Abstract
OBJECTIVES
Prothrombin complex concentrates (PCCs) are sometimes used as ‘off label’ for excessive bleeding after cardiopulmonary bypass (CPB). The main objective of this study was to retrospectively evaluate the clinical and biological efficacy of PCC in this setting.
METHODS
We reviewed the charts of all patients who had undergone cardiac surgery under CPB in our institution for 2 years. Patients treated for active bleeding with haemostatic therapy were identified. Chest tube blood loss was quantified postoperatively in the first 24 h. Coagulation parameters were recorded at intensive care unit admission and in the patient's first 24 h. Thromboembolic complications were also ascertained.
RESULTS
Seventy-seven patients out of the 677 studied (11.4%) were included: PCC was solely administered in 24 patients (group I), fresh frozen plasma in 26 (group II) and both in 27 (group III). The mean dose of PCC was 10.0 UI/kg ± 3.5 for group I vs 14.1 UI/kg ± 11.2 for group III (P = 0.09). Initial blood loss in the first hour was different between the three groups (P = 0.05): 224 ± 131 ml for group I, 369 ± 296 ml for group II and 434 ± 398 ml for group III. Only group I vs group III presented a significant difference (P = 0.02). Variations of blood loss over time were no different according to the treatment groups (P = 0.12). Reductions in blood loss expressed in percentage showed no difference between the three groups after 2 h: 54.5% (68.6–30.8) for group I; 45.0% (81.6–22.2) for group II; 57.6 (76.0–2.1) for group III; (P = 0.89). Re-exploration for bleeding involved 1 patient in group I (4%), 2 in group II (8%) and 10 in group III (37%) (P = 0.002). Except for fibrinogen, variations of prothrombin time, activated partial thromboplastin time and platelets with time were not different according to the treatment groups. Cerebral infarction occurred in one patient in group II.
CONCLUSIONS
Administration of low-dose of PCC significantly decreased postoperative bleeding after CPB.
Keywords: Prothrombin complex concentrates, Bleeding, Cardiopulmonary bypass, Thromboembolic complications
INTRODUCTION
Excessive bleeding after cardiac surgery is one of the major causes associated with increased morbidity and mortality [1]. Transfusion of allogeneic blood products and surgical re-exploration are frequently required [1, 2]. Such bleeding could be related to altered haemostasis mainly due to dilution of factors [3, 4], and to surgical trauma. Initially indicated for treating haemophilia B, the use of prothrombin complex concentrates (PCCs) has been advocated for the rapid correction of coagulation deficits [5]. PCCs are not yet recommended by guidelines, reviews or algorithms [6–9]. However, in some countries, PCCs are used in addition to or instead of fresh frozen plasma (FFP) [9]. Moreover, the European Medicines Agency core summary of product characteristics for PCC gives ‘treatment of bleeding and perioperative prophylaxis of bleeding in acquired deficiency of the prothrombin complex coagulation factors’ as an indication [10]. The rationale behind this is that clinicians believe the level of coagulation in FFP is relatively low, and too large a volume of FFP is needed to correct the coagulopathy [9, 11]. PCCs also offer a number of advantages over FFP. These include a lower infusion volume, ambient storage, rapid reconstitution, immediate availability, lack of blood group specificity and a better safety profile [12].
It is acknowledged that administrating PCC for managing massive bleeding is currently based on low levels of evidence [13]. Although PCCs have demonstrated some efficacy in animal research [14], reports of utilizing PCC postoperatively after cardiopulmonary bypass (CPB) are sparse [5, 11, 15, 16]. Recently, the first-line administration of fibrinogen concentrate and/or PCC combined with point-of-care testing was associated with a decreased intraoperative incidence of blood transfusion [17]. Furthermore, the incidence of massive transfusion, re-exploration and of thrombotic/thromboembolic events also decreased [17].
The main objective of this study was to retrospectively evaluate the clinical and biological efficacy of PCC administered alone or in combination with FFP in bleeding patients after cardiac surgery performed under CPB. The second objective was to record the postoperative outcome in terms of postoperative complications and transfusion requirements.
PATIENTS AND METHODS
After approval from our institutional review board, we reviewed the charts of all patients who had undergone cardiac surgery under CPB in our institution from January 2009 to December 2010. Patients who received PCC and/or FFP in the immediate postoperative period were identified and represented the studied population. Coronary artery bypass grafting (CABG) was exclusively performed using internal thoracic artery. In triple-vessel-disease patients, both internal thoracic arteries were used in a Y fashion with multiple sequential side-to-side coronary anastomoses [18].
Anaesthetic management
Anaesthesia was induced with etomidate 0.3 mg/kg, sufentanyl 0.15 μg/kg and atracurium 0.5 mg/kg. For maintenance of anaesthesia, sevoflurane was titrated to an end-tidal concentration of 1–2% until the institution of CPB. During CPB, anaesthesia was maintained with the infusion of propofol (50 mg/kg) or midazolam (0.05–0.1 mg/kg), and sufentanyl (0.2–0.5 mg/kg). Boluses of atracurium were administered when indicated.
The CPB circuit was primed with ∼1.4 l Ringer lactate solution, 500 ml sodium bicarbonate 1.4% and 10 000 UI heparin. The patients received heparin (300 UI/kg) in order to maintain an activated clotting time (ACT) >400 s. The ACT was maintained >400 s during CPB by supplemental heparin. After CPB, heparin was reversed by the administration of protamine sulphate (100 UI protamine/100 UI heparin). Additional protamine sulphate was given if the ACT was above 130 s. Patients were kept at normothermia throughout the procedure (>36°C).
Red blood cells were administered in order to maintain haematocrit values above 28% in general and above 30% in patients with CABG or redux procedure. FFP and platelet concentrate were administered according to clinical bleeding and blood sample results. Administration of tranexamic acid and PCC was left to the discretion of the attending physician. Aspirin was not discontinued before surgery and clopidrogel was discontinued 5 days before surgery.
Postoperative management
All patients were admitted to the intensive care unit (ICU) and no changes to standard practice were made during the study period (analgesia, weaning from mechanical ventilation, volume expansion). Anticoagulation was begun 6 h after ICU admission if bleeding was controlled. Low-molecular weight heparin and aspirin were prescribed for all patients after CABG or bioprothesis aortic valve replacement; in other procedures, heparin was chosen.
The decision for transfusion of blood products (varying combinations of PCC, FPP, platelets and erythrocytes) was based on the clinician's judgment with or without guidance from laboratory tests. However, despite the absence of an algorithm, the management of bleeding was similar among the eight attending ICU physicians. In case of detectable anti-Xa activity in bleeding patients, 2500 UI of protamine sulphate was infused over 20 min. PCC or FFP were administered when prothrombin time (PT) ≤50%. Platelets were transfused when platelet count ≤70 × 109/l. Haemoglobin level was maintained ≥8 g/dl. The decision to re-explore was at the discretion of the individual surgeon and attending physician.
Octaplex® (Octapharma AG, Lachen, Switzerland) was the sole PCC available in our institution. It is a solvent/detergent-treated and nanofiltered PCC containing factors II, VII, X and proteins C and S at a concentration of 10–40 IU/ml. Octaplex also contained factor IX (20–31 IU/ml) and heparin 0.2–0.5 IU/IU factor IX. The product was supplied as 500 IU factor IX (20 ml) vials [19].
In our hospital, the cost of 500 UI of Octaplex® (one vial) is 140.00 Euros. In France, the cost of one packed red cells is 183.84 Euros, one FFP unit is 97.21 Euros, the first platelet unit is 75.02 Euros and it is 37.50 Euros for the following units.
Data collection
The following preoperative data were abstracted from all charts by means of a standardized form: age, sex, body mass index (BMI), comorbid cardiovascular conditions (hypertension, diabetes, arrhythmia, thromboembolic events), anticoagulant and anti-platelet therapies, type of procedure, haemoglobin level, coagulation parameters (PT, activated partial thromboplastin time (aPTT), platelet count, fibrinogen level), Euroscore and Simplified Acute Physiology Score (SAPS II). PT results are reported as an activity percentage. To obtain the activity percentage expression, a saline dilution curve was constructed with normal pool plasma and the patient's result was expressed as the percentage of normal plasma yielding the same PT in seconds. SAPS II is a severity of disease classification system.
Preoperative data included: duration of intervention, duration of CPB, aortic clamp time, heparin and protamine doses, crystalloids and hetastarchs infusion (Voluven®, Fresenius Kabi France, Louviers, France), transfusion requirement, tranexamic acid, PCC and cathecholamine use and postoperative ACT.
Postoperative data included: need of re-exploration, ICU transfusion requirement, ICU and hospital stays and death. Coagulation parameters were recorded at ICU admission (T1), 24 h later (T4) and at different times after ICU admission (T2 and T3). Postoperative complications were ascertained from a detailed chart review according to the operational definitions reported below. It is acknowledged that the retrospective identification of postoperative complications depends on the detail and completeness of the medical record. To minimize this factor, the analysis was limited to postoperative complications thought to be clinically significant (see below), and thus unlikely to be omitted from the medical record.
Definitions of postoperative complications
Postoperative complications were defined as those occurring within 30 days after the surgical procedure.
Acute myocardial infarction: ECG evidence of ≥1 new Q waves, left bundle-branch block or new pathological R waves; significant elevation of troponine Ic, plus a clinical picture of haemodynamic instability that gives rise to the suspicion of myocardial infarction or graft occlusion.
Cerebral infarction: new focal neurological deficit, either transient but present >24 h, or permanent.
Pulmonary embolism: documented by pulmonary arteriogram or autopsy, or supported by a ventilation/perfusion radioisotope scan showing ‘high probability’ of pulmonary embolism.
Other clinically symptomatic thrombotic events: signs or suspicion of clinically significant thrombotic event confirmed by ultrasonography or CT scan positive finding.
Pericardial effusion: diagnosed by echocardiography or thoracic CT scan.
Mediastinitis: defined as a deep wound infection associated with sternal osteomyelitis with or without infected retrosternal space.
Other infections: according to the definitions of the Centers for Disease Control.
Acute respiratory distress syndrome: acute onset with a PaO2/FIO2 ≤200 mmHg and bilateral infiltrates seen on frontal chest radiograph, with no clinical evidence of left atrial hypertension.
Statistical analysis
Data were entered and analysed using Statview 5.0 statistical packages (SAS Institute Inc., SAS Campus Drive, Cary, NC, USA). We expressed continuous variables as the mean (±SD) or as the median (25th–75th percentiles) if their distribution was skewed. Chi-square tests were used to compare proportions and rates. Comparisons between the three groups were performed by analysis of variance (ANOVA) followed by a Scheffe-F-test for post hoc comparisons of quantitative variables. The Kruskal–Wallis test was used to analyse continuous variables with a non-normal distribution. Statistical significance was defined as a P-value of 0.05 or less.
RESULTS
Patients' characteristics
During the study period, 677 patients underwent cardiac surgery with CPB. Seventy-seven patients (11.4%), treated for active bleeding with haemostatic therapy during the postoperative period were identified: PCC was solely administered in 24 patients (group I), FFP in 26 patients (group II) and both in 27 patients (group III). Patients' characteristics are summarized in Table 1. Except for body mass index, there was no difference between the three groups.
Table 1:
Patients' characteristics
| Variables | Group I (n = 24) | Group II (n = 26) | Group III (n = 27) | ANOVA P-value |
|---|---|---|---|---|
| Age (years) | 64 ± 13* | 72 ± 14 | 73 ± 10 | 0.04 |
| Male gender, n (%) | 18 (75) | 18 (69) | 15 (55) | 0.32 |
| Body mass index | 27.0 ± 3.6* | 26.0 ± 4.1 | 24.6 ± 3.0 | 0.05 |
| Comorbidities | ||||
| Hypertension, n (%) | 19 (79) | 18 (69) | 14 (52) | 0.10 |
| Diabetes, n (%) | 6 (25) | 6 (23) | 5 (18) | 0.85 |
| Arrythmia, n (%) | 3 (12) | 3 (11) | 6 (22) | 0.50 |
| Previous thromboembolic events | 2 (8) | 1 (4) | 1 (4) | 0.72 |
| Aspirin use | 12 (50) | 19 (73) | 14 (52) | 0.17 |
| Clopidogrel use | 5 (21) | 6 (23) | 7 (26) | 0.91 |
| Oral anticoagulants use | 3 (12) | 1 (4) | 4 (15) | 0.39 |
| Heparin use | 4 (17) | 5 (19) | 5 (18) | 0.88 |
| Type of procedure, n (%) | ||||
| CABG | 9 (38) | 9 (35) | 7 (26) | 0.4 |
| Valve | 13 (54) | 11 (42) | 13 (48) | |
| CABG + valve | 2 (8) | 6 (23) | 5 (19) | |
| Miscellaneous | 0 | 0 | 2 (7) | |
| No prior cardiac surgery | 24 (100) | 24 (92) | 26 (96) | 0.37 |
| Preoperative laboratory data | ||||
| Prothrombin time (%) | 95 ± 13 | 93 ± 18 | 91 ± 23 | 0.74 |
| aPTT (sec) | 32 ± 3 | 35 ± 6 | 34 ± 6 | 0.12 |
| Platelets (× 106/l) | 238 ± 79 | 211 ± 54 | 219 ± 68 | 0.35 |
| Fibrinogen (g/l) | 4.0 ± 1.1 | 4.2 ± 1.2 | 3.7 ± 1.2 | 0.35 |
| Haemoglobin (g/dl) | 14.2 ± 4.2 | 13.3 ± 1.4 | 13.4 ± 1.4 | 0.42 |
| EuroSCORE | 5.6 ± 2.7 | 6.7 ± 2.3 | 6.7 ± 2.5 | 0.20 |
| SAPS II | 28 ± 10 | 30 ± 10 | 34 ± 10 | 0.16 |
CABG: coronary artery bypass grafting; aPTT: activated partial thromboplastin time; SAPS II: simplified acute physiology score II.
*P = 0.05 group I vs group III.
Perioperative management was similar between the three groups except for a lower hetastarchs infusion and less red blood cell transfusion in group I (Table 2).
Table 2:
Perioperative data
| Variables | Group I (n = 24) | Group II (n = 26) | Group III (n = 27) | P-value |
|---|---|---|---|---|
| Duration of intervention (min) | 310 ± 50* | 317 ± 68 | 361 ± 100 | 0.04 |
| Duration of CPB (min) | 88 ± 34 | 90 ± 26 | 102 ± 46 | 0.36 |
| Aortic clamp time (min) | 66 ± 39 | 67 ± 24 | 78 ± 31 | 0.26 |
| Heparin dose (UI/kg) | 513 ± 115 | 459 ± 97 | 540 ± 155 | 0.07 |
| Protamine dose (UI/kg) | 653 ± 168 | 712 ± 339 | 773 ± 203 | 0.22 |
| Crystalloids infusion (ml/kg) | 9.5 ± 4.2 | 9.5 ± 7.0 | 12.6 ± 8.7 | 0.19 |
| Hetastarchs infusion (ml/kg) | 5 (0–6)** | 6 (0–14) | 7 (1–10) | 0.03 |
| Red blood cells units | 0 (0–1)** | 1 (0–2) | 2 (0–3) | 0.03 |
| FFP units | 0 (0–0) | 0 (0–0) | 0 (0–3) | 0.23 |
| Platelets units | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.74 |
| Tranexamic acid use, n (%) | 15 (62) | 10 (38) | 16 (59) | 0.17 |
| Dose of tranexamic acid, mg/kg | 37.2 ± 9.0 | 33.0 ± 15.3 | 30 ± 11.2 | 0.24 |
| PCC use, n (%) | 6 (25) | 13 (50) | 11 (41) | 0.19 |
| Dose of PCC, UI/kg | 13.5 ± 8.2 | 12.5 ± 5.5 | 12.2 ± 5.8 | 0.90 |
| Catecholamine use, n (%) | 0 | 1 (4) | 4 (15) | 0.08 |
| Postoperative ACT (sec) | 131 ± 24 | 140 ± 18 | 135 ± 18 | 0.43 |
CPB: cardiopulmonary bypass; FFP: fresh frozen plasma; PCC: prothrombin complex concentrates; ACT: activated clotting time.
*P = 0.06 for group I vs group III.
**P = 0.004 for group I vs group III.
Effect of prothrombin complex concentrates on blood loss
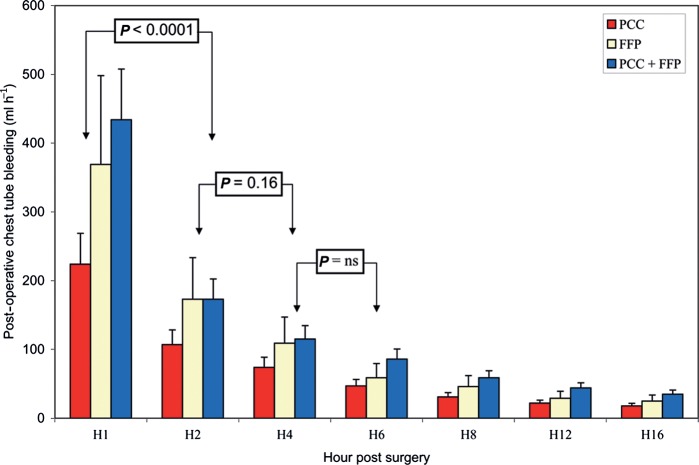
The course of the chest tube blood loss is reported in Fig. 1. Initial blood loss at H1 was different between the three groups: (P = 0.05) 224 ± 131 ml for group I, 369 ± 296 ml for group II and 434 ± 398 ml for group III. Only group I vs group III presented a significant difference (P = 0.05) for blood loss at H1. When expressed in ml kg−1 h−1 and as median, the initial blood loss was as follows: 2.6 (1.4–4.1) for group I, 3.2 (2.2–8.2) for group II and 4.6 (2.8–7.8) for group III. Only group I vs group III presented a significant difference (P = 0.02). Variations of blood loss over time were not different according to the treatment groups (P = 0.12). Reductions in blood loss expressed in percentage showed no difference between the three groups at H2: 54.5% (68.6–30.8) for group I; 45.0% (81.6–22.2) for group II; 57.6 (76.0–2.1) for group III; (P = 0.89) and at H4: 33.0% (62.5–0.0) for group I; 36.7% (59.1–2.8) for group II; 36.5 (66.4–2.4) for group III; (P = 0.98).
Figure 1:
Course of chest tube blood loss. Initial blood loss at H1 was different between the three groups (P = 0.05). Only group I vs group III presented a significant difference (P = 0.05) for blood loss at H1. Variations of blood loss over time were not different according to the treatment groups (P = 0.12). Decrease in blood loss was significantly different between H1 and H2 (P < 0.0001). After H2, no significant changes were demonstrated: P = 0.16 between H2 and H4.
Volume chest drains at H4 were non-significantly different between the three groups (P = 0.06): 475 ± 279 ml for group I, 667 ± 244 ml for group II and 970 ± 1122 ml for group III. Volume chest drains at H24 were markedly different between the three groups (P = 0.009): 923 ± 411 ml for group I, 1250 ± 441 ml for group II and 1656 ± 1148 ml for group III. Only group I vs group III presented a significant difference (P = 0.009) for blood loss at H24.
PCC administered in the ICU mainly occurred during the first 2 h (Fig. 2). The mean dose of PCC was 10.0 UI/kg ± 3.5 for group I vs 14.1 UI/kg ± 11.2 for group III (P = 0.09). FFP were administered mainly between H2 and H4 (Fig. 2). Median units were 3.0 (2.0–4.0) for group II vs 3.0 (2.0–4.0) for group III (P = 0.58).
Figure 2:
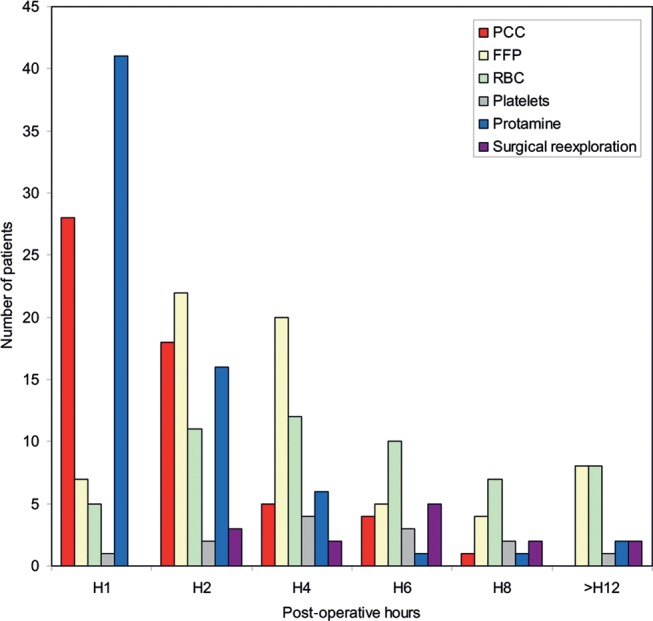
Timing of administration of haemostatic treatment in the immediate postoperative period. PCC: prothrombin complex concentrates; FFP: fresh frozen plasma, RBC: red blood cells.
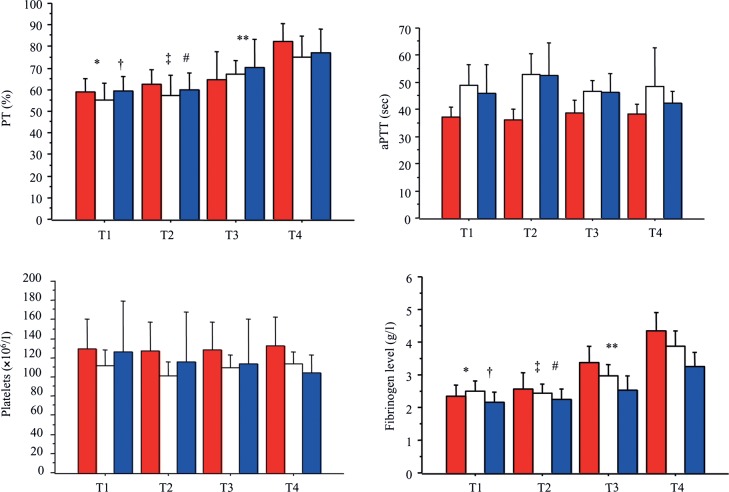
Course of biological parameters
The course of the biological parameters (PT, aPTT, platelets, fibrinogen) is depicted in Fig. 3. The second blood sample (T2) was harvested at 7.1 ± 5.4 h for group I, 5.2 ± 3.5 h for group II and 5.1 ± 2.8 h for group III after ICU admission (P = 0.16). The third blood sample (T3) was harvested at 11.1 ± 5.5 h for group I, 12.0 ± 6.2 h for group II and 10.3 ± 4.9 h for group III after ICU admission (P = 0.67).
Figure 3:
Course of biological parameters. T1: at ICU admission; T2: 6 h ± 4 after ICU admission; T3: 11 h ± 5 after ICU admission; T4: 24 h after ICU admission. Red boxes: prothrombin complex concentrates (group I); White box: fresh frozen plasma (group II); blue boxes: prothrombin complex concentrates and fresh frozen plasma (group III). Prothrombin time (PT). Course of PT varied over time: *T1 vs T3 (P = 0.008); †T1 vs T4 (P < 0.0001); ‡T2 vs T3 (P = 0.05); #T2 vs T4 (P = 0.0001); **T3 vs T4 (P = 0.006). Variations of PT over time were not different according to the treatment groups. There was no difference between groups for PT. Activated partial thromboplastin time (aPTT). Course of aPTT did not vary over time. Variations of aPPT over time were not different according to the treatment groups. Values of aPTT were different between group I vs group II (P = 0.04). Platelet count. Course of platelet count did not vary over time. Variations of platelet count with time were not different according to the treatment groups. There was no difference between groups for platelet count. Fibrinogen. Course of fibrinogen varied over time: *T1 vs T3 (P < 0.0001); †T1 vs T4 (P < 0.0001); ‡T2 vs T3 (P < 0.0001); #T2 vs T4 (P < 0.0001); **T3 vs T4 (P < 0.0001). Variations of fibrinogen over time were different according to the treatment groups (P < 0.0001). Values of fibrinogen were different between group I vs group III (P = 0.03).
Except for fibrinogen, variations of PT, aPTT and platelets over time were not different according to the treatment groups. There was no difference between groups for PT and platelet number. A difference was shown between group I and group II for aPTT values (P = 0.04) and between group I and III for fibrinogen level (P = 0.03). The course of PT and fibrinogen varied with time, contrary to aPTT and platelet number at the different points of time studied.
Outcome
Outcome data are summarized in Table 3. Re-exploration for bleeding was higher in group III (37%) compared with group I (4%) and group II (8%). Cerebral infarction occurred in one patient in group II. Mean cost-related administration of blood products and PCC was substantially different among the three groups: 261 ± 162 Euros for group I, 684 ± 455 Euros for group II and 1281 ± 772 Euros for group III (P < 0.0001).
Table 3:
Outcome
| Variables | Group I (n = 24) | Group II (n = 26) | Group III (n = 27) | P-value |
|---|---|---|---|---|
| Re-exploration for bleeding, n (%) | 1 (4)* | 2 (8)† | 10 (37) | 0.002 |
| ICU transfusion | ||||
| Red blood cells, n | 0 (0–0)# | 2.0 (0–3.0)‡ | 3.0 (0.5–5.0) | <0.0001 |
| FFP, n | — | 3 (2–4) | 3 (2–4) | 0.58 |
| Platelets, units | 0 (0–0) | 0 (0–0) | 0 (0–7) | 0.10 |
| First 24-h colloids/crystalloids infusion in ICU (ml/kg) | 10 (0–16) | 11 (0–16)** | 6 (0–13) | 0.43 |
| Thromboembolic events, n (%) | 0 | 1 (4) | 0 | 0.37 |
| Pericardial effusion, n (%) | 8 (33) | 4 (15) | 12 (44) | 0.07 |
| Pulmonary oedema, n (%) | 1 (4) | 1 (4) | 2 (7) | 0.81 |
| ARDS, n (%) | 0 | 0 | 1 (4) | 0.39 |
| Mediastinitis, n (%) | 3 (12) | 2 (8) | 2 (7) | 0.78 |
| Other infections, n (%) | 7 (29) | 7 (27) | 77 (27) | 0.97 |
| Duration of MV (hours) | 6.0 (4.5–12.0) | 14.5 (8.0–19.0)†† | 12.0 (7.2–20.0)‡‡ | 0.02 |
| ICU stay (days) | 3 (2–6) | 3 (2–4) | 3 (2–4) | 0.82 |
| Hospital stay (days) | 12 (10–15) | 15 (12–23) | 12 (9–20) | 0.12 |
| Death, n (%) | 0 | 1 (4) | 3 (11) | 0.19 |
ICU: intensive care unit; FFP: fresh frozen plasma; ARDS: acute respiratory distress syndrome; MV: mechanical ventilation.
*Group I vs group III: P = 0.004.
†Group II vs group III: P = 0.01.
#Group I vs group II: P < 0.0007.
‡Group I vs group III: P < 0.0001.
**Group II vs group III: P = 0.002.
††Group I vs group II: P = 0.02.
‡‡Group I vs group III: P = 0.01.
DISCUSSION
Our results show that PCC significantly decreased postoperative bleeding after CPB. No thromboembolic complications related to PCC were noted in this series.
CPB is a traumatic procedure that is associated with platelet and coagulation defects, inflammation and increased fibrinolysis [3, 4]. In a porcine model after CPB, circulating levels of FII, FVII, FIX and FX declined from baseline by 32% [14]. Similar results were reported in patients after CPB [4]. Excessive dilution of blood volume led to a critical lowering of the clotting factor concentration and to postoperative bleeding [3, 8].
The incidence of excessive bleeding (11.4%) was similar to a previous study [20] and slightly higher than that of the Papworth group (8%) [2]. The first point to state was the presence of abnormal bleeding post-CPB. When available, different definitions were provided in the literature. However, the threshold of ≥200 ml/h or ≥2 ml/kg/h was retained in one randomized study [21], and in the development of the Papworth bleeding risk score [2]. In our study, the group receiving PCC alone (group I) has a mean blood loss of 224 ml or a median of 2.6 ml/kg in the first hour.
The present study indicates that PCC infusion reduces postoperative bleeding after cardiac surgery, in ∼50% of our patient study (group I). An analysis of the effects of postoperative administration of PCC must take into account the intraoperative use of PCC and the treatment of the residual heparin effect. Only 50% of patients received PCC intraoperatively and doses of PCC were similar among the three groups. Moreover, despite the intraoperative administration of PCC, all patients experienced abnormal postoperative bleeding. In an ‘in vivo’ large-animal CPB model, PCC was effective in correcting dilutional coagulopathy and reducing diffuse bleeding [14]. In a porcine model, PCC also increased peak thrombin generation to a level higher than baseline [14].
Ten years ago, two case reports described the successful use of PCC in patients with liver dysfunction undergoing cardiac surgery [11]. More recently, clinical and biological evidence on the use of PCC has been provided by different studies after general or cardiac surgery [9, 15–17, 22] and in ICUs [23]. A decrease in the international normalized ratio was noted 2–4 h after infusion [11, 22, 23]. Bleeding ceased in almost all patients (96–100%) after infusion of PCC [22, 23]. In a randomized trial [15] in patients undergoing CPB surgery, when PCC was compared with FFP for reversing oral anticoagulants, PCC reversed anticoagulation safely, faster and with less bleeding [15]. However, multifactorial deficiencies in massive bleeding may not be fully restored by PCC. Importantly, fibrinogen levels should be restored to at least 1.5 g/l, because hypofibrinogenemia is an important cause of bleeding in surgical patients [8, 9, 12, 21].
A similar decrease in postoperative bleeding was also observed when FPP was administered alone or in combination with PCC. To date, only transfusion of FFP was recommended in the setting of active bleeding that may be related to substantial reductions in coagulation factor levels [6, 8]. Intriguingly, there is a relative paucity of high-quality evidence reporting the outcome of administrating FFP in a perioperative setting, despite the long period of its usage [24]. Interestingly, FFP and PCC were prescribed in combination with the most severely bleeding patients. Such a strategy could be of value to reduce blood products administration and avoid fluid overload, but should be confirmed in future prospective studies.
Our patients received a mean dose of 10–15 IU/kg of factor IX. A dose of 30 IU/kg was generally used to reverse even over-anticoagulated patients [5] and in critically ill bleeding patients [22, 23]. In the setting of CPB, a dose of around 10–15 UI/kg was generally infused [15, 17]. This could potentially reflect the reluctance of the attending physician to administer the recommended doses of PCC in the context of cardiac surgery due to the fear of thromboembolic complications. This ‘low-dose’ regimen may be a rational approach to avoid the potential thrombogenic side effects of PCC while preserving their effects on bleeding.
Historically, the major drawback of PCC has been the risk of thrombotic complications [5]. Available evidence indicates that the accumulation of factor II could be the primary determinant of the thrombotic risk associated with frequent doses of PCC [25]. Three major risk factors of thrombogenicity have been mentioned [5]: the presence of chronic liver disease, rapid infusion and large repetitive doses of PCC and the quality of PCC. This risk has decreased with the improvement in the quality of coagulation factor concentrates and a more judicious use of these products [5, 12, 13, 25]. The majority of four-factor PCC used in Europe (such as Octaplex®) contain balanced amounts of the coagulation factors II, VII, IX and X, as well as protein C, S and Z [19]. Fraser et al. [16] reported no thrombotic complications in a series of 60 patients after CPB treated with a dose of 500 IU. Sorensen et al. [25] have published an overview of studies that have reported thrombotic complications. In all these cases, patients receiving PCC had an increased risk of thrombosis due to medical history or current illness [25]. There have been no reports of thrombotic complications with PCC in the treatment of perioperative bleeding, although only a few studies have been published in these settings [25]. To date, although thousands of patients have been treated (with average dose of 2000 units), there have been no proven cases of thromboembolism [25]. Our study supported such assertions with the use of low doses (10–15 IU/kg).
Our study has several limitations, mainly due to its retrospective design and the small number of patients treated. Postoperative complications were determined by a chart review. However, all severe complications—particularly thromboembolic complications—should have been detected, as they would greatly affect the postoperative prognosis. Lastly, lack of an algorithm for coagulation management could have influenced the results.
In conclusion, the administration of low doses of PCC significantly decreased postoperative bleeding after CPB. In this small series of patients, no thromboembolic complications-related PCC infusions were recorded. This study provides enough significant results to plan a prospective study on the use of PCC after cardiac surgery under CPB.
Funding
This study support was provided solely from institutional and/or departmental sources.
Conflict of interest: none declared.
REFERENCES
- 1.Dacey LJ, Munoz JJ, Baribeau YR, Johnson ER, Lahey SJ, Leavitt BJ, et al. Reexploration for hemorrhage following coronary artery bypass grafting. Incidence and risk factors. Arch Surg. 1998;133:442–7. doi: 10.1001/archsurg.133.4.442. [DOI] [PubMed] [Google Scholar]
- 2.Wuylsteke A, Pagel C, Gerrard C, Reddy B, Nashef S, Aldam P, et al. The Papworth bleeding risk score: a stratification scheme for identifying cardiac surgery patients at risk of excessive early postoperative bleeding. Eur J Cardiothorac Surg. 2011;39:924–31. doi: 10.1016/j.ejcts.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Bull BS, Hay KL, Herrmann PC. Postoperative bypass bleeding: a bypass-associated dilutional [BAD] coagulopathy? Blood Cells Mole Dis. 2009;43:256–9. doi: 10.1016/j.bcmd.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Ternstöm L, Radulovic V, Karlsson M, Baghaei F, Hyllner M, Bylock A, et al. Plasma activity of individual coagulation factors, hemodilution and blood loss after cardiac surgery: a prospective observational study. Thromb Res. 2010;126:e128–33. doi: 10.1016/j.thromres.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 5.Key NS, Negrier C. Coagulation factor concentrates: past, present, and future. Lancet. 2007;370:439–48. doi: 10.1016/S0140-6736(07)61199-4. [DOI] [PubMed] [Google Scholar]
- 6.Practice guidelines for perioperative blood transfusion and adjuvant therapies. An updated report by the American Society of Anesthesiologists task force on perioperative blood transfusion and adjuvant therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 7.The Society of thoracic surgeons blood conservation guideline task force, the Society of cardiovascular anesthesiologists special task force on blood transfusion. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of thoracic surgeons blood conservation guideline task force, the Society of cardiovascular anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83:S27–86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 8.Despotis G, Eby C, Lublin DM. A review of transfusion risks and optimal management of perioperative bleeding with cardiac surgery. Transfusion. 2008;48:2S–30S. doi: 10.1111/j.1537-2995.2007.01573.x. [DOI] [PubMed] [Google Scholar]
- 9.Levi M, Fries D, Gombotz H, van der Linden Ph, Nascimento B, Callum JL, et al. Prevention and treatment of coagulopathy in patients receiving massive transfusions. Vox Sanguinis. 2011;101:154–74. doi: 10.1111/j.1423-0410.2011.01472.x. [DOI] [PubMed] [Google Scholar]
- 10.European Medicines Agency. Core SPC for human prothrombin complex products CPMP/BPWG/3735/02 http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003518.pdf. 12 January 2012, date last accessed)
- 11.Stuklis RG, O'Shaughnessy DF, Ohri SK. Novel approach to bleeding in patients undergoing cardiac surgery with liver dysfunction. Eur J Cardiothorac Surg. 2001;19:219–20. doi: 10.1016/s1010-7940(00)00641-2. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka KA, Szlam F. Treatment of massive bleeding with prothrombin complex concentrate: argument for. J Thromb Haemost. 2010;8:2589–91. doi: 10.1111/j.1538-7836.2010.04052.x. [DOI] [PubMed] [Google Scholar]
- 13.Godier A, Susens S, Samama C-M. Treatment of massive bleeding with prothrombin complex concentrate: argument against. J Thromb Haemost. 2010;8:2592–5. doi: 10.1111/j.1538-7836.2010.04062.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaspereit F, Hoffmann S, Pragst I, Dickneite G. Prothrombin complex concentrate mitigates diffuse bleeding after cardiopulmonary bypass in a porcine model. Br J Anaesth. 2010;105:576–82. doi: 10.1093/bja/aeq216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demeyre R, Gillardin S, Arnout J, Strengers PFW. Comparison of fresh frozen plasma and prothrombin complex concentrate for the reversal of oral anticoagulants in patients undergoing cardiopulmonary bypass surgery: a randomized study. Vox Sanguinis. 2010;99:251–60. doi: 10.1111/j.1423-0410.2010.01339.x. [DOI] [PubMed] [Google Scholar]
- 16.Fraser TA, Corke CF, Mohajeri M, Stevenson L, Campbell PJ. A retrospective audit of the use of prothrombinex-HT for refractory bleeding following adult cardiac surgery. Crit Care Resusc. 2006;8:141–5. [PubMed] [Google Scholar]
- 17.Görlinger K, Dirkmann D, Hanke AA, Kamler M, Kottenberg E, Thielmann M, et al. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery. A retrospective, single-center cohort study. Anesthesiology. 2011;115:1179–91. doi: 10.1097/ALN.0b013e31823497dd. [DOI] [PubMed] [Google Scholar]
- 18.Azmoun A, Ramadan R, Al-Attar N, Kortas C, Ghostine S, Caussin C, et al. Exclusive internal thoracic artery grafting in triple-vessel-disease patients: angiographic control. Ann Thorac Surg. 2007;83:2098–102. doi: 10.1016/j.athoracsur.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Kalina U, Bickhard H, Schulte S. Biochemical comparison of seven commercially available prothrombin complex concentrates. Int J Clin Pract. 2008;62:1614–22. doi: 10.1111/j.1742-1241.2008.01859.x. [DOI] [PubMed] [Google Scholar]
- 20.Nuttall GA, Oliver WC, Santrach PJ, Bryant S, Dearani JA, Schaff HV, et al. Efficacy of a simple intraoperative transfusion algorithm for nonerythrocyte component utilization after cardiopulmonary bypass. Anesthesiology. 2001;94:773–81. doi: 10.1097/00000542-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson M, Ternström L, Hyllner M, Baghaei F, Flinck A, Skrtic S, et al. Prophylactic fibrinogen infusion reduces bleeding after coronary artery bypass surgery. A prospective randomised pilot study. Thromb Haemost. 2009;102:137–44. doi: 10.1160/TH08-09-0587. [DOI] [PubMed] [Google Scholar]
- 22.Schick KS, Fertmann JM, Jauch K-W, Hoffmann JN. Prothrombin complex concentrate in surgical patients: retrospective evaluation of vitamin K antagonist reversal and treatment of severe bleeding. Crit Care. 2009;13:R191. doi: 10.1186/cc8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staudinger T, Frass M, Rintelen C, Quehenberger P, Wagner O, Stoiser B, et al. Influence of prothrombin complex concentrates on plasma coagulation in critically ill patients. Intensive Care Med. 1999;25:1105–10. doi: 10.1007/s001340051019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozek-Langenecker S, Sorensen B, Hess JR, Spahn DR. Clinical effectiveness of fresh frozen plasma compared with fibrinogen concentrate: a systematic review. Crit Care. 2011;15:R239. doi: 10.1186/cc10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorensen B, Spahn DR, Innerhofer P, Spanngl M, Rossaint R. Clinical review: prothrombin complex concentrates—evaluation of safety and thrombogenicity. Crit Care. 2011;15:201. doi: 10.1186/cc9311. [DOI] [PMC free article] [PubMed] [Google Scholar]