Abstract
Disturbed flow patterns, including reversal in flow direction, are key factors in the development of dysfunctional endothelial cells (ECs) and atherosclerotic lesions. An almost immediate response of ECs to fluid shear stress is the increase in cytosolic calcium concentration ([Ca2+]i). Whether the source of [Ca2+]i is extracellular, released from Ca2+ intracellular stores, or both is still undefined, though it is likely dependent on the nature of forces involved. We have previously shown that a change in flow direction (retrograde flow) on a flow-adapted endothelial monolayer induces the remodeling of the cell-cell junction along with a dramatic [Ca2+]i burst compared with cells exposed to unidirectional or orthograde flow. The heterotrimeric G protein-α q and 11 subunit (Gαq/11) is a likely candidate in effecting shear-induced increases in [Ca2+]i since its expression is enriched at the junction and has been previously shown to be activated within seconds after onset of flow. In flow-adapted human ECs, we have investigated to what extent the Gαq/11 pathway mediates calcium dynamics after reversal in flow direction. We observed that the elapsed time to peak [Ca2+]i response to a 10 dyn/cm2 retrograde shear stress was increased by 11 s in cells silenced with small interfering RNA directed against Gαq/11. A similar lag in [Ca2+]i transient was observed after cells were treated with the phospholipase C (PLC)-βγ inhibitor, U-73122, or the phosphatidylinositol-specific PLC inhibitor, edelfosine, compared with controls. Lower levels of inositol 1,4,5-trisphosphate accumulation seconds after the onset of flow correlated with the increased lag in [Ca2+]i responses observed with the different treatments. In addition, inhibition of the inositol 1,4,5-trisphosphate receptor entirely abrogated flow-induced [Ca2+]i. Taken together, our results identify the Gαq/11-PLC pathway as the initial trigger for retrograde flow-induced endoplasmic reticulum calcium store release, thereby offering a novel approach to regulating EC dysfunctions in regions subjected to the reversal of blood flow.
Keywords: shear stress; endothelium; G protein-α; inositol 1,4,5-trisphosphate receptor
disturbed laminar shear stress patterns, including retrograde flow, are known promoters of atherogenic signaling. These variations of hemodynamic parameters may occur naturally as a result of an increased vascular tone in the downstream resistant vessel beds, the geometrical nature of the vessels (anatomical curvature), or by the increase in diastolic retrograde flow often exacerbated with aging, hypertension, or obesity (40). It was recently shown that even at rest, older subjects exhibit greater brachial retrograde flow compared with younger subjects (30). Changes in the nature of the pulsatile laminar flow in straight segments of the vasculature may lead to the appearance of a net flow reversal in regions where the endothelium has geometrically adapted to shear by inclining in the direction of flow. Correlations between increased retrograde arterial blood flow and the severity of the congestive heart failure state or an acceleration of atherosclerotic lesion formation have been established (12, 16). In fact, increases in magnitude of retrograde shear were shown to induce dose-dependent reduction in endothelial function in vivo (38). Recently, surgical procedures such as the use of intra-aortic balloon pumping, the device most frequently used in circulatory support, or the commonly performed bypass surgeries also expose autologous vessels to nonnative flow reversal, which often leads to the development of neointimal hyperplasia (5, 6, 23).
The consequences of sustained retrograde flow include increased endothelial cell (EC) permeability and atherogenic gene expression (31). However, studies of early responses to shear stress in ECs may be key to understanding endothelium dysfunction. It was shown that flow-adapted ECs respond to sudden changes in flow direction by a restructuring of the cell-cell junction along with a rapid and significantly higher intracellular calcium concentration ([Ca2+]i) than cells subjected to orthograde flow (24). Changes in [Ca2+]i occur promptly and elevations in [Ca2+]i have profound effects upon a host of cellular processes, including cell growth and differentiation, cell motility and contraction, intercellular coupling, and apoptosis and necrosis. Nonetheless, the molecular mechanisms linking changes in junctional inclination and the initiation of the [Ca2+]i transients in response to reversal of flow remain to be determined.
The heterotrimeric G protein subunits-α q and 11 (Gαq/11) are abundantly expressed at the endothelial cell-cell junction and are activated upon shear stress within seconds (15, 13, 29). The activation of G proteins may be direct (13) or indirect by flow-induced activation of G protein-coupled receptor (7). Upon receptor coupling, Gαq/11 is known to activate β-isoforms of phospholipase C (PLC-β) and initiate inositol lipid signaling, which leads to calcium release from inositol 1,4,5-trisphosphate (IP3)-regulated intracellular stores (17). Studies have shown that inhibition of Gαq/11 significantly reduces systemic blood pressure and neointima formation in ferric chloride-induced carotid injury (19). Blocking of Gαq was also shown to prevent pressure overload myocardial hypertrophy in transgenic mice (1). Furthermore, shear-induced nitric oxide production and Ras activation were shown to be altered by pertussis toxin-sensitive G proteins (14, 21). Nonetheless, no causal link has been clearly established to address the prominent role of Gαq/11 in triggering the initial intracellular responses to shear. Phospholipase dependence of calcium mobilization was first suggested in bovine ECs subjected to mechanical stimulation (10). Although, mechanical stimulation was shown in the complete absence of external calcium by promoting calcium release from internal stores in ECs (9, 28, 25), a new class of plasmalemmal calcium channels, the transient receptor potential cation (TRPC) channels, has recently been described as being implicated in triggering calcium dynamics from extracellular pool (42). It is still unclear whether the source of calcium is extracellular, released from intracellular calcium stores, or both upon flow reversal in ECs.
To address to what extent the Gαq/11-PLC pathway participates in initiating retrograde flow-induced calcium responses on flow-adapted monolayers, RNA interference techniques and inhibitory drugs were employed. Here we demonstrated the cooperative involvement of Gαq/11 and PLC in mediating primary flow-induced calcium responses. The specific targeting of the IP3 receptor (IP3R) was sufficient to entirely block calcium transient in response to retrograde flow.
MATERIALS AND METHODS
Cell cultures and treatments.
Primary human coronary artery endothelial cells (HCAECs) were purchased from Lonza Walkersville (Walkersville, MD) and Cell Applications (San Diego, CA) and grown in complete EC growth media (EGM-2, Lonza). Before all experimental procedures, HCAECs were serum-starved overnight in ATP-free EBM-2 (Lonza), supplemented with 1% bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, MO) to establish quiescence in the monolayer. The same starvation media was used for perfusion after overnight CO2 equilibration. In some experiments, cells were treated with 100 nM final concentration of histamine dihydrochloride (Tocris Bioscience, Ellisville, MO) as a positive control for the activation of Gαq/11 in ECs (32). The PLC inhibitors edelfosine and U-73122 (Tocris Bioscience) were resuspended on the day of experiment in water or DMSO (0.1% final) and used at 25 and 5 μM final, respectively, in the perfusion media during both the flow adaptation and experimental step flow. Concentrations of these inhibitors were chosen based on their abilities to significantly reduce, but not completely, histamine-induced calcium responses. Higher concentrations or longer exposure induced cytotoxicity during preincubation time as also observed by Sobolewski and colleagues (35). The inactive U-73122 analog, U-73343, was purchased from Enzo Life Sciences (Farmingdale, NY) and used at same final concentration. The IP3R blocker xestospongin C (XeC) was purchased from Wako Chemical (Richmond, VA), and each batch was resuspended on the day of experiment in DMSO (0.5% final) at 10 μM final in perfusion media.
Silencing of Gαq/11 was performed using a small interfering (si)RNA target sequence common for both human Gαq and Gα11 (siGαq/11, sense: 5′-AAGATGTTCGTGGACCTGAA-3′). siRNA (375 nM) was combined with Lipofectamine siRNAmax (Invitrogen) according to the manufacturer's protocol and dropped onto subconfluent cells for 4 h before cells were reseeded at higher density onto fibronectin-coated glass slides or coverslips for 2 days, allowing cells to reestablish confluency and reform cell-cell junctions. Transfection of a nontargeting siRNA (siCTRL, Ambion, Foster City, CA) was used as a control.
Shear-stress exposure.
HCAEC monolayers were used in a parallel plate flow microchamber as described in Melchior and Frangos (24). A syringe pump delivered orthograde or retrograde flow rate via a computer-controlled PHD 2000 syringe pump (Harvard Apparatus) holding a 100-ml CO2 gas-tight prewarmed syringe (SGE analytical science). Flow adaptation consisted of a preconditioning orthograde flow involving a 5-min slowly ramped-up flow to 5 dyn/cm2, followed by a 20-min steady-flow period at 5 dyn/cm2, and finally a 5-min ramped down flow. Cells then rested for 2 min, allowing time for the user to switch valves, and then were exposed to 10 dyn/cm2 step retrograde flow according to experimental conditions. Sham controls were flow adapted but not exposed to retrograde flow. Static control slides were only removed from the incubator immediately before harvest.
Intracellular calcium measurement.
Cells were incubated overnight in starvation medium (1% BSA) and then incubated for 1 h with 15 ng/ml of the free intracellular calcium chelator fluo-4 AM (excitation, 494 nm; and emission, 516 nm), supplemented with 2.5 mM probenicid, 20 mM HEPES, 0.042% Pluronic F-127, and 0.1% BSA (Sigma) to facilitate the calcium indicator loading (all from Invitrogen, unless specified). Cells were then rinsed with HBSS containing calcium and incubated at 34°C for 30 min for equilibration in ATP-free media before mounting slides onto the flow chamber. Picture sequences were acquired continuously (8-bit unidirectional scan; time lapse, 1.57 s) under a confocal fluorescent microscope (Zeiss Pascal LSM5, Carl Zeiss), equipped with a ×40 Plan-NeoFluar/1.3 numerical aperture oil immersion objective, and the mean intensity of each selected cells was calculated using Volocity 5 software (Improvision/PerkinElmer, Waltham, MA). To control for cell-to-cell variation in dye loading, all fluorescence measurements were expressed as a ratio (F/F0) of dynamic fluorescence intensity (F) to the average of basal fluorescence intensity measured in the 2-min period before onset of flow (F0). A value of amplitude exceeding a maximum F/F0 ratio above 1.5 was defined as a positive Ca2+ response. Internal controls showed there was limited photobleaching of the fluo-4 AM during the time course of our experiments.
IP3 measurement.
IP3 was detected on a fluorescence polarization reader (GeniosPro, Tecan) using the HitHunter IP3-FP assay from DiscoveRx (Fremont, CA) following the manufacturer's instructions. Briefly, HCAEC slides subjected to a 10-s retrograde flow were immediately quenched in perchloric acid and put in competition with an IP3 fluorescent tracer to tumble with an IP3 binding protein. The polarized signal (in mP) is inversely proportional to the amount of IP3, and measurements from triplicates were plotted on a standard curve run in parallel for each individual experiment. Concentration values were readjusted according to the final volume of lysate harvested for each samples and to a ×40 dilution factor to match our samples to the IP3 standard concentration provided by the manufacturer. Each sample was measured in triplicate, and each well was an average of 25 readings.
Western blot analysis.
To determine Gαq/11 expression after siRNA silencing, a separate set of slides also starved overnight in 1% BSA were scraped off at the time of experiments and cells were pelleted with ice-cold PBS, resuspended in 200 μl lysis buffer [consisting of 60 mmol/l octyl glucoside, 50 mM Tris·HCl (pH 7.5), 50 μmol/l EGTA, 125 mmol/l NaCl, 2 mmol/l dithiothreitol, 2 mmol/l Na3VO4, and protease inhibitors], and incubated on ice for 30 min. Lysates were centrifuged (14 000 g, 20 min, 4°C), and the detergent-soluble supernatant fractions were retained. Crude lysates were separated on NuPAGE 4–12% Bis-Tris gels (Invitrogen) and transferred to polyvinylidene difluoride membrane (Millipore, Temecula, CA). Membranes were blocked for 1 h with 5% BSA in Tris-buffered saline with 0.1% (vol/vol) Tween 20 (TBST) and then incubated with a primary antibody overnight in 3% BSA-TBST at 4°C. β-Tubulin antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and a rabbit polyclonal antibody (LJBI clone47) was produced in-house against the common COOH-terminal regions of both Gαq and Gα11. Bound primary antibodies were detected by horseradish peroxidase-conjugated secondary antibodies anti-rabbit IgG (Cell Signaling Technology, Danvers, MA). Band intensity was quantified on unsaturated X-ray film by a digital image analyzer (Bio-Rad, Hercules, CA).
Statistics.
Data are expressed as means ± SE from at least three independent experiments. Statistical comparisons between groups were performed using a one-tailed paired Student's t-test. A difference of P < 0.05 was judged as significant and indicated on bar graphs with an asterisk.
RESULTS
Delayed calcium response to retrograde flow in Gαq/11-silenced ECs.
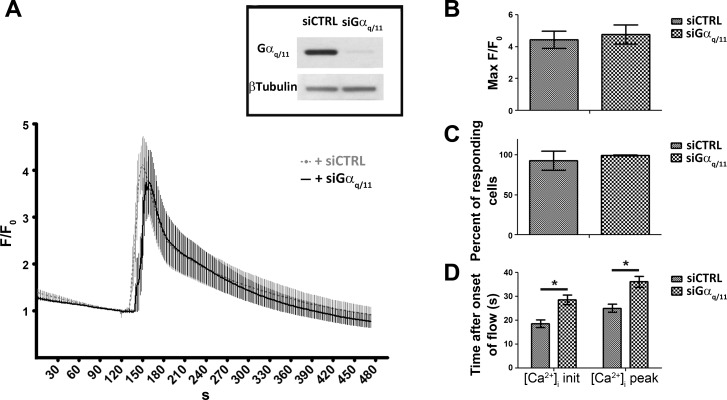
To address to what extent the Gαq/11-PLC pathway participates in initiating retrograde flow-induced calcium responses, low-passage HCAEC monolayers were transfected with a custom-designed siRNA targeting a common sequence of both human the Gαq and Gα11 sequences. Western blot analysis on lysates harvested at time of experiments showed a >95% Gαq/11 protein reduction compared with cells transfected with a nontargeting control siRNA (Fig. 1A, inset). Neither siRNA showed any cytotoxic effect within the 48 h following the transfection, and the integrity of the EC monolayer was confirmed by platelet endothelial cell adhesion molecule-1 immunostaining (data not shown). Application of a sudden 10 dyn/cm2 retrograde step flow on flow-adapted Gαq/11-silenced cells did not significantly reduce the magnitude of [Ca2+]i response (Fig. 1, A and B, maximum F/F0: siGαq/11, 4.8 ± 0.7, n = 6; and siCTRL, 4.5 ± 0.5, n = 8, P = 0.35). Because individual cells within an EC monolayer may not be transfected uniformly, the ratio of cells responding to retrograde flow with a [Ca2+]i increase was assessed and found not to be significantly different between the two groups (Fig. 1C; percentage of cells responding: siCTRL, 93.3 ± 12.3, n = 8; and siGαq/11, 99.5 ± 0.7, n = 6). However, the two transfected groups showed differences in calcium dynamics at both the initiation of the calcium response and at the time to peak after onset of flow. Cytosolic calcium responses at both burst and peak were further delayed by 10 and 11 s, respectively, between the siCTRL and siGαq/11-transfected cells (Fig. 1, A and D; siCTRL [Ca2+]i peak, 24.8 s ± 1.6, n = 8; siGαq/11 [Ca2+]i peak, 35.8 s ± 2.4, n = 6; siCTRL [Ca2+]i initiation, 18.3 s ± 1.7, n = 8; and siGαq/11 [Ca2+]i burst, 28.4 s ± 2.0, n = 6).
Fig. 1.
Increased latency in retrograde flow-induced intracellular calcium concentration ([Ca2+]i) responses in G protein-α q and 11 subunit (Gαq/11)-silenced endothelial cells. A: human coronary artery endothelial cells (HCAECs) were transfected with either a nontargeting small interfering (si)RNA (siCTRL) or a siRNA specially designed against a common region of the human Gαq and Gα11 nucleotide sequence (siGαq/11). Knockdown of protein expression was monitored by Western blot analysis 48 h after transfection, and experiments were considered when the ratio of Gαq/11 to β-tubulin protein expression was >95% of basal control (inset). After an initial 30-min period of gradual ramp-up and down flow, flow-adapted HCAEC monolayers were subjected to a 10 dyn/cm2 step retrograde flow for 1 min (indicated as “Flow”). All fluorescence measurements were expressed as a ratio (F/F0) of dynamic fluorescence intensity (F) to the average of basal fluorescence intensity measured in the 2-min period before onset of flow (F0). F/F0 values are means ± SE of n = 6 for siGαq/11 (solid black) and n = 8 for siCTRL (dotted gray) experiments, and each individual experiment consists of an average of F/F0 measurements from 24 cells for each 302 time points. Data were acquired every 1.6 s over a 5-min period. B: averages of F/F0 peak values during the flow period for each siRNA-transfected group of experiments. C: percentage of cells responding to retrograde flow with increased [Ca2+]i. Cells were considered responders when their maximum F/F0 exceeded 1.5 after onset of flow. D: time to initiation (init) and time to peak of [Ca2+]i responses. At both time points, significant lags in calcium transient were observed when cells were silenced with siGαq/11 (*P < 0.05).
Lags in flow-induced calcium transient are PLC dependent.
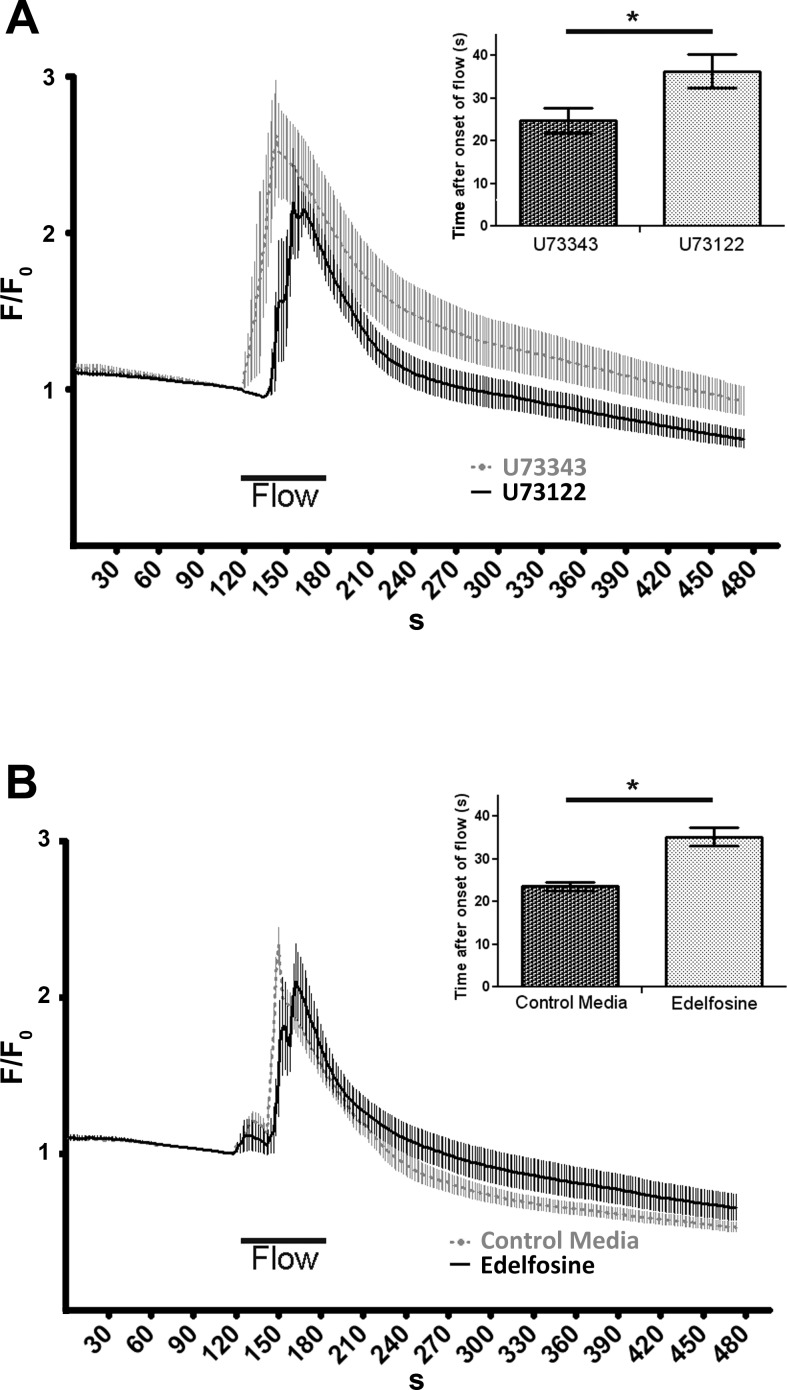
One target of the Gαq/11 subunit is the membrane-bound PLC-β (17). Using both U-73122, a nonselective inhibitor of the PLC, and the phosphatidylinositol-specific PLC specific inhibitor edelfosine, we observed a similar lag in calcium response to retrograde flow compared with their respective control [Fig. 2A: U-73122 [Ca2+]i peak, 36.3 s ± 3.9, n = 6; U-73343 [Ca2+]i peak, 24.8 s ± 3.0, n = 6; delay (U-73122 vs. U-73343) = 11.4 s; P = 0.019; and Fig. 2B: edelfosine [Ca2+]i peak, 35.2 s ± 2.1, n = 6; control media [Ca2+]i peak, 23.6 s ± 1.0, n = 8; delay (edelfosine vs. control) = 11.6 s; P = 0.00008].
Fig. 2.
Changes in retrograde flow-induced calcium dynamics after phospholipase C (PLC) inhibition. A: increased latency in [Ca2+]i responses to retrograde flow after pretreatment with 5 μM of the broad PLC inhibitor U-73122 (solid black) compared with its analog U-73433 (dotted gray). [Ca2+]i responses were monitored as in Fig. 1, retrograde flow (“flow”) was applied on HCAEC monolayers for 1 min at 10 dyn/cm2. F/F0 values are means ± SE; for both groups, n = 6 experiments in which 24 cells were monitored for each individual experiment. B: lags in time to peak of [Ca2+]i response was also observed when perfusion media included 25 μM of the phosphatidylinositol-specific PLC inhibitor edelfosine (dotted gray, average of n = 6 experiments) compared with regular perfusion media (solid black, n = 8 experiments). Insets: averages ± SE of time to peak [Ca2+]i responses for each treatment. *P < 0.05.
Gαq/11/PLC-dependent flow-induced IP3 levels.
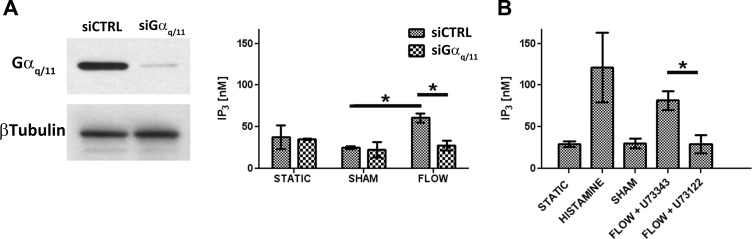
Differences in the time to peak rather than the magnitude of the [Ca2+]i responses in siGαq/11 or PLC inhibitor-treated cells could imply a slower accumulation of a secondary messenger such as IP3, which is produced in cells by PLC-mediated hydrolysis of phosphatidylinositol-4,5-biphosphate. To verify this hypothesis, we measured IP3 accumulation in flow-adapted cells subjected to a 10-s retrograde flow (seconds before onset of the [Ca2+]i burst in control cells) and compared it with a positive control exposed to 30 s of 100 nM histamine (Fig. 3). IP3 levels were significantly increased in flow-induced siCTRL samples compared with the respective sham-treated cells [Fig. 3A, IP3 (in nM) per 5.105 cells, ShamsiCTRL, 25.32 ± 1.79, n = 5, compared with FlowsiCTRL, 60.89 ± 5.26, n = 6, P = 0.00034]. However, IP3 levels remained at their respective sham levels when cells were silenced with siGαq/11 (ShamsiGαq/11, 22.79 ± 9.22, n = 5, compared with FlowsiGαq/11, 27.81 ± 5.90, n = 6, P = 0.104; FlowsiCTRL vs. FlowsiGαq/11, P = 0.00096). Similarly, treatment with the PLC inhibitor U-73122 reduced retrograde flow-induced IP3 levels compared with slides treated with the U-73433 analog [Fig. 3B; IP3 (in nM) per 5.105 cells; FlowU-73343, 81.61 ± 11.1, n = 7 vs. FlowU-73122, 29.13 ± 10.8, n = 7, P = 0.00395].
Fig. 3.
Effect of Gαq/11-PLC pathway blocking on flow-induced intracellular inositol 1,4,5-trisphosphate (IP3) concentration. A: HCAECs transfected with either siGαq/11 or siCTRL were assessed for Gαq/11 and β-tubulin protein content by Western blot analysis (left) or harvested for IP3 intracellular concentration measurements (right) after cells were either subjected to 10 s of a 10 dyn/cm2 retrograde flow, subjected to flow adaptation only (sham), or left under static conditions. B: similar measurements were performed after 10 s of retrograde flow and treatment with 5 μM of either U-73122 or its inactive analog U-73343. Addition of 100 μM of histamine for 30 s was used as positive control. *P < 0.05.
Inhibition of IP3R abrogates all retrograde flow-induced calcium response.
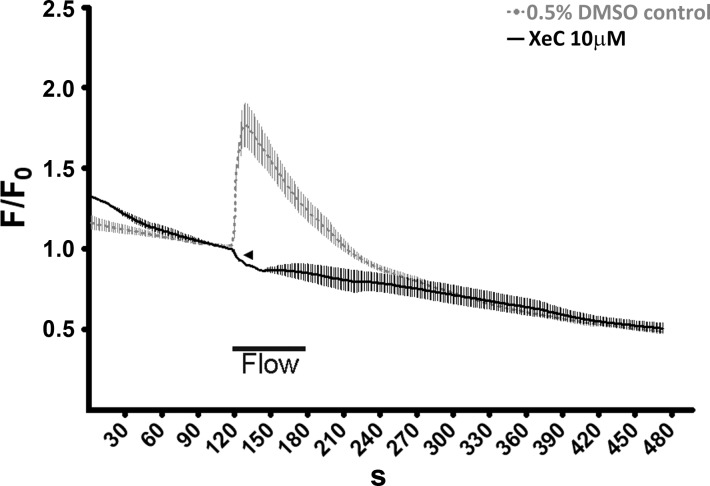
Because [Ca2+]i responses in Gαq/11/PLC-inhibited cells were only delayed and not reduced in magnitude, we investigated the level of participation of the IP3 pathway in triggering retrograde flow-induced [Ca2+]i responses using the potent IP3R blocker XeC. Surprisingly, 10 μM of XeC completely abrogated retrograde flow-induced [Ca2+]i responses compared with the 0.5% DMSO control perfusion media (Fig. 4). Interestingly, whereas XeC-treated cells did not show any burst of [Ca2+]i, a few cells noticeably showed a reduction from the basal cytosolic calcium after being subjected to retrograde flow (Fig. 4, arrowhead, and supplemental video S1), indicating a possible mechanism of flow-induced intracellular calcium extrusion, which was previously masked by the IP3R response.
Fig. 4.
Abrogation of reversal flow-induced calcium transient after IP3 receptor inhibition. [Ca2+]i response to retrograde flow were measured as described in Fig. 1 in HCAEC monolayers with 10 μM of xestospongin C (XeC; solid black, average of n = 3 experiments) or an equivalent amount of DMSO carrier (dotted gray, average of n = 4 experiments) in the perfusion media. XeC completely abrogated retrograde flow-induced [Ca2+]i responses. Calcium extrusion was detected in some cells at the onset of flow (arrowhead) as exemplified in supplemental video S1.
DISCUSSION
Using a model of flow-adapted arterial ECs, we addressed the role of the Gαq/11-PLCβ pathway in modulating intracellular calcium responses to retrograde flow. Using both RNA silencing methods and an inhibitory drug approach, we observed an increased latency in triggering [Ca2+]i responses and decreased levels of IP3 at onset of flow. Moreover, blocking IP3R completely inhibited calcium transients. We conclude that Gαq/11-PLCβ activation is the primary pathway in triggering endoplasmic reticulum (ER) calcium release in response to retrograde shear stress in ECs.
In our model of retrograde shear stress on flow-adapted arterial cell monolayer, calcium transients consistently peaked around 25 s after onset of flow. Latency in building up calcium responses was first described to occur within 1 min of shear stress on bovine ECs (3), in which IP3 was found to precede and peak after 20–30 s of flow in human umbilical vein endothelial cells (4, 27). In more recent studies, a significant lag between the onset of fluid-flow and [Ca2+]i increase was also observed in intercalated, renal, and bone cells subjected to shear stress (20, 22, 33, 34, 41). Although likely to be a function of the nature of the force applied, this lag time differs between cell types, but the peak occurrence was typically found to be between 10 and 30 s after stimulus. Our data showed that reduced expression of Gαq/11 or PLC inhibition consistently increased the time to peak calcium transients by 11 s, but the peak value was only slightly attenuated. Similar increased lags in calcium response were observed in bone cells treated with lipid raft-disrupting agent (41). Interestingly, Kou and colleagues (20) observed a 10-s increase in response time when lowering oscillatory shear stress in osteoblasts. The existence of an equivalent delay in calcium response can also be observed with a reduction of shear magnitude, and an activation threshold of the applied shear stress was suggested (2). In a model of mechanical forces applied by micropipettes on isolated ECs, the calcium response also reaches a maximum within 10–20 s and was further delayed by used of membrane calcium channel blockers (35). Overall, these data and ours strongly suggest that mechanically induced calcium responses require a threshold for activation and that any reduction in mechanical cue or treatment aiming at reducing accumulation of a second messenger only delays the threshold required for the calcium response.
To assess the participation of the second messenger IP3 in triggering calcium release upon retrograde flow stimulation, cells were harvested seconds before initiation of flow-induced [Ca2+]i responses. Levels of IP3 were found to be reduced when the Gαq/11-PLC pathway was compromised. In these experiments, it is hypothesized that inhibition of this pathway, albeit partial, allowed minute levels of IP3 to accumulate to later reach a threshold for release of intracellular calcium stores. In fact, G protein-mediated IP3 accumulation to a threshold for calcium release was previously shown in neuronal cells (39). In a similar study in Purkinje cells, calcium dynamics were shown to be threshold dependent on IP3 concentration, which in turn was dependent on Gαq activation (11).
Blocking of the IP3R completely abrogated retrograde-flow [Ca2+]i responses in flow-adapted arterial cell monolayers. There are reports of the nonspecific action of XeC, showing it as an equally potent blocker of the endoplasmic reticulum calcium pump (8). Whether this is the case in our experiments, our results still indicates that retrograde flow-induced calcium responses uniquely involve ER calcium store release rather than the induction of extracellular calcium influx through plasmalemmal nonselective calcium channels. This would be a unique feature of retrograde shear stress forces on arterial ECs. Indeed, other mechanical cues such as hypoosmotic stress have shown participation of extracellular calcium through stretch activation of the superfamily of TRPC channels (18, 36). With the use of mechanical vibration in ECs, it was shown that calcium influx starts first at regions of impact through membrane stretch-activated channels and then is followed by ER calcium release (26). Another study in TRPC6-overexpressing HEK293 cells suggests that simultaneous mechanical forces and receptor activation may synergistically amplify TRPC activation, but a 10 dyn/cm2 shear force alone does not trigger any TRPC6 current (18). In our model of retrograde flow, it is suggested that the mechanical stimuli is likely due to membrane tension accumulation and repositioning of the cell-cell junction (24). With regard to our observations, this mechanism appears to uniquely involve ER calcium release, and it is suggested that plasmalemmal calcium channels activation, if any, is secondary upon retrograde-induced flow. Supporting this hypothesis, it was recently demonstrated that TRPC1 requires IP3 as a prerequisite for activation (37).
Although the role of Gαq/11 was previously demonstrated on shear-induced nitric oxide production and Ras activation (14, 21), no study had yet established a direct link between Gαq/11-PLCβ pathway and early molecular events occurring at onset of flow stimulation in ECs. Our results here establish for the first time a causal link between Gαq/11 activation and retrograde flow-induced [Ca2+]i responses. The calcium response can be ascribed to the Gαq/11-PLCβ pathway activation and restricted to ER calcium release in ECs. Although only delayed, calcium transients are significantly affected by the disruption of the Gαq/11-PLCβ pathway. In this regard, Gαq/11 can be considered as the primary component of the observed calcium transients evoked by retrograde shear stress.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute MERIT Award R37-HL-040696.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
B.M. and J.A.F. conception and design of research; B.M. performed experiments; B.M. analyzed data; B.M. and J.A.F. interpreted results of experiments; B.M. prepared figures; B.M. drafted manuscript; B.M. and J.A.F. edited and revised manuscript; B.M. and J.A.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge Nathaniel dela Paz for assistance in providing healthy HCAEC cultures and for review and criticism of the manuscript.
REFERENCES
- 1. Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science 280: 574– 577, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Allen FD, Hung CT, Pollack SR, Brighton CT. Serum modulates the intracellular calcium response of primary cultured bone cells to shear flow. J Biomech 33: 1585– 1591, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Ando J, Komatsuda T, Kamiya A. Cytoplasmic calcium response to fluid shear stress in cultured vascular endothelial cells. In Vitro Cell Dev Biol 24: 871– 877, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Bhagyalakshmi A, Berthiaume F, Reich KM, Frangos JA. Fluid shear stress stimulates membrane phospholipid metabolism in cultured human endothelial cells. J Vasc Res 29: 443– 449, 1992 [DOI] [PubMed] [Google Scholar]
- 5. Bia D, Zocalo Y, Armentano R, de Forteza E, Cabrera-Fischer E. Acute increase in reversal blood flow during counterpulsation is associated with vasoconstriction, and changes in the aortic mechanics. Conf Proc IEEE Eng Med Biol Soc 2007: 3986– 3989, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Bia D, Zocalo Y, Armentano R, Camus J, Forteza E, Cabrera-Fischer E. Increased reversal, and oscillatory shear stress cause smooth muscle contraction-dependent changes in sheep aortic dynamics: role in aortic balloon pump circulatory support. Acta Physiol (Oxf) 192: 487– 503, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Chachisvilis M, Zhang YL, Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci USA 103: 15463– 15468, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Smet P, Parys JB, Callewaert G, Weidema AF, Hill E, De Smedt H, Erneux C, Sorrentino V, Missiaen L. Xestospongin C is an equally potent inhibitor of the inositol 1,4,5-trisphosphate receptor, and the endoplasmic-reticulum Ca2+ pumps. Cell Calcium 26: 9– 13, 1999 [DOI] [PubMed] [Google Scholar]
- 9. Demer LL, Wortham CM, Dirksen ER, Sanderson MJ. Mechanical stimulation induces intercellular calcium signaling in bovine aortic endothelial cells. Am J Physiol Heart Circ Physiol 264: H2094– H2102, 1993 [DOI] [PubMed] [Google Scholar]
- 10. Diamond SL, Sachs F, Sigurdson WJ. Mechanically induced calcium mobilization in cultured endothelial cells is dependent on actin, and phospholipase. Arterioscler Thromb 14: 2000– 2006, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Doi T, Kuroda S, Michikawa T, Kawato M. Inositol 1,4,5-trisphosphate-dependent Ca2+ threshold dynamics detect spike timing in cerebellar Purkinje cells. J Neurosci 25: 950– 961, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gharib M, Beizaie M. Correlation between negative near-wall shear stress in human aorta, and various stages of congestive heart failure. Ann Biomed Eng 31: 678– 685, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress, and phospholipid composition. Proc Natl Acad Sci USA 95: 2515– 2519, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gudi S, Huvar I, White CR, McKnight NL, Dusserre N, Boss GR, Frangos JA. Rapid activation of Ras by fluid flow is mediated by Galpha(q), and Gbetagamma subunits of heterotrimeric G proteins in human endothelial cells. Arterioscler Thromb Vasc Biol 23: 994– 1000, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Gudi SR, Clark CB, Frangos JA. Fluid flow rapidly activates G proteins in human endothelial cells Involvement of G proteins in mechanochemical signal transduction. Circ Res 79: 834– 839, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Hoi Y, Zhou YQ, Zhang X, Henkelman RM, Steinman DA. Correlation between local hemodynamics, and lesion distribution in a novel aortic regurgitation murine model of atherosclerosis. Ann Biomed Eng 39: 1414– 1422, 2011 [DOI] [PubMed] [Google Scholar]
- 17. Hubbard KB, Hepler JR. Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cell Signal 18: 135– 150, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Inoue R, Jensen LJ, Jian Z, Shi J, Hai L, Lurie AI, Henriksen FH, Salomonsson M, Morita H, Kawarabayashi Y, Mori M, Mori Y, Ito Y. Synergistic activation of vascular TRPC6 channel by receptor, and mechanical stimulation via phospholipase C/diacylglycerol and phospholipase A2/omega-hydroxylase/20-HETE pathways. Circ Res 104: 1399– 1409, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Kawasaki T, Taniguchi M, Moritani Y, Uemura T, Shigenaga T, Takamatsu H, Hayashi K, Takasaki J, Saito T, Nagai K. Pharmacological properties of YM-254890, a specific G(alpha)q/11 inhibitor, on thrombosis, and neointima formation in mice. Thromb Haemost 94: 184– 192, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Kou S, Pan L, van Noort D, Meng G, Wu X, Sun H, Xu J, Lee I. A multishear microfluidic device for quantitative analysis of calcium dynamics in osteoblasts. Biochem Biophys Res Commun 408: 350– 355, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Kuchan MJ, Jo H, Frangos JA. Role of G proteins in shear stress-mediated nitric oxide production by endothelial cells. Am J Physiol Cell Physiol 267: C753– C758, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow, and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998– F1012, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Lu X, Kassab GS. Nitric oxide is significantly reduced in ex vivo porcine arteries during reverse flow because of increased superoxide production. J Physiol 561: 575– 582, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Melchior B, Frangos JA. Shear-induced endothelial cell-cell junction inclination. Am J Physiol Cell Physiol 299: C621– C629, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Niggel J, Sigurdson W, Sachs F. Mechanically induced calcium movements in astrocytes, bovine aortic endothelial cells, and C6 glioma cells. J Membr Biol 174: 121– 134, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Nishitani WS, Saif TA, Wang Y. Calcium signaling in live cells on elastic gels under mechanical vibration at subcellular levels. PLoS One 6: e26181, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nollert MU, Eskin SG, McIntire LV. Shear stress increases inositol trisphosphate levels in human endothelial cells. Biochem Biophys Res Commun 170: 281– 287, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Oike M, Droogmans G, Nilius B. Mechanosensitive Ca2+ transients in endothelial cells from human umbilical vein. Proc Natl Acad Sci USA 91: 2940– 2944, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Otte LA, Bell KS, Loufrani L, Yeh JC, Melchior B, Dao DN, Stevens HY, White CR, Frangos JA. Rapid changes in shear stress induce dissociation of a G alpha(q/11)-platelet endothelial cell adhesion molecule-1 complex. J Physiol 587: 2365– 2373, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Padilla J, Simmons GH, Fadel PJ, Laughlin MH, Joyner MJ, Casey DP. Impact of aging on conduit artery retrograde, and oscillatory shear at rest and during exercise: role of nitric oxide. Hypertension 57: 484– 489, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Passerini AG, Milsted A, Rittgers SE. Shear stress magnitude, and directionality modulate growth factor gene expression in preconditioned vascular endothelial cells. J Vasc Surg 37: 182– 190, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Pollock WK, Wreggett KA, Irvine RF. Inositol phosphate production, and Ca2+ mobilization in human umbilical-vein endothelial cells stimulated by thrombin and histamine. Biochem J 256: 371– 376, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Praetorius HA, Spring KR. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens 12: 517– 520, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Rydholm S, Zwartz G, Kowalewski JM, Kamali-Zare P, Frisk T, Brismar H. Mechanical properties of primary cilia regulate the response to fluid flow. Am J Physiol Renal Physiol 298: F1096– F1102, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Sobolewski P, Kandel J, Klinger AL, Eckmann DM. Air bubble contact with endothelial cells in vitro induces calcium influx, and IP3-dependent release of calcium stores. Am J Physiol Cell Physiol 301: C679– C686, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation, and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA 103: 16586– 16591, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tai K, Hamaide MC, Debaix H, Gailly P, Wibo M, Morel N. Agonist-evoked calcium entry in vascular smooth muscle cells requires IP3 receptor-mediated activation of TRPC1. Eur J Pharmacol 583: 135– 147, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow, and shear rate acutely impair endothelial function in humans. Hypertension 53: 986– 992, 2009 [DOI] [PubMed] [Google Scholar]
- 39. Wang SS, Alousi AA, Thompson SH. The lifetime of inositol 1,4,5-trisphosphate in single cells. J Gen Physiol 105: 149– 171, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu M, Fannin J, Rice KM, Wang B, Blough ER. Effect of aging on cellular mechanotransduction. Ageing Res Rev 10: 1– 15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xing Y, Gu Y, Xu LC, Siedlecki CA, Donahue HJ, You J. Effects of membrane cholesterol depletion, and GPI-anchored protein reduction on osteoblastic mechanotransduction. J Cell Physiol 226: 2350– 2359, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yin J, Kuebler WM. Mechanotransduction by TRP channels: general concepts, and specific role in the vasculature. Cell Biochem Biophys 56: 1– 18, 2010 [DOI] [PubMed] [Google Scholar]