Abstract
OBJECTIVES
We report the assessment and the activities for the first year of our airborne circulatory support mobile unit (CSMU) in the French Caribbean.
METHODS
From January 2010 to June 2011, 12 patients (mean age = 35.7 years; range: 15–62 years; sex ratio = 1:1) were attended outside Martinique by our CSMU and transferred to our unit by air.
RESULTS
Eight patients had acute respiratory distress syndrome and were assisted by veno-venous extra corporeal membrane oxygenation (ECMO) four had refractory cardiogenic shock, assisted by extra corporeal life support (ECLS). The average air transfer distance for patients was 912 km (range: 198–1585 km). The average flying time was 124 min (range: 45–255 min). The aircraft used were heliciopter, military transport or private jet. The setting-up of assistance devices and transfer of patients was uneventful. One patient subsequently benefited from heart transplantation after long-term circulatory support. One patient died under ECMO support after 51 days of assistance and another died on the 60th day after withdrawal of ECLS.
CONCLUSIONS
CSMUs can be very efficient in providing support to patients in refractory shock, when remote from a cardiac surgery centre. The airborne transfer of patients on ECMO/ECLS can be achieved safely, even over long distances.
Keywords: Circulatory support, ECMO, ECLS, Transportation, ARDS, Heart failure
Introduction
Extra corporeal membrane oxygenation (ECMO) is usually available only in specialized centres for cardiac surgery. Given the success rate of such techniques, it is worth considering whether this technique should not be more widespread. Some specialists certainly believe it should be available in all intensive care units or cardiology departments. At a large number of technical centres, including those handling small volumes, it had become obvious over the last few years—and especially since the outbreak of H1N1—that supply creates its own demand in health care. We can therefore contemplate making such an outstanding technique commonplace. The question: is this relevant, justified, realistic and desirable?
Guadeloupe, Saint Martin, Saint Barthelemy, Martinique and French Guiana (Guyane) are French overseas territories in the Caribbean area, hundreds of miles from each other and very far (more than 7000 km or 4400 miles) from the French motherland. The cardiac surgery department of the Fort de France University Hospital (Martinique, French West Indies) developed a circulatory support mobile unit (CSMU) to care for people from the French Caribbean with severe cardiogenic or pulmonary distress, who are highly catecholamine-dependent and unsuitable for conventional transportation. These patients' transfers under ECMO support were carried out by air.
This study aims to evaluate the feasibility of such a procedure and report the preliminary results and feedback of our experience, developed in a region which is unusual in terms of its socio-economic contrast, geographic isolation, remoteness and insularity: the Caribbean.
PATIENTS AND METHODS
Study population
This study reviewed all patients suffering from cardiocirculatory shock resistant to conventional critical care treatment, or respiratory failure resistant to advanced ventilation strategies, who were brought to our centre by air between January 2010 and June 2011 under ECMO support implanted by our CSMU outside Martinique. There were 12 such cases, among which four were assisted for cardiogenic shock and eight for respiratory failure (Table 1). In the same period, 43 patients were supported by ECMO in our centre for the same pathologies.
Table 1:
Study population
| Median / n | Range | |
|---|---|---|
| Age (years) | 42 | 15–63 |
| Male | 6 | |
| Veno-arterial ECMO (n = 4) | ||
| Age (years) | 58 | 55–63 |
| Male (n) | 2 | |
| Blood lactate (mmol/l) | 8.7 | 4–12 |
| Troponin | 10 | 8–12 |
| Bilirubin (μmol/l) | 26.5 | 20–33 |
| ASAT (UI/L) | 972 | 432–2230 |
| ALAT (UI/L) | 621 | 203–1867 |
| Prothrombin activity (%) | 53 | 41–65 |
| Creatinine (μmol/l) | 125 | 61–193 |
| Dobutamine (μg/kg/min) | 22.5 | 0–30 |
| Epinephrine (μg/kg/min) | 0.7 | 0–1.2 |
| Norepinephrine (μg/kg/min) | 0.5 | 0–0.75 |
| Veno-venous ECMO (n = 8) | ||
| Age (years) | 22 | 15–43 |
| Male (n) | 4 | |
| pH | 7.2 | 7.12–7.35 |
| PaO2 (mmHg) | 63.8 | 45–84 |
| PaCO2 (mmHg) | 68.7 | 57–85 |
| PaO2/FiO2 | 65 | 50–84 |
| TV (ml/kg) | 4.875 | 4.61–5.14 |
| PEEP (cmH2O) | 16 | 12–20 |
| NO (ppm) | 12 | 0–15 |
Inclusion criteria
We included all patients who were treated with emergency ECMO outside Martinique between January 2010 and June 2011 and, when stabilized, subsequently repatriated under ECMO support to our institution by the mobile unit (no patient implanted by our team was left in a remote institution).
Table 2 summarizes the criteria (based on the medical literature) used by our mobile unit for evaluating the need for ECMO. All patients supported by ECMO satisfied these criteria. Contra-indications criteria used for ECMO-implantation outside our institution were as follows: cardiopulmonary resuscitation in progress (very disappointing results despite the feasibility of implantation [1]); cardiac arrest without neurologic status evaluation; age greater than 65 years; terminal malignancy.
Table 2:
Indications criteria
| Criteria to discuss veno-venous ECMO |
|---|
| Acute circulatory failure with the following conditions: |
| (1) Systolic arterial pressure <90 mmHg or mean arterial pressure <50 mmHg |
| (2) Despite filling ≥1000 ml |
| (3) Inotropic support: |
|
| (4) Cardiogenic shock |
|
| Criteria to discuss veno-arterial ECMO |
| Acute respiratory failure with 1 of the following conditions: |
| (1) Refractory hypoxemia with PaO2 / FiO2 < 150 |
|
| (2) PlatP >35 cmH2O |
|
| (3) pH < 7.15 |
|
The decision to implant an ECMO was taken by a cardiac surgeon of the mobile unit, after discussion with the referent's cardiologist and our institution's critical care anaesthesiologists.
The circulatory support mobile unit.
The circulatory support mobile unit (CSMU) is a part of the cardiac surgery department. We have developed a telephone network linking all the emergency medical services, cardiology intensive care units and general intensive care units of the French West Indies, French Guiana and our ICU. We have round-the-clock monitoring and each of these services can contact our mobile unit to consider indications of the need for ECMO.
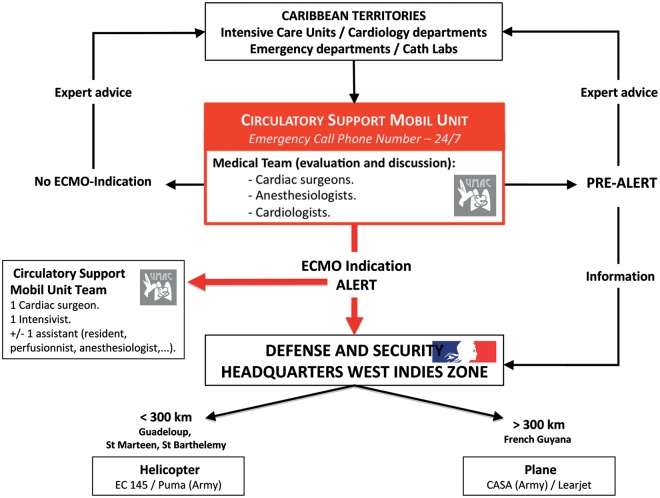
When the indication is validated, the three-person team that will implant the ECMO and repatriate the patient is assembled: a cardiac surgeon, an intensivist and an assistant (a perfusionist, a nurse, or an intern). Our mobile units have agreed activation procedures (Fig. 1) with intensive care mobile units and Defence and Security Headquarters West Indies zone, in order to reach the patient and to repatriate him or her to our unit at the Fort de France University Hospital as quickly as possible.
Figure 1:
Alert procedure.
Logistics
The means of transport used depended on the destination. According to our procedures, if the patient was in a hospital less than 300 km from our centre, we used a helicopter. In other situations, a fixed-wing aircraft was used.
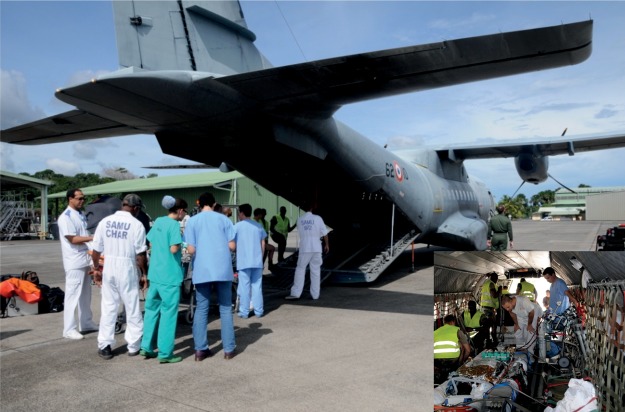
In the procedures established with the security headquarters French West Indies zone (État Major de Zone de Défense et de Sécurité), when requiring a helicopter, we used primarily the Civil Security's EC-145 Eurocopter (Eurocopter, Marignane, France). If this aircraft was not available, we used a Puma SA332 military type (Eurocopter, Marignane, France). For fixed-wing aircraft, we used military twin-engine transport types such as the CASA CN235 (Airbus Military, EADS, Leyde, Netherlands) (Fig. 2) or a private jet (Learjet, Bombardier, Quebec, Canada) according to availability.
Figure 2:
CASA : military aircraft.
In order to make the activation procedure easier, our mobile unit called the security headquarters directly. In a few minutes, the officer on duty organized all the logistics with our institution's intensive care mobile unit.
ECMO circuit and set-up
The extracorporeal system consisted of PVC tubing, a membrane oxygenator (Quadrox Bioline, Jostra-Maquet, Orléans, France; Medos, Le Locle, Suisse), a centrifugal pump (Rotaflow, Jostra-Maquet, Orléans, France; Levitronix, Waltham, MA, USA) and arterial (16–20 Fr) and venous (24–28 Fr) femoral cannulae (Edwards, France). When a veno-arterial configuration was chosen, vessels were surgically controlled through a transversal incision and cannulae were inserted using the Seldinger technique and an additional 7-Fr cannula was inserted distally into the femoral artery. In veno-venous configuration, an arterial cannula was inserted in the right jugular vein and the venous cannula was inserted in a femoral vein.
RESULTS
The results are summarized in Table 4 below.
Table 4:
Results
| Median / n | Range | |
|---|---|---|
| Distance of transfer (km) | 912 | 198–1585 |
| Flying time of transfer (min) | 124 | 45–255 |
| Aircraft used | ||
| Puma helicopter | 3 | |
| EC 145 helicopter | 2 | |
| Learjet | 3 | |
| CASA twin-engine transport | 4 | |
| Serious event during the transfer | 0 | |
| Time of ECMO-assist | 12 | 4–51 |
| ECMO weaning | 11 | |
| Hospital discharge | 10 | |
| Veno-arterial ECMO (n = 4) | ||
| Etiology of the cardiogenic shock | ||
| myocardial infarction | 2 | |
| dilated cardiomyopathy | 1 | |
| tight mitral stenosis | 1 | |
| Time of ECMO-assist | 6.8 | 4–10 |
| ECMO weaning | 4 | |
| Hospital discharge | 3 | |
| LVAD implantation | 1 | |
| Heart transplantation | 1 | |
| Veno-venous ECMO (n = 8) | ||
| Etiology of the ARDS | ||
| infectious pneumopathy | 4 | |
| H1N1 | 1 | |
| Crush syndrome | 1 | |
| sickle cell disease | 1 | |
| inhalation | 1 | |
| Time of ECMO-assist | 15 | 4–51 |
| ECMO weaning | 7 | |
| Hospital discharge | 6 |
No patient implanted by our team was left in a remote institution. Eight patients had acute respiratory distress syndrome [H1N1 (n = 1); infection (n = 4); inhalation (n = 1); crush syndrome (n = 1), sickle cell disease (n = 1)] and were assisted by veno-venous ECMO, carried out with percutaneous cannulation.
Four patients had refractory cardiogenic shock [dilated cardiomyopathy (n = 1); tight mitral stenosis (n = 1); myocardial infarction (n = 2)], and were assisted by veno-arterial ECMO, implanted via a surgical open approach.
The average air transfer distance for patients on ECMO was 912 km (range: 198–1585 km), with an average flying time of 124 min (range: 45–255 min). The aircraft used were military or civil types: Puma helicopter (n = 3), CASA twin-engine transport (n = 4), EC145 helicopter (n = 2) or Learjet private jet (n = 3). Implementation of ECMO and transfer of patients were uneventful.
The mean duration of support was 12 days (range: 4–51). Eleven patients were weaned from ECMO. One patient subsequently benefited from heart transplantation after long-term circulatory support (HeartMate II). One patient died under ECMO support after 51 days of assistance and another died on the 60th day after removal of ECMO (septic shock on infectious colitis). No leg ischaemia or major bleeding were observed in our study population.
DISCUSSION
Between January 2010 and June 2011, our CSMU supported 12 patients in therapeutic impasse, located several hundred kilometres from our centre. All patients were repatriated by air on ECMO in good conditions and 10 were saved.
In the early interventions, we had to respond rapidly to unusual requests: young patients had respiratory failure refractory to conventional resuscitation and appeared to be eligible for support by ECMO. These patients had been hospitalized several hundred kilometres from our centre and were not actually transferable by conventional means. As a result, we decided to fly out to assist these patients and then repatriate them to our centre by air. As this type of request was becoming more frequent, we developed a mobile unit.
Airborne transfers of patients under ECMO support are not unique. Similar experiments have already been reported in the literature, especially during the H1N1 flu epidemic [2, 7–11]. The results achieved by our mobile unit are good and quite similar to those observed in the literature and encourage us to continue this activity [2, 8, 12]. We believe that these results are due in part to technical mastery of circulatory support, partly to organization and optimization of logistics transfers and, finally, to the setting-up of a regional ‘network’ of multidisciplinary collaboration. The telecommunication network between emergency departments and intensive care units in the French Caribbean, protocols and strict criteria for indications are an important part of the mechanism that determines the success of interventions.
Our delivery times have reduced considerably since the beginning of our experience (Table 5). Indeed, we have worked with local staff on the establishment of procedures to reduce delays in providing in-flight direction by air traffic control in mobilizing air transport so that, at the present time, we can take care of a patient in Guadeloupe (198 km) within 90 minutes of the call and another in Saint-Martin/Saint-Barthelemy in 180 minutes. For patients of French Guiana, 1585 km distant, the delays are longer (<6 hours) and variable depending on the aircraft available (jet / airliner / military transport).
Table 5:
Time between call, team departure and ECMO start
| Time (hours) |
||||
|---|---|---|---|---|
| Call to Departure | Transportation | Call to ECMO start | ||
| Guadeloupe (198 km) | Before | 6h00 | 0h55 | 8h40 |
| After | 1h00 | 0h47 | 2h32 | |
| French Guiana (1585 km) | Before | 8h45 | 3h25 | 14h25 |
| After | 3h00 | 4h00 | 8h07 | |
The literature reports many incidents relating to the transfer of patients on ECMO [2, 7, 13]. Since the beginning of our experience, no serious incident is to be deplored. First of all, hardware footprint is a challenge that the industry has gradually solved by offering ECMO pumps that are increasingly compact. The first mobile device described by Bennett weighed 69 kg [14]. Today, there are commercial pumps specially designed for hospital transfers, weighing only a few kilograms and with aviation approval [15]. Indeed, one of the main problems posed by air transfer of patients is the weight of the material. This problem is particularly important for helicopter transfers [2]. To reduce payload to a minimum, we decided to limit the team to three people (surgeon and intensivist, plus perfusionist, nurse or intern) and set up an exact list of equipment to board which never exceeds 35 kg (Table 3).
Table 3:
Material characteristics
| Materials | Characteristics | Weight (Kg) |
|---|---|---|
| Surgical equipment | 60 cm×40 cm×30 cm | 10 |
| Transportation device | 80 cm×40 cm×40 cm | 5 |
| ECMO device | 60 cm×40 cm×30 cm | 17–20 |
| MAQUET Rotaflow | ||
| Pump | 230–240 V, 60 Hz, 220 W | 15 |
| Emergency pump | manual | 3 |
| Fixing kit | 2 | |
| LEVITRONIX Centrimag | ||
| Pump | 100–240 V, 50/60 Hz, 120 W | 6 |
| Emergency pump | electric | 5 |
| Fixing kit | 6 | |
| Total weight | 32–35 |
Issues can arise over the duration of the pump power supplies [6]. On longer transfers, inadequate running time would be a serious and annoying problem. We therefore use a converter to connect to the aircraft's internal supply.
Among the difficulties inherent in aircraft, we were apprehensive about vibration [2], especially during helicopter transfers. In fact, we have encountered no problems related to this. We also had no problems related to takeoff or landing. Indeed, in our experience the phenomena of acceleration and deceleration do not seem to have any impact on either venous drainage or the speed of the pump. Finally, a theoretical problem was variations in atmospheric pressure. With altitude, the decrease in atmospheric pressure results in decreased oxygen transfer rate through the membrane [2]. In practice, no significant change was observed. The flights were carried out below 5000 feet (1524 m) true altitude and cabin altitude and, at this altitude, desaturation is negligible at only 3% to 4% [2].
The development of mobile units as described could be expensive [8, 12]. But, in the Caribbean region, many medical evacuations are already air-delivered to our centre, so it would not really increase costs. The procedures implemented with the Defence and Security Headquarters reduce this cost to zero for interventions because they offer free emergency helicopter deployment.
To us, the circulatory support mobile unit can be very efficient in providing support to patients in refractory shock when they are remote from a cardiac surgery centre. Transfer of patients on ECMO by air can be achieved safely, even for long distances, and is no longer an obstacle. In our opinion, good results in circulatory support depend on the experience of the centres. So rather than multiplying the centres that can assist patients with ECMO, it is probably much better to organize a multidisciplinary regional network for the management of these patients with specialized teams and a complete care package. This would enable this technique, streamlined and operating within strict indications, to spread to the entire population, reducing health costs and without the uncontrolled development of technology that would put patients at risk.
Appendix: Conference discussion
Dr G. Gerosa(Padua, Italy): I have a couple of questions. The first relates to technical logistics and organization. In your manuscript you stated that you have a 24/7 on-call team. In terms of cost, does that mean that this team is devoted only to ECMO rescue, or is it the daily on-call team which means that it can be absorbed by other duties?
You have only four patients supported by ECMO due to cardiogenic shock. Now, are you removing the intra-aortic balloon pump before the transfer of the patients on the airplane or the helicopter? And second, you had two patients treated because of myocardial infarction. How long were those patients supported by ECMO? Did you remove the femoral cannula and do you move to the axillary cannulation over time? And finally, left ventricular venting is sometimes an issue with these patients; how did you deal with that problem?
Dr Lebreton: Concerning the first question, we do not have a team dedicated for ECMO: it's not possible. You speak of the cost and that is a really important factor, so we organize this team with the minimum of people. There is only one surgeon and one assistant, who can be a nurse, an intern, or a perfusionist: it depends on the situation. But it's not always the case. We have a very small team to implant ECMO because, if not, this creates a lot of problems in our department for the cardiac surgery programme. And I think it's really important to be organized like that.
Regarding the second question about venoarterial ECMO: in this presentation there were only four ECMO for this situation, but now there are more ECMO than that. Because for the first time we have been called for air transport; the intensive care unit called us for that. But now, with time, they also call us, and the cardiology units call us—for myocardial infarction, for example. But in this situation, most of the time we have the call early and we can repatriate the patient to Martinique before ECMO is needed.
When we put venoarterial ECMO in our population, we try to have assistance time and their ECMO support of less than one week, because after that I think there are a lot of complications. And for the moment, it's possible in our experience. It has been necessary to convert an axillary cannulation for one patient after this patient had been weaned for ECMO.
Dr Gerosa: So, two questions. One question was about intra-aortic balloon pump catheter. Because when you are transferring your patient by helicopter, by airplane, you are removing the balloon catheter, and after that you have inserted the ECMO, the intra-aortic balloon counterpulsation catheter. Are you removing the counterpulsation catheter prior to transfer?
Dr Lebreton: No. For all of the patients where we have been called, there was no counterpulsation: not for these patients. But, if we were in this situation, from Guiana it's not a problem because we take aircraft—military aircraft—and there is a place in the plane. And for Guadeloupe, in helicopter transfer, we have already transferred patients and they are on intra-aortic balloon pump without problem.
Dr J. Gummert (Bad Oeynhausen, Germany): I have one question for you. Actually, it seems to be positive selection of these severely ill patients. If you compared to other networks, you certainly preselect those patients that have probably a much higher chance to survive. In our region, for instance, we try to enable the cardiologists to put in the ECMO to save time and therefore we accept many more patients under resuscitation conditions. What do you think, would that be an option, to distribute those cannulas to those other places so as to enable them to put in the ECMO right away and then call you?
Dr Lebreton: Well, I think it's really important to select the patient to have good results. So what we have done in our protocols is that the centres call us early. Because I think it's important to be called when it's possible to do something. After, it's too late. And in the past it was like that. So when they call us, most of the time we have time to do what it's possible to do. And for the other part of your question, I think that a good result of the technique depends on a lot of things. Somebody who implants an ECMO once every six months, I think it's not good. So we prefer not to put on pump overseas.
REFERENCES
- 1.Le Guen M, Nicolas-Robin A, Carreira S, Raux M, Leprince P, Riou B, et al. Extracorporeal life support following out-of-hospital refractory cardiac arrest. Crit Care. 15:R29. doi: 10.1186/cc9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haneya A, Philipp A, Foltan M, Mueller T, Camboni D, Rupprecht L, et al. Extracorporeal circulatory systems in the inter-hospital transfer of critically ill patients: experience of a single institution. Ann Saudi Med. 2009;29:110–4. doi: 10.4103/0256-4947.51792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossaint R, Pappert D, Gerlach H, Lewandowski K, Keh D, Falke K. Extracorporeal membrane oxygenation for transport of hypoxemic patients with severe ARDS. Br J Anaesth. 1997;78:241–6. doi: 10.1093/bja/78.3.241. [DOI] [PubMed] [Google Scholar]
- 4.Midla GS. Extracorporeal circulatory systems and their role in military medicine: a clinical review. Mil Med. 2007;172:523–6. doi: 10.7205/milmed.172.5.523. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann M, Philipp A, Schmid FX, Dorlac W, Arlt M, Bein T. From Baghdad to Germany: use of a new pumpless extracorporeal lung assist system in two severely injured US soldiers. ASAIO J. 2007;53:e4–6. doi: 10.1097/MAT.0b013e3180574b37. [DOI] [PubMed] [Google Scholar]
- 6.Huang SC, Chen YS, Chi NH, Hsu J, Wang CH, Yu HY, et al. Out-of-centre extracorporeal membrane oxygenation for adult cardiogenic shock patients. Artif Organs. 2006;30:24–8. doi: 10.1111/j.1525-1594.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 7.Linden V, Palmer K, Reinhard J, Westman R, Ehren H, Granholm T, et al. Inter-hospital transportation of patients with severe acute respiratory failure on extracorporeal membrane oxygenation: national and international experience. Intensive Care Med. 2001;27:1643–8. doi: 10.1007/s001340101060. [DOI] [PubMed] [Google Scholar]
- 8.D'Ancona G, Capitanio G, Chiaramonte G, Serretta R, Turrisi M, Pilato M, et al. Extracorporeal membrane oxygenator rescue and airborne transportation of patients with influenza A (H1N1) acute respiratory distress syndrome in a Mediterranean underserved area. Interact CardioVasc Thorac Surg. 12:935–7. doi: 10.1510/icvts.2010.260448. [DOI] [PubMed] [Google Scholar]
- 9.Davies A, Jones D, Bailey M, Beca J, Bellomo R, Blackwell N, et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–95. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 10.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quinones-Falconi F, Bautista E, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–9. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 11.Ciapetti M, Cianchi G, Zagli G, Greco C, Pasquini A, Spina R, et al. Feasibility of inter-hospital transportation using extra-corporeal membrane oxygenation (ECMO) support of patients affected by severe swine-flu(H1N1)-related ARDS. Scand J Trauma Resusc Emerg Med. 19:32. doi: 10.1186/1757-7241-19-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gariboldi V, Grisoli D, Tarmiz A, Jaussaud N, Chalvignac V, Kerbaul F, et al. Mobile extracorporeal membrane oxygenation unit expands cardiac assist surgical programs. Ann Thorac Surg. 90:1548–52. doi: 10.1016/j.athoracsur.2010.06.091. [DOI] [PubMed] [Google Scholar]
- 13.Wagner K, Sangolt GK, Risnes I, Karlsen HM, Nilsen JE, Strand T, et al. Transportation of critically ill patients on extracorporeal membrane oxygenation. Perfusion. 2008;23:101–6. doi: 10.1177/0267659108096261. [DOI] [PubMed] [Google Scholar]
- 14.Bennett JB, Hill JG, Long WB, 3rd, Bruhn PS, Haun MM, Parsons JA. Interhospital transport of the patient on extracorporeal cardiopulmonary support. Ann Thorac Surg. 1994;57:107–11. doi: 10.1016/0003-4975(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 15.Arlt M, Philipp A, Voelkel S, Camboni D, Rupprecht L, Graf BM, et al. Hand-held minimised extracorporeal membrane oxygenation: a new bridge to recovery in patients with out-of-centre cardiogenic shock. Eur J Cardiothorac Surg. 40:689–94. doi: 10.1016/j.ejcts.2010.12.055. [DOI] [PubMed] [Google Scholar]