance imaging. Eur J Cardiothorac Surg. 2008;34:766–70. doi: 10.1016/j.ejcts.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 11.Kamohara K, Fukamachi K, Ootaki Y, Akiyama M, Cingoz F, Ootaki C, et al. Evaluation of a novel device for left atrial appendage exclusion: the second-generation atrial exclusion device. J Thorac Cardiovasc Surg. 2006;132:340–6. doi: 10.1016/j.jtcvs.2006.04.021. [DOI] [PubMed] [Google Scholar]