Abstract
1α,25(OH)2D3, the active form of vitamin D3, has been reported to regulate the cell biology of skeletal muscle. However, there has been some controversy about the expression of the vitamin D receptor (VDR) and thus the potential role of vitamin D3 in skeletal muscle. In this study, we isolated and sequenced the full-length Vdr and Cyp27b1 transcripts in C2C12 myoblasts and myotubes. Western blots and immunocytochemistry confirmed protein expression in both myoblasts and myotubes clearly demonstrating that C2C12 cells express VDR and CYP27B1. To determine the vitamin D3 action, we found that C2C12 myoblasts treated with either 1α,25(OH)2D3 or 25(OH)D3 inhibited cell proliferation and this was associated with increased Vdr expression. The observation that treatment of C2C12 myoblasts with the inactive form of vitamin D3, [25(OH)D3], inhibited proliferation suggested that CYP27B1 was functionally active. We used small interfering RNA to knock down Cyp27b1 in myoblasts, and cells were treated with 25(OH)D3. The growth-suppressive effects of 25(OH)D3 were abolished, suggesting that CYP27B1 in myoblasts is necessary for the ability of 25(OH)D3 to affect cell proliferation. Finally, we analyzed expression of VDR and CYP27B1 in regenerating skeletal muscle in vivo. We found that expression of VDR and CYP27B1 increased significantly at day 7 of regeneration, and these results confirm the expression of Vdr and Cyp27b1 in vivo and suggest a potential role for vitamin D3 in skeletal muscle regeneration following injury.
Keywords: 1α-hydroxylase; 1α,25(OH)2D3; 25(OH)D3; muscle regeneration; vitamin D receptor
vitamin d is a pleiotropic hormone that has been implicated in a wide number of physiological and biochemical functions such as calcium homeostasis and regulation of cell proliferation and differentiation (4). The inactive form of vitamin D3 [25(OH)D3] must be converted to the bioactive metabolite 1α,25(OH)2D3 before it can activate the nuclear vitamin D receptor (VDR), which is a member of the ligand-induced transcription factor family. Once 1α,25(OH)2D3 binds VDR, the ligand-occupied VDR forms a complex with the retinoid X receptor (RXR) and the VDR:RXR heterodimer binds to specific DNA sequences and recruits coregulators for transcriptional modulation of VDR downstream target genes (4), including calcium-binding protein (19), and bone-related genes (12). In addition to the role in calcium and phosphate regulation, most of the literature reports an inhibitory effect of 1α,25(OH)2D3 on cell proliferation in various cell types, i.e., G-8 (mouse myoblasts; Ref. 27), MCF-7 (breast cancer cells; Ref. 21), C3H 10T1/2 (multipotent mesenchymal stem cells; Ref. 2), and C4-2 (prostate cancer cells; Ref. 26). Lack of functional VDR or hypovitaminosis D has been implicated for an array of pathological conditions including cancer, multiple sclerosis, rickets (4), rheumatoid arthritis (10), and muscle weakness (15).
Recent studies (14) have demonstrated that skeletal muscle cells are a direct target for vitamin D action. The bioactive metabolite of vitamin D3, 1α,25(OH)2D3 inhibits proliferation of C2C12 cells (14) and modulates expression of C2C12 cell differentiation (13, 14). VDR immunoreactivity has been reported in myoblasts from chicken (8, 9, 32), mouse G-8 (27), mouse C2C12 (7, 13, 14), and rat H9c2 (27), and it was also detected in skeletal muscle tissue of various species including chicken (32), mouse (13), and human (5). Muscles from the VDR knockout mice are smaller than wild-type mice (13). However, due to concerns regarding antibody specificity there is still some controversy about whether VDR immunoreactivity in skeletal muscle truly represents authentic VDR (29, 30).
If expressed in skeletal muscle, VDR is only activated by the bioactive 1α,25(OH)2D3. The production of 1α,25(OH)2D3 from 25(OH)D3 is mediated by the activity of the mitochondrial 1α-hydroxylase encoded by the gene Cyp27b1. The kidney has long been viewed as the primary site expressing CYP27B1 and thus is the key organ that produces 1α,25(OH)2D3 (33). However, CYP27B1 was also reported to be expressed in various tissues besides the kidney including skin, lymph nodes, hair follicles, colon epithelium, islets of the pancreas, medulla of adrenal gland, and neurons of the cerebral cortex (34), bone (1), and colon (6). In addition, CYP27B1 expression has also been detected in nonrenal cells that express VDR, i.e., primary mammary epithelial cells (16) and primary human osteoblasts (3) raising the possibility that bioactive 1α,25(OH)2D3 might be formed locally and activate VDR in target cells. Whether skeletal muscle cells express CYP27B1 and whether 25(OH)D3 can be used as a precursor for 1α,25(OH)2D3 to regulate VDR activity in skeletal muscle are currently unknown.
In this study, we investigated 1) whether VDR and CYP27B1 are expressed in vitro (C2C12 myoblasts and myotubes); 2) whether Cyp27b1 expression is functionally active to modulate the effects of 25(OH)D3 and 3) whether VDR and CYP27B1 are expressed during skeletal muscle regeneration when there is high satellite cell activity. Our results demonstrate that Vdr and Cyp27b1 are constitutively expressed in both myoblasts and myotubes. Furthermore, we determined that 25(OH)D3 like 1α,25(OH)2D3 treatment inhibits proliferation of C2C12 myoblasts and that this is associated with significant upregulation of VDR. Knockdown of Cyp27b1 resulted in the cells being insensitive to 25(OH)D3 and confirmed a functional role for CYP27B1 in muscle cells. Lastly, we determined that both VDR and CYP27B1 are significantly expressed in vivo during regeneration of skeletal muscle. These results confirm that skeletal muscle in vivo does express VDR and CYP27B1, especially during regeneration, and this opens the possibility of vitamin D3 contributing to regulation of cell proliferation during injury repair.
MATERIALS AND METHODS
Cell culture.
The mouse muscle cell line C2C12 was obtained from ATCC (Manassas, VA). Cells were grown in growth medium consisting of DMEM (Invitrogen), 10% FBS, and antibiotics (100 U/ml penicillin and 100 μg/ml of streptomycin; Cellgro). When myoblast reached 60–70% confluence, cells were trypsinized and seeded on a six-well plate (Corning) at an initial plating density of 5 × 104 cells/well to use for the cell proliferation assay, real-time PCR, and small interfering (si)RNA transfection. For immunocytochemistry and 5′-bromo-2-deoxyuridine (BrdU) incorporation assay, cells were plated on an eight-well chamber slide (Lab-Tek, Fisher Scientific) at a density of 6×103 cells/well. For myotube studies, C2C12 myoblasts were grown to ∼70% confluence on an eight-well chamber slide and then the media were switched to DMEM plus 2% horse serum (Invitrogen). Differentiation medium was replaced every 2 days and myotube formation was allowed for 5 days. 1,25(OH)2D3 (20 nM, final concentration; Sigma), 25(OH)D3 (2 μM, final concentration; Sigma), or vehicle (absolute ethanol) with the final concentration of ethanol at 0.1% were used in the present study to determine the effects of vitamin D3.
Animals.
All animal procedures were performed in accordance with institutional guidelines for the care and use of laboratory animals as approved by the Animal Care and Use Committee of the University of Kentucky. Mice (C57BL/6), aged 12 wk, were housed in a temperature- and humidity-controlled room in standard cage with a 12:12-h light-dark cycle and fed with food and water ad libitum. Muscle regeneration was induced by an injection of 1.2% BaCl2 to the tibialis anterior muscles as previously described (22). The regenerating muscles were collected at day 7 following injection. Muscles from control mice served as uninjured controls for this study.
RNA isolation and real-time PCR.
Trizol reagent (Life Technologies) was used to isolate the total RNA from C2C12 cell cultures. SuperScript III first-strand synthesis (Invitrogen) was used to generate the first-strand cDNA according to the manufacturer's instruction. Real-time PCR was performed as previously described (28). Expression of Vdr and Cyp27b1 mRNAs were analyzed using Applied Biosystems 7500 real-time PCR system (Applied Biosystems). Input RNA was normalized to Rpl26 (ribosomal protein L26) based on our previous studies (35). Data were analyzed using ABI 7500 software version 2.03 (Applied Biosystems) using the 2−ΔΔCT method. The sequences for the primers used for real-time PCR in this study are shown in the Table 1.
Table 1.
Primer sequences for real-time PCR
| Genes | Accession Number | Primers (5′-3′) | Size, bp |
|---|---|---|---|
| Cyp27b1 | NM_010009.2 | Forward: CAGCTTCCTGGCTGAACTCT | 93 |
| Reverse: GACCATATTGGCCCGTACC | |||
| Rpl26 | NM_009080.2 | Forward: CGAGTCCAGCGAGAGAAGG | 200 |
| Reverse: GCAGTCTTTAATGAAAGCCGTG | |||
| Vdr | NM_009504.4 | Forward: CACCTGGCTGATCTTGTCAGT | 93 |
| Reverse: CTGGTCATCAGAGGTGAGGTC |
Vdr and Cyp27b1 cloning.
The RNA samples from untreated C2C12 myoblasts and myotubes were reverse transcribed to cDNA and PCR amplified using the Vdr primers (forward) 5′-GTCAAAAAGCTGCTGCCGA-3′ and (reverse) 5′-ACCCTGGTCAGGAGATCTCATT-3′; and Cyp27b1 primers (forward) 5′-CACGCTAGCGACTCCCCAAACACAGACATGA-3′ and (reverse) 5′-CACCTCGAGACAAAGACCCCATCCTGTCTTG-3′. Based on the Genbank sequences for mouse Vdr and Cyp27b1, the predicted full-length PCR products span from 140 and 15 bp upstream of the start codon to the stop codon and are ≈1,400 and 1,595 bp for Vdr and Cyp27b1, respectively. The PCR products were separated on a 5% acrylamide gel, stained with SYBR Safe DNA stain (Invitrogen), and cloned into pcDNA3.1 plus vector, and both strands were sequence verified from ACGT.
Cell proliferation assay.
The proliferation of C2C12 myoblasts was determined by counting the cell number over time as well as BrdU incorporation assays. Four independent experiments were carried out for each condition. Cell counting was determined in duplicates for each experiment using a hemocytometer. The percent change of cell number from day 1 to day 4 after treatment was compared with initial number of cells plated at day 0 (5×104 cells/well). For the BrdU incorporation assay, cells were incubated with 10 μM (final concentration) of BrdU (Sigma) in sterile PBS (Cellgro) for 1 h at 37°C and 5% CO2.
Immunocytochemistry.
All steps were performed at room temperature (RT). For double immunostaining of VDR and rhodamine-phalloidin (F-actin), C2C12 cells were washed with PBS and fixed with 4% paraformaldehyde (PFA; Electron Microscopy Sciences) for 10 min and followed by 0.1% of Triton X-100 (Bio-Rad, USA) for 5 min. Nonspecific reactivity was blocked with 10% normal donkey serum (Jackson Immunoresearch) for 30 min. Primary rabbit polyclonal anti-VDR (1:100) (Santa Cruz Biotechnology) was applied and incubated for 1 h, and then donkey anti-rabbit (1:500; Invitrogen) secondary antibody was applied for 1 h. After several washes with PBS, cells were postffixed with 4% PFA for 5 min and quenched with 0.1 M glycine (Fisher) for 5 min. Then, cells were permeabilized with 0.1% Triton X-100 in PBS for 1 min followed by incubation with rhodamine-phalloidin (Invitrogen) (1:100) for 15 min. Thereafter, cells were washed with PBS and counterstained with an anti-fade solution containing DAPI (Vector Labs).
For anti-OxPhos complex V (ATP synthase, F1F0) a subunit (F1 complex) and CYP27B1 double immunostaining, cells were fixed as described above and blocked with 10% normal goat serum (Jackson Immunoresearch), primary mouse monoclonal anti-OxPhos complex V (1:250; Invitrogen) and rabbit polyclonal anti-CYP27B1 (1:100; Santa Cruz Biotechnology) were applied together and incubated for 1 h, washed with PBS, and then goat anti-mouse Texas Red (Rockland Immunochemicals; 1:200) and goat anti-rabbit Alexa Fluor 488 (1:500; Invitrogen) secondary antibodies were applied together for 1 h. After several washes with PBS, cells were counterstained with anti-fade solution containing DAPI. For VDR or CYP27B1 double immunostaining with myosin heavy chain (MF20), the protocol used was similar as described above except to the mouse anti-myosin antibody MF20 (supernatant) was used (Developmental Studies Hybridoma Bank). BrdU staining was performed as previously described (28) with minor modification on addition of the antifade containing DAPI. Images were taken with Zeiss laser scanning microscope (LSM5 Live; Jena, Germany).
Immunohistochemistry.
Muscle and kidney samples were collected from animals, covered with optimal cutting temperature compound, and frozen in isopentane (Sigma) precooled with liquid nitrogen. Samples were serial sectioned at 10-μm thickness with a cryostat (Microm HM 525; Thermo Scientific). For immunostaining, all steps were performed at RT. Muscle sections were air dried for 10 min, rehydrated with PBS, permeabilized with 0.5% Triton X-100 for 5 min, and blocked with Mouse IgG blocking reagent (Vector Labs) for 1 h. Sections were washed twice with PBS and further blocked with 10% normal goat serum (Jackson Immunoresearch) for 1 h. Primary rabbit polyclonal anti-VDR (1:50; Santa Cruz Biotechnology), rabbit polyclonal anti-CYP27B1 (1:50; Santa Cruz Biotechnology), and mouse monoclonal anti-dystrophin (1:00; Sigma) antibodies were incubated for 1 h. Different host species (rabbit and mouse) antibodies were incubated together in the same sections, i.e., VDR and dystrophin/CYP27B1 and dystrophin for double immunostaining. After three washes with PBS, goat anti-rabbit Alexa Fluor 488 (1:500; Invitrogen) and goat anti-mouse Texas Red (1:200; Rockland Immunochemicals) secondary antibodies were applied for 1 h in a dark humidified chamber and then washed three times with PBS containing 0.01% Tween 20 (Fisher). Sections were fixed with 4% PFA for 10 min, washed twice with PBS, and mounted with antifade containing DAPI. For kidney sections, the procedures for VDR and CYP27B1 immunostaining were the same as muscle sections except Mouse IgG blocking reagent, primary mouse monoclonal anti-dystrophin antibody, and goat anti-mouse Texas Red secondary antibody did not apply. Images were captured with Zeiss Axio Imager. M1 microscope and processed with AxioVision Rel software (v4.8).
Western blot analysis.
Immunoblotting was used to evaluate protein levels of VDR and CYP27B1 with procedures as previously described (28) with modifications; all steps were performed at RT except noted. Briefly, 25–50 ug of protein were separated on a SDS-PAGE gel and transferred to Immobilon-FL transfer membrane (PVDF) with a Semi-Dry Transfer System (Bio-Rad Laboratories) for 30 min. Membranes were incubated with the Odyssey blocking buffer (Bio-Rad Laboratories) for 1 h. Primary antibodies, rabbit VDR (1:250) (Santa Cruz Biotechnology), rabbit CYP27B1 antibody (1:400; Santa Cruz Biotechnology), and mouse γ-tubulin (1:4,000; Sigma) were used. Primary antibodies were incubated in Odyssey blocking buffer and PBS (1:1) plus 0.2% Tween 20 for overnight at 4°C (VDR and CYP27B1) or 1 h at RT (γ-tubulin). After several washes with PBS plus 0.1% Tween 20, secondary antibodies, anti-rabbit (VDR and CYP27B1) or anti-mouse (γ-tubulin; 1:7,500, Molecular Probe) were applied in Odyssey blocking buffer and PBS (1:1) plus 0.2% Tween 20 and 0.01% SDS for 45 min. Then, the membrane was washed 5 min × 4 with PBS plus 0.1% Tween 20. Protein expression was detected with the Odyssey LI-COR system (LI-COR). Band density was analyzed using the Odyssey software version 3.0 (LI-COR). Control 293T cell lysates (Santa Cruz Biotechnology) were used as a negative control for VDR protein expression, and mouse kidney lysate was used as a positive control for CYP27B1 protein expression in this study.
Transfection of C2C12 cells with Cyp27b1 siRNA.
siRNA transfection was performed as previously described with minor modification (28). Briefly, predesigned Cyp27b1 siRNA (Santa Cruz Biotechnology) or scrambled siRNA (Invitrogen) were prediluted in OPTI-MEM medium (Invitrogen) containing Lipofectamine 2000 (Invitrogen). Lipofectamine-siRNA complexes were added into each well immediately after cells were plated in DMEM + 5% FBS (no antibiotic; final concentration of each siRNA was 10 nM). After 6 h of incubation, the media was replaced with regular growth medium (DMEM supplemented with 10% FBS and antibiotic) for 18 h.
Statistical analysis.
Data are presented as means ± SE. Significant differences among groups were analyzed by using either two-way ANOVA or Kruskal-Wallis with Student-Newman-Keuls test or independent t-test. Data were analyzed with SigmaPlot version 11.0 Build 11.0.0.75, and the level of statistical significance was set with α level of P < 0.05.
RESULTS
Expression of Vdr in C2C12 myoblasts and myotubes.
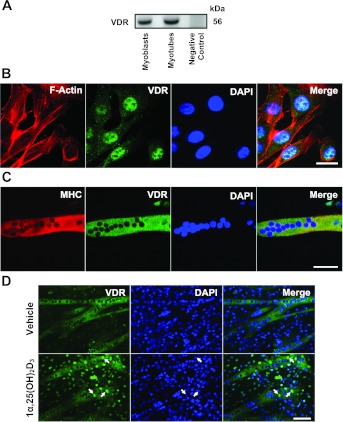
To clarify the expression of Vdr in myoblasts and myotubes, first we took RNA samples isolated from C2C12 myoblasts and myotubes to clone and sequence the full-length Vdr mRNA. The amplified cDNAs obtained from the samples were the appropriate size (≈1.4 kb), and following DNA sequencing we confirmed that the full-length Vdr mRNA is expressed in myoblasts and myotubes (Fig. 1). Once confirmed, we then examined whether the VDR protein was expressed using both Western blots and immunocytochemistry. Western blot analysis revealed that the predicted 56-kDa VDR protein was detected in both C2C12 myoblasts and myotubes, with no detectable signal from lysates of 293T cells (negative control; Fig. 2A). Immunocytochemistry demonstrated that VDR was detected in both C2C12 myoblasts and myotubes (Fig. 2, B and C). We noted that localization was primarily found in the nucleus of C2C12 myoblasts (Fig. 2B), while in C2C12 myotubes, we detected VDR expression in the cytoplasm (Fig. 2C). However, when C2C12 myotubes were treated with 1,25(OH)2D3, we did observe a translocation of VDR to the nucleus within 24 h after 1,25(OH)2D3 treatment (Fig. 2D, arrows). Taken together, these findings confirm that VDR is expressed in both C2C12 myoblasts and myotubes and suggest that there is potential for differential localization.
Fig. 1.
Presence of vitamin D receptor (Vdr) transcript in C2C12 cells. The 1.4 kb of Vdr transcript encoding the full-length mouse Vdr was amplified from C2C12 myoblasts and myotubes, and the identity of the cloned Vdr was confirmed by DNA sequencing.
Fig. 2.
VDR expression in C2C12 myoblasts and myotubes. VDR expression was determined using Western blot analysis and immunocytochemistry. A: Western blot analysis revealed the expression of VDR in C2C12 myoblasts and myotubes, respectively. Control 293T cell lysate was used as a negative control for VDR protein expression, and 25 μg of protein were loaded for each sample. B: VDR is predominantly localized to the nucleus of cultured C2C12 myoblasts by being colocalized with DAPI (nuclear staining), and C2C12 myoblasts were colabeled with phalloidin to illustrate actin-filament system. Representative images at ×1,000 magnification are shown along with the size bar of 20 μm. C: VDR is expressed in newly formed C2C12 myotube as determined by the colocalization of myosin heavy chain (MHC) expression. Representative images at ×630 magnification are shown along with the size bars of 50 μm. D: C2C12 myoblasts (70% confluency) were cultured in differentiation medium for 5 days to induce myotube formation and then cells were treated with either vehicle or 1,25(OH)2D3 (20 nM) for 24 h. Arrows indicated increased translocation of VDR to the nucleus of C2C12 myotubes after 1,25(OH)2D3 treatments. Images were taken at ×200 magnification; bar = 100 μm.
Treatment of C2C12 cells with 1,25(OH)2D3 and 25(OH)D3 leads to inhibition of myoblast proliferation.
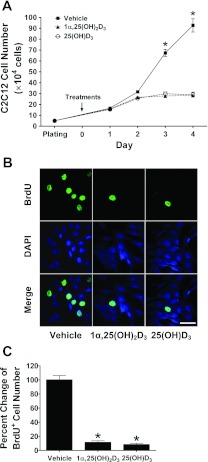
Our first set of experiments was to test the effect of the active form of vitamin D3, 1,25(OH)2D3 on C2C12 myoblast proliferation. We found that myoblast number did not increase significantly after 2 days of treatment with 1,25(OH)2D3 (Fig. 3A; P < 0.001). In these experiments, we also treated a set of C2C12 myoblasts with the inactive precursor, 25(OH)D3 as a negative control, but we were surprised to find that myoblast number was also inhibited in a manner similar to that found with 1,25(OH)2D3 (Fig. 3A, P < 0.001). To further test whether the decreased cell count following either 1,25(OH)2D3 or 25(OH)D3 treatment was due to inhibition of proliferation, we performed a BrdU incorporation assay. As seen in the representative image (Fig. 3B), treatment with either 1,25(OH)2D3 or 25(OH)D3 resulted in significant decreases in BrdU+ cells on day 2 (Fig. 3C; P < 0.001). Taken together, these results confirm that treatment with 1,25(OH)2D3 inhibits myoblast proliferation and identify that treatment of C2C12 myoblasts with the inactive form of vitamin D3, 25(OH)D3 can inhibit myoblast proliferation in a similar manner to 1,25(OH)2D3.
Fig. 3.
1α,25(OH)2D3 and 25(OH)D3 inhibit proliferation of C2C12 myoblasts in culture. C2C12 myoblasts were cultured and treated with vehicle or vitamin D3 reagents in the DMEM growth medium containing 10% FBS. A: 1α,25(OH)2D3 (20 nM) and 25(OH)D3 (2 μM) blocked C2C12 cell proliferation as determined by cell counting after 0–4 days of treatment. A change in the cell number from the day 0 is presented. Values are means ± SE (n = 4 independent experiments). Statistical analysis of the data using two-way ANOVA with Tukey's post hoc test indicates the presence of significant differences among the groups (*P < 0.001). B: 1α,25(OH)2D3 (20 nM) and 25(OH)D3 (2 μM) inhibited C2C12 cell proliferation as determined for 5′-bromo-2-deoxyuridine positive (BrdU+) incorporation. C2C12 cells were exposed to 10 μM of BrdU for 1 h (pulse labeling) after treated with vehicle, 1α,25(OH)2D3 (20 nM), or 25(OH)D3 (2 μM) for 47 h. Cells were then processed for immunostaining for BrdU and DAPI, and the merged images are shown. Representative images of BrdU+ cells at ×630 magnification from each treatment are shown along with the size bar of 50 μm. C: number of BrdU+ cells was expressed as the percentage of those in the vehicle-treated group (n = 4, independent experiments). Statistical analysis of the data using Kruskal-Wallis with Student-Newman-Keuls test indicates that both 1α,25(OH)2D3 (20 nM)- and 25(OH)D3 (2 μM)-treated groups have significantly lower BrdU+ cells compared with the vehicle-treated group (*P < 0.001).
25(OH)D3 treatment leads to upregulation of VDR expression.
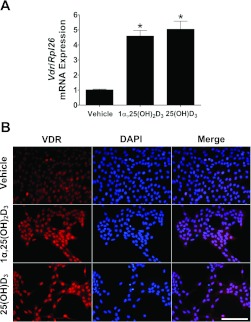
The observation that 25(OH)D3 treatment inhibited myoblast proliferation was a surprise. To begin to address potential mechanisms, we asked whether 25(OH)D3 treatment also affected expression of Vdr. In this set of experiments, we determined the effect of 25(OH)D3 or 1,25(OH)2D3 on the expression of Vdr at both the mRNA and protein levels. As seen in Fig. 4A, treatment of C2C12 myoblasts with a single dose of 25(OH)D3 (2 μM) within 24 h after C2C12 cells were plated resulted in approximately equal to a fivefold increase in Vdr mRNA expression and these changes were observed through day 4. The increase in Vdr mRNA levels was similar in magnitude to that found when myoblasts were treated with 1,25(OH)2D3 (20 nM), suggesting the possibility of a common mechanism. Consistent with the changes in Vdr mRNA expression, we detected an increased expression of VDR in myoblasts as seen in the representative images in Fig. 4B. From these results, we have determined that 25(OH)D3 treatment of C2C12 myoblasts induces similar changes in Vdr mRNA and VDR protein expression to 1α,25(OH)2D3 treatment.
Fig. 4.
25(OH)D3 treatment increased VDR expression in C2C12 myoblasts. C2C12 myoblasts were cultured and treated with vehicle or vitamin D3 reagents in the DMEM growth medium containing 10% FBS. A: culturing C2C12 myoblasts in the presence of 1α,25(OH)2D3 (20 nM) or 25(OH)D3 (2 μM) results in upregulation of Vdr mRNA expression (fold change) as determined by real-time PCR. Values are means ± SE (n = 4, independent experiments). Statistical analysis using Kruskal-Wallis with Student-Newman-Keuls test indicates significant differences among groups (*P < 0.001). B: immunolocalization of VDR in cultured C2C12 myoblasts showed markedly increased in the intensity of nuclear VDR immunoreactivity in the presence of 1α,25(OH)2D3 (20 nM) or 25(OH)D3 (2 μM). Representative images at ×200 magnification are shown along with the size bar of 200 μm.
Expression of Cyp27b1 in C2C12 myoblasts and myotubes.
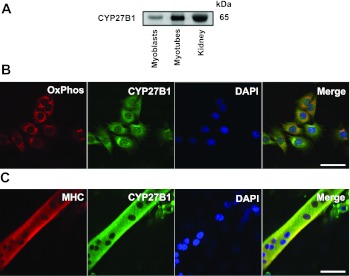
Our observation that treatment of cells with 25(OH)D3 led to the inhibition of C2C12 proliferation and upregulation of VDR expression suggested that C2C12 cells may express Cyp27b1, the gene encoding 1α-hydroxylase enzyme that is required for conversion of 25(OH)D3 to 1α,25(OH)2D3. To examine whether Cyp27b1 is expressed in C2C12 cells, we first cloned the 1.6-kb Cyp27b1 mRNA transcript from C2C12 myotubes. The sequence analysis confirmed that C2C12 cells express the full-length Cyp27b1 transcript (Fig. 5). Next, we analyzed CYP27B1 protein expression using Western blots and immunocytochemistry to confirm our cloning and sequence analysis. As seen in Fig. 6A, CYP27B1 protein is detected in C2C12 myoblasts and myotubes at the expected molecular mass (65 kDa) similar to that detected in lysates from the mouse kidney. Since CYP27B1 has previously been reported to be localized to the mitochondria (23), we used immunocytochemistry to determine if CYP27B1 protein localizes to mitochondria in C2C12 myoblasts. Representative images are presented in Fig. 6B and demonstrate that CYP27B1 protein is detected in the cytoplasm of myoblasts, and compared with the localization of OxPhos complex V (ATP synthase, F1F0) a subunit (F1 complex, a subunit), we do detect some overlap, suggesting CYP27B1 may be found in mitochondria. We also evaluated the localization of CYP27B1 in C2C12 myotubes, and this is presented in Fig. 6C. In myotubes, cells positive for myosin heavy chain, we detected CYP27B1 in the cytoplasm. These results confirm that Cyp27b1 is expressed in C2C12 cells at both the mRNA and protein levels.
Fig. 5.
Presence of Cyp27b1 transcript in C2C12 cells. The 1.6 kb of Cyp27b1 transcript encoding the full-length mouse Cyp27b1 was amplified from C2C12 myotubes, and the identity of the cloned Cyp27b1 was confirmed by DNA sequencing.
Fig. 6.
CYP27B1 expression in C2C12 myoblasts and myotubes. CYP27B1 expression in C2C12 cells was determined using immunocytochemistry. A: Western blot analysis revealed the expression of CYP27B1 in C2C12 myoblasts and myotubes, respectively. Mouse kidney extracted was used as a positive control for CYP27B1 protein expression, 25 μg of protein were loaded for each sample. B: mitochondrial localization of C2C12 myoblasts was clarified by subjected to immunocytochemical staining for OxPhos complex V (ATP synthase, F1F0) a subunit (F1 complex). Overlap between OxPhos complex V and CYP27B1 was detected in cultured C2C12 myoblasts suggests mitochondrial localization of CYP27B1. C: CYP27B1 was expressed in C2C12 myotube as determined by the colocalization of MHC expression. Representative images (B and C) at × 630 magnification are shown along with the size bars of 50 μm.
Effects of siRNA-mediated knockdown of Cyp27b1 on myoblast proliferation after 25(OH)D3 treatment.
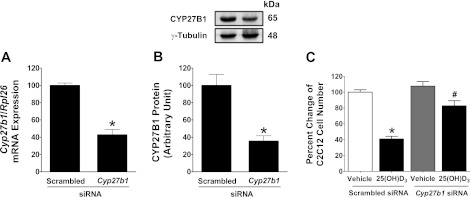
To test whether the presence of CYP27B1 mediated the inhibitory effects of 25(OH)D3 treatment on myoplast proliferation, we knocked down Cyp27b1 using siRNA. We found that treatment of C2C12 myoblasts with siRNA to Cyp27b1 resulted in a 60% reduction in Cyp27b1 mRNA levels by 24 h (Fig. 7A; P < 0.001) and protein levels were down 65% by 72 h (Fig. 7B; P < 0.001). Once we confirmed effective knockdown, we tested whether Cyp27b1 was necessary for mediating the effect of 25(OH)D3 on myoblast proliferation. As expected, treatment of C2C12 cells with scrambled siRNA did not alter the decreased proliferation effect induced by treatment with 25(OH)D3. In contrast, we found that targeted knockdown of Cyp27b1 resulted in a twofold increase in cell number compared with cells treated with scrambled siRNA (Fig. 7C; P < 0.001). These results provide direct evidence that Cyp27b1 is biologically active in C2C12 myoblasts and functions to convert 25(OH)D3 to 1α,25(OH)2D3.
Fig. 7.
Small interfering (si)RNA-mediated knockdown of Cyp27b1 reverses an inhibitory effect of 25(OH)D3 on C2C12 cell proliferation. A: Cyp27b1 mRNA expression was substantially decreased when cells were transfected with Cyp27b1 siRNA prior 25(OH)D3 treatment. B: CYP27B1 protein expression was significantly decreased as shown by Western blot analysis and this change related with its mRNA suppression when cells were transfected with Cyp27b1 siRNA, and 50 μg of protein were loaded for each sample. Graph was plotted from the normalized band density of CYP27B1 with γ-tubulin. Values are means ± SE in A and B (n = 4, independent experiments). Statistical analyses of the data using independent t-test indicate a significant difference between the groups transfected with scrambled vs. Cyp27b1 siRNA (*P < 0.001). C: inhibitory effects of 25(OH)D3 on C2C12 cell proliferation were reversed when cells were transfected with Cyp27b1 siRNA before treatment with 25(OH)D3. Values are means ± SE (n = 4, independent experiments). Statistical analysis using Kruskal-Wallis with Student-Newman-Keuls test indicates *,#P < 0.001 compared with scrambled siRNA (vehicle) and scrambled siRNA [25(OH)D3] (n = 4 separate experiments).
VDR and CYP27B1 expression in control and regenerating muscles in vivo.
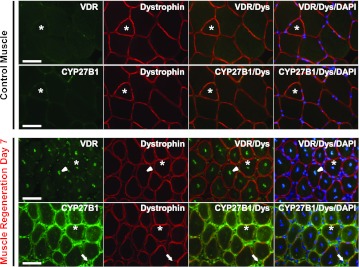
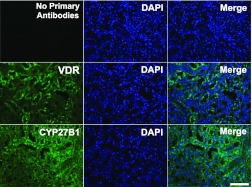
The expression of Vdr in adult skeletal muscle has been a point of controversy in the field (30), and very little is known about the expression of Cyp27b1 in skeletal muscle. Based on our in vitro work and the literature showing that vitamin D3 treatment is most commonly known to have effects on cell proliferation, we studied expression of Vdr and Cyp27b1 in actively regenerating skeletal muscle. BaCl2 treatment was used to induce regeneration in mouse skeletal muscle for these studies and the serial sections were used to demonstrate the coexpression of VDR and CYP27B1 in the same muscle fibers. We found that expression of VDR and CYP27B1 were increased significantly in the regenerating tibialis anterior muscle compared with nonregenerating control muscle (Fig. 8). In control muscle, VDR and CYP27B1 were detected but levels were very low. In contrast, significant VDR expression was detected in the central nuclei of newly regenerating muscle fibers (arrowhead). In the same fibers, we also detected significant expression of CYP27B1 in the cytoplasm of the fibers as well as in the extracellular matrix (arrow). The specificity of antibodies that we used for both VDR and CYP27B1 staining in muscle sections was confirmed with use on mouse kidney sections (Fig. 9). Taken together, our results clearly demonstrate that both VDR and CYP27B1 are upregulated within regenerating muscle fibers in vivo.
Fig. 8.
Expression of VDR and CYP27B1 in control and regenerating skeletal muscles. Serial sections at 10-μm thickness were used to demonstrate the localization and expression of VDR and CYP27B1 expression in control and regenerating muscle fibers day 7. Muscle regeneration was induced to TA muscle by 1.2% BaCl2. VDR and CYP27B1 were expressed at barely detectable in control but the expression was significantly increased in regenerating muscle fibers. Strong positive of VDR expression was found in the nuclei (arrowhead) of regenerating muscle fibers (dystrophin-stained) and CYP27B1 was clearly detected in the cytoplasm of VDR-positive regenerating muscle fibers and extracellular matrix space (arrow). Asterisks denoted an example of the same muscle fibers in these serial sections. Images are shown at ×400 magnification with bars = 50 μm.
Fig. 9.
VDR and CYP27B1 expression in mouse kidney. VDR and CYP27B1 were counterstained with DAPI to show nuclear/cytoplasmic localization in the mouse kidney section. Images were captured at ×200 magnification; bar = 100 μm.
DISCUSSION
In the present study, we examined the expression of VDR and CYP27B1 in C2C12 cells in vitro and regenerating skeletal muscle in vivo. The main findings of this work include 1) VDR and CYP27B1 are expressed in C2C12 myoblasts and myotubes and regenerating skeletal muscle in vivo; and 2) 25(OH)D3 treatment inhibits myoblast proliferation and increases expression of VDR in a similar manner to 1α,25(OH)2D3 and this is likely mediated by the function of Cyp27b1. The novelty of this work is the discovery of CYP27B1 bioactivity in C2C12 cells and our observation of significant expression of both VDR and CYP27B1 in regenerating muscle in vivo. These results suggest that both 1α,25(OH)2D3 and 25(OH)D3 can contribute to regulation of satellite cells, and this may hold particular significance during repair of injured skeletal muscle.
VDR has been reported to be expressed in myoblasts (7–9, 13, 14, 27, 32) and skeletal muscle (5, 13, 32); however, this has been controversial because the specificity of various VDR antibodies has been questioned (29, 30). Here, we combined the use of Western blot analysis, immunocytochemistry, PCR cloning, and DNA sequencing to validate the expression of Vdr in C2C12 cells (myoblasts and myotubes). We found that C2C12 myoblasts expressed VDR protein primarily in the nucleus, and we confirmed the presence of the full-length Vdr mRNA by cloning and DNA sequencing. We also detected VDR expression in C2C12 myotubes, but unlike myoblasts, most of the VDR was found in the cytoplasm. Regulation of VDR localization between nuclear and cytosolic compartments in cells has been reported to be regulated by RXR through translocation of unliganded VDR (25). Increases in RXR expression are associated with increased cytoplasmic localization of VDR. Thus a potential explanation for the differential localization of VDR between C2C12 myoblasts and myotubes could be due to the increased expression of Rxr that occurs following differentiation into myotubes (11). However, when C2C12 myotubes were treated with 1α,25(OH)2D3, we did detect increased nuclear levels of VDR, indicating that in the presence of vitamin D3 the VDR is translocated to the nucleus in C2C12 myotubes.
One of the surprising findings of this study was the effects of the inactive form of vitamin D3, 25(OH)D3, on myoblast proliferation. This observation prompted our experiments to determine whether Cyp27b1 was expressed and, if so, whether it was functional in C2C12 myoblasts. The suppressive effects of 25(OH)D3 were detected within 2 days after treatment. In addition, upregulation of both Vdr mRNA and VDR protein was similar to that seen with 1α,25(OH)2D3 treatment, suggesting that 25(OH)D3 was converted to 1α,25(OH)2D3 via CYP27B1 and acts through VDR. Consistent with our findings, previous studies (16) using human mammary epithelial cells demonstrated that 25(OH)D3 is metabolized by CYP27B1 and downstream effects are exerted through VDR.
Data from our CYP27B1 knockdown experiments revealed the important biological function of CYP27B1 in myoblast proliferation. However, it should be noted that we did use a high dose of 25(OH)D3 (2 μM) compared with 1α,25(OH)2D3 (20 nM) to mimic the effects on cell proliferation. This potentially reflects the low expression of CYP27B1 in C2C12 myoblasts leading to a relatively low potency for production 1α,25(OH)2D3. Indeed, the low conversion rate of 25(OH)D3 to 1α,25(OH)2D3 argues for muscle to act only as a local site for autocrine/paracrine production of 1α,25(OH)2D3 rather than a site for systemic production. Our findings are consistent with results reported for neonatal rat cardiac myocytes in which ∼10% of 25(OH)D3 was converted to 1α,25(OH)2D3 (31). However, we cannot rule out the potential for a direct effect of 25(OH)D3 as an agonist for VDR since the agonistic action of 25(OH)D3 has been recently demonstrated in CYP27B1−/− cells (20). This is an important area requiring further study in muscle cells.
While our validation of Vdr expression and discovery of Cyp27b1 expression in C2C12 cells were important, we were unsure what the translation of these results from a muscle cell line were for in vivo muscle. Thus our final set of studies pursued the potential expression of VDR and CYP27B1 in adult skeletal muscle. We found that both VDR and CYP27B1 were significantly expressed at day 7 during skeletal muscle regeneration. VDR expression was primarily detected in the myonuclei, and CYP27B1 was cytoplasmic in these newly formed fibers. These results are exciting as they confirm the expression of VDR in muscle and add new data that CYP27B1 can be expressed in adult skeletal muscle. The significant increase in both VDR and CYP27B1 expression with regeneration does not change the question of VDR expression in control muscle, but it does confirm that they can be expressed in skeletal muscle in response to challenges. These results suggest that both VDR and CYP27B1 may hold play an essential role during muscle regeneration to repair existing muscle fibers or reform new muscle fibers in response to muscle injury.
There have been results from human studies (24) that vitamin D3 treatment does lead to improvements in skeletal muscle function; however, the field is controversial as other studies (17, 18) have shown no improvement of skeletal muscle strength and function in the elderly after vitamin D3 treatment. The results from the present study help contribute to the field by confirming the expression of VDR in skeletal muscle especially during regeneration and repair. In addition, we found that like VDR, skeletal muscle expresses the key enzyme 1-α hydroxylase encoded by gene Cyp27b1 and expression of CYP27B1 can likely function to convert 25(OH)D3 to the active form of vitamin D3. Our findings raise the possibility that skeletal muscle may be able to function as a local site for conversion of 25(OH)D3 to 1α,25(OH)2D3 for physiologically relevant effects on muscle cells during the regenerative process.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR-45617 and AR-045617-09S2 (to K. A. Esser).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.S., O.-K.P.-S., and K.A.E. conception and design of research; R.S. and X.Z. performed experiments; R.S. analyzed data; R.S., X.Z., O.-K.P.-S., and K.A.E. interpreted results of experiments; R.S. prepared figures; R.S. drafted manuscript; R.S., X.Z., O.-K.P.-S., and K.A.E. edited and revised manuscript; R.S., X.Z., O.-K.P.-S., and K.A.E. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. John J. McCarthy for discussions that contributed to the development of this project.
REFERENCES
- 1. Anderson PH, O'Loughlin PD, May BK, Morris HA. Modulation of CYP27B1 and CYP24 mRNA expression in bone is independent of circulating 1,25(OH)2D3 levels. Bone 36: 654–662, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Artaza JN, Sirad F, Ferrini MG, Norris KC. 1,25(OH)2vitamin D3 inhibits cell proliferation by promoting cell cycle arrest without inducing apoptosis and modifies cell morphology of mesenchymal multipotent cells. J Steroid Biochem Mol Biol 119: 73–83, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atkins GJ, Anderson PH, Findlay DM, Welldon KJ, Vincent C, Zannettino AC, O'Loughlin PD, Morris HA. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1 alpha,25-dihydroxyvitamin D3. Bone 40: 1517–1528, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab 21: 375–384, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stahelin HB, Dick W. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J 33: 19–24, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Bises G, Kallay E, Weiland T, Wrba F, Wenzl E, Bonner E, Kriwanek S, Obrist P, Cross HS. 25-Hydroxyvitamin D3-1alpha-hydroxylase expression in normal and malignant human colon. J Histochem Cytochem 52: 985–989, 2004 [DOI] [PubMed] [Google Scholar]
- 7. Buitrago C, Costabel M, Boland R. PKC and PTPalpha participate in Src activation by 1alpha,25OH2 vitamin D3 in C2C12 skeletal muscle cells. Mol Cell Endocrinol 339: 81–89, 2011 [DOI] [PubMed] [Google Scholar]
- 8. Buitrago C, Vazquez G, De Boland AR, Boland RL. Activation of Src kinase in skeletal muscle cells by 1, 1,25-[OH(2)]-vitamin D(3) correlates with tyrosine phosphorylation of the vitamin D receptor (VDR) and VDR-Src interaction. J Cell Biochem 79: 274–281, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Capiati D, Benassati S, Boland RL. 1,25(OH)2-vitamin D3 induces translocation of the vitamin D receptor (VDR) to the plasma membrane in skeletal muscle cells. J Cell Biochem 86: 128–135, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Cutolo M, Otsa K, Uprus M, Paolino S, Seriolo B. Vitamin D in rheumatoid arthritis. Autoimmun Rev 7: 59–64, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Downes M, Mynett-Johnson L, Muscat GE. The retinoic acid and retinoid X receptors are differentially expressed during myoblast differentiation. Endocrinology 134: 2658–2661, 1994 [DOI] [PubMed] [Google Scholar]
- 12. Drissi H, Pouliot A, Koolloos C, Stein JL, Lian JB, Stein GS, van Wijnen AJ. 1,25-(OH)2-vitamin D3 suppresses the bone-related Runx2/Cbfa1 gene promoter. Exp Cell Res 274: 323–333, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Endo I, Inoue D, Mitsui T, Umaki Y, Akaike M, Yoshizawa T, Kato S, Matsumoto T. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 144: 5138–5144, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Garcia LA, King KK, Ferrini MG, Norris KC, Artaza JN. 1,25(OH)2 vitamin D3 stimulates myogenic differentiation by inhibiting cell proliferation and modulating the expression of promyogenic growth factors and myostatin in C2C12 skeletal muscle cells. Endocrinology 152: 2976–2986, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Janssen HC, Samson MM, Verhaar HJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr 75: 611–615, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Kemmis CM, Salvador SM, Smith KM, Welsh J. Human mammary epithelial cells express CYP27B1 and are growth inhibited by 25-hydroxyvitamin D-3, the major circulating form of vitamin D-3. J Nutr 136: 887–892, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Kenny AM, Biskup B, Robbins B, Marcella G, Burleson JA. Effects of vitamin D supplementation on strength, physical function, and health perception in older, community-dwelling men. J Am Geriatr Soc 51: 1762–1767, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Latham NK, Anderson CS, Lee A, Bennett DA, Moseley A, Cameron ID. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: the Frailty Interventions Trial in Elderly Subjects (FITNESS). J Am Geriatr Soc 51: 291–299, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Lips P. Vitamin D physiology. Prog Biophys Mol Biol 92: 4–8, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Lou YR, Molnar F, Perakyla M, Qiao S, Kalueff AV, St-Arnaud R, Carlberg C, Tuohimaa P. 25-Hydroxyvitamin D(3) is an agonistic vitamin D receptor ligand. J Steroid Biochem Mol Biol 118: 162–170, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Marchionatti AM, Picotto G, Narvaez CJ, Welsh J, Tolosa de Talamoni NG. Antiproliferative action of menadione and 1,25(OH)2D3 on breast cancer cells. J Steroid Biochem Mol Biol 113: 227–232, 2009 [DOI] [PubMed] [Google Scholar]
- 22. McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development 138: 3657–3666, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakamura Y, Eto TA, Taniguchi T, Miyamoto K, Nagatomo J, Shiotsuki H, Sueta H, Higashi S, Okuda KI, Setoguchi T. Purification and characterization of 25-hydroxyvitamin D3 1alpha-hydroxylase from rat kidney mitochondria. FEBS Lett 419: 45–48, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Pfeifer M, Begerow B, Minne HW, Suppan K, Fahrleitner-Pammer A, Dobnig H. Effects of a long-term vitamin D and calcium supplementation on falls and parameters of muscle function in community-dwelling older individuals. Osteoporos Int 20: 315–322, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Prufer K, Barsony J. Retinoid X receptor dominates the nuclear import and export of the unliganded vitamin D receptor. Mol Endocrinol 16: 1738–1751, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Rohan JN, Weigel NL. 1Alpha,25-dihydroxyvitamin D3 reduces c-Myc expression, inhibiting proliferation and causing G1 accumulation in C4-2 prostate cancer cells. Endocrinology 150: 2046–2054, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25-dihydroxyvitamin D3 receptors and activities in muscle. J Biol Chem 260: 8882–8891, 1985 [PubMed] [Google Scholar]
- 28. Srikuea R, Esser KA, Pholpramool C. Leukaemia inhibitory factor is expressed in rat gastrocnemius muscle after contusion and increases proliferation of rat L6 myoblasts via c-Myc signalling. Clin Exp Pharmacol Physiol 38: 501–509, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Y, Becklund BR, DeLuca HF. Identification of a highly specific and versatile vitamin D receptor antibody. Arch Biochem Biophys 494: 166–177, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Wang Y, DeLuca HF. Is the vitamin d receptor found in muscle? Endocrinology 152: 354–363, 2011 [DOI] [PubMed] [Google Scholar]
- 31. Wu-Wong JR, Chen YW, Nakane M, Wolf M. Differential effects of vitamin d receptor agonists on gene expression in neonatal rat cardiomyocytes. Cardiovasc Drugs Ther 25: 215–222, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Zanello SB, Collins ED, Marinissen MJ, Norman AW, Boland RL. Vitamin D receptor expression in chicken muscle tissue and cultured myoblasts. Horm Metab Res 29: 231–236, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Zehnder D, Bland R, Walker EA, Bradwell AR, Howie AJ, Hewison M, Stewart PM. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in the human kidney. J Am Soc Nephrol 10: 2465–2473, 1999 [DOI] [PubMed] [Google Scholar]
- 34. Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab 86: 888–894, 2001 [DOI] [PubMed] [Google Scholar]
- 35. Zhang X, Patel SP, McCarthy JJ, Rabchevsky AG, Goldhamer DJ, Esser KA. A noncanonical E-box within the MyoD core enhancer is necessary for circadian expression in skeletal muscle. Nucleic Acids Res 40: 3419–3430, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]