Abstract
PIKfyve is an essential mammalian lipid kinase with pleiotropic cellular functions whose genetic knockout in mice leads to preimplantation lethality. Despite several reports for PIKfyve-catalyzed synthesis of phosphatidylinositol 5-phosphate (PtdIns5P) along with phosphatidylinositol-3,5-biphosphate [PtdIns(3,5)P2] in vitro and in vivo, the role of the PIKfyve pathway in intracellular PtdIns5P production remains underappreciated and the function of the PIKfyve-synthesized PtdIns5P pool poorly characterized. Hence, the recently discovered potent PIKfyve-selective inhibitor, the YM201636 compound, has been solely tested for inhibiting PtdIns(3,5)P2 synthesis. Here, we have compared the in vitro and in vivo inhibitory potency of YM201636 toward PtdIns5P and PtdIns(3,5)P2. Unexpectedly, we observed that at low doses (10–25 nM), YM201636 inhibited preferentially PtdIns5P rather than PtdIns(3,5)P2 production in vitro, whereas at higher doses, the two products were similarly inhibited. In cellular contexts, YM201636 at 160 nM inhibited PtdIns5P synthesis twice more effectively compared with PtdIns(3,5)P2 synthesis. In 3T3L1 adipocytes, human embryonic kidney 293 and Chinese hamster ovary (CHO-T) cells, levels of PtdIns5P dropped by 62–71% of the corresponding untreated controls, whereas those of PtdIns(3,5)P2 fell by only 28–46%. The preferential inhibition of PtdIns5P versus PtdIns(3,5)P2 at low doses of YM201636 was explored to probe contributions of the PIKfyve-catalyzed PtdIns5P pool to insulin-induced actin stress fiber disassembly in CHO-T cells, GLUT4 translocation in 3T3L1 adipocytes, and induction of aberrant cellular vacuolation in these or other cell types. The results provide the first experimental evidence that the principal pathway for PtdIns5P intracellular production is through PIKfyve and that insulin effect on actin stress fiber disassembly is mediated entirely by the PIKfyve-produced PtdIns5P pool.
Keywords: insulin-regulated F-actin disassembly, insulin-regulated GLUT4 translocation, vacuolation phenotype, phosphoinositide profiles, Dok proteins
the phosphorylated derivatives of phosphatidylinositol (PtdIns), called phosphoinositides (PIs), are membrane-anchored phospholipids of eukaryotic cells in which the inositol head-group could be reversibly phosphorylated at positions D3, D4, and/or D5, singly or in all possible combinations to yield seven PIs: PtdIns3P, PtdIns4P, PtdIns5P, PtdIns(3,4)P2, PtdIns(3,5)P2, PtdIns(4,5)P2, and PtdIns(3,4,5)P3. The metabolism of PIs is tightly controlled in time and space by the coordinated action of various kinases and phosphatases (1, 3, 7, 27, 36, 38, 53). Although present in cells only in minute quantities, PIs are indispensable and versatile membrane-anchored signaling molecules that regulate diverse and essential cell processes. A great deal of knowledge about the functions of PIs has been gathered through the use of small-molecule inhibitors of the kinases, the best example being the fungal toxin wortmannin (4, 50, 52). Wortmannin powerfully and irreversibly inhibits class I and class III PI3-kinases (PI3K) at a low nanomolar concentration (IC50 of 5–10 nM), to which other kinases appear to be insensitive. The specificity and potency of wortmannin and the synthetic inhibitor LY294002 have made them widely used probes to uncover the cellular functions of PI3K. Importantly, as the PI3K pathway has now been found dysregulated in various cancers, arrest of this pathway is the subject of intensive research with several classes of new inhibitors currently in clinical trials (29, 57).
PIKfyve belongs to an evolutionarily ancient gene family of PtdIns(3,5)P2-synthesizing kinases that, except for plants, are presented by a single gene in eukaryotic genomes (30, 47, 48). In mammals, PIKfyve constitutes the only pathway for PtdIns(3,5)P2 biosynthesis from PtdIns3P. At least in mammalian cells, PIKfyve also synthesizes PtdIns5P from PtdIns, but its contribution to the total cellular PtdIns5P pool is unknown (39). Recent findings for preimplantation lethality of the mouse model with systemic pikfyve knockout brought to light the essentiality of PIKfyve for life in mammals (14). Although direct information about the cause of this early lethality is still unavailable, studies in mouse embryonic fibroblasts (MEFs) derived from PIKfyveflox/flox embryos and rendered null for pikfyve by Cre-induced excision of the LoxP flanked region have pointed to arrested mitogenesis (14). In addition to DNA synthesis, PIKfyve has been implicated as a key regulator in myriad cellular processes, including endocytic and exocytic membrane trafficking, stress- or hormone-induced signaling, ion channel activity, filamentous actin fiber remodeling, nuclear transport, gene transcription, and cell cycle progression (46). However, lack of suitable tools for dissociating the two PIKfyve lipid kinase activities makes it unclear whether the functional outcomes are associated with selective changes in PIKfyve-generated PtdIns(3,5)P2, PtdIns5P, or both. This issue is particularly relevant for PtdIns5P, because in addition to PIKfyve, PtdIns5P levels might be controlled by type II PIPK kinases [phosphatidylinositol 5-phosphate 4-kinases, (PIP4K2s)] that consume PtdIns5P to produce PtdIns(4,5)P2 (11). Additionally, with the possibility for members of the phosphatase families of PtdIns(4,5)P2 4-phosphatases and myotubularins (MTMs and MTMRs) to make PtdIns5P by PtdIns(4,5)P2 and PtdIns(3,5)P2 turnover, respectively, the role of PIKfyve in PtdIns5P biosynthesis from PtdIns in intact cells is elusive and a matter of continuing debate (47, 49, 51, 54, 59). Furthermore, in Chinese hamster ovary (CHO-T) cells and 3T3L1 adipocytes, PtdIns5P is upregulated in response to insulin and implicated in insulin-regulated F-actin stress fiber disassembly and GLUT4 translocation (37, 43). However, whether these functions are supported, entirely or in part, by the PtdIns5P pool dependent on PIKfyve or other enzymes is yet to be directly examined.
The YM201636 compound is the first specific cell-permeable small-molecule inhibitor of PIKfyve, characterized recently (22). It powerfully inhibits PIKfyve-catalyzed PtdIns(3,5)P2 synthesis in vitro, with an IC50 of 33 nM. Concordantly, PtdIns(3,5)P2 production is efficiently arrested in intact cells, with only 20% of control levels remaining subsequent to acute treatment with 800 nM YM201636 (22). By contrast, the enzymes synthesizing PtdIns(4,5)P2, i.e., type 1α PIP5K1 and type 2γ PIP4K2 are largely insensitive, with in vitro IC50 values, >100-fold greater than that for PIKfyve (22). Notably, the inhibition of PIKfyve-catalyzed PtdIns5P synthesis has not been investigated (22). As expected, YM201636 (or its analog MF4) has become a widely used probe to uncover new insights of PIKfyve's functional relevance to cell processes (6, 8, 9, 21, 22, 24, 34). The reported dysfunction in endocytic membrane trafficking, secretory-cargo export, autophagy, retroviral assembly, micropinocytosis, glucose transport, and cell invasiveness has been merely attributed to suppressed PIKfyve-dependent PtdIns(3,5)P2 synthesis (6, 8, 9, 21, 22, 24, 34). Whereas this might be correct, the potential contribution of reduced PtdIns5P levels through PIKfyve inhibition by YM201636 has not been taken into account. Here, we have addressed the effect of YM201636 against PIKfyve-catalyzed PtdIns5P and PtdIns(3,5)P2 synthesis in a comparative manner. Unexpectedly, we observed that YM201636 not only inhibits PtdIns5P synthesis both in vitro and in cell contexts, but at low doses, it is more potent toward PtdIns5P rather than PtdIns(3,5)P2 production. This preferential inhibition was further explored to establish a selective role of the PIKfyve-catalyzed PtdIns5P pool in the cellular effect of insulin on actin stress fiber disassembly.
MATERIALS AND METHODS
Materials.
YM201636 (pyridofuropirimidine compound [6-amino-N-(3-(4-(4-morpholinyl)pyrido [3′,2′:4,5] furo[3,2-d]pyrimidin-2-yl)phenyl)-3-pyridine carboxamide]) was purchased from Symansis NZ (Timaru, New Zealand) and used as recommended by the manufacturer. Insulin was kindly provided by Eli Lilly (Indianapolis, IN). The NH2-terminal anti-PIKfyve antibodies (R7069) were previously characterized (42). Anti-phosphotyrosine antibody (4G10), anti-phosphoThr308-Akt, or anti-Akt antibodies were from Cell Signaling (Beverly, MA). Rabbit (M-276) and goat Dok1 antibody (M19) was from Santa Cruz (Santa Cruz, CA). [γ-32P]ATP (6,000 Ci/mmol) and myo-[2-3H]inositol (22.5 Ci/mmol) were from Perkin Elmer (Boston, MA). All other standard reagents were purchased from Sigma.
Cell cultures and treatments.
Mouse 3T3-L1 fibroblasts, purchased from ATCC (Manassas, VA) were differentiated into adipocytes following a standard differentiation protocol detailed elsewhere (15). 3T3L1 adipocytes were used between days 5 and 7 following the initiation of the differentiation program. CHO-T cells, stably expressing the human insulin receptor, were maintained in Ham's F-12 medium, containing 10% fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin sulfate. Human embryonic kidney (HEK)293 cell line stably expressing PIKfyveWT was generated using Tet-Off/Tet-On gene expression system exactly as described previously (19). A clone DB4 that expressed in the absence of doxycycline induction approximately twofold above the endogenous PIKfyve levels was used in this study. Before experiments and unless otherwise stated, adipocytes were serum deprived for 3 h in low-glucose DMEM, whereas the CHO-T, for 12 h. Cells were incubated with indicated concentrations of YM201636 for 30 min at 37°C, and where indicated, this was followed by stimulation with insulin (100 nM) for 10 min at 37°C.
Fluorescence microscopy.
CHO-T cells were fixed in 4% formaldehyde and stained with rhodamine-phalloidin (Molecular Probes) to visualize F-actin cytoskeleton. Coverslips were mounted on slides using the Slow Fade Antifade Kit (Molecular Probes, Eugene, OR). Fluorescence analysis was performed in a Nikon Eclipse TE 200 inverted fluorescence microscope using a 60×1.4 oil immersion lens. Images were captured with a SPOT RT Slider charge-coupled device camera (Diagnostic Instruments) mounted on the microscope. Phase-contrast images were obtained by a Hoffman Modulation Contrast System with 40× objective on a Nikon Eclipse TE 200. For quantitation of actin stress fibers, individual CHO-T cells (150–200 cells/per condition) were inspected. Those exhibiting parallel actin fibers that colocalized with the nucleus were scored positive, whereas those showing actin staining in the periphery were scored negative for F-actin stress fibers, as detailed elsewhere (43).
Immunoblotting.
Following treatments, cell lysates were collected in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris·HCl buffer, pH 8.0, containing 150 mM NaCl, 1% Nonidet P-40, 0.5% Na deoxycholate), supplemented with 1× protease (1 mM phenylmethylsulphonylfluoride, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 1 μg/ml pepstatin, and 1 mM benzamidine) and 1× phosphatase inhibitor mixtures [(in mM) 50 NaF, 10 Na pyrophosphate, 25 Na β-glycerophosphate, and 2 Na metavanadate]. Immunoblotting with the indicated antibodies was performed following protein separation by SDS-PAGE and electrotransfer as specified elsewhere (15).
Lipid kinase assay and thin-layer chromatography analyses.
These were performed following previously published protocols (42). Briefly, cell lysates collected in RIPA buffer containing 1× protease inhibitor mixture were clarified by centrifugation (14,000 g, 15 min, 4°C) and then subjected to immunoprecipitation with anti-PIKfyve antisera (16 h, 4°C), with protein A-sepharose added during the last 1.5 h of incubation. Beads were washed once with RIPA buffer, twice with 50 mM HEPES (pH 7.4), 1 mM EDTA, 150 mM NaCl, three times with 100 mM Tris·HCl (pH 7.5), 500 mM LiCl, twice with 10 mM Tris·HCl (pH 7.5), 100 mM NaCl, 1 mM EDTA and twice with “assay buffer” as described (42). Kinase reactions containing 100 μM PtdIns [sonicated before use in 20 mM HEPES (pH 7.5), 1 mM EDTA] was first preincubated for 15 min (37°C) in the presence of YM201636 (100 nM) or vehicles. The kinase assay was then carried out for 15 min at 37°C with 15 μM ATP and [γ-32P]ATP (30 μCi). Lipids were extracted, spotted on a thin-layer chromatography (TLC) glass plate (Whatman, K6 silica gel 60A, 250 μm) and then resolved by a chromatographic solvent system composed of either chloroform/methanol/water/30% amonia (90:90:20:7) or n-propanon/2 M acetic acid (65:35) as described previously (42) and specified in the figure legends. Generated radioactive products were detected by autoradiography.
Myo-[2-3H]inositol cell labeling and HPLC analyses.
Cells were labeled with myo-[2-3H]inositol following our previous protocols (14, 17, 44). Briefly, cells (35–60 mm dishes) were maintained for 12–22 h in glucose- and inositol-free DMEM, containing 10% dialyzed FBS, and 5 μg/ml transferrin, 2 mM pyruvate, 25 mM HEPES (pH 7.4), 100 units/ml penicillin, and 100 mg/ml streptomycin. Cells were labeled for 26–40 h with 25 μCi/ml myo-[2-3H]inositol in glucose- and inositol-free DMEM, containing 5 μg/ml transferrin, 2 mM pyruvate, 25 mM HEPES (pH 7.4), and 10% dialyzed FBS or 0.5% BSA as indicated in the figure legends. CHO-T cells received 2.5 mM glucose during labeling since they were sensitive to prolonged glucose starvation. Following treatments, specified in the figure legends, lipids were extracted, deacylated, and analyzed by HPLC (Waters 5215) on a 5-micron Partisphere SAX column (Whatman). [32P]GroPIns5P, [32P]GroPIns3P, [32P]GroPIns(4,5)P2, and [32P]GroPIns(3,5)P2 prepared by enzymatic synthesis with [γ-32P]-ATP were co-injected as internal HPLC standards as described elsewhere (14, 17, 44). Fractions were collected every 0.25 min and analyzed for [3H] and [32P] radioactivity after the addition of scintillation mixture. Data evaluation and documentation was performed by Microsoft Excel. Individual peak radioactivity was quantified by area integration and presented as a percentage of the summed radioactivity from the [3H]-GroPIns3P, -4P, -5P, -(3,5)P2, and -(4,5)P2 peaks (“total radioactivity”).
Other methods.
Protein concentration was determined by bicinchoninic acid protein assay kit (Pierce). Protein or lipid levels were quantified from the intensity of the immunoblot bands/TLC spot by laser scanning densitometry (Epson V700) and UN-SCAN-IT software (Silk Scientific). Several films of different exposure times were quantified to assure the signals were within the linear range. In addition, the radioactivity of the lipid spot was quantified by radioactive counting of the silica gel scraped from the TLC plate. Data are expressed as means ± SE. Statistical analysis was performed by Student's t-test for independent samples and a one-tail t-test for paired samples. Confidence intervals (95%) were calculated as means ± 2.92 × SE. The 2.92 coefficient is the one-tail 0.05 t value at degree of freedom equal to 2. P < 0.05 was considered as significant.
RESULTS
Preferential inhibition of in vitro PIKfyve-catalyzed PtdIns5P versus PtdIns(3,5)P2 synthesis at low doses of YM201636.
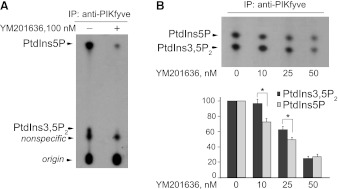
TLC analyses revealed that short preincubation of PIKfyve, immunopurified from 3T3L1 adipocyte lysates, with the YM201636 compound at 100 nM, inhibited nearly completely both PtdIns5P and PtdIns(3,5)P2 products, generated during the in vitro PIKfyve lipid kinase reaction (Fig. 1A). Such in vitro inhibition was previously noted (21) but whether it proceeded in a selective manner remained to be addressed. Considering previous findings that lysines at position 1999 and 2000 from the proposed substrate activation loop in the PIKfyve catalytic domain support differentially PIKfyve-catalyzed PtdIns5P and PtdIns(3,5)P2 synthesis in vitro (18), we reasoned that YM201636 at lower doses might differentially inhibit the two PIKfyve activities. To test this, we conducted similar in vitro lipid kinase assays but used lower concentrations of YM201636 (0–50 nM). In the TLC resolution we applied the n-propanol/2 M acetic acid rather than the chloroform/methanol/ammonia/water solvent system used in Fig. 1A. Under the former conditions, the radioactive spots corresponding to PtdIns5P and PtdIns(3,5)P2 products are devoid of tailing and are well separated from comigrating unspecific components, making it amenable for quantitative analyses, as noted previously by our laboratory (42). Unexpectedly, we observed that at 10 and 25 nM of YM201636, there was a greater inhibition (by ∼20–25%) of in vitro PtdIns5P production rather than PtdIns(3,5)P2 production (Fig. 1B). Quantification of the radioactive spots from four experiments revealed that PtdIns5P is inhibited with IC50 of ∼25 nM, whereas for PtdIns(3,5)P2, this value is ∼25% higher, with the difference between the two values reaching statistical significance (Fig. 1B). However, at 50 nM, the YM201636 inhibited similarly both PIKfyve activities, as evidenced by the similar degree of reduction of intensity/radioactivity of the 32P-PtdIns5P and 32P-PtdIns(3,5)P2 spots (Fig. 1, A and B). These data indicate, first, that YM201636 inhibits selectively and potently not only in vitro PIKfyve-catalyzed PtdIns(3,5)P2 production as previously reported (22) but also PIKfyve-catalyzed PtdIns5P production and, second, that the IC50 concentration for inhibition of PtdIns5P synthesis is ∼25% lower compared with that for PtdIns(3,5)P2 synthesis.
Fig. 1.
YM201636 preferentially inhibits PIKfyve-catalyzed PtdIns5P synthesis vs. PtdIns(3,5)P2 production in vitro. A: cleared cell lysates derived from 3T3L1 adipocytes were immunoprecipitated (IP) with anti-PIKfyve antibodies and then washed, preincubated with YM201636 (100 nM) or with vehicle for 15 min, and subjected to lipid kinase assay in the presence [γ-32P]ATP (15 min). Extracted lipids were resolved by thin-layer chromatography (TLC) developed with chloroform/methanol/water/ammonia solvent system, as described in materials and methods. Shown is a representative autoradiogram of a TLC plate of 3 independent experiments with similar results. B: anti-PIKfyve immunoprecipitates were incubated with the indicated concentrations of YM201636 and subjected to lipid kinase assay as in A. Extracted lipids were resolved by TLC developed with the n-propanol/2M acetic acid solvent system, as described in materials and methods. Shown is a representative autoradiogram of a TLC plate (top) and quantitation from 4 independent experiments, calculated as a percentage of the corresponding control value (only vehicle) and presented as means ± SE (bottom). *Statistically significant difference (P < 0.05) of the inhibition of PtdIns(3,5)P2 vs. PtdIns5P synthesis.
YM201636 inhibits preferentially PtdIns5P versus PtdIns(3,5)P2 synthesis in intact cells.
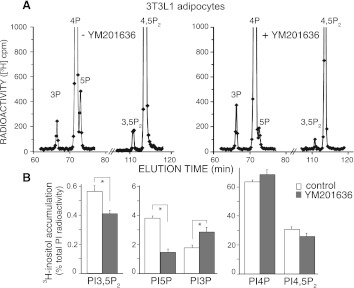
To address whether this greater inhibition of the in vitro PIKfyve activity for PdtIns5P versus PtdIns(3,5)P2 synthesis observed at low doses of the YM201636 compound is translated to differential reduction of these PIs in intact cells, we examined the PI profiles by HPLC under conditions that allow simultaneous quantification of steady-state levels of both PtdIns5P and PtdIns(3,5)P2. To this end, we performed 24- to 40-h metabolic cell labeling experiments with myo-[2-3H]inositol rather than short labeling with [32P]orthophosphate. Under these conditions both PtdIns5P and PtdIns(3,5)P2 are readily detectable by our routine protocol for HPLC separation, as revealed previously (14, 44). Subsequent to labeling and before lipid extraction, cells were treated shortly with 160 nM YM201636, a concentration that is approximately fivefold below the required dose for a decrease of PtdIns(3,5)P2 production by 80% in NIH3T3 cells (22). As illustrated in Fig. 2A, such analysis in fully differentiated 3T3L1 adipocytes revealed that whereas the inhibition of [3H]PtdIns(3,5)P2 production was only minor, that of [3H]PtdIns5P was much more pronounced under these conditions. Quantitation from three independent labeling experiments and HPLC inositol-head group analyses in 3T3L1 adipocytes showed that at 160 nM, YM201636 decreased intracellular [3H]PtdIns(3,5)P2 levels to only 71.3 ± 2.5% of the steady-state levels in the control untreated cells. In contrast, under these conditions, the [3H]PtdIns5P levels dropped to as low as 37.3 ± 2.3% of the corresponding [3H]PtdIns5P levels in nontreated control cells (Fig. 2). Importantly, this preferential inhibition of PtdIns5P versus PtdIns(3,5)P2 production was statistically significant as revealed by analyses using 95% confidence intervals (Table 1). Consistent with slightly inhibited PtdIns(3,5)P2 synthesis from PtdIns3P, we observed a commensurate increase (by ∼33%) in [3H]PtdIns3P (Fig. 2, A and B). In agreement with previous studies in NIH3T3 cells, where YM201636 at 800 nM decreased PtdIns(4,5)P2 levels by 20% (22), in 3T3L1 adipocytes subjected to 160 nM of YM201636, we also observed a decrease, although not statistically significant, in levels of [3H]PtdIns(4,5)P2 versus nontreated cells (Fig. 2B and Table 1). As there was a trend for commensurate increases in [3H]PtdIns4P levels under these conditions (Fig. 2), we concluded that slight inhibition of type 1 PIP5Ks that make PtdIns(4,5)P2 from PtdIns4P may account for the changes in this cell type. Together, these data indicate that in 3T3L1 adipocytes, the YM201636 compound at 160 nM not only inhibits PIKfyve-catalyzed intracellular synthesis of PtdIns5P but it does so with nearly twofold greater inhibitory efficiency as compared with that of PtdIns(3,5)P2.
Fig. 2.
In 3T3L1 adipocytes, YM201636 inhibits more powerfully PtdIns5P than PtdIns(3,5)P2 synthesis. 3T3L1 fibroblasts, differentiated to adipocytes on 60-mm dishes, were subjected to inositol and glucose starvation (22 h). Cells were then labeled with myo-[2-3H]inositol (40 h) in the presence of 10% dialyzed FBS and treated with YM201636 (160 nM) or vehicle (DMSO) in DMEM for 40 min. Lipids were extracted, deacylated, and fractionated by HPLC as described in materials and methods. Shown are the radioactivity from the HPLC profiles of a representative experiment (A) and the quantitation from 3 independent experiments, calculated as a percentage of the total PI radioactivity and presented as means ± SE (B). The total PI radioactivity was obtained by summing the counts within the elution times corresponding to the indicated [3H]GroPIns peaks. *P < 0.001, differences in PtdIns(3,5)P2, PtdIns5P, and PtdIns3P in treated vs. untreated 3T3L1 adipocytes.
Table 1.
Effect of YM201636 on the steady-state levels of PIs in indicated cell lines
| PI Species | 3T3L1 Adipocytes, % of control | HEK293 Cells (DB4), % of control | CHO-T Cells, % of control |
|---|---|---|---|
| PtdIns5P | 37.3 ± 2.3* | 29.4 ± 1.7* | 38.0 ± 3.9* |
| P value | 0.0006 | 0.0003 | 0.0016 |
| 95% CI | (30.58–44.02)Δ | (24.44–34.36)Δ | (26.61–49.39)Δ |
| PtdIns(3,5)P2 | 71.3 ± 2.5* | 53.2 ± 3.5* | 70.5 ± 3.7* |
| P value | 0.01 | 0.002 | 0.002 |
| 95% CI | (64.00–78.60) | (43.98–63.42) | (59.70–81.3) |
| PtdIns3P | 137.3 ± 5.9* | 168.6 ± 16.2* | 99.3 ± 3.8 |
| P value | 0.012 | 0.016 | 0.85 |
| PtdIns4P | 108.0 ± 4.3 | 104.2 ± 4.4 | 101.0 ± 5.7 |
| P value | 0.33 | 0.59 | 0.59 |
| PtdIns(4,5)P2 | 86.0 ± 5.7 | 100.9 ± 5.8 | 102.2 ± 5.2 |
| P value | 0.16 | 0.92 | 0.41 |
Values are means ± SE; n = 3 per each cell line. Data are presented as a percentage of the corresponding PI control measured in the absence of YM201636. Myo-[2-3H]inositol-labeled cell lines were treated with 160 nM of YM201636 for 40 min in DMEM as described in materials and methods. Lipids were extracted and resolved by HPLC.
Statistically significant difference vs. respective not-treated controls; Δstatistically significant difference (P < 0.05%) of PtdIns5P vs. PtdIns(3,5)P2 inhibition in each cell line, as evidenced by the nonoverlapping 95% confidence intervals (95% CI) of the 2 lipids. PtdIns3P, phosphatidylinositol 3-phosphate; PtdIns4P, phosphatidylinositol 4-phosphate; PtdIns(4,5)P2, phosphatidylinositol-4,5-bisphosphate.
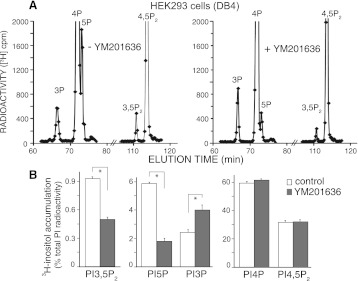
To reveal whether this differential inhibition by low doses of YM201636 found in 3T3L1 adipocytes could be reproduced in other cell types, we subjected to similar analyses a HEK293 cell line, stably expressing PIKfyve at a level approximately twofold higher than that of the endogenous protein. Contrary to a 2-h labeling with [32P]orthophosphate where we previously were unable to detect a clear peak of [32P]PtdIns5P in quiescent HEK293 cells (20, 39), under the conditions of cell labeling with myo-[2-3H]inositol for 24 h, we observed a readily quantifiable peak of steady-state levels of PtdIns5P along with that of PtdIns(3,5)P2 (Fig. 3A). Importantly, consistent with the observations in differentiated 3T3L1 adipocytes demonstrated above (Fig. 2), YM201636 at 160 nM also preferentially inhibited intracellular [3H]PtdIns5P versus [3H]PtdIns(3,5)P2 synthesis in this cell type (Fig. 3A). Thus, quantification from three independent labeling experiments and HPLC analyses in the HEK293 cell line revealed that whereas the YM201636 compound reduced accumulated levels of [3H]PtdIns(3,5)P2 by less than half compared with [3H]PtdIns(3,5)P2 in control untreated cells, there was statistically significant greater reduction in the [3H]PtdIns5P levels (Fig. 3B and Table 1). Under these conditions, [3H]PtdIns3P levels were increased as would be expected due to inhibited PIKfyve-catalyzed PtdIns(3,5)P2 synthesis from PtdIns3P (Fig. 3B). Levels of [3H]PtdIns4P and [3H]PtdIns(4,5)P2 remained practically unaffected (Fig. 3B and Table 1).
Fig. 3.
In HEK293 cells, YM201636 inhibits preferentially PtdIns5P vs. PtdIns(3,5)P2 synthesis. A HEK293 cell line (DB4), stably expressing PIKfyve at ∼2-fold above the endogenous levels, seeded on 35-mm dishes was subjected to inositol and glucose starvation (22 h). Cells were then labeled with myo-[2-3H]inositol (26 h) in the presence of 10% dialyzed FBS and treated with YM201636 (160 nM) or vehicle in DMEM for 40 min. Lipids were extracted, deacylated, and fractionated by HPLC as described in materials and methods. Presented are the radioactivity from the HPLC profiles of a representative experiment (A) and the quantitation from 3 independent experiments, calculated as a percentage of the total PI radioactivity and presented as means ± SE (B). *P < 0.001, differences in PtdIns(3,5)P2, PtdIns5P, and PtdIns3P in treated vs. untreated cells.
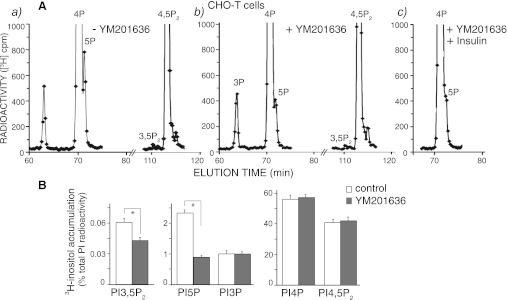
Similar analyses in transformed CHO-T cells, expressing the human insulin receptor, yielded analogous results. Thus, whereas levels of PtdIns5P in [32P]orthophosphate-labeled cells were undetectable, buried within the descending arm of the abundant PtdIns4P (20, 43), the currently applied conditions of cell metabolic labeling with myo-[2-3H]inositol allowed for a clearly detectable and quantifiable shoulder of steady-state [3H]PtdIns5P levels in this cell type (Fig. 4A, a). Treatment with 160 nM of YM201636 markedly reduced the [3H]PtdIns5P accumulation (Fig. 4A, b). More importantly, similar to 3T3L1 adipocytes and HEK293 cells, in CHO-T cells we also observed that at 160 nM, YM201636 produced nearly twofold greater inhibition of PtdIns5P production than that of PtdIns(3,5)P2 (Fig. 4B and Table 1). [3H]PtdIns3P, [3H]PtdIns4P, and [3H]PtdIns(4,5)P2 levels remained practically unchanged by the inhibitor (Fig. 4B and Table 1). At 160 nM, YM201636 also profoundly blunted the effect of insulin (Fig. 4A, c) that was shown previously to elevate PtdIns5P in this cell type (37, 43).
Fig. 4.
In Chinese hamster ovary (CHO-T) cells, YM201636 inhibits preferentially PtdIns5P vs. PtdIns(3,5)P2 synthesis. CHO-T cells, stably expressing the human insulin receptor, seeded on 35-mm dishes were subjected to inositol and glucose starvation (12 h) as indicated in materials and methods. Cells were then labeled with myo-[2-3H]inositol (26 h) in the presence of 0.5% BSA and 2.5 mM glucose and treated with YM201636 (160 nM) or vehicle in DMEM for 40 min in the absence (a and b) or presence of 100 nM insulin for the last 10 min of incubation (c). Lipids were extracted, deacylated, and fractionated by HPLC. Presented are the radioactivity from the HPLC profiles of a representative experiment (A) and the quantitation from 3 independent experiments, calculated as a percentage of the total PI radioactivity and presented as means ± SE (B). *P < 0.001, differences in PtdIns(3,5)P2 and PtdIns5P in treated vs. untreated cells.
YM201636 as a probe for dissecting between PtdIns5P and PtdIns(3,5)P2 mediated cellular functions of PIKfyve.
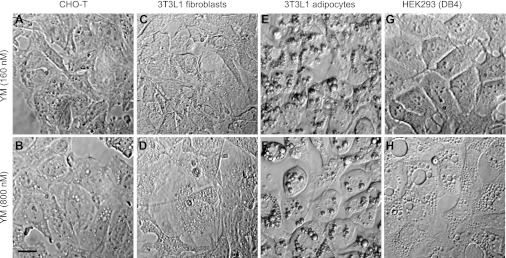
We next sought to explore this differential inhibition of the two PIKfyve lipid kinase activities by YM201636 at low doses and dissect between the PtdIns5P- and PtdIns(3,5)P2-mediated cellular responses. Formation of cellular vacuoles is a well-known phenomenon observed upon perturbation of PIKfyve lipid kinase activity by various means (20, 47). Because this aberrant cell phenotype is specifically associated with PIKfyve mutants with defective PtdIns(3,5)P2 activity and is selectively reversed by exogenously delivered PtdIns(3,5)P2, the formation of aberrant cell vacuoles became a signature of, and a sensitive functional measure for, localized reduction in PtdIns(3,5)P2 (18, 47). However, the threshold levels of PtdIns(3,5)P2 permissive for normal endomembrane homeostasis are unknown. On the other hand, an insulin-regulated transient increase of PtdIns5P production in a PI3K-independent manner has been linked to the well-established PI3K-independent insulin-regulated disassembly of F-actin stress fiber, but the involvement of PIKfyve-catalyzed PtdIns5P production is elusive (43). The preferential inhibition of PIKfyve-catalyzed PtdIns5P versus PtdIns(3,5)P2 production illustrated in Figs. 2–4 indicates that YM201636 might be a beneficial tool to directly address these questions. We first inspected whether the three cell types used herein will acquire the defective vacuolation phenotype at 160 nm of YM201636, a concentration that inhibited PtdIns(3,5)P2 synthesis by 28% to 46% (Table 1). Morphological evaluation of numerous fields of cells in several independent experiments revealed that none of the cell types acquired the aberrant vacuolation phenotype under these conditions (Fig. 5). These data are consistent with our recent observation in MEFs derived from PIKfyve+/− embryos, where the 35–40% reduction in steady-state levels of PtdIns(3,5)P2 (and PtdIns5P for that matter) did not result in aberrant cell vacuoles (14). However, at 800 nM, YM201636 triggered formation of profound vacuoles in HEK293 and CHO-T cells (Fig. 5), consistent with previous data in 3T3NIH fibroblasts for appearance of dramatic vacuoles concomitant with ∼80% reduction in PtdIns(3,5)P2 levels (22). These data indicate that the cellular vacuolation phenotype is associated only with major ablation of the PtdIns(3,5)P2 pool (by >50%). It should be emphasized that, unlike the precursor 3T3L1 fibroblasts, terminally differentiated 3T3L1 adipocytes were without apparent vacuoles at 800 nM of YM201636, despite the dramatic decrease in PtdIns(3,5)P2 (Fig. 5 and not shown). This observation is reminiscent of previous findings indicating that of the multiple cell types tested, only differentiated 3T3L1 adipocytes failed to display the vacuolation phenotype upon expression of the kinase-deficient dominant-negative PIKfyveK1831E mutant (19, 20), the reasons for which remain to be clarified.
Fig. 5.
YM201636 (YM) at 800 nM, but not at 160 nM, triggers aberrant vacuolation phenotype. CHO-T (a and b), 3T3L1 fibroblasts (c and d), differentiated 3T3L1 adipocytes (e and f), and HEK293 DB4 cells (g and h) were treated with YM201636 at the indicated concentrations for 40 min and fixed. Images were obtained by Hoffman modulation. Pronounced vacuoles are seen only at 800 nM but not at 160 nM of YM201636. In 3T3L1 adipocytes aberrant vacuolation morphology is not apparent at either concentration. Scale bar, 10 μm.
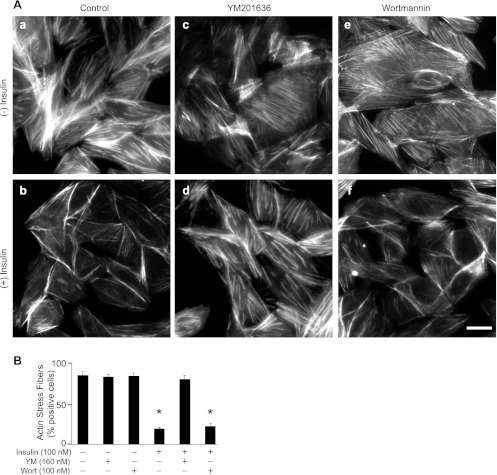
We next monitored the insulin-regulated F-actin remodeling in CHO-T cells pretreated with 160 nM of YM201636, conditions that substantially depleted the PIKfyve-maintained PtdIns5P pool and the insulin-dependent increment, whereas the reduction in PtdIns(3,5)P2 was only minor (Table 1). Remarkably, at 160 nM, YM201636 profoundly blocked the loss of F-actin stress fibers in response to insulin without affecting the F-actin stress fibers in quiescent cells (Fig. 6A). The specificity of the effect was underscored by the control treatment with low doses of PI3K inhibitor wortmannin (Fig. 6A) that, contrary to YM201636, failed to significantly affect the insulin-induced loss of stress fibers, as reported previously (26, 43). Quantitation of cells positive for actin stress fibers indicated that the YM201636 inhibited insulin-induced stress fiber breakdown in a vast majority of the cells (Fig. 6B). Together, these data indicate that PIKfyve-catalyzed PtdIns5P mediates insulin action on F-actin stress fiber disassembly and suggest that this pool may be entirely responsible for this effect of insulin.
Fig. 6.
Insulin-induced disassembly of actin stress fibers is arrested by YM201636 but not by wortmannin (Wort). A: CHO-T cells were serum-starved for 12 h and incubated for 30 min with vehicle (a and b), YM201636 (160 nM; c and d), and wortmannin (100 nM; e and f). Cells were stimulated with 100 nM of insulin for 10 min (b, d, and f) and then stained for F-actin as described in material and methods. Note the disappearance of thick actin filaments crossing the middle of the cell upon insulin stimulation of control and wortmannin-treated (b and f) but not in YM201636-treated (d) cells. B: quantitation of positive cells for F-actin stress fibers, presented as percentage of the total number of inspected cells (counted 150 cells per condition per experiment in 2 separate experiments). Cells displaying at least 2 parallel thick actin fibers crossing nucleus were scored positive. Data are presented as means ± SE. *Statistically significant difference in comparison with the vehicle-treated cells (P < 0.001). Scale bar, 10 μm.
Dok1 is tyrosine-phosphorylated independently of PIKfyve-produced PtdIns5P.
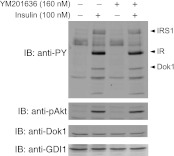
The downstream of tyrosine kinase (Dok1) protein, together with the closely related Dok2, is shown to be typrosine phosphorylated upon activation of a number of receptor tyrosine kinases in various cell types (28). In CHO-T cells, insulin receptor stimulation has been shown to tyrosine phosphorylate the endogenous Dok1/2 proteins (33, 56). It is intriguing that Dok1 and Dok2 have been recently suggested to operate downstream of PtdIns5P based on findings for increased tyrosine phosphorylation upon expression of IpgD, a bacterial inositol polyphosphate 4-phosphatase that elevates PtdIns5P due to PtdIns(4,5)P2 turnover (12). Because insulin action in CHO-T cells increases PtdIns5P levels as measured by both PtdIns5P mass assay and HPLC [(37, 43), and this study], we asked whether the Dok proteins are the downstream effectors of the PIKfyve-controlled PtdIns5P pool, mediating the signal from activated insulin receptor to F-actin stress fiber breakdown. To address this question, we inspected the effect of YM201636 on protein tyrosine phosphorylation in CHO-T cells in response to insulin stimulation. Western blotting with anti-phosphotyrosine antibodies presented in Fig. 7 documented that tyrosine phosphorylated forms of IR and IRS1 seen in the insulin-treated condition were not significantly altered upon treatment with YM201636 at 160 nM, consistent with intact proximal insulin signaling. However, the tyrosine phosphorylation of Dok1 remained unchanged under these conditions (Fig. 7). The insulin-regulated Akt activation, revealed by phosphoAkt antibodies, remained unaffected (Fig. 7). Cell treatment with higher concentrations of YM201636 (800 nM) also did not alter the tyrosine phosphorylation status of IR/IRS1 or Dok1 (data not shown). These data are consistent with the notion that the Dok proteins are unlikely to be the downstream effector of PIKfyve-catalyzed PtdIns5P in CHO-T cells in response to insulin.
Fig. 7.
YM201636 does not alter tyrosine phosphorylation of Dok1. CHO-T cells were serum-starved for 12 h and incubated for 30 min with vehicle or YM201636 at 160 nM. Cells were then stimulated with insulin (100 nM) for 10 min. Cell lysates were collected in the presence of protease and phosphatase inhibitors. Equal amounts of protein (75 μg) were analyzed by SDS-PAGE and immunoblotting with anti-phosphotyrosine (anti-PY), anti-phosphoThr308-Akt (pAkt), anti-Dok, and anti-GDI1 antibodies (GDI1 is used as a loading control) as indicated, with a stripping step between the antibodies. Shown are chemiluminescence detections of a representative blot out of 3 independent experiments with similar results. Note that the insulin-dependent tyrosine phosphorylation of all phospho-proteins, including Dok1, remains unaltered under these conditions. IRS, insulin receptor substrate.
DISCUSSION
Concurrent quantification of intracellular PtdIns5P and PtdIns(3,5)P2 levels is typically challenging and is conducted only in a few studies (14, 39, 44, 58). The obstacles are mainly related to the poor separation of PtdIns5P from the abundant PtdIns4P by conventional HPLC analyses on one hand and the very low steady-state levels of PtdIns(3,5)P2 on the other. Additional methodology to quantify intracellular levels of PtdIns5P has been developed using either the ability of PIP4Ks to convert PtdIns5P to PtdIns(4,5)P2 that is subsequently measured (so called “mass assay”) or expanded bed volume of the HPLC column, achieving a baseline separation of PtdIns5P from PtdIns4P (2). However, although improving PtdIns5P quantification, these two approaches either prohibit (the mass assay) or compromise PtdIns(3,5)P2 codetection (the increased column bed volume) (5, 31, 37, 39). These technical limitations greatly hamper studies on the regulation of PIKfyve activity that makes PtdIns5P and PtdIns(3,5)P2 both in vitro and in vivo as we established in previous studies from our laboratory (14, 39, 42, 48). As a result, intracellular production of PtdIns5P from PtdIns by PIKfyve remained underappreciated and controversial for years, with PtdIns(3,5)P2 breakdown by myotubularins considered as a central pathway in PtdIns5P production (46, 47). The paucity for coincident detection of intracellular PtdIns5P and PtdIns(3,5)P2 was presumably the reason for characterizing the recently identified pyridofuropyrimidine compound, YM201636, the only specific PIKfyve inhibitor known to date, as an inhibitor for only PtdIns(3,5)P2 synthesis (22). In this study, we took advantage of our ability to obtain simultaneously unambiguous radioactive peaks for both PIKfyve lipid products that could be quantified at high efficiency (14, 44) and investigated the relative inhibitory potency of the YM201636 compound toward PtdIns(3,5)P2 and PtdIns5P synthesis. We demonstrate here not only that PIKfyve-catalyzed PtdIns5P is powerfully inhibited by YM201636 at the low nanomolar range but that in several cellular contexts, this inhibition occurs at nearly twofold greater efficiency compared with that for PtdIns(3,5)P2 (Figs. 1–4). The higher inhibitory doses of YM201636 against type I and type II PIPKs, together with the inability of these kinases to modulate PtdIns5P levels upon heterologous expression (22, 35) and the findings herein (Figs. 2–4 and Table 1), are consistent with the notion that YM201636-inhibitable PIKfyve-catalyzed PtdIns5P biosynthesis accounts for the vast majority of the total intracellular PtdIns5P pool. The current data coupled with our recent findings that PtdIns5P levels exceed by many fold those of PtdIns(3,5)P2, yet both lipids decrease equally in MEFs of PIKfyveWT/KO (14), make PtdIns5P production from PtdIns(3,5)P2 highly unlikely and indicate that direct PIKfyve-catalyzed synthesis from PtdIns is the principal mechanism for PtdIns5P production in quiescent mammalian cells. We next exploited the higher sensitivity of PtdIns5P versus PtdIns(3,5)P2 intracellular production to YM201636-dependent inhibition and provided the first experimental evidence for a central role of the PIKfyve-generated PtdIns5P pool in insulin-regulated F-actin depolymerization in CHO-T cells.
In the absence of structural information for the PIKfyve catalytic domain, the molecular basis for the observed differential arrest in the two PIKfyve lipid kinase activities is a matter of speculation. One potential mechanism may involve the ligand interaction with specific residues from the PIKfyve catalytic domain, selectively supporting PtdIns5P synthesis. Previous findings that Lys2000 within this region supports preferentially PtdIns5P over PtdIns(3,5)P2 synthesis is consistent with such prediction (18). However, other PIKfyve-specific regions within the catalytic domain must also be important as inferred by the inability of YM201636 to block the catalytic activity of the PIKfyve yeast ortholog Fab1 (22). Alternatively or additionally, it is highly likely that PtdIns5P and PtdIns(3,5)P2 are produced by discrete subfractions of PIKfyve. It is now clear that the intracellular PtdIns(3,5)P2 synthesis is executed by the PIKfyve complex that incorporates the ArPIKfyve-Sac3 subcomplex (41); the triple interaction is typically referred to as the PAS complex, for the first letters of the three proteins (16, 40). It is possible that PtdIns5P might be synthesized by a PIKfyve subpopulation that is independent of the PAS complex. This is supported by quantitative analyses of numerous coimmuoprecipitation assays in different cell types revealing a substantial portion of PIKfyve unrelated to the PAS complex (41). Hence, PIKfyve within the PAS complex might be less accessible for YM201636 inhibition. Future structural studies combined with molecular modeling will certainly enlighten the basis for the selective inhibition of PtdIns5P versus PtdIns(3,5)P2 by YM201636, reported herein.
Insulin action relays two types of signals to modulate F-actin dynamics in insulin-sensitive cells, one that is PI3K dependent and another that proceeds in a PI3K-independent manner (13, 23, 55). In previous studies we have implicated PtdIns5P in the PI3K-independent molecular mechanisms of F-actin stress fiber disassembly that operates in CHO-T cells in response to insulin (43). However, whether PIKfyve is the enzyme responsible for the elevated PtdIns5P levels and the insulin-regulated loss of actin stress fibers has remained a matter of speculation due to the complex PtdIns5P enzymology (43). The data presented herein for profoundly inhibited PIKfyve-dependent PtdIns5P production and a commensurate arrest of the insulin-response on F-actin stress fiber disassembly at 160 nM of YM201636 (Fig. 6) provide strong evidence that PIKfyve-catalyzed PtdIns5P mediates the insulin action on F-actin disassembly in CHO-T cells. However, at these low doses, the inhibitor does not suffice to arrest insulin-regulated GLUT4 surface translocation in 3T3L1 adipocytes, as demonstrated previously by our laboratory (21) and confirmed herein (data not shown). Rather, such an arrest occurs only at 800 nM of YM201636, a concentration that significantly reduces PtdIns(3,5)P2 levels as well (22). These data are consistent with the notion that PIKfyve-controlled PtdIns(3,5)P2 has a predominant role versus PtdIns5P in insulin action on GLUT4 translocation. The reported strong arrest of this process by the triple tandem probe of the PHD domain derived from the tumor suppressor ING2, which binds PtdIns5P, in addition to PtdIns4P and PtdIns3P, although to a lesser degree (10, 43), could be a result of a deranged overall PI lipid profile, adversely affecting GLUT4 translocation (45).
The potential role of the tyrosine phosphorylated Dok proteins in linking elevated PIKfyve-catalyzed PtdIns5P to insulin action on F-actin stress fiber disassembly has also been addressed in the current study through the usage of the YM201636 compound at low doses. Our inability to detect YM201636-dependent changes in insulin-induced Dok1 tyrosine phosphorylation under conditions of ∼75% reduction of PtdIns5P yet preserved proximal insulin signaling (Figs. 4, A–C, and 7) suggests that the PIKfyve-produced PtdIns5P pool is likely unrelated to the molecular mechanisms operating upstream of Dok tyrosine phosphorylation. Previous observations for increased Dok tyrosine phosphorylation in several mammalian cell types upon expression of the S. flexneri virulence factor IpgD could be related to adverse side effects in the overall PI metabolism, whereby, in addition to the rise of PtdIns5P and massive PtdIns(4,5)P2 hydrolysis, there is an increase of higher 3′-PIs due to activation of the PI3K pathway (12, 32).
Intriguingly, PIKfyve has been recently associated with certain human cancers and tumor cell invasiveness. Experimental support for such a connection is data for PIKfyve-assisted EGFR nuclear trafficking in human bladder carcinoma cells, promoting gene regulation and cell cycle progression (25). These findings are corroborated by the recent demonstration for lethality of the pikfyve-null mouse embryos as early as the preimplantation stage due to impaired cell proliferation (14). Of significant interest are also data for PIKfyve somatic mutations present in all of the examined seven ovarian adenocarcinoma samples (http://www.sanger.ac.uk). Furthermore, a recent study indicates reduced cell invasive capacity of the NPM-ALK oncogene, a constitutively active tyrosine kinase found in anaplastic large cell lymphomas, upon inactivation of PIKfyve by YM201636 at 400 nM (8). Whether PIKfyve-catalyzed PtdIns5P and/or PtdIns(3,5)P2 production underlie this effect and play a role in metastatic cancers remain key questions in future studies. In conclusion, the present study demonstrating differential YM201636 inhibition of the two lipid products generated by PIKfyve together with previous observations for the selective role of Lys2000 and Lys1999 from the PIKfyve catalytic region in PtdIns5P and PtdIns(3,5)P2 synthesis, respectively (18), indicate that the two lipid kinase activities of PIKfyve engage distinct amino-acid residues. We also demonstrate here that the PIKfyve-synthesized PtdIns5P and PtdIns(3,5)P2 mediate diverse functions that could be segregated and individually studied. Development of a second generation of small-molecule inhibitors of PIKfyve, exhibiting more significant differential specificity toward PtdIns5P versus PtdIns(3,5)P2 synthesis, will certainly facilitate further research into the molecular mechanisms of PIKfyve's pleiotropic physiological functions in health and disease.
GRANTS
This project was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-58058 and by the American Diabetes Association (to A. Shisheva).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.S., O.C.I., C.F., and K.D. performed experiments; D.S., O.C.I., C.F., and A.S. analyzed data; D.S., O.C.I., and A.S. interpreted results of experiments; D.S. and O.C.I. prepared figures; D.S., O.C.I., and C.F. edited and revised manuscript; D.S., O.C.I., C.F., K.D., and A.S. approved final version of manuscript; A.S. conception and design of research; A.S. drafted manuscript.
ACKNOWLEDGMENTS
We thank Linda McCraw for outstanding secretarial assistance. A. Shisheva thanks the late Violeta Shisheva for many years of support.
REFERENCES
- 1.Astle MV, Seaton G, Davies EM, Fedele CG, Rahman P, Arsala L, Mitchell CA. Regulation of phosphoinositide signaling by the inositol polyphosphate 5-phosphatases. IUBMB Life 58: 451–456, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Backer JM. New methods for capturing the mystery lipid, PtdIns5P. Biochem J 428: e1–e2, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balla T, Szentpetery Z, Kim YJ. Phosphoinositide signaling: new tools and insights. Physiology (Bethesda) 24: 231–244, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpenter CL. Cantley Phosphoinositide kinases LC Curr Opin Cell Biol 8: 153–158, 1996 [DOI] [PubMed] [Google Scholar]
- 5.Coronas S, Lagarrigue F, Ramel D, Chicanne G, Delsol G, Payrastre B, Tronchere H. Elevated levels of PtdIns5P in NPM-ALK transformed cells: implication of PIKfyve. Biochem Biophys Res Commun 372: 351–355, 2008 [DOI] [PubMed] [Google Scholar]
- 6.de Lartigue J, Polson H, Feldman M, Shokat K, Tooze SA, Urbe S, Clague MJ. PIKfyve regulation of endosome-linked pathways. Traffic 10: 883–893, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature 443: 651–657, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Dupuis-Coronas S, Lagarrigue F, Ramel D, Chicanne G, Saland E, Gaits-Iacovoni F, Payrastre B, Tronchere H. The nucleophosmin-anaplastic lymphoma kinase oncogene interacts, activates and uses PIKfyve to increase invasiveness. J Biol Chem 286: 32105–32114, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes F, Chen K, Ehrlich LS, Jin J, Chen MH, Medina GN, Symons M, Montelaro R, Donaldson J, Tjandra N, Carter CA. Phosphoinositides direct equine infectious anemia virus gag trafficking and release. Traffic 12: 438–451, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gozani O, Karuman P, Jones DR, Ivanov D, Cha J, Lugovskoy AA, Baird CL, Zhu H, Field SJ, Lessnick SL, Villasenor J, Mehrotra B, Chen J, Rao VR, Brugge JS, Ferguson CG, Payrastre B, Myszka DG, Cantley LC, Wagner G, Divecha N, Prestwich GD, Yuan J. The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell 114: 99–111, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Grainger DL, Tavelis C, Ryan AJ, Hinchliffe KA. The emerging role of PtdIns5P: another signalling phosphoinositide takes its place. Biochem Soc Trans 40: 257–261, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Guittard G, Gerard A, Dupuis-Coronas S, Tronchere H, Mortier E, Favre C, Olive D, Zimmermann P, Payrastre B, Nunes JA. Cutting edge: Dok-1 and Dok-2 adaptor molecules are regulated by phosphatidylinositol 5-phosphate production in T cells. J Immunol 182: 3974–3978, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab 5: 237–252, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Ikonomov OC, Sbrissa D, Delvecchio K, Xie Y, Jin JP, Rappolee D, Shisheva A. The phosphoinositide kinase PIKfyve is vital in early embryonic development: preimplantation lethality of PIKfyve−/− embryos but normality of PIKfyve+/− mice. J Biol Chem 286: 13404–13413, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ikonomov OC, Sbrissa D, Dondapati R, Shisheva A. ArPIKfyve-PIKfyve interaction and role in insulin-regulated GLUT4 translocation and glucose transport in 3T3–L1 adipocytes. Exp Cell Res 313: 2404–2416, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikonomov OC, Sbrissa D, Fenner H, Shisheva A. PIKfyve-ArPIKfyve-Sac3 core complex: contact sites and their consequence for Sac3 phosphatase activity and endocytic membrane homeostasis. J Biol Chem 284: 35794–35806, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikonomov OC, Sbrissa D, Ijuin T, Takenawa T, Shisheva A. Sac3 is an insulin-regulated phosphatidylinositol 3,5-bisphosphate phosphatase: gain in insulin responsiveness through Sac3 down-regulation in adipocytes. J Biol Chem 284: 23961–23971, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikonomov OC, Sbrissa D, Mlak K, Kanzaki M, Pessin J, Shisheva A. Functional dissection of lipid and protein kinase signals of PIKfyve reveals the role of PtdIns 3,5-P2 production for endomembrane integrity. J Biol Chem 277: 9206–9211, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Ikonomov OC, Sbrissa D, Mlak K, Shisheva A. Requirement for PIKfyve enzymatic activity in acute and long-term insulin cellular effects. Endocrinology 143: 4742–4754, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Ikonomov OC, Sbrissa D, Shisheva A. Mammalian cell morphology and endocytic membrane homeostasis require enzymatically active phosphoinositide 5-kinase PIKfyve. J Biol Chem 276: 26141–26147, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Ikonomov OC, Sbrissa D, Shisheva A. YM201636, an inhibitor of retroviral budding and PIKfyve-catalyzed PtdIns(3,5)P2 synthesis, halts glucose entry by insulin in adipocytes. Biochem Biophys Res Commun 382: 566–570, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jefferies HB, Cooke FT, Jat P, Boucheron C, Koizumi T, Hayakawa M, Kaizawa H, Ohishi T, Workman P, Waterfield MD, Parker PJ. A selective PIKfyve inhibitor blocks PtdIns(3,5)P2 production and disrupts endomembrane transport and retroviral budding. EMBO Rep 9: 164–170, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang ZY, Chawla A, Bose A, Way M, Czech MP. A phosphatidylinositol 3-kinase-independent insulin signaling pathway to N-WASP/Arp2/3/F-actin required for GLUT4 glucose transporter recycling. J Biol Chem 277: 509–515, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Kerr MC, Wang JT, Castro NA, Hamilton NA, Town L, Brown DL, Meunier FA, Brown NF, Stow JL, Teasdale RD. Inhibition of the PtdIns(5) kinase PIKfyve disrupts intracellular replication of Salmonella. EMBO J 29: 1331–1347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J, Jahng WJ, Di Vizio D, Lee JS, Jhaveri R, Rubin MA, Shisheva A, Freeman MR. The phosphoinositide kinase PIKfyve mediates epidermal growth factor receptor trafficking to the nucleus. Cancer Res 67: 9229–9237, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight JB, Yamauchi K, Pessin JE. Divergent insulin and platelet-derived growth factor regulation of focal adhesion kinase (pp125FAK) tyrosine phosphorylation, and rearrangement of actin stress fibers. J Biol Chem 270: 10199–10203, 1995 [DOI] [PubMed] [Google Scholar]
- 27.Lindmo K, Stenmark H. Regulation of membrane traffic by phosphoinositide 3-kinases. J Cell Sci 119: 605–614, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Mashima R, Hishida Y, Tezuka T, Yamanashi Y. The roles of Dok family adapters in immunoreceptor signaling. Immunol Rev 232: 273–285, 2009 [DOI] [PubMed] [Google Scholar]
- 29.McNamara CR, Degterev A. Small-molecule inhibitors of the PI3K signaling network. Future Med Chem 3: 549–565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michell RH, Heath VL, Lemmon MA, Dove SK. Phosphatidylinositol 3,5-bisphosphate: metabolism and cellular functions. Trends Biochem Sci 31: 52–63, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Morris JB, Hinchliffe KA, Ciruela A, Letcher AJ, Irvine RF. Thrombin stimulation of platelets causes an increase in phosphatidylinositol 5-phosphate revealed by mass assay. FEBS Lett 475: 57–60, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Niebuhr K, Giuriato S, Pedron T, Philpott DJ, Gaits F, Sable J, Sheetz MP, Parsot C, Sansonetti PJ, Payrastre B. Conversion of PtdIns(4,5)P2 into PtdIns(5)P by the S. flexneri effector IpgD reorganizes host cell morphology EMBO J 21: 5069–5078, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noguchi T, Matozaki T, Inagaki K, Tsuda M, Fukunaga K, Kitamura Y, Kitamura T, Shii K, Yamanashi Y, Kasuga M. Tyrosine phosphorylation of p62(Dok) induced by cell adhesion and insulin: possible role in cell migration. EMBO J 18: 1748–1760, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osborne SL, Wen PJ, Boucheron C, Nguyen HN, Hayakawa M, Kaizawa H, Parker PJ, Vitale N, Meunier FA. PIKfyve negatively regulates exocytosis in neurosecretory cells. J Biol Chem 283: 2804–2813, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Roberts HF, Clarke JH, Letcher AJ, Irvine RF, Hinchliffe KA. Effects of lipid kinase expression and cellular stimuli on phosphatidylinositol 5-phosphate levels in mammalian cell lines. FEBS Lett 579: 2868–2872, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Roth MG. Phosphoinositides in constitutive membrane traffic. Physiol Rev 84: 699–730, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Sarkes D, Rameh LE. A novel HPLC-based approach makes possible the spatial characterization of cellular PtdIns5P and other phosphoinositides. Biochem J 428: 375–384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sasaki T, Takasuga S, Sasaki J, Kofuji S, Eguchi S, Yamazaki M, Suzuki A. Mammalian phosphoinositide kinases and phosphatases. Prog Lipid Res 48: 307–343, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Sbrissa D, Ikonomov OC, Deeb R, Shisheva A. Phosphatidylinositol 5-phosphate biosynthesis is linked to PIKfyve and is involved in osmotic response pathway in mammalian cells. J Biol Chem 277: 47276–47284, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Sbrissa D, Ikonomov OC, Fenner H, Shisheva A. ArPIKfyve homomeric and heteromeric interactions scaffold PIKfyve and Sac3 in a complex to promote PIKfyve activity and functionality. J Mol Biol 384: 766–779, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sbrissa D, Ikonomov OC, Fu Z, Ijuin T, Gruenberg J, Takenawa T, Shisheva A. Core protein machinery for mammalian phosphatidylinositol 3,5-bisphosphate synthesis and turnover that regulates the progression of endosomal transport. Novel Sac phosphatase joins the ArPIKfyve-PIKfyve complex J Biol Chem 282: 23878–23891, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Sbrissa D, Ikonomov OC, Shisheva A. PIKfyve, a mammalian ortholog of yeast Fab1p lipid kinase, synthesizes 5-phosphoinositides. Effect of insulin J Biol Chem 274: 21589–21597, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Sbrissa D, Ikonomov OC, Strakova J, Shisheva A. Role for a novel signaling intermediate, phosphatidylinositol 5-phosphate, in insulin-regulated F-actin stress fiber breakdown and GLUT4 translocation. Endocrinology 145: 4853–4865, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Sbrissa D, Shisheva A. Acquisition of unprecedented phosphatidylinositol 3,5-bisphosphate rise in hyperosmotically stressed 3T3–L1 adipocytes, mediated by ArPIKfyve-PIKfyve pathway. J Biol Chem 280: 7883–7889, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Shisheva A. Phosphoinositides in insulin action on GLUT4 dynamics: not just PtdIns(3,4,5)P3. Am J Physiol Endocrinol Metab 295: E536–E544, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shisheva A. PIKfyve and its lipid products in health and in sickness. Curr Top Microbiol Immunol 362: 2012 [DOI] [PubMed] [Google Scholar]
- 47.Shisheva A. PIKfyve: partners, significance, debates and paradoxes. Cell Biol Int 32: 591–604, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shisheva A. PIKfyve: the road to PtdIns 5-P and PtdIns 3,5-P2. Cell Biol Int 25: 1201–1206, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Tronchere H, Laporte J, Pendaries C, Chaussade C, Liaubet L, Pirola L, Mandel JL, Payrastre B. Production of phosphatidylinositol 5-phosphate by the phosphoinositide 3-phosphatase myotubularin in mammalian cells. J Biol Chem 279: 7304–7312, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Ui M, Okada T, Hazeki K, Hazeki O. Wortmannin as a unique probe for an intracellular signalling protein, phosphoinositide 3-kinase. Trends Biochem Sci 20: 303–307, 1995 [DOI] [PubMed] [Google Scholar]
- 51.Ungewickell A, Hugge C, Kisseleva M, Chang SC, Zou J, Feng Y, Galyov EE, Wilson M, Majerus PW. The identification and characterization of two phosphatidylinositol-4,5-bisphosphate 4-phosphatases. Proc Natl Acad Sci USA 102: 18854–18859, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol 13: 195–203, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Vicinanza M, D′Angelo G, Di Campli A, De Matteis MA. Function and dysfunction of the PI system in membrane trafficking. EMBO J 27: 2457–2470, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walker DM, Urbe S, Dove SK, Tenza D, Raposo G. Clague Characterization of MTMR3 MJ an inositol lipid 3-phosphatase with novel substrate specificity. Curr Biol 11: 1600–1605, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Watson RT, Kanzaki M, Pessin JE. Regulated membrane trafficking of the insulin-responsive glucose transporter 4 in adipocytes. Endocr Rev 25: 177–204, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Wick MJ, Dong LQ, Hu D, Langlais P, Liu F. Insulin receptor-mediated p62dok tyrosine phosphorylation at residues 362 and 398 plays distinct roles for binding GTPase-activating protein and Nck and is essential for inhibiting insulin-stimulated activation of Ras and Akt. J Biol Chem 276: 42843–42850, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene 27: 5497–5510, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Zolov SN, Chow CY, Slutsky SG, Richardson SC, Piper RC, Yang B, Nau JJ, Westrick RJ, Morrison SJ, Meisler MH, Weisman LS. Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. Proc Natl Acad Sci USA 104: 17518–17523, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zou J, Marjanovic J, Kisseleva MV, Wilson M, Majerus PW. Type I phosphatidylinositol-4,5-bisphosphate 4-phosphatase regulates stress-induced apoptosis. Proc Natl Acad Sci USA 104: 16834–16839, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]