Abstract
B-type natriuretic peptide (BNP) response early after a tetralogy of Fallot's repair remains unclear. BNP was measured pre- and post-operatively (immediately, day 1) in 18 children undergoing corrective repair with concurrent echocardiography (pre-, post-op day 1) to assess right ventricular (RV) systolic dysfunction, restrictive physiology, wall motion and pulmonary regurgitation (PR). In the first 24 h postoperatively, BNP rose acutely in all patients (mean 34.9 vs 144.4 vs 716.9 pg/ml at pre-op, days 0 and 1; P < 0.001). Immediate postoperative BNP correlated with preoperative haematocrit (rho = 0.52, P = 0.03) and inversely with preoperative oxygen saturation (rho = −0.63, P = 0.007). All patients showed reduced RV systolic function and abnormal wall motion with at least moderate PR in six patients (33.3%) and restrictive physiology in four (24%). Subsequent BNP expression (post-op day 1) correlated with a low RV fractional area change (rho = −0.51, P = 0.04), high oxygen extraction ratio (rho = 0.56, P = 0.02) and high central venous pressure (rho = 0.79, P < 0.001). The LV function and wall motion remained preserved in all patients. The mechanism of BNP expression is likely to be multi-factorial in the presence of a complex postoperative RV physiology in tetralogy of Fallot. An acute BNP response in the early postoperative period reflects an important physiological role and may be used as an adjunct biomarker to assess the RV function.
Keywords: Tetralogy of Fallot, Natriuretic peptides, B-type, Right ventricle
INTRODUCTION
B-type natriuretic peptide (BNP) is synthesized by cardiac myocytes in response to abnormal ventricular wall stress and loading conditions, acting principally by increasing sodium and water excretion and the relaxation of vascular resistance to limit the cardiac workload. Studies on BNP in the perioperative period of congenital heart surgery have remained scarce [1, 2]. Although the right ventricle (RV) in the early postoperative period is classically associated with restrictive RV physiology after tetralogy of Fallot's tetralogy repair, we had also shown previously that early systolic RV function is also impaired with evidence of a dyssynchronous RV wall motion [3, 4]. Therefore, tetralogy of Fallot in the early post-operative period represents a model of complex RV physiology characterized by abnormal contractility, relaxation, wall motion and loading conditions [3–5]. Each of these abnormal physiologies can potentially affect BNP expression, and their relationships with BNP in the early postoperative period remains unknown.
MATERIALS AND METHODS
This single-institutional study was undertaken prospectively in the Royal Hospital for Sick Children, Glasgow with approval from the Glasgow West Research Ethics Committee.
Patients
Eighteen tetralogy of Fallot patients undergoing elective surgical repair were studied (Table 1). The median age at corrective repair was 21.4 months (interquartile range 8.7–25.7 and range 5.5–148.1 months). Standard repair was performed by VSD closure and resection of RV infundibular muscles via transtricuspid and transpulmonary routes. If required (n = 9, 50%), the transannular incision was kept to a minimum with placement of a monocusp patch to limit pulmonary regurgitation (PR). Six (33%) patients had subvalvar patches to enlarge the RV outflow tract. An RV to pulmonary artery conduit was used in one patient with pulmonary atresia. Epicardial echocardiography was routinely performed intraoperatively to exclude any residual defect.
Table 1:
Perioperative characteristics of patients
| n = 18 | |
|---|---|
| Preoperative factors | |
| Age (mean, SD) months | 27.8, 34.1 |
| Gender | 11 males, 61% |
| Weight (mean, SD) kg | 11.8, 6.5 |
| Haematocrit level % (median, Q1–Q3) | 41.70 (37.58–48.08) |
| O2 saturation (median, Q1–Q3) | 87.50 (73.75–92.00) |
| Intraoperative factors | |
| Cross-clamp time (mean, SD) min | 85.7, 26.8 |
| Bypass time (mean, SD) min | 180.6, 76.1 |
| Postoperative factors (POD1) | |
| Central venous pressure (CVP, mmHg) (mean, SD) | 12.8, 3.5 |
| Mixed venous oxygen saturation (mean, SD) | 62.6, 8.4 |
| Lactate (mean, SD) | 1.47, 0.50 |
| Inotrope score (mean, SD) | 10.81, 6.50 |
| RV end-diastolic area index (median, Q1–Q3) | 7.63 (6.36–9.04) |
| PR presence (n, %) | 12, 66.7% |
| PR severity (mild:moderate–severe) | 6 mild, 6 mod-sev |
| Restrictive physiology (n, %) | 5 |
| Critical care stay (median, Q1–Q3, days) | 5.83 (3.63–9.46) |
| Ventilation time (median, Q1–Q3, h) | 102.4 (23.0–147.0) |
Blood sampling protocol
Blood samples were collected preoperatively (immediately after induction of general anaesthesia), on postoperative day 0 (within the first 2 h after surgery) and postoperative day 1 (between 16 and 24 h postoperatively). One millilitre of whole blood was obtained and stored in EDTA (potassium ethylene diamine tetra-acetic acid) tubes. These samples, stored at room temperature, were centrifuged within 4 h or within 24 h when they were stored at 4°C. Each sample was centrifuged at 3000g (4°C, 15 min) and stored at −20°C. The BNP level (pg/ml) was quantified by Chemiluminescent Microparticle Immunoassay (CMIA) using Architect i2000 analysers (Abbott Diagnostics).
Echocardiography protocol
Echocardiography was performed preoperatively following anaesthetic induction and on postoperative day 1 with concurrent blood sampling to measure the following:
biventricular systolic function using tissue Doppler imaging: real-time peak systolic myocardial velocities were measured at the triscuspid annulus [STV], mitral annulus [SMV] and basal septum [SS]. The mean velocity (cms−1) was obtained from three cardiac cycles. The postoperative fractional area change, % [(RV area diastole − RV area in systole)/RV area in diastole × 100%] was calculated from an apical four-chamber view,
restrictive RV physiology, defined as the antegrade flow across the pulmonary artery coincident with the atrial systole in all respiratory cycles [5],
PR severity was assessed by the width of the regurgitant jet and the level of the diastolic retrograde flow seen in the para-sternal short-axis RVOT view: branch PA (severe PR), main PA (moderate) and RVOT (mild) [4, 6]. RV dimension was quantified by the RV end-diastolic area index (RVEDAI) [4].
RV dyssynchrony: segmental dyssynchrony scored using the previously described method. It represents the total number of dyssynchronous segments in the RV free wall (0–3 segments) and the interventricular septum (0–3 segments) as assessed using tissue Doppler imaging [4].
Those patients with a significant residual RV outflow tract obstruction (RVOTO) postoperatively were excluded from subsequent analyses.
Postoperative monitoring and outcome measures
All patients had routine monitoring and measurement of lactate and mixed venous oxygen saturation (SvO2). As SvO2 is a function of systemic arterial oxygen saturation (SaO2), which can be affected by various factors including right-to-left intra-cardiac shunt and lung pathology, the oxygen extraction ratio [OER=(SaO2 − SvO2)/SaO2] represents a better indicator of systemic perfusion [7]. Oxygen saturation was measured from concurrent blood sampling taken from the peripheral arterial line and the central venous catheter [7]. The inotrope score was calculated based on Wernovsky et al. [8]. Outcome measures also included the duration of ventilation and critical care (intensive and high dependency unit) stay.
Statistical analysis
Comparisons of BNP levels over time were done using Friedman tests with follow-up Wilcoxon tests between time points if appropriate, to determine where difference exists. Relationships between continuous variables were investigated using the Spearman's rank correlation (rho). Statistical analyses were performed using Minitab® Statistical Software Release 15 at a significance level of 5%.
RESULTS
Perioperative B-type natriuretic peptide
The median preoperative BNP was 23.5 pg/ml (interquartile range 12.8–39.3), and, by using a cut-off point of 30 pg/ml, 39% (7/18 patients) had elevated BNP. Four patients (22%) had very low BNP values of 9 pg/ml preoperatively.
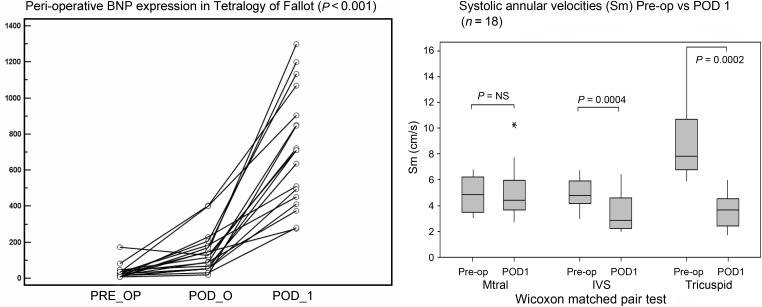
Following surgery, the median BNP (interquartile range) was elevated on postoperative day 0 (122.0, 54.8–191.8) (5-fold) and (711.5, 443–946.8) on POD1 (40-fold rise) (P < 0.001) (Fig. 1, left). The Wilcoxon test showed a significant rise between pre-operative vs POD0 BNP (P < 0.0001) and POD0 vs POD1 BNP (P < 0.0001).
Figure 1:
(Left) BNP levels for each individual patient from the preoperative day to post-op days 0 (POD 0) and 1 (POD 1) showing an initial rise immediately after surgery and a further rise on postoperative day 1 (P < 0.001). (Right) Boxplots showing perioperative systolic annular velocities at mitral, tricuspid and basal septum.
One patient had a significant residual RV outflow tract obstruction (peak gradient of 74.9 mmHg) and was excluded from subsequent postoperative analysis (vs median 18.8 mmHg, interquartile range 11.9–30.0 in the remaining 17 patients).
Relationship between B-type natriuretic peptide and preoperative characteristics
There were no correlations between preoperative BNP and age, weight, haematocrit, oxygen saturation, BT shunt, systolic annular velocities (mitral, tricuspid), RV/septal wall thickness or post-operative BNP release (days 0 and 1).
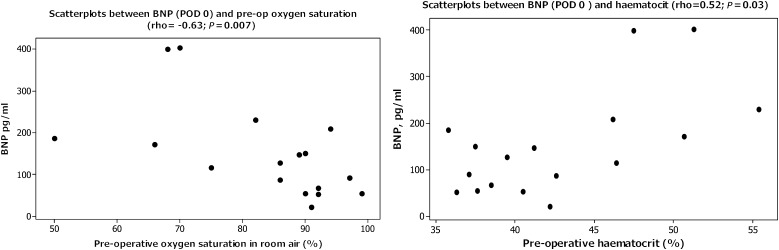
Immediate postoperative BNP was found to correlate with preoperative haematocrit (rho = 0.52, P = 0.03) and inversely with the preoperative oxygen saturation in room air (rho = −0.63, P = 0.007) (Fig. 2).
Figure 2:
Scatterplots showing correlation between immediate postoperative BNP release and preoperative oxygen saturation (Spearman rho = −0.63, P = 0.007) (left); and preoperative haematocrit (Spearman rho = 0.52, P = 0.03) (right).
Relationship between postoperative right ventricular function and B-type natriuretic peptide
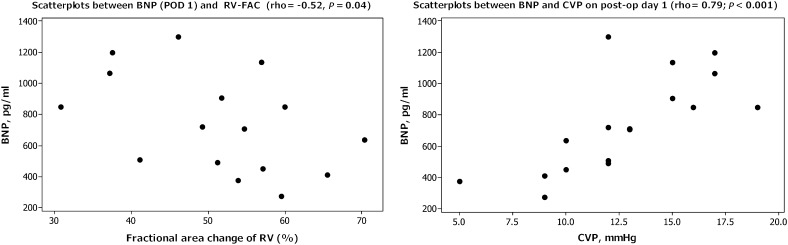
Postoperative systolic velocities were reduced at the tricuspid annulus and the basal septum but remained preserved at the mitral annulus (Fig. 1, right). By assessing the RV systolic function using the fractional area change, a lower postoperative (day 1) FACRV correlated with a higher postoperative day 1 BNP (rho = −0.51, P = 0.04), preoperative haematrocrit (rho = −0.50, P = 0.05), longer cardiopulmonary bypass time (rho = −0.57, P = 0.02) and higher lactate level (rho = −0.59, P = 0.016) (Fig. 3, left).
Figure 3:
Scatterplots showing correlation between BNP and fractional area change of RV (left) and CVP (right) on post-op day 1.
Restrictive RV physiology was seen in four (24%) patients and there was no difference in the BNP release on postoperative day 0 (P = 0.37) or day 1 (P = 0.43) in these patients.
Relationship between pulmonary regurgitation and B-type natriuretic peptide
Two-thirds of the patients (n = 12) developed PR: six (50%) had mild and six (50%) had moderate-to-severe degrees. The BNP level was not related to the presence of PR (711.5 without PR vs 849.0 pg/ml with PR; P = 0.8) or RVEDAI (P = 0.96). There were also no relations with the degree of PR (mild: 711.5, moderate: 637, severe PR: 959 pg/ml; P = 0.76). The BNP increased significantly from pre- to postoperative (days 0 and 1) in all PR severity groups: absent (P = 0.002), mild (P = 0.006) and moderate–severe (P = 0.007).
Relationship between abnormal wall motion and B-type natriuretic peptide
Postoperatively (day 1), all patients developed significant RV-septal wall dyssynchrony (median 2 segments vs 0 pre-op, P < 0.0001). The LV free wall motion remained synchronous pre- and postoperatively. The degree of RV dyssynchrony did not correlate with postoperative day 0 (P = 0.58) or day 1 (P = 0.09) BNP release.
Relationships between postoperative B-type natriuretic peptide and outcome measures
Postoperative day 1 BNP correlated with the oxygen extraction ratio (rho = 0.56, P = 0.02) and central venous pressure (rho = 0.79, P < 0.001) (Fig. 3, right panel). There were no correlations between postoperative BNP (day 0 and day 1) and lactate level, inotrope score, ventilation time or length of intensive care unit stay.
DISCUSSION
Preoperative B-type natriuretic peptide response in congenital heart patients
It is recognized that BNP in healthy individuals differs according to age groups and genders. Healthy neonates in the first 2 weeks of life express higher levels of BNP, which slowly regress with age [9]. In all healthy paediatric subjects (n = 152) more than 2 weeks old, BNP values were <32.7 pg/ml [9]. Koch and Singer [9] demonstrated that there were only gender differences in BNP values in those older than 10-years (girls have higher BNP than boys). Therefore, between 2-weeks and 10-years old, BNP values are generally stable without the influence of age and gender. As such, we did not need to perform any age or gender adjustment when comparing the BNP values in our study.
By taking 30 pg/ml as the cut-off for normal, ∼60% of Fallot's children had normal BNP levels preoperatively. It is recognized that the BNP level in the RV pressure overloading condition is significantly lower compared with left ventricular or biventricular volume overloading; the reason behind this remains unclear [10]. In fact, four patients (22%) in this study had a very low BNP value of 9 pg/ml. The production of BNP depends on ventricular wall tension, and it is possible that in tetralogy of Fallot, the RV wall stress represents a balance between severity of RVOTO and ‘off-loading’ by a non-restrictive VSD. It is clearly evident from this study that tetralogy of Fallot can present with normal BNP or even a very low BNP level preoperatively despite RV pressure overloading. Unlike the adult population with suspected LV failure where BNP can be used as a guide for subsequent imaging investigations, a normal BNP does not exclude congenital heart disease in the paediatric population, and this is especially true for tetralogy of Fallot patients.
B-type natriuretic peptide expression in tetralogy of Fallot after corrective surgery
BNP rose significantly in the first 24 h following a corrective Fallot's repair. The initial BNP rise was followed by a more dramatic BNP response 16–24 h later. Unlike ANP, the basal storage of BNP is minimal and requires time for gene activation and de novo synthesis [11]. This may explain why a peak level is not seen immediately after surgery. A later BNP rise could also be due to the progressive decline in ventricular function and cardiac output with a typically maximal nadir at 9–12 h following the cardiopulmonary bypass [8].
The immediate postoperative BNP level correlated with pre-operative haematocrit and inversely with preoperative oxygen saturation. The underlying pathophysiology remains conjectural, but possible explanations include a longer ischaemic time, a greater degree of RV dysfunction and a larger loading shift after the relief of more severe RVOTO in patients with greater cyanosis preoperatively. Cyanosis could increase myocellular susceptibility to oxygen-mediated ischaemic–reperfusion injury during surgery and therefore cause a greater degree of right heart dysfunction [12]. Our data showed that cyanotic patients demonstrated a higher degree of RV systolic dysfunction, and postoperative BNP correlated significantly with postoperative RV fractional area change. BNP was also associated with a higher CVP and oxygen extraction ratio. The latter indicates lower mixed venous oxygen saturation and poorer systemic perfusion. As LV function remained preserved on postoperative day 1, the two parameters would reflect the degree of RV dysfunction.
In a late Fallot's study, BNP correlated with severity of PR, and significantly diminished RV following pulmonary valve replacement [13]. Our data suggested that PR per se is not responsible for stimulating BNP production as acute BNP rises were also seen in the absence of PR. This early postoperative BNP level is several folds higher than previously reported late after TOF repair. Unlike the late Fallot's study, an acute BNP rise in the early postoperative period is associated with additional stressors, including cardiopulmonary bypass and ischaemic–reperfusion injury. The degree of postoperative fractional area change in RV in our patients was associated with the length of cardiopulmonary bypass time and level. The acute elevation of BNP following cardiac surgery has been linked to its cytoprotecitve role, including coronary vasodilatation and salvage from irreversible ischaemic–reperfusion injury following the cardiopulmonary bypass in addition to its natri-diuretic properties [14]. A significant BNP elevation despite the correction of abnormal loading in patients with VSD/ASD suggests that the cardiopulmonary bypass may be a potential stimulus to BNP production, which may play an important protective role against cardiopulmonary bypass induced injury in the perioperative period [1, 14].
CONCLUSION
The early postoperative RV is characterized by abnormal contractility, relaxation, wall motion and loading conditions in tetralogy of Fallot, while the LV function and wall motion remains preserved in all patients. Although we did not show any relationship between PR and BNP, it cannot be excluded in this study.
The postoperative RV physiology in tetralogy of Fallot is complex, and the mechanism of BNP expression is likely to be multifactorial. An acute BNP response in the early postoperative period reflects an important physiological role and may be used as an adjunct biomarker to assess the RV function.
Funding
Funding was provided by the Yorkhill Children's Foundation.
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
We wish to thank the following individuals for their assistance in this study: F. Lyall, J. Pollock, K. MacArthur, N. Morton, S. Lilley, P. Galloway (Royal Hospital for Sick Children, Glasgow) and N. Yonan (University of Manchester).
REFERENCES
- 1.Mainwaring RD, Parise C, Wright SB, Juris AL, Achtel RA, Fallah H. Brain natriuretic peptide levels before and after ventricular septal defect repair. Ann Thorac Surg. 2007;84:2066–9. doi: 10.1016/j.athoracsur.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 2.Hsu J-H, Keller RL, Chikovani O, Cheng H, Hollander SA, Karl TR, et al. B-type natriuretic peptide levels predict outcome after neonatal cardiac surgery. J Thorac Cardiovasc Surg. 2007;134:939–45. doi: 10.1016/j.jtcvs.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Peng EW, McCaig D, Pollock JC, MacArthur K, Lyall F, Danton MH. Myocardial expression of heat shock protein 70i protects early postoperative right ventricular function in cyanotic tetralogy of Fallot. J Thorac Cardiovasc Surg. 2011;141:1184–91. doi: 10.1016/j.jtcvs.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 4.Peng EW, Lilley S, Knight B, Sinclair J, Lyall F, Macarthur K, et al. Synergistic interaction between right ventricular mechanical dyssynchrony and pulmonary regurgitation determines early outcome following tetralogy of Fallot repair. Eur J Cardiothorac Surg. 2009;36:694–702. doi: 10.1016/j.ejcts.2009.02.061. [DOI] [PubMed] [Google Scholar]
- 5.Cullen S, Shore D, Redington A. Characterization of right ventricular diastolic performance after complete repair of tetralogy of Fallot. Restrictive physiology predicts slow postoperative recovery. Circulation. 1995;91:1782–9. doi: 10.1161/01.cir.91.6.1782. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg SJ, Allen HD. Quantitative assessment by Doppler echocardiography of pulmonary or aortic regurgitation. Am J Cardiol. 1985;56:131–5. doi: 10.1016/0002-9149(85)90581-8. [DOI] [PubMed] [Google Scholar]
- 7.Martin J, Shekerdemian LS. The monitoring of venous saturations of oxygen in children with congenitally malformed hearts. Cardiol Young. 2009;19:34–9. doi: 10.1017/S1047951109003539. [DOI] [PubMed] [Google Scholar]
- 8.Wernovsky G, Wypij D, Jonas RA, Mayer JE, Jr, Hanley FL, Hickey PR, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226–35. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 9.Koch A, Singer H. Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89:875–8. doi: 10.1136/heart.89.8.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantinotti M, Vittorini S, Storti S, Prontera C, Murzi M, De Lucia V, et al. Diagnostic accuracy and clinical relevance of brain natriuretic peptide assay in pediatric patients with congenital heart diseases. J Cardiovasc Med (Hagerstown) 2009;10:706–13. doi: 10.2459/JCM.0b013e32832c15fb. [DOI] [PubMed] [Google Scholar]
- 11.Hall C. Essential biochemistry and physiology of (NT-pro)BNP. Eur J Heart Fail. 2004;6:257–60. doi: 10.1016/j.ejheart.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 12.del Nido PJ, Mickle DA, Wilson GJ, Benson LN, Coles JG, Trusler GA, et al. Evidence of myocardial free radical injury during elective repair of tetralogy of Fallot. Circulation. 1987;76:V174–9. [PubMed] [Google Scholar]
- 13.Dodge-Khatami A, Buchel EV, Knirsch W, Kadner A, Rousson V, Dave HH, et al. Brain natriuretic peptide and magnetic resonance imaging in tetralogy with right ventricular dilatation. Ann Thorac Surg. 2006;82:983–8. doi: 10.1016/j.athoracsur.2006.03.038. [DOI] [PubMed] [Google Scholar]
- 14.Koch A, Kitzsteiner T, Zink S, Cesnjevar R, Singer H. Impact of cardiac surgery on plasma levels of B-type natriuretic peptide in children with congenital heart disease. Int J Cardiol. 2007;114:339–44. doi: 10.1016/j.ijcard.2006.01.022. [DOI] [PubMed] [Google Scholar]