Abstract
OBJECTIVES
To reduce the complications associated with cardiopulmonary bypass (CPB) during cardiac surgery, many modifications have been made to conventional extracorporeal circulation systems. This trend has led to the development of miniaturized extracorporeal circulation systems. Cardiac surgery using conventional extracorporeal circulation systems has been associated with significantly reduced microcirculatory perfusion, but it remains unknown whether this could be prevented by an mECC system. Here, we aimed to test the hypothesis that microcirculatory perfusion decreases with the use of a conventional extracorporeal circulation system and would be preserved with the use of an miniaturized extracorporeal circulation system.
METHODS
Microcirculatory density and perfusion were assessed using sublingual side stream dark-field imaging in patients undergoing on-pump coronary artery bypass graft (CABG) surgery before, during and after the use of either a conventional extracorporeal circulation system (n = 10) or a miniaturized extracorporeal circulation system (n = 10). In addition, plasma neutrophil gelatinase-associated lipocalin and creatinine levels and creatinine clearance were assessed up to 5 days post-surgery to monitor renal function.
RESULTS
At the end of the CPB, one patient in the miniaturized extracorporeal circulation-treated group and five patients in the conventional extracorporeal circulation-treated group received one bag of packed red blood cells (300 ml). During the CPB, the haematocrit and haemoglobin levels were slightly higher in the miniaturized extracorporeal circulation-treated patients compared with the conventional extracorporeal circulation-treated patients (27.7 ± 3.3 vs 24.7 ± 2.0%; P = 0.03; and 6.42 ± 0.75 vs 5.41 ± 0.64 mmol/l; P < 0.01). The density of perfused vessels with a diameter <25 µm (i.e. perfused vessel density) decreased slightly in the conventional extracorporeal circulation-treated group from 16.4 ± 3.8 to 12.8 ± 3.3 mm/mm2 (P < 0.01) and remained stable in the miniaturized extracorporeal circulation-treated group (16.3 ± 2.7 and 15.2 ± 2.9 mm/mm2 before and during the pump, respectively). Plasma neutrophil gelatinase-associated lipocalin levels were increased following the use of extracorporeal circulation in both groups, and no differences were observed between the groups. Plasma creatinine levels and creatinine clearance were not affected by CABG surgery or CPB.
CONCLUSIONS
The results from this relatively small study suggest that the use of the miniaturized extracorporeal circulation system is associated with a statistically significant (but clinically insignificant) reduction in haemodilution and microcirculatory hypoperfusion compared with the use of the conventional extracorporeal circulation system.
Keywords: Extracorporeal circulation circuit, Coronary artery bypass grafting, Cardiopulmonary bypass, Stream dark-field imaging, Microcirculation
INTRODUCTION
Since the first cardiopulmonary bypass (CPB) performed by Dennis et al. [1] in a 6-year old patient with a complex congenital heart defect, there have been many developments in heart–lung devices, oxygenators, filtration systems, blood pumps and surgical techniques. Presently, CPB remains the most commonly used method permitting surgical intervention to manage coronary artery disease. However, several complications can occur during the procedure, including systemic inflammatory response, neurocognitive decline, renal dysfunction, pulmonary dysfunction, gaseous microemboli, multiple organ failure and death [2–4]. Most complications are attributed to severe haemodilution, low blood pressure and the contact of the blood with air and the artificial surface of the bypass circuit during CPB. In an attempt to avoid or reduce these complications, off-pump coronary artery bypass (OPCAB) surgery has been introduced [4]. Regardless of a clinician's high level of experience with OPCAB, significant haemodynamic collapse, difficulty placing the graft due to movements of the heart, incomplete myocardial revascularization and high rates of repeat revascularization have been reported with OPCAB [5]. These problems have limited the use of OPCAB in cardiac surgery; therefore, CPB remains the method preferred by most cardiac surgeons to manage complicated cardiac operations [6]. To eliminate or reduce the complications associated with the CPB procedure, many modifications and refinements have been made to the conventional extracorporeal circulation (cECC) systems, which have led to the development of the less invasive and more biocompatible miniaturized extracorporeal circulation systems (mECC). mECC systems aim to minimize the priming volume and reduce the blood-foreign surface and blood–air contact area by removing the venous reservoir and omitting cardiotomy suction [6]. The advantages of using these mECC systems include reduced haemodilution [7–10], lower systemic inflammatory response [8], reduced activation of coagulation cascades [11], lower incidence of microbubbles [12], reduced postoperative morbidity [13] and blood conservation [14]. Furthermore, mECCs have been reported to decrease peri- and postoperative blood loss [14, 15] and thus, to decrease the need for blood transfusions [14–16].
With respect to the effects of CPB at the microcirculatory level, the haemodilution caused by cECC has been shown to significantly reduce capillary density by reducing blood viscosity [17, 18]. De Backer et al. conducted a study comparing patients undergoing cardiac surgery with and without ECC (on- vs off-pump surgery); the patients subjected to ECC exhibited the most decreased proportion of perfused microvessels and the most substantial increase in lactate levels [17]. This result has been confirmed by a study by Atasever et al., which showed that on-pump and off-pump CABG surgical procedures were associated with distinct alterations in sublingual microcirculatory perfusion and haemoglobin (Hb) oxygenation. Although on-pump surgery caused a fall-out of capillaries (reduced oxygen diffusion), off-pump surgery caused a cessation of the capillary flow during luxation (reduced oxygen convection) [18]. Thus, it has been established that cardiac surgery and the use of cECC systems are associated with disturbances of the microcirculation. However, no studies have investigated whether the miniaturization of ECC systems prevents these disturbances at the microcirculatory level.
The main aim of the present study was, therefore, to test the hypothesis that microcirculatory perfusion would decrease with the use of a cECC system (as shown in [17, 18]) and that microcirculatory perfusion would be preserved with the use of an mECC system. To this end, we measured the microcirculatory density and perfusion using sublingual side stream dark-field (SDF) imaging in patients undergoing on-pump coronary artery bypass graft (CABG) surgery before, during and after the use of either a cECC system or an mECC system. Furthermore, we tested whether CABG and CPB were associated with altered renal function by measuring the plasma neutrophil gelatinase-associated lipocalin (NGAL) and creatinine levels and creatinine clearance up to 5 days post-surgery.
MATERIALS AND METHODS
Patients
The study design was approved by the medical ethics committee of the Academic Medical Center of the University of Amsterdam. Written informed consent was obtained from all participating patients. Twenty patients undergoing CABG surgery were included in the study and were randomly allocated into two groups; in one group, a conventional ECC system (cECC; n = 10) was used, and in the other group, a miniaturized ECC system (mECC; n = 10) was used. Two surgeons participated in the study (not listed as coauthors); one performed the operations on the patients in the cECC group, and the other performed the operations on the patients in the mECC group. Both surgeons were experienced in the CPB systems applied. The same perfusionist (E.W.S.) was present during all of the surgeries. Patient exclusion criteria were an age <18 years, the withdrawal of consent, a recent oral surgery, vasculitis, preexisting renal dysfunction and a revision surgery.
Anaesthetic and surgical procedure
Before surgery, patients received 1–2 mg lorazepam, 2 g paracetamol and proton pump inhibitors. Furthermore, patients received 500 ml Ringer's lactate, 500 ml 6% voluven/tetraspan and cefamandol before induction. Anaesthesia was induced with sufentanil or fentanyl, and muscle relaxation was established by pancuronium and/or rocuronium. Patients were intubated endotracheally and ventilated with 100% oxygen. Arterial oxygen saturation was monitored by pulse oximetry. Swan-Ganz catheters were used to monitor pulmonary artery pressure (PAP) and the systolic, diastolic and mean arterial blood pressures (MAP). Patients received one dose of 300 IU per kg body weight heparin with a targeted activated clotting time (ACT) of >480 s.
The heart was exposed through median sternotomy, and the grafts used for revascularization were obtained from the saphenous vein, the mammary artery or the radial artery. Cardioplegia was established by an infusion of a potassium chloride solution, either directly in the pump circulation (blood cardioplegia) or diluted as cardioplegic fluid and administered through a separate pump unit.
Extracorporeal circulation
The cECC system employed in this study was a Stöckert S5 (Sorin Group, Italy) with roller pumps and a PrimO2x oxygenator (Sorin Group) with a membrane surface of 1.8 m2. The circuit was equipped with a hard-shell venous reservoir and a cardiotomy reservoir with cardiotomy suction. The tubing in this circuit was uncoated. The system was primed with 1650 ml of fluids, consisting of 1100 ml Ringer's lactate, 300 ml albumin (20%), 200 ml mannitol (20%), 50 ml NaHCO3− (8.4%), 10 000 IU heparin and magnesium sulphate (20%, 12 mmol).
The mECC system used in this study was a Physio coated Dideco ECC.O (Sorin Group) with an oxygenator membrane surface of 1.1 m2 [6]. A centrifugal pump actively drained the venous side and pumped the blood through the oxygenator and the arterial outlet. The circuit did not incorporate a cardiotomy reservoir, cardiotomy suction or a hard-shell venous reservoir. A collapsible soft-shell overflow bag was used to regulate the volume. The entire inner surface of the system was coated with a biocompatible phosphorylcholine coating. The circuit was primed with 500 ml Tetraspan (6%). At the initiation of perfusion, all of the fluids were removed from the system by retrograde autologous priming, antegrade autologous priming or ante-retrograde autologous priming.
Before initiating the extracorporeal perfusion, all of the patients received 150 IU/kg bodyweight heparin. The perfusion fluids were maintained at 39°C, and body temperature (measured rectally) was maintained between 35.5 and 36.5°C. For both ECC systems, the nominal pump flow was maintained at a cardiac index of 2.6 l/m2 per minute.
The use of a cell saver system was the standard procedure in the treatment of all patients. Shed blood suctioned from the operation field was processed in the cell saver and returned to the patient at the termination of extracorporeal perfusion, or earlier if necessary.
Blood samples and assessments
Arterial blood samples were obtained before, during and after the use of the ECC. The samples were analysed for haematocrit (Hct), Hb, lactate and whole blood viscosity. Hb and lactate were measured immediately after sampling in an automatic blood gas analyzer (RAPIDlab 1265, Bayer Health Care LLC, Tarrytown, NY, USA). Hct was read on a manual Hct scale after centrifugation (Hettich Hematokrit D-2700, Andreas Hettich GmbH & Co. KG, Tuttlingen, Germany). Whole blood viscosity was measured using a cone and plate viscometer (Contraves Low-shear 30, Contraves, AG Zürich).
The assessment of renal injury and renal function
Plasma NGAL (a marker of renal injury), plasma creatinine and creatinine clearance were measured before surgery; before, during and immediately after the use of the ECC; and up to 5 days after surgery. Plasma NGAL levels were measured by a sandwich enzyme-linked immunosorbent assay as described previously [19]. In short, plasma samples were stored in aliquots at −80°C. Monoclonal antibodies from Antibodyshop (Antibodyshop, BioPorto Diagnostics, Gentofte, Denmark) were used. The recombinant protein lipocalin was obtained from R&D Systems Europe Ltd (Abingdon, UK). The detection limit for NGAL in serum was 3.9 ng/ml. The intra-assay variation was <10%. Creatinine clearance was calculated by the Cockcroft and Gault [20] equation
Microcirculatory imaging
Sublingual microcirculatory density and perfusion were peri-operatively monitored using side SDF imaging. In SDF imaging, a central light guide is surrounded by concentrically placed light-emitting diodes to provide the SDF illumination. The lens system at the core of the light guide is optically isolated from the illuminating outer ring to prevent contamination of the microcirculatory image by the tissue surface reflections. Light from the illuminating outer ring of the SDF probe penetrates the tissue and illuminates the tissue-embedded microcirculation by scattering. Because the green light is absorbed only by Hb, RBCs are depicted as dark, moving globules against a bright background. To improve the imaging of moving structures such as the flowing RBCs, the illumination provided by the Leeds pulses in synchrony with the CCD frame rate. This stroboscopic imaging partially prevents the blurring of the moving features, such as the flowing RBCs, and the motion-induced blurring of the capillaries [21].
To comply with a recently published consensus report on the evaluation of the microcirculation [22], the SDF probe, covered by a sterile disposable cap, was gently placed on the sublingual tissue surface to avoid pressure artefacts. At each time point, five different sublingual sites were monitored for >20 s per site with adequate focus and contrast. The SDF images were analysed for the density of perfused vessels with a diameter <25 µm [i.e. perfused vessel density; PVD (mm vessel/mm2 image)] that serves as a surrogate value for the functional capillary density [22]. The SDF image analysis was performed using a computer software package (Automated Vascular Analysis Software (AVA) (Microvision Medical BV, Amsterdam, Netherlands). In addition, the microvascular flow index [MFI (AU)] in these small vessels, which provides an index for the microcirculatory blood flow velocity, was analysed semi-quantitatively as described previously [22].
Data collection and statistical analysis
Measurements were performed at three designated time points: (T1) before the initiation of ECC and just after the administration of heparin; (T2) during ECC and 10 min after total cardioplegia; and (T3) after the termination of ECC and 10 min after the administration of protamine. At these time points, haemodynamic parameters, blood samples and sublingual microcirculatory recordings were acquired.
The statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA). A comparative analysis of the values obtained in various groups and at various time points was performed using a two-way analysis of variance. All data are presented as the mean ± SD, and the differences between values were considered statistically significant at P < 0.05, which is denoted with an asterisk for P < 0.05 when comparing groups (e.g. cECC-treated vs mECC-treated) and with a hash symbol for P < 0.05 when comparing time points (e.g. During vs Before).
RESULTS
Patient characteristics
The patient characteristics and surgical characteristics are reported in Table 1. At the end of the CPB procedure, one patient in the mECC-treated group and five patients in the cECC-treated group received a bag of packed red blood cells (300 ml). After the CPB procedure, the patients treated with the mECC system received 241 ± 68 ml of blood from the cell saver system, and the patients treated with the cECC system received 232 ± 129 ml of blood from the cell saver system.
Table 1:
Patient characteristics
| cECC group | mECC group | P-value | |
|---|---|---|---|
| Age (years) | 64 ± 8 | 66 ± 9 | 0.50 |
| Gender (male:female) | 9:1 | 9:1 | — |
| Weight (kg) | 87 ± 19 | 83 ± 14 | 0.59 |
| Body mass index (kg/m2) | 28 ± 4 | 27 ± 3 | 0.74 |
| Hypertension (number of patients) | 3 | 2 | — |
| LV dysfunction (number of patients) | 2 | 2 | — |
| Diabetes (non-insulin dependent) (number of patients) | 2 | 1 | — |
| Hypercholesterolemia (number of patients) | 3 | 2 | — |
| Anastomoses (number) | 2.8 ± 1.0 | 2.8 ± 0.6 | 1.00 |
| Duration of CPB (min) | 119 ± 14 | 71 ± 16 | <0.01 |
| Duration of aortic clamping (min) | 81 ± 22 | 44 ± 15 | <0.01 |
| Period spent in ICU (days) | 2.2 ± 1.8 | 1.5 ± 0.7 | 0.26 |
cECC: conventional extracorporeal circulation circuit; mECC: minimized extracorporeal circulation circuit; LV: left ventricle; CPB: cardiopulmonary bypass; ICU: intensive care unit.
Macrocirculatory parameters
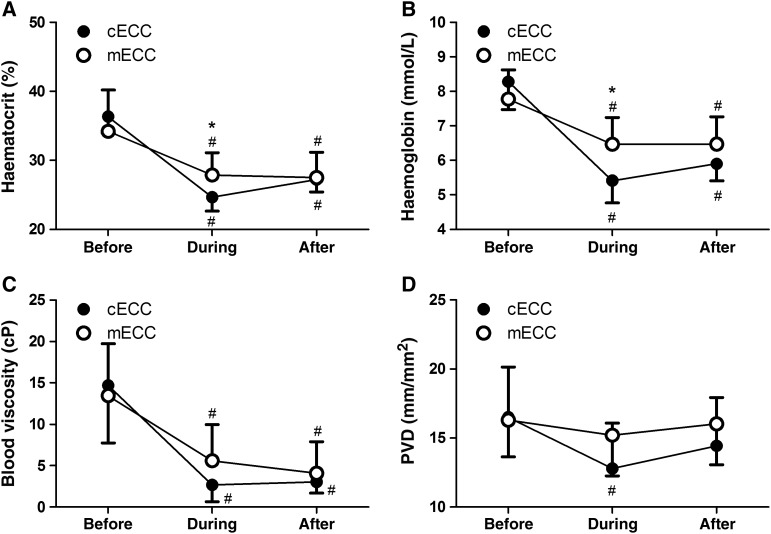
Systemic parameters before, during and after the use of the ECC systems are presented in Table 2. During the use of the ECC, the Hct (27.7 ± 3.3 vs 24.7 ± 2.0%; P = 0.03) and Hb (6.42 ± 0.75 vs 5.41 ± 0.64 mmol/l; P < 0.01) levels were slightly higher in the mECC-treated group compared with those in the cECC-treated group (Fig. 1). Viscosity decreased at the onset of extracorporeal circulation in a similar manner in both groups.
Table 2:
Systemic parameters before, during and after the use of the extracorporeal circulation circuit
| cECC group | mECC group | P-value | |
|---|---|---|---|
| Haematocrit (%) | |||
| Before | 36.3 ± 2.4 | 34.2 ± 6.0 | 0.33 |
| During | 24.7 ± 2.0 | 27.7 ± 3.3 | 0.03 |
| After | 27.2 ± 1.8 | 27.5 ± 3.7 | 0.84 |
| Haemoglobin (mmol/l) | |||
| Before | 8.28 ± 0.81 | 7.78 ± 0.84 | 0.19 |
| During | 5.41 ± 0.64 | 6.42 ± 0.75 | <0.01 |
| After | 5.90 ± 0.49 | 6.43 ± 0.77 | 0.08 |
| Whole blood viscosity (mPa s) | |||
| Before | 14.7 ± 7.0 | 13.5 ± 6.3 | 0.69 |
| During | 2.7 ± 2.0 | 5.4 ± 4.6 | 0.12 |
| After | 3.0 ± 1.4 | 4.1 ± 3.8 | 0.43 |
| Lactate (mmol/l) | |||
| Before | 1.35 ± 0.52 | 1.18 ± 0.32 | 0.37 |
| During | 1.77 ± 0.54 | 1.55 ± 0.32 | 0.25 |
| After | 1.64 ± 0.48 | 1.40 ± 0.37 | 0.21 |
| MAP (mmHg) | |||
| Before | 71 ± 5 | 74 ± 9 | 0.40 |
| During | 63 ± 8 | 65 ± 6 | 0.63 |
| After | 66 ± 3 | 71 ± 7 | 0.04 |
| PAP (mmHg) | |||
| Before | 20.0 ± 3.2 | 18.1 ± 4.0 | 0.23 |
| During | 8.1 ± 1.8 | 9.0 ± 2.6 | 0.39 |
| After | 19.2 ± 4.0 | 18.8 ± 2.0 | 0.78 |
| Heart rate (bpm) | |||
| Before | 60 ± 8 | 60 ± 8 | 0.84 |
| During | — | — | — |
| After | 76 ± 10 | 85 ± 16 | 0.17 |
cECC: conventional extracorporeal circulation circuit; mECC: minimized extracorporeal circulation circuit; MAP: mean arterial pressure; PAP: pulmonary arterial pressure.
Figure 1:
Haematocrit (A), haemoglobin level (B), blood viscosity (C) and PVD (D) before, during and after the use of the extracorporeal circulation circuit. cECC: conventional extracorporeal circulation circuit; mECC: minimized extracorporeal circulation circuit *P < 0.05 vs cECC; #P < 0.05 vs BEFORE.
Lactate levels were similar in both groups. MAP was similar before and during the use of the ECC, but afterwards, MAP was slightly higher in the mECC-treated group (71 ± 7 vs 66 ± 3 mmHg; P = 0.04). After the use of the ECC, the heart rate was higher in the mECC-treated group, but not to a statistically significant degree (85 ± 16 vs 76 ± 10 bpm; P = 0.17).
Microcirculatory parameters
The density of perfused vessels with a diameter <25 µm (i.e. PVD), which serves as a surrogate value for the functional capillary density [22] before, during and after application of the ECC, is depicted in Fig. 1. During the application of the ECC, the PVD decreased slightly in the cECC-treated group from 16.4 ± 3.8 to 12.8 ± 3.3 mm/mm2 (P < 0.01) and remained stable in the mECC-treated group (16.3 ± 2.7 and 15.2 ± 2.9 mm/mm2 before and during the use of the pump, respectively). The MFI (=3) remained normal throughout the entire procedure in both groups.
Renal injury and renal function parameters
Table 3 presents the plasma NGAL levels, the plasma creatinine concentrations and the creatinine clearance rates before surgery; before, during and immediately after the use of the ECC; and up to 5 days after surgery. Plasma NGAL levels were significantly higher after the cessation of the ECC in both groups compared with levels before the application of the ECC, but the levels did not differ between the mECC-treated group and the cECC-treated group. Plasma creatinine levels and creatinine clearance rates were unaffected by CABG surgery and by the use of the ECCs.
Table 3:
Plasma NGAL levels, plasma creatinine concentration and creatinine clearance rates before surgery, before, during and immediately after the use of the ECC, and up to 5 days after surgery
| cECC group | mECC group | P-value | |
|---|---|---|---|
| NGAL | |||
| Before | 172 ± 87 | 128 ± 56 | 0.19 |
| During | 140 ± 45 | 154 ± 50 | 0.53 |
| After | 256 ± 94 | 190 ± 85 | 0.12 |
| Plasma creatinine | |||
| Before surgery | 80 ± 13 | 83 ± 10 | 0.51 |
| Before pump | 80 ± 10 | 80 ± 12 | 0.97 |
| During pump | 80 ± 9 | 79 ± 11 | 0.84 |
| After pump | 82 ± 11 | 80 ± 11 | 0.70 |
| Day 1 | 81 ± 16 | 83 ± 20 | 0.87 |
| Day 2 | 80 ± 25 | 78 ± 17 | 0.86 |
| Day 3 | 80 ± 18 | 80 ± 16 | 0.91 |
| Day 4 | 77 ± 19 | 82 ± 16 | 0.49 |
| Day 5 | 80 ± 15 | 79 ± 12 | 0.82 |
| Creatinine clearance | |||
| Before surgery | 109 ± 38 | 97 ± 35 | 0.48 |
| Before pump | 114 ± 39 | 96 ± 31 | 0.26 |
| During pump | 115 ± 41 | 95 ± 30 | 0.21 |
| After pump | 113 ± 41 | 93 ± 29 | 0.20 |
| Day 1 | 113 ± 44 | 96 ± 34 | 0.32 |
| Day 2 | 118 ± 41 | 102 ± 41 | 0.39 |
| Day 3 | 117 ± 45 | 97 ± 34 | 0.27 |
| Day 4 | 112 ± 42 | 102 ± 36 | 0.55 |
| Day 5 | 118 ± 39 | 95 ± 24 | 0.13 |
Creatinine clearance was estimated with the Cockcroft and Gault equation: [(140−age)× weight × 1.23/plasma creatinine]. cECC: conventional extracorporeal circulation circuit; mECC: minimized extracorporeal circulation circuit.
DISCUSSION AND CONCLUSIONS
In the present study, we aimed to test the hypothesis that microcirculatory perfusion would decrease with the use of a conventional ECC (cECC) system and that this would be preserved when applying a miniaturized ECC (mECC) system. To this end, we measured the microcirculatory density and perfusion using sublingual SDF imaging in patients undergoing on-pump CABG surgery before, during and after the use of either a cECC system or a mECC system. In addition, we tested whether CABG and CPB would be associated with an altered renal function. Our main findings were that [1] the use of the mECC system was associated with a statistically significant (but clinically insignificant) reduction in haemodilution and microcirculatory hypoperfusion compared with the those levels associated with the use of the cECC system and that [2] the plasma NGAL levels were increased following the use of the ECC in both groups with no differences between the groups.
Progressive haemodilution has been shown to cause microcirculatory dysfunction and to depress microcirculatory oxygen delivery via a diminished capillary density. To determine the role of cECC in microcirculatory alterations, De Backer et al. performed a study comparing patients undergoing cardiac surgery with cECC or OPCAB (off-pump) and patients undergoing non-cardiac surgery (thyroidectomy). The proportion of perfused microcirculatory vessels decreased most in the patients undergoing cardiac surgery with cECC, and these patients exhibited the most substantial increase in lactate, reflecting tissue hypoxia, compared with the other two groups of patients [17]. This finding agrees with the findings of a study by Atasever et al. [18] showing that on-pump cardiac surgery resulted in a fall out of capillaries and consequently a decreased oxygen extraction, while off-pump surgery resulted in a cessation of flow during luxation. This phenomenon indicates a differential response at the microcirculatory level in which on-pump surgery mainly affects the diffusive component of the microcirculation (capillary density), while luxation mainly affects the convective component of the microcirculation (capillary flow). In agreement with these studies, we found that PVD was significantly reduced during CPB with the use of the cECC but not with the use of the mECC. This result might best be explained by the differences observed in Hct and Hb levels. Neither the cECC nor the mECC system altered the microvascular flow dynamics observed in the present study. It should be noted that although the PVD decreased significantly in the cECC-treated group and not in the mECC-treated group, the PVD during CPB did not differ between the two groups to a statistically significant degree, likely because of the small sample size per group. Thus, a larger study is required to confirm these findings.
In addition to haemodilution, many other factors during CPB could disturb the microcirculatory perfusion. Blood contact with the ECC tubing activates the coagulation cascade [11, 12]. Fibrin and fibrinogen are deposited onto the exposed surfaces and cause thrombin adherence and activation. Platelet dysfunction and thrombocytopenia may occur because of platelet activation receptor depletion through contact with the tubing, platelet adhesion to the tubing and subsequent platelet depletion from the body as well as platelet damage from shear force within the circuit. Furthermore, haemodilution may aggravate this by changing the concentration of thrombocytes, coagulation factors and inhibition factors. Here, we found that during the use of the ECC, the Hct was only 12% higher in the mECC-treated group than that in the cECC-treated group (27.7 ± 3.3 vs 24.7 ± 2.0%). The PVD was 19% higher in the mECC-treated group than that in the cECC-treated group (15.2 ± 2.9 vs 12.8 ± 3.3 mm/mm2), which suggests that factors other than haemodilution affect microcirculatory perfusion during CABG surgery and CPB. However, the design of the present study precluded the identification of other such factors.
A strong association has been found between altered microcirculation and poor clinical outcome [22]. However, most of these studies have been conducted in intensive care unit patients; it remains unclear how they relate to microcirculatory dysfunction and the clinical outcome in other conditions such as CABG surgery. Although the PVD was significantly reduced in the cECC-treated group during CPB, the reduction was relatively small; because no cut-off values for PVD have been established to date, it is difficult to view this result from a clinical perspective. Furthermore, in the studies comparing on-pump and off-pump cardiac surgery, only small differences in the degree of the microcirculatory alterations have been observed. Therefore, De Backer et al. [17] stated that the ECC system may only play a minor role among all of the other factors that affect the microcirculation, including anaesthetic agents, medication and the inflammatory activation that occurs during surgery. Improvements in ECCs, however, may still have a clinical benefit because of the large numbers of patients undergoing on-pump surgery. Viewed from this perspective, mECC systems may be a valuable tool in cardiac surgery.
Recent clinical studies have indicated that the use of an ECC system may be associated with depressed organ function and poor outcome following CABG surgery [23, 24]. In this respect, the kidneys are especially susceptible due to their particularly complex microvascular organization and their high demand for oxygen [25]. The pathophysiology of the kidney injury related to CPB is multifactorial. Potential mechanisms involved include haemodilution, low cardiac output, hypotension, non-pulsatile flow, renal hypoperfusion, activation of systemic inflammation and coagulation and microemboli. The use of mECC systems may decrease the inflammatory response and reduce the injury marker levels of the heart, lung, kidney and intestine compared with levels observed after the use of cECC systems [15, 16]. In a prospective observational study that included 9080 patients undergoing cardiac surgery with CPB, Karkouti et al. [23] showed that the risk of acute renal failure necessitating renal replacement therapy increased as Hct levels deviated in either direction from ‘moderate’ haemodilution levels (i.e. Hct of 21–25%). In our study, however, the Hct levels of both groups remained within this moderate haemodilution range, which might explain why we observed only a small rise in plasma NGAL levels and no change in creatinine clearance.
This study has some shortcomings. First, 10 patients per group is a relatively small sample size. Therefore, the differences between groups and time points might not have reached statistical significance because the study was underpowered. However, in a previous study by our group in which we investigated the effects of blood transfusion at the microcirculatory level in patients undergoing CABG surgery, 10 subjects per group was sufficient to observe statistically significant differences in microcirculatory density. A post hoc power analysis for the values obtained for the PVD (last time point: difference in mean = 1.6, σ = 3.5, power ≥ 0.80, P ≤ 0.05) and NGAL (difference in mean = 66, σ = 94, power ≥ 0.80, P ≤ 0.05) indicates that study populations of 40 per group and 18 per group for PVD and NGAL, respectively, would be required to reach statistical significance with the differences observed in this study. Second, only the sublingual mucosal microcirculation was analysed; the condition of this vascular bed may not be representative of the condition of the microcirculation in other parts of the body. In fact, it has been established that the microcirculation can be heterogeneously altered between and within organs [22]. Conversely, several studies have confirmed that sublingual microcirculation is representative of a patient's overall microcirculatory condition [17, 22]. Third, the length of the CPB and the duration of the aortic clamping period were significantly longer in the cECC-treated group compared with those durations in the mECC-treated group. As a result, the ‘before’ and ‘during’ time points are similar for both groups (i.e. before the initiation of the ECC and just after the administration of heparin; and during ECC and 10 min after total cardioplegia), but the ‘after’ time point is significantly later for the cECC-treated group. However, this difference did not seem to affect our results; the most important differences observed between the groups (in Hct and Hb levels and PVD) were only present during the use of the ECC and not afterward. Furthermore, although only one time point before CPB and only one after CPB might not be enough to track all of the changes occurring during surgery, all of the observed changes were quite small and additional time points are unlikely to contribute much information. Fourth, differences in the priming fluid applied, inconsistent use of autologous priming and differences in the type of cardioplegia as well as a lack of data regarding the central venous pressure, the mixed venous oxygen saturation and the vasopressor and inotropic requirements make it difficult to identify the exact effects of the different CPB systems on the studied parameters.
In conclusion, the results from this relatively small study suggest that the use of the mECC system is associated with a statistically significant (but clinically insignificant) reduction in haemodilution and microcirculatory hypoperfusion compared with the use of the cECC system.
Funding
This research was financially supported by the Landsteiner Foundation for Blood Research (Grant 2006-0621).
Conflict of interest: Can Ince is the inventor of SDF technology that is commercialized by MicroVision Medical. He has been a consultant for this company in the past but has had no contact with the company for more than 2 years. He also has no competing interests in MicroVision Medical, Cytometrics or Braedius Scientific other than his commitment to promote the importance of microcirculation in the care of critically ill patients. All of the other authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
We would like to thank Van Zwieten for his constructive feedback with regard to the study design and the manuscript.
REFERENCES
- 1.Dennis C, Spreng DS, Jr, Nelson GE, Karlson KE, Nelson RM, Thomas JV, et al. Development of a pump-oxygenator to replace the heart and lungs; an apparatus applicable to human patients, and application to one case. Ann Surg. 1951;134:709–21. doi: 10.1097/00000658-195113440-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bical OM, Fromes Y, Gaillard D, Fischer M, Ponzio O, Deleuze P, et al. Comparison of the inflammatory response between miniaturized and standard CPB circuits in aortic valve surgery. Eur J Cardiothorac Surg. 2006;29:699–702. doi: 10.1016/j.ejcts.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 3.Ascione R, Lloyd CT, Underwood MJ, Lotto AA, Pitsis AA, Angelini GD. Inflammatory response after coronary revascularization with or without cardiopulmonary bypass. Ann Thorac Surg. 2000;69:1198–204. doi: 10.1016/s0003-4975(00)01152-8. [DOI] [PubMed] [Google Scholar]
- 4.Elahi MM, Khan JS, Matata BM. Deleterious effects of cardiopulmonary bypass in coronary artery surgery and scientific interpretation of off-pump's logic. Acute Card Care. 2006;8:196–209. doi: 10.1080/17482940600981730. [DOI] [PubMed] [Google Scholar]
- 5.Arom KV, Flavin TF, Emery RW, Kshettry VR, Janey PA, Petersen RJ. Safety and efficacy of off-pump coronary artery bypass grafting. Ann Thorac Surg. 2000;69:704–10. doi: 10.1016/s0003-4975(99)01510-6. [DOI] [PubMed] [Google Scholar]
- 6.Valtonen M, Vahasilta T, Kaila-Keinanen T, Kuttila K. New mini-extracorporeal circulation system (ECC.O) is a safe technique in coronary surgery. Scand Cardiovasc J. 2007;41:345–50. doi: 10.1080/14017430701446933. [DOI] [PubMed] [Google Scholar]
- 7.Perthel M, Klingbeil A, El-Ayoubi L, Gerick M, Laas J. Reduction in blood product usage associated with routine use of mini bypass systems in extracorporeal circulation. Perfusion. 2007;22:9–14. doi: 10.1177/0267659106075660. [DOI] [PubMed] [Google Scholar]
- 8.Fromes Y, Gaillard D, Ponzio O, Chauffert M, Gerhardt MF, Deleuze P, et al. Reduction of the inflammatory response following coronary bypass grafting with total minimal extracorporeal circulation. Eur J Cardiothorac Surg. 2002;22:527–33. doi: 10.1016/s1010-7940(02)00372-x. [DOI] [PubMed] [Google Scholar]
- 9.Schöttler J, Lutter G, Boning A, Soltau D, Bein B, Caliebe D, et al. Is there really a clinical benefit of using minimized extracorporeal circulation for coronary artery bypass grafting? J Thorac Cardiovasc Surg. 2008;56:65–70. doi: 10.1055/s-2007-989336. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-Rahman U, Martens S, Risteski P, Ozaslan F, Riaz M, Moritz A, et al. The use of minimized extracorporeal circulation system has a beneficial effect on hemostasis—a randomized clinical study. Heart Surg Forum. 2006;9:E543–8. doi: 10.1532/HSF98.20051110. [DOI] [PubMed] [Google Scholar]
- 11.Wippermann J, Albes JM, Hartrumpf M, Kaluza M, Vollandt R, Bruhin R, et al. Comparison of minimally invasive closed circuit extracorporeal circulation with conventional cardiopulmonary bypass and with off-pump technique in CABG patients: selected parameters of coagulation and inflammatory system. Eur J Cardiothorac Surg. 2005;28:127–32. doi: 10.1016/j.ejcts.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 12.Perthel M, Kseibi S, Sagebiel F, Alken A, Laas J. Comparison of conventional extracorporeal circulation and minimal extracorporeal circulation with respect to microbubbles and microembolic signals. Perfusion. 2005;20:329–33. doi: 10.1191/0267659105pf828oa. [DOI] [PubMed] [Google Scholar]
- 13.Mazzei V, Nasso G, Salamone G, Castorino F, Tommasini A, Anselmi A. Prospective randomized comparison of coronary bypass grafting with minimal extracorporeal circulation system (MECC) versus off-pump coronary surgery. Circulation. 2007;116:1761–7. doi: 10.1161/CIRCULATIONAHA.107.697482. [DOI] [PubMed] [Google Scholar]
- 14.Perthel M, El-Ayoubi L, Bendisch A, Laas J, Gerigk M. Clinical advantages of using mini-bypass systems in terms of blood product use, postoperative bleeding and air entrainment: an in vivo clinical perspective. Eur J Cardiothorac Surg. 2007;31:1070–5. doi: 10.1016/j.ejcts.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 15.Biancari F, Rimpiläinen R. Meta-analysis of randomized trials comparing the effectiveness of miniaturized versus conventional cardiopulmonary bypass in adult cardiac surgery. Heart. 2009;95:964–9. doi: 10.1136/hrt.2008.158709. [DOI] [PubMed] [Google Scholar]
- 16.Alevizou A, Dunning J, Park JD. Can a mini-bypass circuit improve perfusion in cardiac surgery compared to conventional cardiopulmonary bypass? Interact CardioVasc Thorac Surg. 2009;8:457–66. doi: 10.1510/icvts.2008.200857. [DOI] [PubMed] [Google Scholar]
- 17.De Backer D, Dubois MJ, Schmartz D, Koch M, Ducart A, Barvais L, et al. Microcirculatory alterations in cardiac surgery: effects of cardiopulmonary bypass and anesthesia. Ann Thorac Surg. 2009;88:1396–403. doi: 10.1016/j.athoracsur.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Atasever B, Boer C, Goedhart P, Biervliet J, Seyffert J, Speekenbrink R, et al. Distinct alterations in sublingual microcirculatory blood flow and hemoglobin oxygenation in on-pump and off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2011;25:784–90. doi: 10.1053/j.jvca.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Kjeldsen L, Koch C, Arnljots K, Borregaard N. Characterization of two ELISAs for NGAL, a newly described lipocalin in human neutrophils. J Immunol Methods. 1996;198:155–64. doi: 10.1016/s0022-1759(96)00153-6. [DOI] [PubMed] [Google Scholar]
- 20.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 21.Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince C. Sidestream Dark Field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Exp. 2007;15:15101–14. doi: 10.1364/oe.15.015101. [DOI] [PubMed] [Google Scholar]
- 22.De Backer D, Hollenberg S, Boerma C, Goedhart P, Büchele G, Ospina-Tascon G, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care. 2007;11:R101. doi: 10.1186/cc6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karkouti K, Beattie WS, Wijeysundera DN, Rao V, Chan C, Dattilo KM, et al. Hemodilution during cardiopulmonary bypass is an independent risk factor for acute renal failure in adult cardiac surgery. J Thorac Cardiovasc Surg. 2005;129:391–400. doi: 10.1016/j.jtcvs.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 24.Habib RH, Zacharias A, Schwann TA, Riordan CJ, Engoren M, Durham SJ, et al. Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: implications on operative outcome. Crit Care Med. 2005;33:1749–56. doi: 10.1097/01.ccm.0000171531.06133.b0. [DOI] [PubMed] [Google Scholar]
- 25.Vermeer H, Teerenstra S, de Sevaux RG, van Swieten HA, Weerwind PW. The effect of hemodilution during normothermic cardiac surgery on renal physiology and function: a review. Perfusion. 2008;23:329–38. doi: 10.1177/0267659109105398. [DOI] [PubMed] [Google Scholar]