Abstract
OBJECTIVES
The aim of the study was to compare diagnostic utility of combined (i.e. transbronchial and transoesophageal) ultrasound imaging with needle biopsy of the mediastinum in lung cancer (LC) staging, (a) by use of a single ultrasound bronchoscope (CUSb) and (b) by using two scopes (CUS).
METHODS
In consecutive LC patients, clinical stage IA-IIIB the CUS or CUSb was performed under mild sedation and, if negative, underwent lung resection with confirmatory systematic lymph node dissection.
RESULTS
From 214 LC patients, 110 underwent CUS and 104 underwent CUSb (618 biopsies); both revealed metastases in 50% of cases. There was ‘minimal N2’ in 11 of 14 false negative patients. Diagnostic sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) of CUS was 91.7%, 98%, 94.6%, 98.2% and 90.7% respectively and of CUSb was 85%, 93.2%, 88.5%, 94.4%, 82%, respectively with no significant difference in yield of CUS vs CUSb (P = 0.255 and P = 0.192). The mean time of CUS (25 ± 4.4 min) was significantly longer as compared to CUSb (14.9 ± 2.3 min) (P < 0.001). No severe complications of either method were observed.
CONCLUSIONS
The combined ultrasound imaging of the mediastinum by use of CUSb is significantly less time-consuming and equally as effective and safe as the use of CUS for LC staging.
Keywords: Combined ultrasound-needle aspiration, Mediastinum, Lung cancer, Staging
INTRODUCTION
Although real-time endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) and endoscopic ultrasound guided fine needle aspiration (EUS-FNA) are techniques providing a minimally-invasive alternative for surgical staging with high efficacy [1–5], mediastinoscopy is still regarded by many authors as a gold standard [6, 7]. Owing the complementary reach of EBUS-TBNA and EUS-FNA in assessing the anterior and posterior regions of the mediastinum, recent studies suggest that complete and accurate mediastinal staging can be achieved by the combination of both procedures [8–12]. This approach of combined ultrasound imaging with use of EBUS and EUS with needle biopsy is also referred to as ‘combined ultrasound needle aspiration’ (CUS-NA) [10].
The use of ultrasound bronchoscope for the transoesophageal imaging and biopsy of mediastinal lymph nodes, together with EBUS-TBNA (termed CUSb), was only reported in a few publications [11, 12]. However, it has never been directly compared to the CUS performed using two ultrasound endoscopes.
MATERIAL AND METHODS
Clinical question
Is there a significant difference in diagnostic yield between CUS-NA and CUSb-NA for mediastinal N staging of lung cancer?
What is the value of CUS and CUSb for T and M staging?
Design
Prospective non-randomized diagnostic study.
Location
Endoscopy Unit, John Paul II Hospital and Department of Thoracic Surgery, Jagiellonian University, Krakow, Poland.
Patients
Inclusion criteria: a group of consecutive lung cancer patients (i) clinical stage IA–IIIB, (ii) with normal-sized or enlarged mediastinal lymph nodes seen on CT scans, and (iii) general condition enabling appropriate pulmonary resection.
Exclusion criteria: lack of patient's consent.
Intervention
All procedures were performed under local anaesthesia and intravenous sedation (fentanyl 0.05–0.1 mg, midazolam 1–5 mg).
At first, the EUS-FNA was performed with patient positioned in the left lateral decubitus position. The EUS-FNA was performed using the GF-UCT160-OL5 videogastroscope (Olympus Medical Systems Corporation, Tokyo, Japan). The videogastroscope's outer diameter is 14.6 mm, it has a 3.7 mm working channel, a 55° optical system and an EU-C60 7.5 MHz ultrasound processor, enabling 20–50 mm depth tissue imaging. For the biopsy a cytological, 80 mm long, 22G needle with guide wire and marking to facilitate its visualization on the ultrasound image was used (NA-200H-8022, Olympus Medical Systems Corporation, Tokyo, Japan).
The EUS-FNA of all lymph nodes ≥5 mm on the short axis were performed (criterion of feasibility of lymph node biopsy according to Annema [4]). The number of biopsied stations in one patient was 1–3 and the number of biopsied nodes in one station was 1–5. After completing EUS-FNA, the patient was turned over onto his back and intravenous sedation was added if necessary.
The EBUS-TBNA was performed using the BF-UC160F-OL8 or BF-UC180F-OL8 videobronchoscope (Olympus Medical Systems Corporation, Tokyo, Japan). The videobronchoscopes are 6.9/7.0 mm wide, have a 2/2.2 mm working channel, a 35° optical system and are connected with the EU-C60 7.5 MHz or the EU-ME1 5–12 MHz ultrasound processor. For the biopsy a cytological, 40 mm long, 22G needle with guide wire and marking to facilitate its visualization on the ultrasound image was used (NA-201SX-4022, Olympus Medical Systems Corporation, Tokyo, Japan).
The EBUS-TBNA of all lymph nodes ≥5 mm on the short axis were performed (criterion of feasibility of lymph node biopsy according to Herth [1]). All biopsies were performed through the macroscopically normal bronchial wall. The number of biopsied stations in one patient was 1–3 and the number of biopsied nodes in one station was 1–5.
The cytological smear of all biopsies was performed and fixed using 96% ethanol and standard haematoxillin-eosin staining was used. Rapid on-site cytology was not performed.
For the CUSb, EBUS-TBNA was performed first, as described above, then the EBUS scope was inserted into the oesophagus and transoesophageal endoscopic ultrasound by EBUS scope was performed. After completing EBUS-TBNA, the patient was not turned over but remained in the supine position and intravenous sedation was added if necessary.
The left adrenal glands were examined by use of EBUS and EUS scope if enlarged on CT scans (M-staging) and the biopsy of adrenals ≥5 mm on the short axis was performed (criterion of feasibility of left adrenal biopsy according to Annema [5]). The examination of potential neoplasmatic infiltration of the aorta, pulmonary artery and left atrium was performed by EBUS and EUS (T-staging). The margin enabling appropriate lung resection was examined by use of EBUS scope.
In patients with negative results of the CUS-NA or CUSb-NA, an appropriate pulmonary resection with the systemic lymph node dissection (SLND) of the mediastinal nodes was performed. The extent of the mediastinal dissection corresponded to the systematic lymph node dissection.
The Mountain–Dresler lymph node classification was used [13].
Statistical analysis
The sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) (including 95% CI) were calculated using GraphPad InStat 3.05 software using the standard definitions.
The classical significance test for difference between two proportions was used for independent and dependent samples. For partially-dependent samples, the bootstrap test based on confidence interval for difference of two proportions was used.
The level of significance was set at ≤0.05.
RESULTS
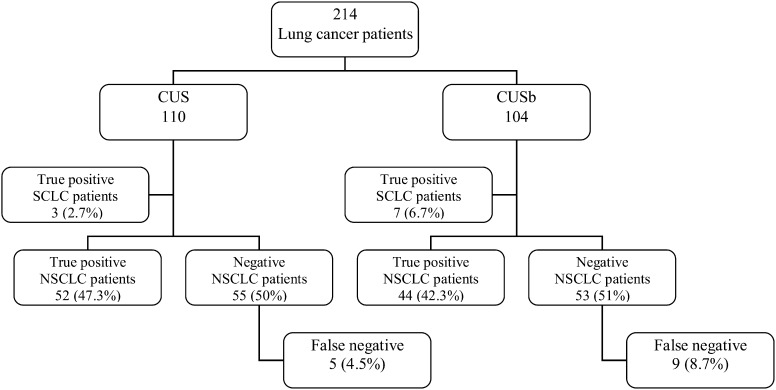
From 01 March 2010 to 31 December 2010, 214 lung cancer patients were enrolled in the study (Fig. 1). Among these, 110 patients underwent CUS and 104 underwent CUSb. In total, 618 mediastinal nodes were biopsied (2.9 nodal station biopsies per patient). In the CUS group, 320 mediastinal stations were biopsied: station 7, n = 134; 2R/4R, n = 67; 2L/4L, n = 88; 8, n = 24; 9, n = 6 and 5, n = 1. In the CUSb group, 298 mediastinal stations were biopsied: station 7, n = 124; 2R/4R, n = 71; 2L/4L, n = 84; 8, n = 12; 9, n = 4 and 5, n = 3.
Figure 1:
A flow chart of consecutive lung cancer patients for staging by use of CUS and CUSb.
The metastatic involvement of the lymph node for the CUS group was confirmed in 200 stations and for the CUSb group in 180 stations (in some patients in both groups, more than one station was involved). The numbers of metastatic nodes in particular stations were for the CUS group: station 7, n = 82; 2R/4R, n = 40; 2L/4L, n = 59; 8, n = 17; 9, n = 2 and for the CUSb group: station 7, n = 78; 2R/4R, n = 38; 2L/4L, n = 56 and 8, n = 8.
In the CUS group, there were 75 men and 35 women in the mean age 64.7 ± 9.5 years and in the CUSb group there were 75 men and 29 women in the mean age 62.7 ± 7.9 years. The mean diameters of the biopsied nodes were 12.7 ± 4.5 mm in the long axis and 8.7 ± 3.3 mm in the short axis. The CUS revealed metastatic nodal involvement in 55/110 patients (50%) and CUSb in 51/104 patients (49%). The prevalence was 60% in both groups. In both groups either CUS or CUSb confirmed nodal metastases of small-cell lung cancer in all 110 patients. In 55 CUS-negative (50%) and 53 (51%) CUSb-negative non-small-cell lung cancer patients, the subsequent SLND revealed metastatic nodes in five patients (4.5%) and in nine patients (8.7%), respectively, but with no statistical difference (P = 0.203). There was ‘minimal N2’ in 11 out of these 14 patients (5.1%)—five in the CUS group and six in the CUSb group—with no predominance for any nodal station (except three patients with right upper lobe tumour, all with false negative results in station 2R/4R) (Table 1). In three patients with false negative results of CUSb, a multilevel N2 was diagnosed by means of SLND (Table 2). Diagnostic sensitivity, specificity, accuracy, PPV and NPV of CUS was 91.7%, 98%, 94.6%, 98.2% and 90.7% respectively and of CUSb was 85%, 93.2%, 88.5%, 94.4% and 82%, respectively. There was no significant difference in sensitivity and NPV of CUS vs CUSb (P = 0.255 and P = 0.192) (Tables 3 and 4).
Table 1:
The non-small-cell lung cancer patients with false-negative results of CUS-NA and CUSb-NA of single mediastinal station
| CUS-NA |
CUSb-NA |
||||
|---|---|---|---|---|---|
| Patient | Tumour location | Metastatic lymph node station | Patient | Tumour location | Metastatic lymph node station |
| 1 | RUL | 4R | 1 | RUL | 2R |
| 2 | LUL | 4L | 2 | RUL | 4R |
| 3 | LUL | 5 | 3 | LUL | 7 |
| 4 | LUL | 7 | 4 | RLL | 7 |
| 5 | LLL | 8 | 5 | LLL | 4L |
| 6 | LLL | 5 | |||
RUL: right upper lobe; LUL: left upper lobe; RLL: right lower lobe; LLL: left lower lobe.
Table 2:
Three non-small-cell lung cancer patients with false-negative results of CUSb-NA and multilevel N2 or N3 disease
| Patient/location of the tumour | Lymph node station | Number and percentage of metastatic lymph nodes in the station |
|---|---|---|
| 1. (2-level N2 disease)/RLL | 9 | 1/4 (25%) |
| 7 | 1/6 (16.7%) | |
| 2. (3-level N3 disease)/LUL | 4R | 2/4 (50%) |
| 7 | 2/5 (40%) | |
| 6 | 2/4 (50%) | |
| 3. (2-level N2 disease)/LLL | 9 | 1/1 (100%) |
| 5 | 1/5 (20%) |
RLL: right lower lobe; LUL: left upper lobe; LLL: left lower lobe.
Table 3:
A comparison of yields per patient of CUS and CUSb techniques
| Diagnostic yield per patient | Bioptic technique |
||
|---|---|---|---|
| CUS | CUSb | P > 0.05 (ns) | |
| Sensitivity | 91.7 | 85 | P = 0.255 (ns) |
| Specificity | 98 | 93.2 | P = 0.248 (ns) |
| Accuracy | 94.6 | 88.7 | P = 0.108 (ns) |
| PPV | 98.2 | 94.4 | P = 0.291 (ns) |
| NPV | 90.7 | 82.7 | P = 0.192 (ns) |
ns: not significant.
Table 4:
A comparison of yields per station of CUS and CUSb techniques
| Diagnostic yield per station/zone | Right paratracheal stations 2R/4R |
Subcarinal station (7) |
Left paratracheal stations 2L/4L |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Bioptic technique | CUS | CUSb | P > 0.05 (ns) | CUS | CUSb | P > 0.05 (ns) | CUS | CUSb | P > 0.05 (ns) |
| Sensitivity | 97.6 | 92.7 | ns | 98.8 | 95.1 | ns | 98.3 | 98.2 | ns |
| Specificity | 100 | 93.3 | ns | 98.0 | 100 | ns | 100 | 96.3 | ns |
| Accuracy | 98.5 | 93.0 | ns | 98.5 | 96.8 | ns | 98.9 | 97.6 | ns |
| PPV | 100 | 95.0 | ns | 98.8 | 100 | ns | 100 | 98.2 | ns |
| NPV | 96.3 | 90.3 | ns | 98.0 | 91.3 | ns | 96.5 | 96.3 | ns |
ns: not significant.
In the CUS group, enlarged left adrenal glands were examined by use of EUS gastroscope in six patients (5.5%), which impacted on surgery in all of them. In two (1.8%) of them metastases were diagnosed (M1) and in four (3.7%) of them benign adenomas were discovered. In the CUSb group, in none of the five patients with enlarged left adrenal glands was the examination performed by use of EBUS scope successful. In the CUS group, the examination changed T-staging in eight patients (7.5%): in three of them aorta infiltration; in one, left atrium infiltration; in one, pulmonary artery infiltration was observed by use of EUS and in the remaining three by use of EBUS. Moreover, in the CUS group, EBUS confirmed sufficient margin enabling appropriate lung resection in the next seven patients (6.4%). In the CUSb group, the examination changed T-staging in five patients (5.1%): in four of them, pulmonary artery infiltration was observed by use of EBUS and, in one, left atrium infiltration. In the CUSb group, EBUS confirmed sufficient margin enabling appropriate lung resection in the next five patients (4.8%).
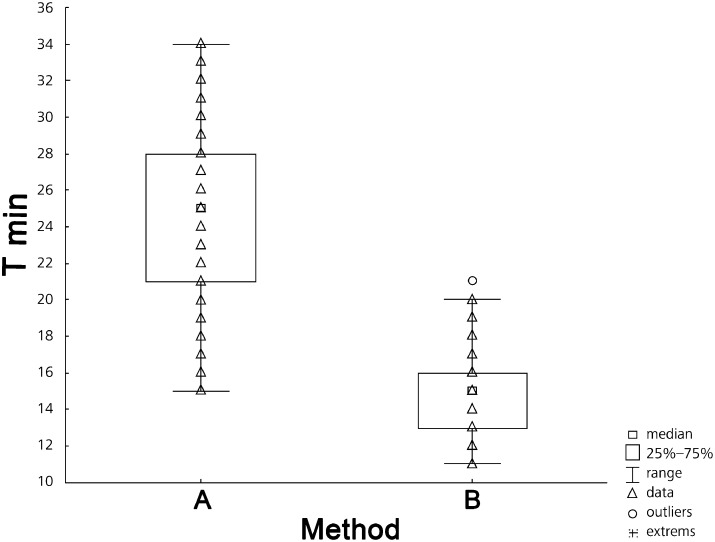
The mean time to carry out CUS (25 ± 4.4 min) was significantly longer, compared to CUSb (14.9 ± 2.3 min) (P <0.001) (Fig. 2).
Figure 2:
A comparison between the time of combined procedures CUS-NA (A) and CUSb-NA (B).
No severe complications of either method were observed. Slight bleeding in the place of biopsy was not considered as a complication. After standard EUS (CUS method) nausea was observed in two patients and weak, self-limiting abdominal pain in three patients.
DISCUSSION
The current ACCP and ESTS guidelines for lung cancer staging suggest that patients with abnormal lymph nodes on CT or PET, or centrally located tumours without mediastinal adenopathy, should undergo invasive staging [6, 7]. Even though the most common invasive method is mediastinoscopy, EBUS-TBNA and EUS-FNA have become reasonable approaches. Several trials utilizing a combination of both methods for lung cancer staging have shown a higher diagnostic yield than each method alone [9, 14, 15]. The main reason for this is that EUS-FNA complements the anterior mediastinal access of EBUS-TBNA and most of the mediastinal lymph node stations (except stations 1, 3a, 5 and 6) can be easily visualized and biopsied. In one prospective trial CUS was presented as a highly effective and safe method in the radiologically-normal mediastinum with significantly higher sensitivity and NPV, as compared to EBUS-TBNA and EUS-FNA alone [10]. There were only a few studies which also presented the superiority of a combination of both methods by use of one EBUS bronchoscope (CUSb) [11, 12]. In the current study we directly compare both combined methods: CUS-NA and CUSb-NA. A diagnostic yield of CUS for N-staging was higher than CUSb but the difference did not reach a level of any significance. There was no significant difference in sensitivity and NPV of CUS (91.7%, 90.7%) versus CUSb (85%, 82%), (P = 0.255 and P = 0.192), respectively. Although both methods seem to be reasonable and highly efficient for N-staging, according to these results a randomized trial is needed. In the current study we found a mean time to carry out CUSb (14.9 ± 2.3 min) significantly lower than CUS (25 ± 4.4 min) (P < 0.001). It results in a positive influence on patients’ comfort and cost-effectiveness of minimally-invasive procedures. The rate of false negative biopsies of CUS-NA was 4.5%, which is lower—but not significantly—than that of CUSb-NA (8.7%), (P = 0.203). In our series there was neither predominance for any nodal station, nor for tumour location, in either method observed. Multilevel N2 disease was missed only in patients in CUSb group. In our study we also discovered the importance of M-staging by use of CUS method, as EUS findings of left adrenal gland impacted on surgery in 5.5% of patients. A diagnosis of adrenal metastases was based on a positive cytological result of biopsy and a diagnosis of benign adenoma was based on negative cytology and, additionally, on typical normoechogenity of the ultrasound images. As the risk of complications related to CUS and CUSb is very low, it is reasonable to perform combined procedures in an outpatient setting.
CONCLUSIONS
The combined ultrasound of the mediastinum by use of a single EBUS bronchoscope (CUSb) is significantly less time-consuming and equally as effective and safe as the use of two endoscopes (CUS) for lung cancer N staging.
Unless the imaging of the left adrenal gland is required, the CUSb technique may become the standard endoscopic approach for mediastinal lung cancer staging.
APPENDIX. CONFERENCE DISCUSSION
Dr F. Detterbeck (New Haven, CT, USA): I have a comment and a question. Artur is a real master at this. I have watched him do this before and I think there are very few people that can manipulate both an endoscope and a bronchoscope – and do this as quickly and as efficiently and as thoroughly. The skill and the thoroughness with which one uses these techniques, as with others, is important and this is a significant advance to show that you can do the same with a bronchoscope in the oesophagus and achieve very good results. My question is, how easily do you think this can be disseminated? How easily can people pick this up and do this really well?
Dr Szlubowski: There have been some papers about the learning curve in EBUS, showing that after 40, maybe 50, endoscopies there is some kind of plateauing of the results. In my experience, there was such a situation because, after two years, I had more false negatives because maybe I performed this kind of staging too fast. But there are papers showing that 50 performances with EBUS is enough to reach some kind of plateau.
The same situation exists with EUS—I mean a standard EUS, of course—if you first have some experience with the ultrasound, with gastroscopy and with performing both, and first EBUS. Then after learning the EUS technique, it is very easy to standardize the performance of EUSb using the bronchoscope because the bronchoscope is adapted to make suction. So you just close the oesophagus; you don't penetrate the stomach and this is the technique of withdrawing of the scope. You just withdraw the scope, slow movements, and it is very easy access, first to stations 7 and 4L, and next for the others. Because I think stage T, it seems to be reasonable not to express that this is for T-staging, but after some experience, we can have some observations about T-stage as well. But it is described in the literature that this is about 78% of sensitivity using only echo-sonography without biopsy. But you have to rely very much on the experience.
Dr S. Bolukbas (Wiesbaden, Germany): I have a technical question. Do you use new needles if you puncture different sites of lymph nodes?
Dr Szlubowski: It depends. You have to think from the beginning when you start the staging from N3, N2, N1 disease. So sometimes if I have the tumour on the right side, I start with EUSb and then make a puncture of station 4L, for example, station 8, and then I can go with the same needle to station 7 using EBUS. But of course, it should be done by using more or less two needles. The first one should be used in the oesophagus and the second one in the trachea and bronchial tree. But these needles are not sufficient to make more than five, six biopsies. So if you want to have a proper biopsying process, you need to change the needle.
Dr Bolukbas: However, if you do punctures at different sites at the mediastinum, there is a possibility of tumour spread. That means you have maybe a single station N2 disease, but with your puncture you might detect multilevel N2 disease and, if you always use a new needle, you can reduce the risk.
Dr Szlubowski: Yes, we made such a comparison and it showed there was no significant difference between using one or both. It depended mainly on that strategy, how do you perform. You have to not forget that you can contaminate the material.
Dr J. Kuzdzal (Krakow, Poland): One short comment regarding what Dr. Detterbeck said. From an historical point of view, the bronchoscope was generally used by pulmonologists and thoracic surgeons, whilst on the other hand, oesophageal endoscopy by gastroenterologists and general surgeons. So it is only from this historical ground difficult for one person to combine both the skills in one hand. But I think that we as thoracic surgeons are a unique population, a unique group of surgeons, who are accustomed to both: oesophageal and bronchial and tracheal endoscopy. So I think we are the most suitable group of people to train and to perform the EBUS and EUS together.
REFERENCES
- 1.Herth FJ, Eberhardt R, Vilmann P, Krasnik M, Ernst A. Real-time endobronchial ultrasound guided transbronchial needle aspiration for sampling mediastinal lymph nodes. Thorax. 2006;61:795–8. doi: 10.1136/thx.2005.047829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szlubowski A, Kuzdzal J, Kolodziej M, Soja J, Pankowski J, Obrochta A, et al. Endobronchial ultrasound-guided needle aspiration in the non-small cell lung cancer staging. Eur J Cardiothorac Surg. 2009;35:332–5. doi: 10.1016/j.ejcts.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Micames CG, McCrory DC, Pavey DA, Jowell PS, Gress FG. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging. A systematic review and metaanalysis. Chest. 2007;131:539–48. doi: 10.1378/chest.06-1437. [DOI] [PubMed] [Google Scholar]
- 4.Annema JT, Versteegh MI, Veselic M, Voigt P, Rabe KF. Endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of lung cancer and its impact on surgical staging. J Clin Oncol. 2005;23:8357–61. doi: 10.1200/JCO.2005.01.1965. [DOI] [PubMed] [Google Scholar]
- 5.Annema JT, Rabe KF. EUS in Non-Small Cell Lung Cancer. Hawes H, Fockens P. Endosonography. Saunders Elsevier. 2006;7:61–72. [Google Scholar]
- 6.Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA. Invasive Mediastinal Staging of Lung Cancer. ACCP Evidence-Based Clinical Practice Guidelines. Chest. 2007;132:202S–20S. doi: 10.1378/chest.07-1362. [DOI] [PubMed] [Google Scholar]
- 7.De Leyn P, Lardinois D, Van Schil P, Rami-Porta R, Passlick B, Zielinski M, et al. ESTS. European trends in preoperative and intraoperative nodal staging: ESTS guidelines. J Thorac Oncol. 2007;2:357–61. doi: 10.1097/01.JTO.0000263722.22686.1c. [DOI] [PubMed] [Google Scholar]
- 8.Herth FJ, Rabe KF, Gasparini S, Annema JT. Transbronchial and transoesophageal (ultrasound-guided) needle aspirations for the analysis of mediastinal lesions. Eur Respir J. 2006;28:1264–75. doi: 10.1183/09031936.00013806. [DOI] [PubMed] [Google Scholar]
- 9.Vilmann P, Puri R. The complete ‘medical’ mediastinoscopy (EUS-FNA + EBUS-TBNA) Minerva Med. 2007;98:331–8. [PubMed] [Google Scholar]
- 10.Szlubowski A, Zieliński M, Soja J, Annema JT, Sośnicki W, Jakubiak M, et al. A combined approach of endobronchial and endoscopic ultrasound-guided needle aspiration in the radiologically normal mediastinum in non-small-cell lung cancer staging–a prospective trial. Eur J Cardiothorac Surg. 2010;37:1175–9. doi: 10.1016/j.ejcts.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Hwangbo B, Lee GK, Lee HS, Lim KY, Lee SH, Kim HY, et al. Transbronchial and transesophageal fine-needle aspiration using an ultrasound bronchoscope in mediastinal staging of potentially operable lung cancer. Chest. 2010;138:795–802. doi: 10.1378/chest.09-2100. [DOI] [PubMed] [Google Scholar]
- 12.Herth FJ, Krasnik M, Kahn N, Eberhardt R, Ernst A. Combined endoscopic-endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes through a single bronchoscope in 150 patients with suspected lung cancer. Chest. 2010;138:790–4. doi: 10.1378/chest.09-2149. [DOI] [PubMed] [Google Scholar]
- 13.Mountain CF, Dresler CM. Regional lymph node classification for lung cancer staging. Chest. 1997;111:1718–23. doi: 10.1378/chest.111.6.1718. [DOI] [PubMed] [Google Scholar]
- 14.Wallace MB, Pascual JM, Raimondo M, Woodward TA, McComb BL, Crook JE, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA. 2008;299:540–6. doi: 10.1001/jama.299.5.540. [DOI] [PubMed] [Google Scholar]
- 15.Szlubowski A, Kużdżał J, Soja J, Hauer J, Hauer Ł, Pankowski J, et al. A combined approach of endobronchial and endoscopic ultrasound-guided needle aspiration in the non-small cell lung cancer staging – a prospective study. Am J Respir Crit Care Med. 2009;179:A1112. [Google Scholar]