Abstract
OBJECTIVES
Empyema is a well-known complication following lung resection. In particular, empyema caused by methicillin-resistant Staphylococcus aureus (MRSA) is difficult to treat. Here, we present our experience of MRSA empyema treated with local irrigation using arbekacin.
METHODS
Six patients consisted of 4 males and 2 females with an average age of 65.7 years. They developed MRSA empyema following lung resection and were treated at our institution between 2007 and 2011. Cases comprised four primary and one metastatic lung cancer, and 1 patient was a living lung transplantation donor. The surgical procedure consisted of four lobectomies, one segmentectomy and one wedge resection. After diagnosis of MRSA empyema, anti-MRSA drugs were administered intravenously in all cases. In addition, arbekacin irrigation at a dose of 100 mg dissolved in saline was performed after irrigation with saline only.
RESULTS
The average number of postoperative days for the diagnosis of MRSA empyema was 13 (range 4–19). The period of irrigation ranged from 6 to 46 days. Arbekacin irrigation did not induce nephrotoxicity or other complications, and no bacteria resistant to arbekacin was detected in the thoracic cavity. We re-operated on 1 case because he had pulmonary fistula and severe wound infection. At the time of removing the thoracic catheter, MRSA in the pleural effusion disappeared completely in 3 patients. The period until MRSA concentration in the pleural effusion became negative after starting arbekacin irrigation ranged from 4 to 9 days. In the remaining cases, in which MRSA did not disappear, the catheter was removed because of no inflammatory reaction after stopping irrigation and clamping the catheters. All patients were discharged from our institution without thoracic catheterization and no patients had relapsed during the follow-up period ranging from 6 to 44 months.
CONCLUSIONS
Irrigation of the thoracic cavity with arbekacin proved to be an effective, safe and readily available method for treating MRSA empyema following lung resection.
Keywords: Methicillin-resistant Staphylococcus aureus, Empyema, Irrigation, Arbekacin, Lung resection
INTRODUCTION
Empyema is a well-recognized complication following lung resection. The frequency of postoperative empyema is 5% [1, 2]. In general, first-line treatment consists of intravenous administration of antibiotics and drainage with or without irrigation of the empyema cavity. However, this treatment often fails, which leads to substantial morbidity and mortality. Several studies have reported the usefulness of the local instillation of antibiotics [3]. Another conservative option is intrapleural administration of fibrinolytic drugs, which is used in an attempt to achieve effective drainage [4]. On the other hand, a recent randomized trial did not demonstrate any advantage of streptokinase for the treatment of empyema [5].
Among bacteria that cause empyema, methicillin-resistant Staphylococcus aureus (MRSA) is difficult to treat because it is resistant to many antibacterial agents. Glycopeptides such as vancomycin and teicoplanin, linezolid and arbekacin are major antibiotics for the treatment of MRSA infection. Arbekacin is a semisynthetic aminoglycoside antibiotic and is not affected by inactivating enzymes produced by MRSA. On the basis of pharmacokinetics/pharmacodynamics, the antibacterial killing effect of arbekacin depends critically on peak concentration (Cmax) [6]. This fact suggests that arbekacin is appropriate for local instillation in cases of localized MRSA infection because local administration is supposed to achieve a much higher drug concentration compared with systemic administration.
In this report, we retrospectively review 6 consecutive cases of MRSA empyema following lung resection, which were treated with arbekacin instillation to the thoracic cavity.
MATERIALS AND METHODS
Patients characteristics and evaluation
Between January 2007 and December 2011, we performed 1017 cases of lung resection for noninfectious disease. During this period, we experienced 6 patients who developed MRSA empyema following lung resection, and irrigated their thoracic cavity with arbekacin dissolved in saline. Patient characteristics are shown in Table 1. Patients comprised 4 males and 2 females, with an average age of 65.7 years (range 45–87). Four cases had primary lung cancer and 1 had metastatic lung cancer. One case was a lung transplantation living donor. Lung resection consisted of four lobectomies, one segmentectomy and one wedge resection.
Table 1:
Patient characteristics
| Patient | Age | Sex | Diagnosis (stage) | Risk factor | Surgery | POD at diagnosis (days) | BT (°C) | Inflammatory marker |
MRSA in PEa | MRSA pneumonia | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WBC | CRP | ||||||||||
| 1 | 67 | M | NSCLC (IB) | None | Lobectomy | 16 | 38.2 | 26.8 | 26.8 | High | No |
| 2 | 87 | M | NSCLC (IB) | HT, TB | Segmentectomy | 4 | 38.6 | 10.3 | 15.3 | Low | Yes |
| 3 | 45 | M | LD | None | Lobectomy | 7 | 39.3 | 13.2 | 32.5 | High | No |
| 4 | 63 | F | NSCLC (IA) | None | Lobectomy | 18 | 37.8 | 16.3 | 21 | Moderate | Yes |
| 5 | 51 | F | NSCLC (IIIA) | None | Lobectomy | 15 | 38.5 | 10.4 | 18.6 | Moderate | No |
| 6 | 81 | M | MLC | DM | Wedge resection | 19 | 37.8 | 12.9 | 9.1 | High | No |
POD: postoperative day; BT: body temperature at diagnosis; WBC: white blood cells (×103/µl) at diagnosis; CRP: C-reactive protein (mg/dl) at diagnosis; MRSA: methicillin-resistant Staphylococcus aureus; PE: pleural effusion; M: male; F: female; NSCLC: non-small-cell lung cancer; LD: living lung transplantation donor; MLC: metastatic lung cancer; HT: hypertension; TB: tuberculosis; DM: diabetes mellitus.
aMRSA concentration in PE at diagnosis of MRSA empyema. High: >50 bacteria per high-power field (magnification ×1000); moderate: 10–50 bacteria per high-power field; low: 1–9 bacteria per high-power field.
MRSA empyema was diagnosed when patients exhibited MRSA in pleural effusion with inflammatory reaction such as hyperthermia, elevation of white blood cell count (WBC) and C-reactive protein (CRP). Sputum culture was performed when patients showed hyperthermia with sputum. When systemic bacteraemia was suspected, blood culture was performed. MRSA concentration was classified as one of the following three categories by microscopy: high, >50 bacteria per high-power field (magnification, ×1000); moderate, 10–50 bacteria per high-power field; low, 1–9 bacteria per high-power field. As a routine surgical prophylaxis, patients undergoing lung resection were administrated cefazolin at 1.0 g at the start time of surgery and two or three doses postoperatively. Surgical prophylaxis was discontinued postoperatively after 24–36 h.
Treatment strategy
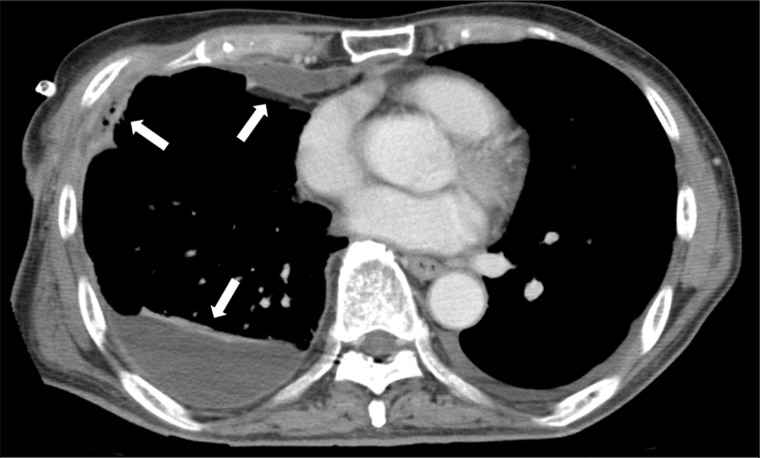
After diagnosis of MRSA empyema, we administrated anti-MRSA drugs intravenously and drained the empyema cavity. If bronchopleural fistula was found by bronchoscopy, surgery or endoscopic intervention was performed [7–9]. If no bronchopleural fistula or severe pulmonary fistula was found, irrigation of the intrathoracic cavity with arbekacin was started. When the presence of multiple isolated regions was suspected on computed tomography scan, urokinase was injected into the thoracic cavity (Fig. 1). Along with evaluation of inflammatory reaction, the effectiveness of irrigation was estimated on the basis of MRSA concentration in the pleural effusion, which was examined once or twice a week. If the MRSA empyema was not improved by the conservative treatment, surgical treatment such as decortication or open-window thoracotomy was considered.
Figure 1:
Multiple isolated regions in the thoracic cavity of Patient 6. Arrows show multiple isolated regions found on computed tomography scan. Efficient drainage or irrigation was prevented.
Irrigation procedure using arbekacin and urokinase
First, we washed the thoracic cavity with 100–500 ml of saline. Next, 100 mg of arbekacin dissolved in 100 ml saline was injected into the thoracic cavity through a thoracic catheter that was clamped for 1 h. We declamped the catheter and performed this irrigation once a day. In cases of pulmonary fistula, the thoracic catheter was not clamped. After 12 h, we added irrigation with saline only.
When the empyema was suspected to contain multiple isolated regions, urokinase was injected into the thoracic cavity once a day (Fig. 1). Initially, 120 000 U of urokinase dissolved in 100 ml saline was injected into the thoracic cavity through a thoracic catheter, which was clamped for 1 h. The catheter was declamped and subsequent irrigation with saline and arbekacin was performed.
RESULTS
The average number of postoperative days for the diagnosis of MRSA empyema was 13 (range 4–19). In all cases, hyperthermia was observed, and WBC and CRP as inflammatory markers were elevated as shown in Table 1. MRSA was detected from the septum in 2 cases, suggesting the co-presence of MRSA pneumonia. No cases of MRSA were revealed by blood culture. The minimum inhibitory concentration (MIC) of arbekacin against MRSA in the pleural effusion ranged from 0.5 to 4 μg/ml with the test for drug sensitivity.
Details of treatment and clinical course are shown in Table 2. In all cases, at least one kind of anti-MRSA drugs was administered intravenously. There were no cases of bronchopleural fistula. Patient 1 received irrigation of the thoracic cavity with saline only for 32 days and received systemic antibiotics for MRSA, but a high MRSA concentration was continuously detected in the pleural effusion. MRSA empyema was finally controlled with arbekacin irrigation. Following this success, we started arbekacin irrigation soon after diagnosing MRSA empyema for subsequent patients. No complications related to arbekacin irrigation and no bacteria resistant to arbekacin appeared were present in any cases. As notable issues, a pulmonary fistula and wound infection, which required surgery, were present in Patient 3. In Patient 6, a minor pulmonary fistula was also observed, which was stopped conservatively for a week during irrigation. Urokinase was injected into the empyema cavity before arbekacin irrigation to destroy the multiple isolated regions in Patients 2 and 6. In addition, the hospital stay of Patient 2 was longer than expected because of intractable aspiration pneumonia.
Table 2:
Treatment and clinical course
| Patient | Systemic administration |
Period of arbekacin irrigation (days) |
MRSA in PEc | Reop | Hospitalization (days) |
Period of FU (months) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic | Duration (days) | Total | Low quantitya | Negativeb | After starting arbekacin irrigation | After removal of catheter | ||||
| 1 | TEIC, arbekacin | 33 | 16 | 7 | 9 | None | No | 22 | 6 | 44 |
| 2 | TEIC, VCM | 14 | 7 | 3 | 4 | None | No | 171 | 137 | 24 |
| 3 | VCM, arbekacin | 54 | 30 | 13 | NA | Low | Yes | 47 | 15 | 24 |
| 4 | VCM | 18 | 46 | 26 | NA | Low | No | 55 | 6 | 21 |
| 5 | VCM | 26 | 6 | 6 | NA | Low | No | 16 | 8 | 36 |
| 6 | TEIC | 17 | 16 | 2 | 9 | None | No | 34 | 13 | 6 |
MRSA: methicillin-resistant Staphylococcus aureus; PE: pleural effusion; Reop: reoperation; TEIC: teicoplanin; VCM: vancomycin; FU: follow-up; NA: not applicable.
aPeriod until MRSA concentration in pleural effusion became low after starting arbekacin irrigation.
bPeriod until MRSA concentration in pleural effusion became negative after starting arbekacin irrigation.
cMRSA concentration in pleural effusion just before the removal of thoracic catheter.
At the time of removing the thoracic catheter, MRSA in the pleural effusion disappeared completely in Patients 1, 2 and 6. The period until MRSA concentration in the pleural effusion became negative after starting arbekacin irrigation ranged from 4 to 9 days. In the remaining cases, in which MRSA did not disappear, the catheter was removed because of no inflammatory reaction after stopping irrigation and clamping the catheters. The period until MRSA concentration in the pleural effusion became low after starting arbekacin irrigation ranged from 2 to 26 days.
Patients were followed up at our outpatient clinic or other hospitals for 6–44 months. No relapse of empyema was diagnosed based on clinical symptom and blood examination values. Patient 6, who was followed for 6 months, died of metastatic lung cancer.
DISCUSSION
Regarding local irrigation with antibiotics for MRSA empyema, only 2 reported cases using vancomycin after pneumonectomy have been reported in the non-English literature in a MEDLINE database search using PubMed [10, 11]. Although arbekacin, whose effect is dependent on Cmax, is considered appropriate for local irrigation, the effects of glycopeptides and linezolid are dependent on the 24-h area under the concentration–time curve/MIC ratio or the time above MIC, indicating that Cmax is not important compared with arbekacin [12, 13]. Additionally, the occurrence of bacteria resistant to arbekacin is less than that of other anti-MRSA drugs [14]. Thus, we consider that arbekacin is more appropriate for local irrigation than other anti-MRSA drugs.
Here, we present our protocol of arbekacin irrigation for MRSA empyema after lung resection. Needless to say, the therapeutic strategy of MRSA empyema should be determined while considering factors such as the presence of a continuous air leakage, size of the dead space and immunocompetence of patients. For example, when a bronchopleural or persistent pulmonary fistula exists, the fistula should be closed by surgery or endobronchial treatment [7–9].
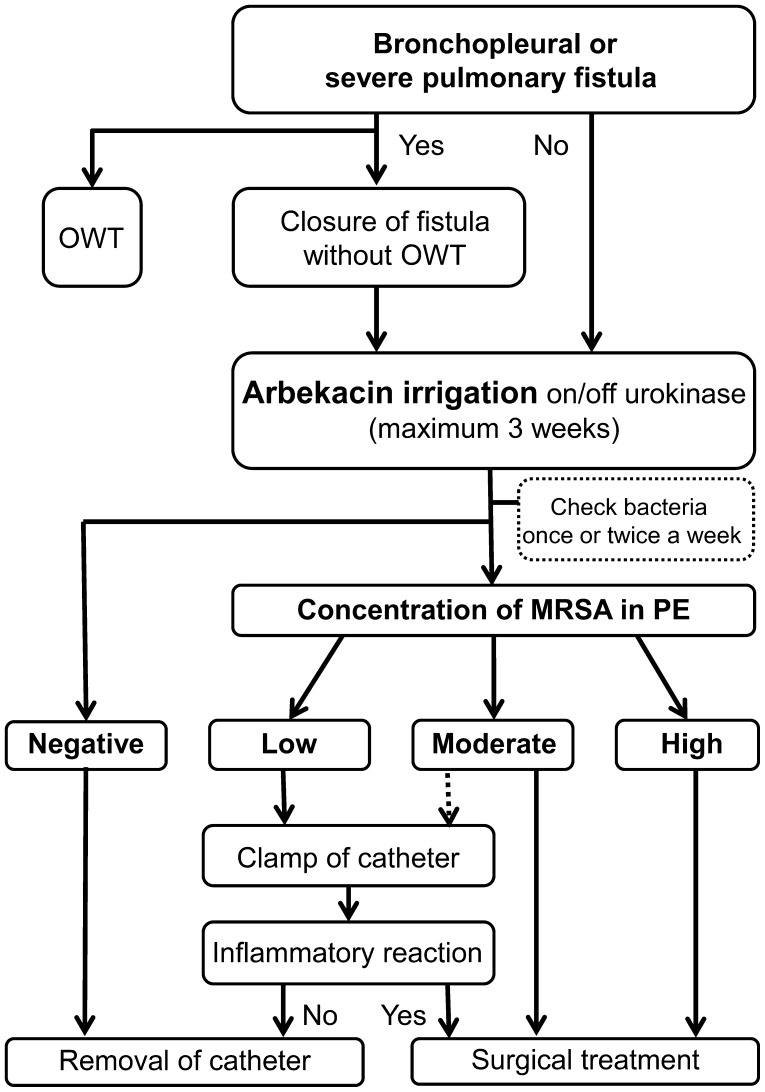
As shown in this study, we consider arbekacin irrigation as an optimal treatment option for MRSA empyema. Based on our experience, we proposed a flowchart of arbekacin irrigation for MRSA empyema in Fig. 2. One of the most challenging issues however, is judging when to stop treatment and remove the catheter: when the MRSA concentration is low but still detectable. Catheters were removed from 3 patients despite the presence of a low MRSA concentration. Patients 3 and 4 were irrigated for >4 weeks. However, on reviewing medical records, the inflammatory reaction of these cases was improved in 2 weeks and MRSA concentration decreased to a low level 4 weeks after starting arbekacin irrigation. These findings suggest that a low MRSA concentration does not have pathological significance. Indeed, the possibility of contamination due to the presence of the catheter is a concern [3]. Of note, MRSA in the pleural effusion of Patient 4 was checked on Days 18 and 26 and showed a moderate and low MRSA concentration, respectively, suggesting a possible reduction in MRSA at 3 weeks. Considering these factors and the length of hospital stay, the appropriate maximum period of arbekacin irrigation is 3 weeks. Even if a low MRSA concentration is detectable after 3 weeks of irrigation, the catheter can be removed when patients do not exhibit an inflammatory reaction. If the MRSA concentration is large or moderate after 3 weeks of irrigation, surgical treatment should be considered. Regarding fibrinolytic drugs, although the usefulness of enzymatic debridement has not been proven [5], it might be useful in some cases [15].
Figure 2:
The flowchart of arbekacin irrigation for MRSA empyema following lung resection. OWT: open-window thoracotomy; PE: pleural effusion. MRSA concentration in PE at diagnosis of MRSA empyema. High: >50 bacteria per high-power field (magnification ×1000); moderate: 10–50 bacteria per high-power field; low: 1–9 bacteria per high-power field.
There are supposed disadvantages of arbekacin. Low pH conditions in the empyema cavity weaken the uptake of aminoglycosides into bacteria [16]. However, the drastic reduction of the bacterial colony in our cases suggested that the first irrigation with saline and direct arbekacin exposure at a high concentration overcame this disadvantage.
In conclusion, irrigation of the thoracic cavity with arbekacin is an effective, safe and readily available method for treating MRSA empyema following lung resection.
ACKNOWLEDGEMENTS
We thank Hans Jiro Becker, University of Cologne, Cologne, Germany, for revising the English of our manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.Deslauriers J, Ginsberg RJ, Piantadosi S, Fournier B. Prospective assessment of 30-day operative morbidity for surgical resections in lung cancer. Chest. 1994;106:329S–30S. doi: 10.1378/chest.106.6_supplement.329s. doi:10.1378/chest.106.6.329S. [DOI] [PubMed] [Google Scholar]
- 2.Mitas L, Horvath T, Sobotka M, Garajova B, Hanke I, Kala Z, et al. Complications in patients undergoing pulmonary oncological surgery. Rozhl Chir. 2010;89:113–7. [PubMed] [Google Scholar]
- 3.Storm HK, Krasnik M, Bang K, Frimodt-Moller N. Treatment of pleural empyema secondary to pneumonia: thoracocentesis regimen versus tube drainage. Thorax. 1992;47:821–4. doi: 10.1136/thx.47.10.821. doi:10.1136/thx.47.10.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moulton JS, Moore PT, Mencini RA. Treatment of loculated pleural effusions with transcatheter intracavitary urokinase. AJR Am J Roentgenol. 1989;153:941–5. doi: 10.2214/ajr.153.5.941. [DOI] [PubMed] [Google Scholar]
- 5.Maskell NA, Davies CW, Nunn AJ, Hedley EL, Gleeson FV, Miller R, et al. UK Controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med. 2005;352:865–74. doi: 10.1056/NEJMoa042473. doi:10.1056/NEJMoa042473. [DOI] [PubMed] [Google Scholar]
- 6.Sato R, Tanigawara Y, Kaku M, Aikawa N, Shimizu K. Pharmacokinetic-pharmacodynamic relationship of arbekacin for treatment of patients infected with methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2006;50:3763–9. doi: 10.1128/AAC.00480-05. doi:10.1128/AAC.00480-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shiraishi Y. Surgical treatment of chronic empyema. Gen Thorac Cardiovasc Surg. 2010;58:311–6. doi: 10.1007/s11748-010-0599-6. doi:10.1007/s11748-010-0599-6. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson JS, Sprenger K, Van Natta T. Closure of a bronchopleural fistula using bronchoscopic placement of an endobronchial valve designed for the treatment of emphysema. Chest. 2006;129:479–81. doi: 10.1378/chest.129.2.479. doi:10.1378/chest.129.2.479. [DOI] [PubMed] [Google Scholar]
- 9.Kurihara M, Kataoka H, Ishikawa A, Endo R. Latest treatments for spontaneous pneumothorax. Gen Thorac Cardiovasc Surg. 2010;58:113–9. doi: 10.1007/s11748-009-0539-5. doi:10.1007/s11748-009-0539-5. [DOI] [PubMed] [Google Scholar]
- 10.Kondo D, Kita Y. A successful case report of conservative treatment of MRSA empyema after right pneumonectomy. Kyobu Geka. 1995;48:592–4. [PubMed] [Google Scholar]
- 11.Kachel T, Pazdzior E, Lisiecka E. A case of empyema after pneumonectomy caused by methicillin-resistant Staphylococcus aureus infection treated successfully with local administration with vancomycin. Pneumonol Alergol Pol. 1999;67:60–4. [PubMed] [Google Scholar]
- 12.Giuliano C, Haase KK, Hall R. Use of vancomycin pharmacokinetic-pharmacodynamic properties in the treatment of MRSA infections. Expert Rev Anti-Infect Ther. 2010;8:95–106. doi: 10.1586/eri.09.123. doi:10.1586/eri.09.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adembri C, Fallani S, Cassetta MI, Arrigucci S, Ottaviano A, Pecile P, et al. Linezolid pharmacokinetic/pharmacodynamic profile in critically ill septic patients: intermittent versus continuous infusion. Int J Antimicrob Agents. 2008;31:122–9. doi: 10.1016/j.ijantimicag.2007.09.009. doi:10.1016/j.ijantimicag.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Tsuchizaki N, Ishino K, Saito F, Ishikawa J, Nakajima M, Hotta K. Trends of arbekacin-resistant MRSA strains in Japanese hospitals (1979 to 2000) J Antibiot (Tokyo) 2006;59:229–33. doi: 10.1038/ja.2006.32. doi:10.1038/ja.2006.32. [DOI] [PubMed] [Google Scholar]
- 15.Misthos P, Sepsas E, Konstantinou M, Athanassiadi K, Skottis I, Lioulias A. Early use of intrapleural fibrinolytics in the management of postpneumonic empyema. A prospective study. Eur J Cardiothorac Surg. 2005;28:599–603. doi: 10.1016/j.ejcts.2005.07.005. doi:10.1016/j.ejcts.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Potts DE, Levin DC, Sahn SA. Pleural fluid pH in parapneumonic effusions. Chest. 1976;70:328–31. doi: 10.1378/chest.70.3.328. doi:10.1378/chest.70.3.328. [DOI] [PubMed] [Google Scholar]