Abstract
OBJECTIVES
Many centres avoid using cardiopulmonary bypass (CPB) for lung transplant due to concerns over aggravated lung reperfusion injury and excessive blood loss. We reviewed our 23-years’ experience of single lung transplantation.
METHODS
A retrospective review of single lung transplants at our institution (1987–2010), examining differences in allograft function and postoperative complications between CPB and non-bypass (non-CPB) cases.
RESULTS
Two hundred and fifty-nine single lung transplants were undertaken. Fifty-three (20.5%) with CPB. There was no difference demographically between the two groups. No difference existed in preoperative PO2/FiO2. At 1 and 24 h, the postoperative PO2/FiO2 ratio was no different (mean 2.95 and 3.24 in non-CPB cases; 3.53 and 3.75 in CPB patients, P = 0.18 and P = 0.34, respectively). Extubation time was not influenced by the use of CPB. Postoperative blood loss was greater in the CPB group. The usage of fresh frozen plasma and platelets was similar (P = 0.64 and 0.41, respectively). More blood was transfused during postoperative care of CPB patients (P = 0.02).
CONCLUSIONS
Fears of poor postoperative lung function after CPB appear unfounded. We could detect no difference in function or extubation time. Although the use of CPB increases postoperative bleeding and the need for transfusion, it may be used safely to facilitate lung transplantation.
Keywords: Lung transplant, Cardiopulmonary bypass
INTRODUCTION
No consensus exists on the place of cardiopulmonary bypass (CPB) in lung transplantation. Practice has changed over time, because of evolving surgical techniques and recipient selection criteria. Many of the first grafts (single lungs) in patients with pulmonary fibrosis did not require CPB. En bloc double lung transplants and single lung transplants for pulmonary hypertension necessitated more frequent use of CPB. With the advent of bilateral sequential lung transplants, and developing surgical techniques, the absolute indication for CPB has lessened. As more lung transplants are performed, and a burgeoning number of institutions involved, divergent practice has stimulated controversy over the indications and value of CPB.
The main potential disadvantage of CPB is an inflammatory response, mediated by the contact of blood with the bypass circuit, with the risk of lung injury and excessive bleeding. This is common to all CPB patients (not just those receiving transplants), and many studies have been conducted to demonstrate the adverse effects [1]. Within the literature, a laboratory study on animals has demonstrated worse early pulmonary graft function [2] in those animals who were transplanted with CPB (even with newer heparin-coated circuits. Proponents of CPB state that an increased cytokine release better correlates with ischaemia and reperfusion rather than the use of CPB itself. Marczin et al. [3] state that microvascular injury caused by hydrostatic stress has caused graft damage, which can be limited by controlling blood flow to the new lung. Those against argue that the systemic inflammatory response of CPB itself has been implicated in lung injury [4]. As the graft is subject to rejection by the host's immune system, unnecessary activation of that system may be significantly detrimental [5].
Other disadvantages of CPB include the need for heparin and the subsequent increased risk of bleeding. In lung transplant recipients, Wang et al. [6] found that the longer CPB time correlated with the use of blood products. Renal and neurological dysfunctions are also possible sequelae. Acute renal failure is particularly important to the transplant population as immunosuppressive medications such as cyclosporin are nephrotoxic and dosage may be restricted in these circumstances [5].
Theoretical arguments clearly exist for and against the use of CPB [3, 5], but the clinical evidence is inconclusive. A recent review article on the use of CPB in double lung transplant patients found ‘conflicting data with some studies showing a significant clinical disadvantage to CPB use, some showing no difference and some showing both depending on the postoperative outcome assessed’ [7].
Prior literature focused on double lung transplants. All studies for this cohort are retrospective. A review concluded: ‘Given that the evidence for using CPB for all elective cases is relatively weak, and the fact that there are strong arguments in the literature for both methods, either would be clinically acceptable’ [7]. A few papers cover all lung transplant patients, failing to differentiate between double and single lung recipients. The exception, Hlozek et al. [8], selects patients with emphysema for sub-group analysis of mortality, 94% of whom had received single lung transplants. He concluded that CPB was safe and effective. While some experimental animal studies have been carried out using a ‘single lung’ model [2], no clinical paper has focused on single lung recipients. There are sufficient differences between single and double lung transplants (e.g. length of surgery, type of incision, likelihood of adhesions and sepsis) to warrant separate analysis. While retrospective, this paper provides a large series of single lung transplants (259 patients), 20.5% of which were carried out on bypass, permitting comparison between the two techniques.
MATERIALS AND METHODS
We retrospectively reviewed data for all adult patients receiving single lung transplants at our institution over the 23-year span of our transplant programme (1987 until July 2010). Patients were identified through our transplant register and information derived from our database and medical notes.
The data were analysed using IBM SPSS v17.0 for Mac. Statistical significance was determined using the Mann–Whitney U-test and defined as a P value < 0.05.
A thoracotomy incision was used for the majority of patients. A small number of the CPB group underwent median sternotomy—for instance in pulmonary hypertension with unilateral pulmonary artery atresia. CPB was available for all cases. Criteria for elective decision to perform CPB were as follows:
inability to give single lung ventilation (small number);
pulmonary hypertension where a single lung transplant was performed for anatomical/surgical reasons and
when the residual lung was small but not transplanted for surgical reasons such as shrunken hemithorax or previous pleurectomy.
The intraoperative decision to use bypass was taken in the following cases:
haemodynamic instability after trial clamping of the pulmonary artery;
progressive rise in pCO2 despite vigorous ventilation of the remaining lung;
loss of one-lung ventilation, preventing satisfactory bronchial anastomosis and
surgical mishap, which occurred very rarely, but could be rescued by resort to CPB.
For CPB patients, 80% were performed with peripheral cannulation through the femoral vessels. This required the hips to be rolled to facilitate exposure of the femoral region. This approach provides an unobstructed operating field for the surgeon. Since 1990 a proportion of patients have received central cannulation. On the right this was a cannula at the front of the incision (or even placed through the chest wall) draining the right atrium. Arterial return was to the ascending aorta exposed behind the SVC. On the left, drainage was in the central pulmonary artery, proximal to the ligamentum (intrapericardial) and passed through the pulmonary valve. Arterial return was through the arch or descending thoracic aorta. Once bypass was initiated, ventilation was discontinued. Hypothermia of ∼32°C was established. Aprotonin was used at the discretion of the surgeon until 2009.
RESULTS
Two hundred and fifty-nine single lung transplants were carried out. Fifty three (20.5%) used CPB. The number requiring CPB has been stable at between 15 and 20% per year. The mean CPB time was 135.2 min (range 62–265). The majority (80%) were carried out using peripheral cannulation of the femoral artery and vein. The remaining 20% used central cannulation. Twelve (23%) were planned (due to poor lung function, poor right ventricular function or in one case due to previous pneumonectomy on the contralateral side). Forty-one (77%) were not planned. Thirty-three (80.5%) of these were due to poor oxygenation or hypercapnia on single lung ventilation. The remainder were due to haemodynamic instability.
The CPB and non-CPB groups were well matched demographically. M:F ratio was 146:115. Age range was 18–66 years. Underlying primary pulmonary pathology was also well matched (Table 1) and has not changed over time. There was no statistical difference between donor ischaemic times: CPB 256.7 min vs non-CPB 241.9 min (P = 0.18).
Table 1:
Recipient primary pulmonary pathology
| Pathology | Non-CPB (n = 206) | CPB (n = 53) |
|---|---|---|
| COPD/alpha 1 def (%) | 98 (47.6%) | 23 (43.3%) |
| IPF (%) | 83 (40.3%) | 25 (47.2%) |
| Other (%) | 25 (12.1%) | 5 (9.4%) |
COPD = Chronic Obstructive Pulmonary Disease IPF = Idiopathic Pulmonary Fibrosis
There was no difference in the preoperative PaO2 (CPB–214/ non-CPB–248). Postoperatively there was no statistical difference in PO2/FiO2 at 1 or 24 h (Fig. 1).
Figure 1:
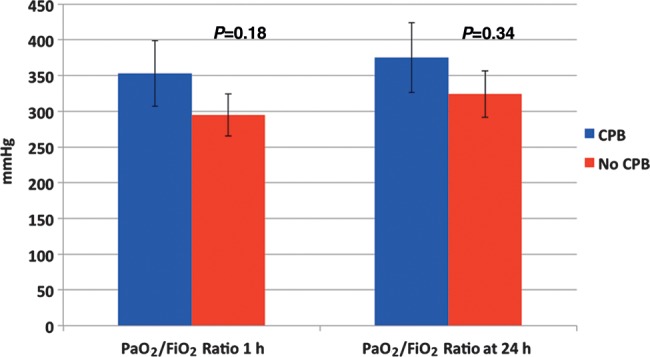
Postoperative PO2/FiO2 ratios.
Extubation time was not influenced by the use of CPB (P = 0.15). Mean 31.3 h (range 6.2–78.3) for CPB patients and mean 26.3 h (range 5.3–60.5) for non-CPB patients (Fig. 2). However, a significant difference did exist in intensive care unit (ITU) stay—CPB patients staying 5.2 days; non-CPB 2.8 (P ≤ 0.01).
Figure 2:
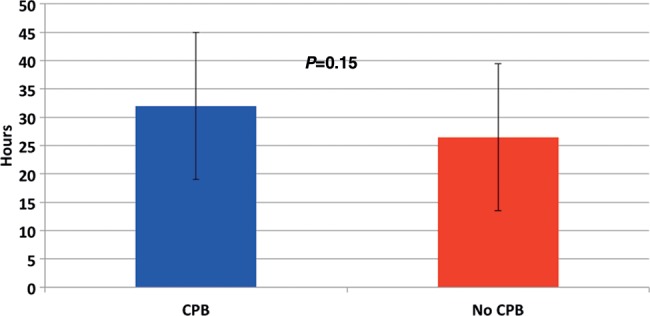
Time to extubation post surgery.
Blood loss and transfusion requirements within the first 24 h were recorded. Mean blood loss was significantly greater in the CPB group (Fig. 3) (P = 0.03). More units of blood [red blood cells (RBCs)] were transfused in the CPB cohort (P = 0.02). However, the two groups received similar quantities of fresh frozen plasma (FFP) and platelets (PLTs).
Figure 3:
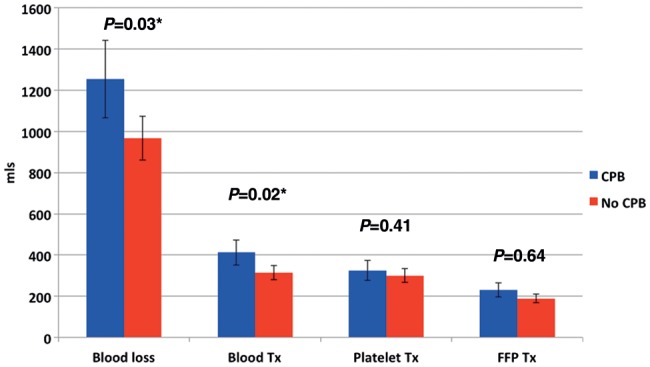
Recipient blood loss and transfusion requirements in the first 24 h.
Our policy is to perform trans-bronchial biopsy (TBB) at 30 days to look for evidence of rejection. No significant difference was observed in biopsy data (Fig. 4) between the rate of rejection in CPB and non-CPB patients (P = 0.99).
Figure 4:
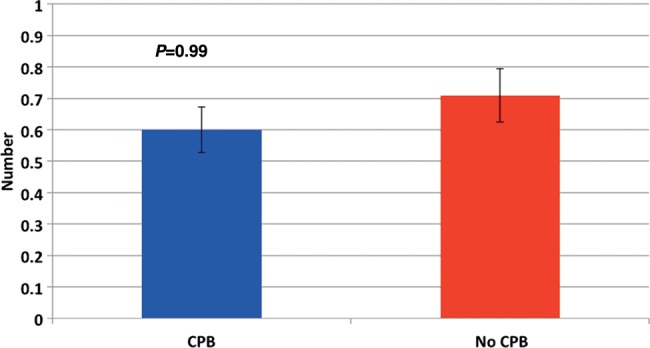
Number of episodes of rejection in the first 30 days.
No difference was shown between the planned and unplanned CPB groups for any of the assessed variables although the numbers in these sub-groups may have been too small to demonstrate a statistical significance.
DISCUSSION
A review of the existing literature shows that for single lung transplants, the use of CPB varies greatly between centres—ranging from 5% [6] to 33% [9]. Our practice (20.5%) lies approximately mid-way. The variability between centres demonstrates lack of consensus that exists on the role of CPB. For instance, Triantafillou et al. [9] argue that the use of CPB is predictable—used in pulmonary hypertensive recipients only, with only 4% of cases requiring urgent conversion to CPB due to ‘surgical mishap’[9]. Dalibon et al. [10], however, had twice as many unplanned to planned CPB cases and state that CPB must be available on standby at any point during the procedure. We would agree with this sentiment. Performing a fifth of our cases on CPB has allowed us to retain CPB-specific skills within the department and provide a comprehensive 24-h service. In the UK, the training in cardiac and thoracic surgery is linked, so all qualified cardiothoracic surgeons should be able to institute CPB, even if they later specialize in thoracic surgery. Most transplant centres have both cardiac and thoracic divisions on site. However worldwide, this is not universally the case and may represent one reason for a reluctance to embrace CPB in some purely thoracic centres running transplant programmes.
A further complicating factor has been the proposed use of extracorporeal membrane oxygenation (ECMO) as an alternative to CPB. None of the patients in this series were on ‘preoperative ECMO’, and with our familiarity with full bypass, we have seen no advantage to intraoperative ECMO. However, advantages presented in the literature include the reduced need for blood transfusion (due to lower anticoagulation requirements) and the ability to continue ECMO postoperatively, reducing the blood flow to the transplanted lung (limiting reperfusion pulmonary oedema). There was no statistical difference between our ischaemic times in the CPB and non-CPB groups. We could find no papers with figures for comparison. Two studies recorded ischaemic time but no breakdown between double and single lung transplant cases [10, 11]. Dalibon et al. [10] did highlight the fact that only unplanned CPB cases had longer ischaemic times when compared with non-CPB cases, emphasizing the fact that an early decision to go on bypass may be beneficial. This is particularly important in the single lung transplant cohort, as it has been suggested that only in single (not double) lung patients can the need for bypass be predicted preoperatively, something that is not systematically carried out at present: ‘For single lung transplantations, 6 min walk, the arterial desaturation/oxygen requirements on exercise, and the right ventricular ejection fraction were all significantly different between the CPB and non-CPB groups (P < 0.001)’ [12].
Our PaO2/FiO2 figures showed no statistical difference between the CPB and non-CPB groups at 1 or 24 h. Hlozek et al. also demonstrated similar results for their combined single and double lung data [8]. Other studies have looked more specifically at acute pulmonary oedema by investigating a number of factors including postoperative chest X-rays. All of these studies combine single and double lung transplant data . Khan et al., in a study spanning 5 years, showed that CPB patients had a greater incidence and severity of acute pulmonary oedema (CPB –70%/no CPB –48% P = 0.03). Its incidence was not related to prolonged ischaemic time, preoperative pulmonary hypertension, the type of lung transplant, underlying lung disease, age or sex of the recipients. It led to prolonged duration of mechanical ventilation (3 vs 2 days P = 0.009) and ITU stay (8 vs 6 days P = 0.03), but did not affect survival measured at 3 years [11]. Dalibon et al. also found the incidence of acute pulmonary oedema greater in the CPB cohort; both planned (81%) and unplanned CPB (50%) compared with non-CPB (34%) P ≤ 0.05. There was no statistical difference between the planned and unplanned CPB groups, suggesting a universally detrimental effect of CPB [10]. Similarly, Aeba et al. [13] looked at early graft dysfunction/injury on chest X-ray and noted that this was more severe in the CPB group, P = 0.034 .
Our study demonstrated no difference in time to extubation. We could find no other studies on single lung transplant patients alone. However, three studies that combined single and double transplant patients all demonstrated the opposite, with longer ventilation times in the CPB group [9, 10, 13]. Despite similar extubation times in our study, we did demonstrate a significantly longer duration of stay in ITU for the CPB patients, possibly because they represent a sicker cohort with more co-morbidities such as poorer preoperative lung and heart function. This is in contrast to Hlozek et al. [8] who for combined single and double lung patients had similar ITU stays in CPB and non-CPB groups.
Our data highlight a greater volume of blood loss in the CPB group. Many studies allude to greater blood loss (most likely due to the requirement for heparinization) but there are no real data available on volumes lost. Instead these studies provide data on transfusion requirements as a surrogate marker. Our CPB patients required significantly more packed RBCs than the non-CPB group. We follow a strict protocol with transfusion given at heamoglobin <8. Usage of PLTs and FFP was also greater but this did not reach statistical significance. Two studies looked specifically at transfusion requirements in lung transplant patients. Triulzi et al. showed a statistical difference in the number of double (92%) compared with single (32%) lung transplant patients who received blood products over a 1-year period P ≤ 0.0001. However, this was probably due to the lower number of single lung transplant patients undergoing CPB. CPB itself was associated with greater transfusion requirements of RBC, PLT and FFP, P = 0.0001. Within the CPB group blood requirements did not differ between the single and double lung recipients [14]. Wang et al. collected data over a 13-year period. Of all CPB patients only 13% were single lung recipients. In this small group, the use of RBCs in the CPB group still reached statistical significance at P ≤ 0.05. There was no difference in PLT or FFP use. For the larger group of single and double lung recipients combined, longer CPB time was predictive of an increased use of each component P = 0.01 [6]. Dalibon et al. (combining data on single and double) showed that whilst there was an increase in the use of RBCs in the CPB group compared to the non-CPB group, no difference existed between the planned and unplanned CPB patients [10]. Hlozek et al. showed an increase in the use of PLTs and FFP, but not RBCs for their CPB patients. However, they stated that: ‘Although CPB led to a significant increase in the use of blood products, the increase was not detrimental nor did it result in any significant postoperative problems’ [8]. Others disagree— suggesting that transplant patients are a unique group, at increased risk of infection from immune-compromise and graft rejection if exposed to additional transfused antibodies [6]. Data on the effect of transfusion on lung transplant recipients are sparse, but there is at least one case report from Australia of transfusion related acute lung injury (TRALI) exclusively in the donor lung following a blood transfusion: ‘Serological testing confirmed the presence of red cell donor antibodies with specificity only for the non-native lung tissues, thus explaining the dramatic unilateral process seen on chest radiograph’ [15]. We have not had any such incidents in our experience.
Our study demonstrated no significance in rejection episodes at 30 days, based on TBB results in the CPB compared with the non-CPB group. We could find no other studies in the literature that provided this for single or combined single and double lung cohorts.
This paper is the first to our knowledge to concentrate solely on single lung transplant recipients. It covers a long time period and includes a comparatively large cohort of single lung patients who underwent CPB, permitting statistical comparison against the non-CPB group. Whilst there are papers that look at both single and double lung transplant patients, they combine the two groups together for most analysis. None of these studies looked at blood loss or 30-day rejection for single or double lung, so our data provide interesting new insights. It is limited by being a retrospective study. Long-term follow-up was limited to 30 days. The time period covered is from the initiation of the lung transplant programme and therefore includes the natural learning curve and subtle modifications to practice that have occurred over time.
In conclusion, we believe that in single lung transplant patients, CPB can be a useful adjunct to surgery. ITU stay was prolonged, blood loss higher and transfusion requirements for RBCs greater, but we found no adverse sequelae—both PaO2/FiO2 (as a marker of early graft dysfunction) and 30-day rejection were similar in the two groups, making it a safe technique. We would therefore advocate its consideration in all patients receiving a single lung transplant especially where concerns are highlighted preoperatively.
Conflict of interest: none declared.
APPENDIX. CONFERENCE DISCUSSION
Dr P. Dartevelle (Le Plessis Robinson, France): My experience is very different. First, I think that single lung transplantation is not a good transplantation in terms of long-term results. Second, in your group of patients, did you have pulmonary hypertension, and do you think that single lung transplantation is a useful treatment in pulmonary hypertension? I would like to tell you that in my experience, we always prefer double lung transplantation because the mortality rate is lower in double lung transplantation than in single lung.
My last question is about the risk of cardiopulmonary bypass. When a patient has had a previous total pleurectomy, do you accept lung transplantation with cardiopulmonary bypass?
Dr Burdett: In response to the first question, we have a major problem with a shortage of organs in the UK, and therefore single lung transplant allows us to be able to transplant a greater number of patients, and that is the policy that we use at our centre.
In terms of pulmonary hypertension, I think if we go back to my table previously, there was another category, and there were a few patients within that, that would have had pulmonary hypertension that could not have double lung transplants for anatomical or surgical reasons. And your last question?
Dr Dartevelle: In patients with complete pleurectomy, do you accept transplantation with cardiopulmonary bypass? It is a question of bleeding.
Dr Burdett: Well, if the pleurectomy was on the other side and you are doing a single lung, it might be an indication for doing a single lung transplant on the other side. And then because you would possibly have a reduced hemithorax and other issues and problems with ventilation, it might actually be beneficial to do that on bypass. I do not know what our figures are for the number that we would perform that way.
Dr Dartevelle: Have you some experience with ECMO instead of cardiopulmonary bypass?
Dr Burdett: No. We have no experience of ECMO with our patients. We do not have any of our patients on ECMO prior to transplant. If we did, then perhaps we would follow them through that way, but we have not had that situation. The literature that I read for single lung transplant is a very small literature base at the moment, and it seems to be very controversial. There were a couple of papers from Southeast Asia that were very positive about it and another paper I read from Europe in which they actually found that complications like bleeding were greater than in the CPB group.
Dr G. Gerosa (Padova, Italy): So, according to your conclusion, you are actually recommending to lower the threshold for its use. Is that correct? According to your experience, what is the ratio between planned and unplanned cardiopulmonary bypass?
Dr Burdett: That is a good question. I do not know that off the top of my head, I am afraid. At the moment, I have not looked at recommending a low threshold rather than a lower threshold, but we should do.
Dr Z. Ahmadi (Tehran, Iran): First of all, you have said that the use of cardiopulmonary bypass during lung transplantation was unpredictable. With the patient in the lateral decubitus position for pneumonectomy, and then suddenly it is decided that cardiopulmonary bypass is necessary, cannulation of the femoral artery and femoral vein is usually very difficult in that position.
And in your presentation, you said that 80% of the cannulations were done peripherally. How do you cannulate the femoral artery and femoral vein in the left lateral decubitus? I think it is very difficult unless you can predict that cardiopulmonary bypass would be necessary, and a guidewire is passed to make cannulation easier.
Dr Burdett: I think in our situation, we always work on the basis that cardiopulmonary bypass might be necessary, and we have a perfusionist in the hospital ready to go. So when we set up the patients, we would set them up with their lower body slightly tilted to one side for exposure of the femoral vessels, so that if they were required, we would be able to cannulate that way. It involves preparing the patient before the operation in case it is necessary.
But, as you said, it might be equally as good to use, as you do, central cannulation once the patient is already on the operating table.
Dr Ahmadi: And the second question is about the dose of heparin that was given. You said that in conventional CPB, usually a high dose of heparin is necessary. But if a centrifugal pump is going to be used instead of a roller pump and it means that ECMO is used, the need for heparin and the consequent bleeding tendency will be very low. Do you have any experience with the use of a centrifugal pump instead of a roller pump in your centre?
Dr Burdett: I believe that all of ours are done on roller pump.
REFERENCES
- 1.Edmunds LH. Inflammatory response in cardiopulmonary bypass. Ann Thorac Surg. 1998;66:S12–16. doi: 10.1016/s0003-4975(98)00967-9. [DOI] [PubMed] [Google Scholar]
- 2.Francalancia NA, Aeba R, Yousem SA, Griffith BP, Marrone GC. Deleterious effects of cardiopulmonary bypass on early graft function after single lung allotransplantation: evaluation of a heparin-coated bypass circuit. J Heart Lung Transplant. 1993;13:498–507. [PubMed] [Google Scholar]
- 3.Marczin N, Royston D, Yacoub M. Pro: lung transplantation should be routinely performed with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2000;14:739–45. doi: 10.1053/jcan.2000.18592. [DOI] [PubMed] [Google Scholar]
- 4.Asimakopoulos G, Smith PLC, Ratnatunga CP, Taylor KM. Lung injury and acute respiratory distress syndrome after cardiopulmonary bypass. Ann Thorac Surg. 1999;68:1107–15. doi: 10.1016/s0003-4975(99)00781-x. [DOI] [PubMed] [Google Scholar]
- 5.McRae K. Con: lung transplantation should not be routinely performed with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2000;14:746–50. doi: 10.1053/jcan.2000.18601. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Kurichi JE, Blumenthal NP, Ahya VN, Christie JD, Pochettino A, et al. Multiple variables affecting blood usage in lung transplantation. J Heart Lung Transplant. 2006;25:533–8. doi: 10.1016/j.healun.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Nagendran M, Maruthappu M, Sugand K. Should double lung transplant be performed with or without cardiopulmonary bypass? Interact CardioVasc Thorac Surg. 2011;12:799–805. doi: 10.1510/icvts.2010.263624. [DOI] [PubMed] [Google Scholar]
- 8.Hlozek CC, Smedira NG, Kirby TJ, Patel A. Cardiopulmonary bypass (CPB) for lung transplantation. Perfusion. 1997;12:107–12. doi: 10.1177/026765919701200205. [DOI] [PubMed] [Google Scholar]
- 9.Triantafillou AN, Pasque MK, Huddleston CB, Pond CG, Cerza RF, Forstot RM, et al. Predictors, frequency and indications for cardiopulmonary bypass during lung transplantation in adults. Ann Thorac Surg. 1994;57:1248–51. doi: 10.1016/0003-4975(94)91367-6. [DOI] [PubMed] [Google Scholar]
- 10.Dalibon N, Geffroy A, Moutafis M, Vinatier I, Bonnette P, Stern M, et al. Use of cardiopulmonary bypass for lung transplantation: a 10-year experience. J Cardiothorac Vasc Anaesth. 2006;20:668–72. doi: 10.1053/j.jvca.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Khan SU, Salloum J, O'Donovan PB, Mascha EJ, Mehta AC, Matthay MA, et al. Acute pulmonary edema after lung transplantation. Chest. 1999;116:187–94. doi: 10.1378/chest.116.1.187. [DOI] [PubMed] [Google Scholar]
- 12.de Hoyos A, Demajo W, Snell G, Miller J, Winton T, Maurer JR, et al. Preoperartive prediction for the use of cardiopulmonary bypass in lung transplantation. J Thorac Cardiovasc Surg. 1993;106:787–96. [PubMed] [Google Scholar]
- 13.Aeba R, Griffith BP, Kormos RL, Armitage JM, Gasior TA, Fuhrman CR, et al. Effect of cardiopulmonary bypass on early graft dysfunction in clinical lung transplantation. Ann Thorac Surg. 1994;57:715–22. doi: 10.1016/0003-4975(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 14.Triulzi DJ, Griffith BP. Blood usage in lung transplantation. Transfusion. 1998;38:12–5. doi: 10.1046/j.1537-2995.1998.38198141492.x. [DOI] [PubMed] [Google Scholar]
- 15.Dykes A, Smallwood D, Kotsimbos T, Street A. Transfusion-related acute lung injury (TRALI) in a patient with a single lung transplant. Br J Haematol. 2000;109:671–8. doi: 10.1046/j.1365-2141.2000.01999.x. [DOI] [PubMed] [Google Scholar]


