Abstract
Small- and intermediate-conductance Ca2+-activated K+ channels (SK3/Kcnn3 and IK1/Kcnn4) are expressed in vascular endothelium. Their activities play important roles in regulating vascular tone through their modulation of intracellular concentration ([Ca2+]i) required for the production of endothelium-derived vasoactive agents. Activation of endothelial IK1 or SK3 channels hyperpolarizes endothelial cell membrane potential, increases Ca2+ influx, and leads to the release of vasoactive factors, thereby impacting blood pressure. To examine the distinct roles of IK1 and SK3 channels, we used electrophysiological recordings to investigate IK1 and SK3 channel trafficking in acutely dissociated endothelial cells from mouse aorta. The results show that SK3 channels undergo Ca2+-dependent cycling between the plasma membrane and intracellular organelles; disrupting Ca2+-dependent endothelial caveolae cycling abolishes SK3 channel trafficking. Moreover, transmitter-induced changes in SK3 channel activity and surface expression modulate endothelial membrane potential. In contrast, IK1 channels do not undergo rapid trafficking and their activity remains unchanged when either exo- or endocytosis is block. Thus modulation of SK3 surface expression may play an important role in regulating endothelial membrane potential in a Ca2+-dependent manner.
Keywords: channel trafficking, endothelium, endocytosis, exocytosis
regulation of vascular tone is critical in controlling blood flow to different vascular beds and organs. Vascular endothelial cells (ECs) play an unequivocal role in modulating vascular tone and tension. It is well known that ECs are capable of generating vasoconstrictors and dilators such as endothelin, nitric oxide (NO), prostacyclin, and endothelium-derived hyperpolarizing factor (EDHF; Refs. 4, 9). Recent functional and biochemical studies (6, 17) have shown that the potassium channels expressed in vascular endothelium, particularly IK1 and SK3 channels, play important roles in the final steps of EDHF activation in resistance arteries. Both IK1 and SK3 channels are voltage independent and gated solely by intracellular Ca2+ (13, 14). Endothelial IK1 and SK3 channel activation results in membrane hyperpolarization of neighboring smooth muscle cells. However, subcellular localization, modulation of surface expression, and differential activation of these channels are not yet fully understood (17).
Recently, it has been shown that IK1 and SK3 channels are localized to different subcellular compartments in ECs. IK1 channels are present in endothelial projections, which go through internal elastic lamina and electrically couple with smooth muscle cells via myoendothelial gap junctions (7, 12, 23). Local Ca2+ transients in the projections activate IK1 channels, the activation of which in turn directly influences the membrane potential of neighboring smooth muscle cells (17). SK3 channels, on the other hand, are localized to caveolae, specialized membrane structure that can either be free in the cytosol or attached to the plasma membrane. Endothelial caveolae form the majority of intracellular vesicles and contain receptors and ion channels that, when fused to the plasma membrane, create signaling microenvironments that are crucial for many endothelial functions (8).
The localization of SK3 channels in caveolae suggests a dynamic process by which removal or incorporation of SK3 channels could regulate endothelial function. We therefore investigated whether trafficking of these channels modulates channel activity in ECs acutely isolated from mouse aorta. With the use of blockers of exocytosis and endocytosis, and caveolar disruption, the results show that SK3 channels undergo caveolae-mediate membrane cycling, while IK1 channels do not cycle. SK3 channel endocytosis is dependent on the GTPase activity of dynamin, and exocytosis is sensitive to the exocytosis blocker N-ethylmaleimide (NEM). Caveolar trafficking significantly alters SK3 channel activity, trafficking, and surface expression, as well as its total plasma membrane surface area of ECs. The combination of SK3 channel trafficking, activity, and surface expression modulates endothelial membrane potential and may play important roles in fine-tuning Ca2+ signaling and vascular tone.
METHODS
Cell isolation.
All procedures were performed in accordance with the animal use protocols approved by the Institutional Animal Care and Use Committee of Oregon Health and Science University. The methods for tissue harvesting and EC isolation were similar to previous studies (18, 30). Briefly, adult C57BL/6J mice were anesthetized with isoflurane and decapitated, followed by perfusion of ice-cold Ca2+ free solution containing (in mM): 55 NaCl, 80 Na+ glutamate, 5.9 KCl, 2 MgCl2, 10 glucose, and 10 HEPES (pH 7.3). Aortic vessels were carefully dissected, cleaned, and then filled with enzymatic solution for 40 min at 37°C in the aforementioned solution with 100 μM Ca2+, 4 mg/ml protease from B. licheniformais, and 1 mg/ml hyaluronidase. Elastase (0.1 mg/ml) was included for the final 10 min of incubation. The vessels were washed several times with ice-cold Ca2+ free solution, cut into small pieces, and gently triturated. Isolated cells were kept in the ice-cold solution, and single ECs were used for recording within 7 h.
Electrophysiology.
Whole cell patch-clamp recordings were performed on isolated ECs using an EPC-9 amplifier (HEKA Instruments, Bellmore, NY) and ITC-16 (HEKA), and data were acquired using Pulse software (HEKA). Whole cell currents were evoked in voltage-clamp mode by applying a voltage ramp (−80 to +50 mV; 200 ms), followed by a voltage step (+20 mV; 200 ms). Membrane capacitance was taken as the whole cell capacitance that was automatically electronically compensated before each series or calculated from hyperpolarizing voltage step from +20 to −60 mV. The membrane capacitance was used as a measure of surface area of the cell. Acquisitions were sampled at 2 kHz and filtered at 1 kHz. Patch pipettes (3–5 MΩ) were filled with different pipette solutions depending on the specific experimental requirements (see Chemicals and solutions). Series resistance was not compensated due to the small amplitude of the recorded currents. In another set of experiments, membrane potential was measured in current-clamp mode using perforated patch without bias current injection.
Chemicals and solutions.
External patch-clamp solution containing the following (in mM): 134 NaCl, 6 KCl, 10 glucose, 15 HEPES, 1 MgCl2, and 2 CaCl2 2 (pH 7.35). Tram-34 (1 μM) or clotrimazole (10 μM) was included in the bath solution to block IK1 channel current, and washout of tram-34 or clotrimazole was used to study trafficking of IK1 channels. Similarly, apamin (300 nM) was used to block SK3 channel current, and washout of apamin was used to study trafficking of SK3 channels. The whole cell pipette solution contained the following (in mM): 4 NaCl, 4 KCl, 133 K+ gluconate, 5 EDTA, 10 HEPES, 5.13 MgCl2, and 1 CaCl2 (pH 7.2). The concentrations of free Mg2+ (1 mM) and Ca2+ (3 μM) were calculated using Patcher's Power Tools (Department of Membrane Biophysics at MPI Biophysical Chemistry in Göttingen, Germany). For voltage-clamp recordings, fresh ATP (4 mM), GTP (0.3 mM), and phosphocreatine (10 mM) were added to the pipette solution daily. For perforated patch recordings in current-clamp mode, patch pipettes were back-filled with pipette solution containing amphotericin B (200 μg/ml). Apamin and phosphocreatine were obtained from Calbiochem (EMD, Philadelphia, PA). Tram-34 was from Alomone (Alomone Labs). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Data and analysis.
Data were analyzed and plotted in IgorPro (WaveMetric). Binning data at 0.5-min intervals generated summary plots. Data are expressed as means ± SE. Paired two sample t-tests were used to determine significance of data from the same cell, and ANOVA with Dunnett's post hoc tests were used to determine significance between groups of data; P < 0.05 was considered significant.
RESULTS
Whole cell recording of IK1 and SK3 currents from aortic ECs.
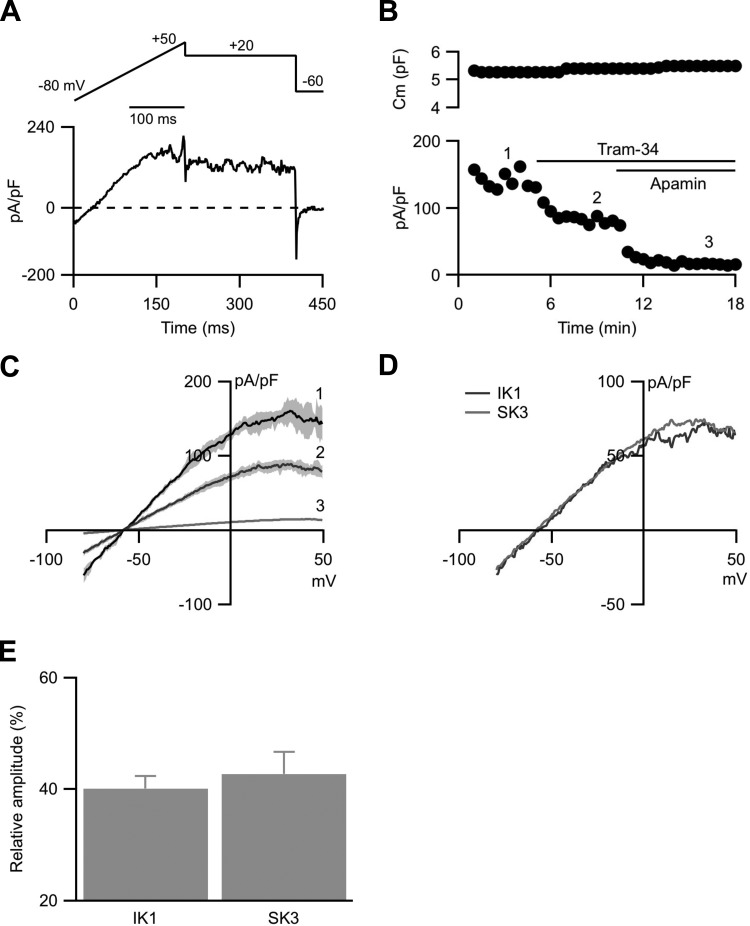
Acutely dispersed mouse aorta preparations contained both ECs and smooth muscle cells. ECs were visually identified by their characteristic phase contrast, rough outline, and appearance. Consistent with the results from previous studies (16) , murine aortic ECs do not express large-conductance BK channels and currents recorded from visually identified ECs were not sensitive to the BK channel blocker 100 nM iberiotoxin or 500 nM paxilline (n = 9; not shown). Endothelial whole cell currents were elicited in the presence of 3 μM internal Ca2+ to activate SK3 and IK1 channels using a voltage-clamp protocol, delivered every 15 s, that consisted of two segments: a 200-ms voltage ramp from −80 to +50 mV followed by a 200-ms voltage step to +20 mV to measure steady-state currents (Fig. 1A). Membrane capacitance, plotted against time of recordings, was stable throughout the experiment under control conditions (Fig. 1B, top; 5.9 ± 7%; n = 7; P = 0.23). Steady-state whole cell currents recorded at +20 mV, normalized to membrane capacitance to obtain current density, were plotted against the time of recordings (Fig. 1B, bottom). Bath application of tram-34 {1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole; 1 μM}, a selective blocker for IK1 channels (32), decreased the current density by 43%. Subsequent application of apamin (300 nM), a selective SK1–3 channel blocker (20, 28), further decreased the current density by an additional 48% (Fig. 1, B and C). It has been shown that these ECs only express SK3, not SK1 or SK2, channels and the residual current was <10% of the total current. Therefore, IK1 and SK3 channel activities are the predominant ion channels contributing approximately equally to endothelial whole cell currents (IK1 = 40 ± 3%, SK3 = 43 ± 5%; n = 7; Fig. 1, D and E).
Fig. 1.
Endothelial whole cell voltage-clamp recording. A: voltage-clamp recording protocol (top) and a representative trace (bottom). Cells were held at −60 mV and a voltage ramp of −80 to +50 mV (200 ms), followed by voltage steps to +20 mV (200 ms) and back to −60 mV (50 ms) were delivered. B: cell membrane capacitance (Cm) plotted against time of recording (top). Time 0 indicates whole cell formation. Capacitance was measured from the hyperpolarizing steps from +20 to −60 mV. Whole cell current density plotted against the time of recording (bottom). Current density was calculated from the steady-state currents recordings at +20 mV, normalized to the membrane capacitance. Numbers on the plot correspond to the representative voltage-ramp current density traces in C. C: representative whole cell current density elicited by −80 to +50 mV voltage-ramp. D: IK1 and SK3 current densities isolated from digital subtraction of the traces in C. E: normalized contribution of IK1 and SK3 currents to the whole cell currents.
Current recovery from SK3 and IK1 channel blockers.
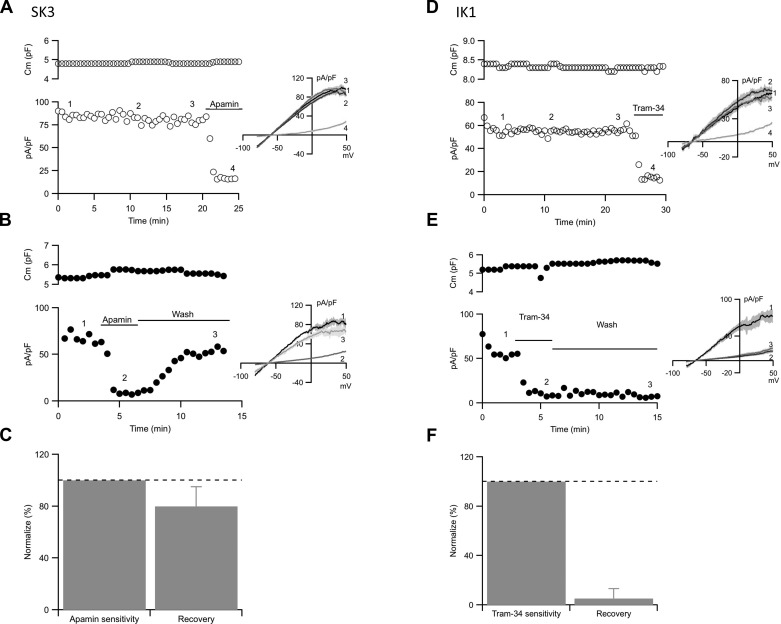
To study SK3 or IK1 channels separately, SK3 channel currents were examined in the presence of tram-34 (1 μM) to block IK1 channels, while IK1 channel currents were recorded in the presence of apamin (300 nM) to block SK3 channels. In both cases, membrane capacitance and current density remained stable throughout the recordings (Fig. 2, A and D). Brief application of apamin (300 nM, 3 min) effectively reduced SK3 current density; however, it recovered following apamin washout from the recording chamber (80 ± 15%, n = 10; Fig. 2, B and C). Since apamin is an effectively irreversible blocker and the membrane capacitance does not change, the results suggest the recovery from apamin block reflects newly trafficked unblocked SK3 channels on the plasma membrane (19, 27). In contrast, IK1 channel activity failed to recover from tram-34 block (5.2 ± 8%, n = 9; Fig. 2, E and F). Similar results were observed with two other unrelated IK1 channel blockers, clotrimazole (10 μM) and charybdotoxin (100 nM). Recovery from these IK1 channel blockers was absent, suggesting that IK1 channels do not traffic over a time course of minutes.
Fig. 2.
SK3 and IK1 current recovery from blockers. A and B: cell membrane capacitance (top) and whole cell SK3 current density (bottom) plotted against experimental time for control cell (A) and recovery from apamin cell (B). C: category plot showing the normalized current density recovery from apamin block. D and E: cell membrane capacitance (top) and whole cell IK1 current density (bottom) plotted against experimental time for control cell (D) and recovery from tram-34 cell (E). F: category plot showing the normalized current density recovery from tram-34 block. Horizontal bars denote the presence and washout of apamin or tram-34 from the bath. Numbers on the plots correspond to the representative traces (insets). Dash lines on the bar graphs show 100% (control).
Blocking exocytosis blocks SK3 current density recovery.
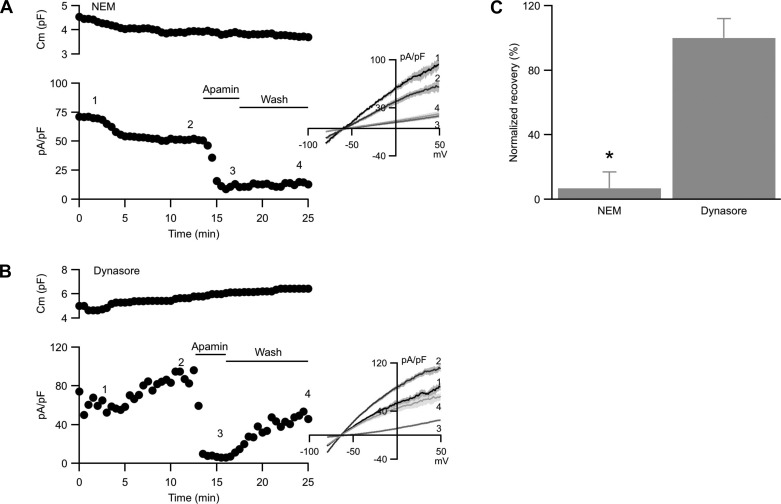
To test whether SK3 current recovery from apamin block reflects exocytosis of unblocked channels, the experiment was repeated while exocytosis was inhibited with NEM to block N-ethylmaleimide-sensitive fusion protein, an activity that is required for vesicular fusion with the plasma membrane (33). When NEM (1 mM) was included in the patch pipette solution and dialyzed into the cell, membrane capacitance slowly decreased over time (Fig. 3A, top; −20 ± 8%; n = 9; P = 0.023), reflecting continual endocytosis of plasma membrane. Whole cell SK3 current density, measured in the presence of tram-34 to inhibit IK1, showed a rapid reduction at ∼2–4 min after whole cell formation that tapered off with time, resulting in reduced current density (Fig. 3A, bottom, between time points 1 and 2; −29 ± 11%, P = 0.013). Since both membrane capacitance and SK3 current density decreased, the reduction in nonnormalized SK3 channel current is actually much greater (−36 ± 15%).
Fig. 3.
SK3 current recovers in N-ethylmaleimide (NEM) but not dynasore. A and B: Cell membrane capacitance (top) and whole cell SK3 current density (bottom) plotted against time of recording from cells dialyzed with 1 mM NEM (A) or bath treated with 50 μM dynasore (B). Horizontal bars denote the presence and washout of apamin from the bath. Numbers on the plot correspond to the representative current density traces shown in the inset. C: normalized averages of SK3 current density recovery from apamin block in the presence of NEM or dynasore. *Statistical significance (P < 0.05, ANOVA) compared with cells not treated with NEM.
Subsequent apamin application significantly reduced SK3 current density. However, with exocytosis blocked, washout of apamin from the recording chamber did not result in SK3 current recovery (7 ± 10%; Fig. 3, A and C). Thus blocking exocytosis resulted in decreased SK3 channel surface expression over time and abolished SK3 channel recovery from apamin block, suggesting that recovery reflects SK3 channel exocytosis.
SK3 current density recovers in the presence of endocytosis blocker.
The results presented above suggest that endothelial SK3 channels may undergo dynamic cycling. This predicts that blocking endocytosis while exocytosis is intact will increase membrane capacitance and allow SK3 current density recovery from apamin block. Therefore, endocytosis was blocked by bath applying dynasore, a membrane permeable dynamin inhibitor that blocks dynamin's GTPase activity and interferes with the scission of vesicles from plasma membrane (31). Dynasore (50 μM) was added after stable baseline recording, ∼4 min after whole cell formation. Contrary to blocking exocytosis, blocking endocytosis resulted in a significant increase in cell capacitance (Fig. 3B, top; 36 ± 17%; n = 10; P < 0.01) and greater SK3 current density (Fig. 3B, bottom, between time points 1 and 2; +35 ± 14%; P < 0.01), suggesting that when endocytosis is blocked, exocytosis continuously deposits SK3 channels on the plasma membrane from vesicles containing high SK3 channel density. In fact, the increased SK3 current density was a result of a substantial increase in raw SK3 current by 78 ± 19%, which outweighed the increased capacitance used for normalization.
Brief apamin application reduced the current density by 92%. However, in contrast to blocking exocytosis, washout of apamin showed a gradual recovery of current density, consistent with the addition of unblocked SK3 channels to the endothelial plasma membrane (Fig. 3, B and C). Comparable results were obtained from cells loaded with the less selective GTPase blocker GDPβS (400 μM; n = 7, not shown). Taken together, the results suggest that endothelial SK3 channels undergo dynamic cycling.
IK1 channels do not cycle.
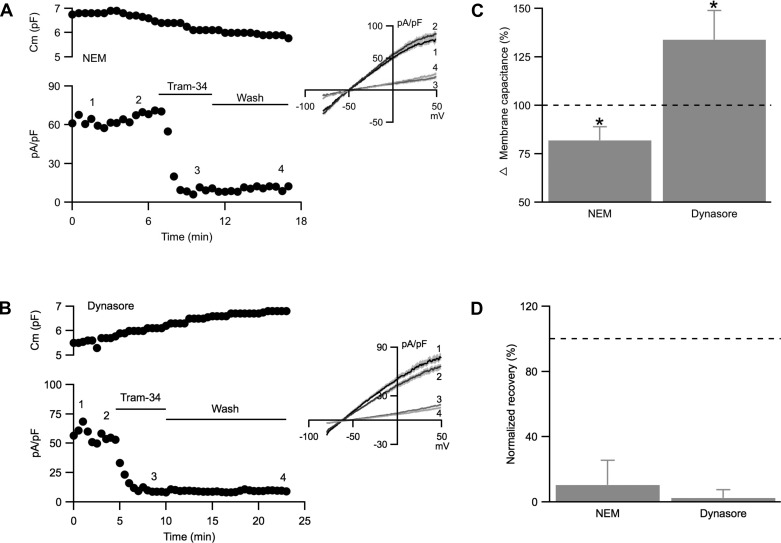
The results presented in Fig. 2, E and F, suggest that unlike SK3 channels, endothelial IK1 channels may not undergo dynamic cycling. Therefore, the effects of NEM and dynasore on endothelial IK1 currents were examined in the presence of apamin (300 nM) to block SK3 channels throughout the experiments. Membrane capacitance recordings from cells loaded with NEM (1 mM) showed a gradual and significant decrease compared with non-NEM dialyzed control (Fig. 4, A, top, and C; −18 ± 7%, n = 9, P = 0.04), reflecting ongoing vesicular endocytosis. Consequently, IK1 current density gradually increased (Fig. 4A, bottom; between time points 1 and 2, +14 ± 6%; P = 0.04), in contrast to the decrease observed for SK3 current density shown in Fig. 3.
Fig. 4.
IK1 current density does not recover in the presence of NEM or dynasore. A and B: cell membrane capacitance (top) and whole cell IK1 current density (bottom) plotted against time of recording from cells dialyzed with NEM (1 mM; A) or with bath application of dynasore (50 μM; B). C: normalized changes in membrane capacitance comparing membrane capacitance from the last 3 min to the first 3 min of recording. D: normalized averages of IK1 current density recovery from tram-34 block in the presence of NEM or dynasore. Horizontal bars denote the presence and washout of tram-34 from the bath. Numbers on the plots correspond to the representative current density traces shown in the inset. Dash lines on the bar graphs show 100% (control). *Statistical significance (P < 0.05, paired t-test).
Blocking endocytosis with dynasore induced a significant increase in membrane capacitance (Fig. 4, B, top, and C; 34 ± 15%; n = 6; P = 0.004), reflecting ongoing vesicular exocytosis, and this was accompanied by reduced IK1 current density (Fig. 4B, bottom; between time points 1 and 2, −16 ± 7%; P = 0.04). Importantly, IK1 current density did not recover following washout of tram-34 from the bath when exocytosis or endocytosis was blocked (Fig. 4D). These results together show that IK1 channels do not undergo dynamic cycling and changes in IK1 current density most likely reflect changes in membrane area. Indeed, the absolute IK1 current, not normalized by capacitance, did not change with NEM or dynasore (control: 104 ± 4%; NEM: 109 ± 7%; dynasore: 98 ± 9%; P = 0.25).
Methyl-β-cyclodextrin abolishes SK3 current density recovery.
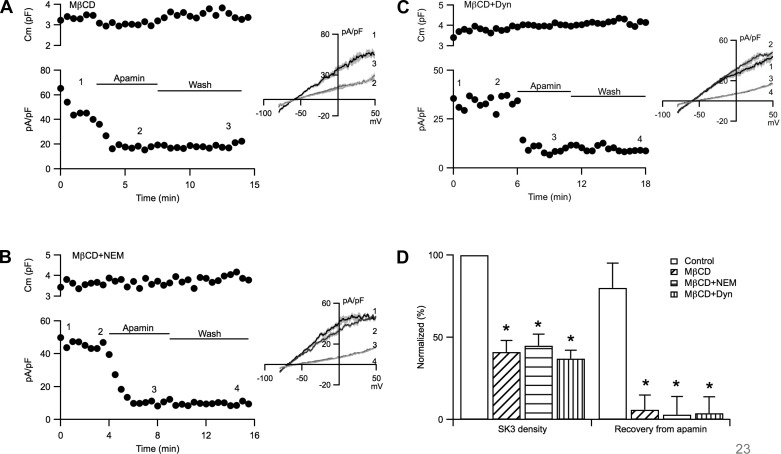
The plasma membrane of ECs is covered with caveolae, major trafficking vesicles required for some endothelial functions (29). Moreover, endothelial SK3 channels are localized to caveolae and they interact with caveolin-1, the major structural component of caveolae (8). The maintenance of caveolar structure requires the presence of membrane cholesterol and exposure to methyl-β-cyclodextrin (MβCD) to remove cholesterol destroys caveolae (1). Therefore, we tested whether disrupting caveolae abolishes endothelial SK3 channel trafficking by incubating ECs in MβCD (1 mM; 40 min at room temperature). The membrane capacitance of MβCD-treated cells, which was also stable with time (normalized change: 2.3 ± 4.3%), was similar to non-MβCD-treated cells (Fig. 5A; MβCD: 5.8 ± 1.0 pF, n = 7; control: 7.0 ± 1.1 pF, n = 10; P = 0.56). Importantly, in the presence of MβCD recovery from apamin block was absent (Fig. 5, A and D). Averages are shown in Fig. 6D; cells in MβCD showed significantly smaller SK3 current density (41 ± 7%; n = 7; P = 0.001), and they did not recover from apamin block (6 ± 9%; n = 7).
Fig. 5.
Methyl-β-cyclodextrin (MβCD) abolishes SK3 current density recovery. A–C: cell membrane capacitance (top) and whole cell SK3 current density (bottom) plotted against time of recording from cells treated with MβCD alone (A), MβCD and dialyzed with NEM (B), or MβCD and dynasore (C). D: normalized averages of SK3 current density and its recovery from apamin block from cells not treated (empty), treated with MβCD alone (hash lines), or treated MβCD dialyzed with NEM (horizontal lines) or in the presence of dynasore (vertical lines). Horizontal bars denote the presence and washout of apamin from the bath. Numbers on the plots correspond to the representative current density traces shown in the inset. *Statistical significance (P < 0.05, ANOVA) compared with cells not treated MβCD.
Fig. 6.
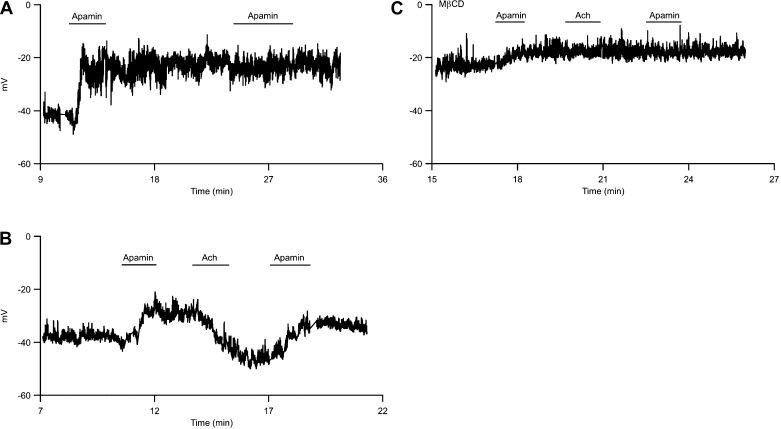
Acetylcholine (Ach) reverses apamin-induced depolarization. A: endothelial membrane potential was recorded using whole cell perforated patch in current-clamp mode and was plotted against time of experiment. Time 0 indicates gigaseal formation. Application of apamin in the bath causes membrane depolarization. B: Ach repolarizes apamin-induced depolarization. C: membrane potential recording from an endothelial cell treated with MβCD. Recording chamber is continually perfused with external recording solution. Horizontal bars indicate brief application of apamin or acetylcholine to the bath.
Recordings were also obtained from cells incubated in MβCD with either NEM (1 mM) in the pipette solution (Fig. 5B) or dynasore (50 μM) in the bath (Fig. 5C) to block exocytosis and endocytosis, respectively. Similar to the results from cells treated with MβCD alone, SK3 current density was significantly smaller than that of control cells not treated with MβCD (Fig. 5D; NEM: 45 ± 7%, n = 6, P = 0.01; dynasore: 37 ± 5%, n = 6, P = 0.003). Moreover, addition of NEM or dynasore to modify vesicular trafficking did not engender recovery from apamin block following MβCD treatment (NEM: 3.0 ± 11%; dynasore: 3.8 ± 10%). These results indicate that MβCD disrupts caveolae, the predominant vesicular trafficking in ECs, required for SK3 channel trafficking and recovery from apamin block.
Acetylcholine induces SK3 channel trafficking.
Acetylcholine (Ach) is a major transmitter in the autonomic nervous system and at the neuromuscular junction. In endothelium-denuded vessels, Ach activates smooth muscle M3-type receptors to cause Ca2+ influx and constriction. However, in the presence of intact endothelium activation of endothelial Ach receptors, leading to Ca2+ influx and SK channel activation, results in vessel relaxation that is attributable to the activation of endothelium-dependent vasoactive pathways (5). To study the effects of SK3 channel trafficking on ECs membrane potential, whole cell perforated patch-clamp recordings were performed on isolated ECs in current-clamp mode under conditions in which intracellular Ca2+ was not perturbed nor buffered (see methods). This recording method is different from the conventional whole cell voltage-clamp recordings shown in Figs. 1–6, in which the cells were dialyzed with 3 μM [Ca2+]i. In control conditions, the resting membrane potential of ECs averaged −37 ± 2 mV (n = 9), and blocking SK3 channels with apamin (300 nM) caused membrane depolarization by 8.4 ± 2.3 mV (Fig. 6A; P = 0.01), indicating the presence of basal SK3 current that contributes to the resting potential. Under these conditions with basal [Ca2+]i, apamin washout did not repolarize membrane potential back to the control baseline. Moreover, subsequent apamin application did not cause further depolarization, suggesting the absence of SK3 channel trafficking in perforated recording conditions (Fig. 6A). However, brief application of Ach (1 μM) to increase [Ca2+]i reversed the apamin-induced membrane depolarization and caused a 9.9 ± 3.0 mV repolarization from the membrane potential measured in the presence of apamin (Fig. 6B; P = 0.0015). Following washout of Ach, apamin again depolarized the membrane potential by 9.6 ± 2.7 mV (P = 0.01). It is interesting that following apamin block Ach did not significantly hyperpolarize membrane potential below the control resting membrane potential. This suggests that Ach induced SK3 cycling that maintained the surface SK3 channel density.
Membrane potential recorded from cells incubated in MβCD to disrupt caveolae (1 mM, 40 min, 22°C) were more depolarized compared with control (Fig. 6C; −26 ± 3 mV; n = 5; P = 0.023). Under these conditions, apamin-induced membrane depolarization was significantly reduced (3.8 ± 3 mV; n = 5), and Ach did not repolarize the membrane potential. In addition, subsequent apamin application did not cause further membrane depolarization. Taken together, these results show that under basal conditions, [Ca2+]i is sufficient to activate a standing SK3 current but not caveolar trafficking. Ach signaling increases [Ca2+]i sufficient to mobilize caveolar trafficking and modulate SK3 channel surface expression and activity.
Ca2+ dependence of membrane internalization.
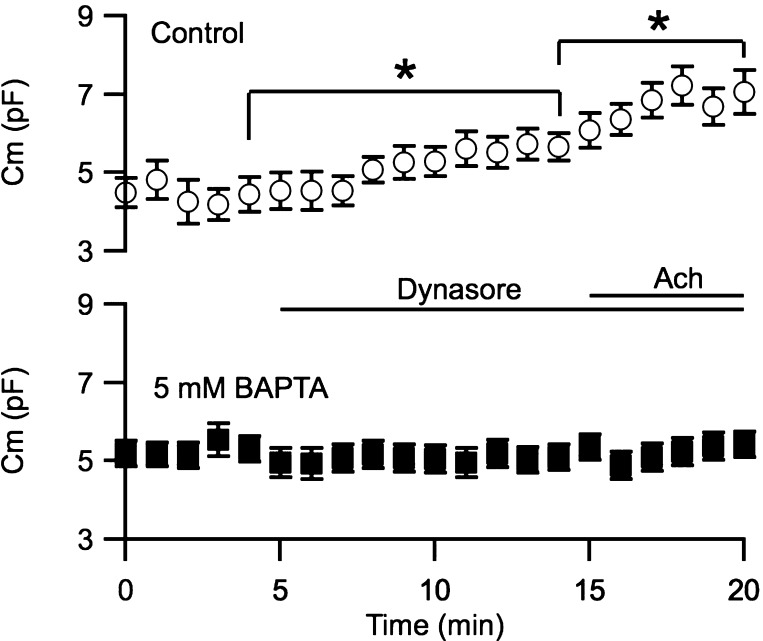
To test the hypothesis that caveolar trafficking is Ca2+-dependent, membrane capacitance was recorded for 20 min in whole cell voltage-clamp configuration. Cells were held at the resting membrane potential, and a −30-mV hyperpolarizing pulse (50 ms) was delivered every 15 s for cell membrane capacitance calculations. Control cells were not dialyzed with the Ca2+ chelator BAPTA (Fig. 7; top), while test cells were dialyzed with 5 mM BAPTA via the patch pipette (Fig. 7, bottom). Similar to the results shown in Fig. 3, in control cells application of 50 μM dynasore to block endocytosis caused a significant increase in membrane capacitance (29.7 ± 6.2%; n = 5; P = 0.02). Subsequent application of 1 μM Ach further increased the capacitance by 21.1 ± 3.7% (P = 0.048). However, membrane accumulation induced by blocking dyamin (membrane accumulates when dynamin is blocked so accumulation is not dynamin dependent) was abolished when the cells were dialyzed with 5 mM BAPTA to chelate [Ca2+]i (Fig. 7, bottom); neither application of dynasore (−2.5 ± 5.6%; n = 8) nor Ach (2.3 ± 1.9%) increased membrane capacitance. Together, these results demonstrate that endothelial caveolar trafficking is dependent on [Ca2+]i.
Fig. 7.
Dynamin-mediated membrane internalization is Ca2+ dependent. Cell membrane capacitance plotted against time of recording from cells not dialyzed (control, top) or dialyzed with 5 mM BAPTA (bottom). Time 0 indicates whole cell formation; 50 μM dynasore were bath-treated at 5 min, followed by 1 μM Ach at 15 min. *Statistical significance (P < 0.05, paired t-test) compared with recordings before application of dynasore or Ach.
DISCUSSION
Pharmacological studies and studies from transgenic animal models have shown that vascular endothelial IK1 and SK3 channels play important roles in modulation of [Ca2+]i and endothelium-dependent vasoregulation of vascular tone (15). While IK1 and SK3 channels, both members of the Kcnn gene family that share the same Ca2+-gating mechanism (13), are structurally and functionally similar, their different subcellular localization and Ca2+ sources in ECs may be important for their distinct contributions to vascular function. To further distinguish between these two Ca2+-activated K+ channels, we show that in acutely isolated aortic ECs SK3 channels undergo Ca2+- and caveolae-dependent trafficking, while IK1 channels do not cycle.
While roles of SK channels in aorta are not well established, previous immunohistochemical studies showed that in resistance vessels IK1 resides in the long protrusions of ECs that penetrate the internal elastic lamina into the smooth muscle cell layers. These endothelial projections form direct electrical coupling with smooth muscle cells via myoendothelial gap junctions. Thus IK1 channel activity may directly influence smooth muscle membrane potential (17). On the other hand, SK3 channels not only coimmunoprecipitate with caveolin-1 but are also localized on the apical side of ECs that is farther away from the smooth muscle compared with IK1 (1). Their activity may directly (via electrical coupling) or indirectly hyperpolarize smooth myocytes via second messengers such as NO and epoxyeicosatrienoic acids. The findings reported here present a further distinction; SK3, but not IK1, channels undergo cycling upon Ca2+-dependent caveloar trafficking. Disrupting caveolae abolishes SK3 channel trafficking and affects its contribution to EC membrane potential.
Caveolae are specialized vesicles that serve a hub for the assembly of signaling complexes, such as Ach receptors, mechanosensitive receptors, transient receptor potential channels, and nitric oxide synthase that are important for endothelial function (8). Caveolae are mobile and are capable of redistributing these signaling complexes, thereby regulating their downstream consequences. By using dynasore and GDPβS, we have demonstrated that the endocytosis of caveolae and SK3 channels is dependent on dynamin's GTPase activity. Furthermore, caveolar, but not clathrin-coated, structures are disrupted by MβCD, which chelates membrane cholesterol molecules that anchor caveolin-1 proteins (21). Caveolin-1 is the main structural component of caveolae and is required for caveolar biogenesis, structure, and function (24). In this regard, SK3 coimmunoprecipitates with caveolin-1 (1) and we now show that disrupting caveolar structure abolishes SK3 channel trafficking (Fig. 5). Surprisingly, disrupting caveolae results in a significant reduction of the SK3 current density, whereas IK1 current density remains unchanged. Together they suggest that MβCD either selectively removes specialized plasma membrane portion containing SK3 channels or causes SK3 channel redistribution (25).
Our findings on SK3 channel trafficking are consistent with previous studies that showed trafficking of SK3 channels in cultured EC lines derived from human microvessels and heterologous expression systems. Gao et al. (10) showed that SK3 channels undergo membrane recycling and intracellular vesicles containing SK3 channels also express the recycling endosomal markers Rab35 and RME-1. On the other hand, IK1 channels undergo only slow internalization and degradation; intracellular pools of IK1 channels colocalize with lysosomes containing Rab7 and ESCRT (2, 3). These biochemical studies overlooked SK channel trafficking on the time scale of 2–12 h. Under our electrophysiological recording conditions on the time scale of <30 min, IK1 channels do not traffic. This discrepancy might be attributable to differences in the time scale of experiments performed or ECs that are 1) acutely isolated or cultured, and/or 2) from aortas or microvessels.
The exact mechanism underlying caveolar trafficking is still not fully understood and remains controversial (11). The present results show that, at least in the case of SK3 channels, endocytosis depends on GTPase activity while exocytosis is NEM sensitive. However, both G proteins and NEM may affect numerous signaling pathways. Consequently, our electrophysiological assays on channel activity and current recovery from blockers may be affected. Identifying the molecular components underlying caveolar and/or SK channel cycling is important to revealing their functions.
The different effects of blocking vesicular cycling on IK1 and SK3 current density highlight the distinct subcellular distributions of IK1 and SK3 channels. Blocking exocytosis with NEM decreased the total membrane surface area as shown by the decreased membrane capacitance. However, while membrane was lost, IK1 current density increased. Since the total IK1 current did not change, this shows that the membrane undergoing endocytosis selectively lacks IK1 channels. Conversely, when exocytosis was blocked, membrane capacitance and SK3 current density both decreased. These results are consistent with the changes in membrane surface area being mediated by caveolar trafficking that selectively include SK3 and exclude IK1 channels. Similar conclusions apply to the results obtained when endocytosis was blocked; IK1 current density decreased, reflecting the constant number of channels present in an expanding membrane, while SK3 current density increased, consistent with additional SK3 channels being inserted as the membrane expands. Taken together, the result show that the relative contributions of IK1 and SK3 channels are subject to different modulation via membrane trafficking and subcellular localization.
Conflicting results from previous studies (5, 26) reported both Ca2+ dependence and independence of caveolar-mediated trafficking of transient receptor potential channels. Nevertheless, it was demonstrated that endothelial caveolar trafficking can be induced through (e.g., Ach) receptor activation (22). Our results show that dialyzing ECs with 3 μM free [Ca2+]i not only activated both IK1 and SK3 channels, it also induced SK3 channel trafficking, evidenced by the current recovery from apamin washout (Figs. 2–4). On the other hand, with the use of perforated patch recording of membrane potential, apamin-induced membrane depolarization is irreversible following apamin washout under conditions that do not increase [Ca2+]i (Fig. 6A). However, a brief application of Ach, which increases [Ca2+]i, activated caveolar trafficking and reversed apamin-induced membrane depolarization (Fig. 6B). The differences between perforated and whole cell patch recordings suggest a differential role for Ca2+ regulation between caveolae trafficking and SK3 and IK1 channel activation. This may reflect the difference in Ca2+ sensitivity or Ca2+ source: the basal [Ca2+]i, sufficient to endow basal SK channel activity, is not sufficient to activate caveolae trafficking. Our BAPTA experiments further confirm that Ach-induced caveolar-mediated membrane cycling is Ca2+ dependent, because cells dialyzed with 5 mM BAPTA did not show Ach-induced membrane cycling.
Furthermore, others have suggested that NO plays a more prominent role in large conduit arteries, such as aortas. Since endothelial nitric oxide synthase activation requires Ca2+, the activation of SK channels that hyperpolarizes endothelial membrane potential may provide a feedforward loop to further increase [Ca2+]i via capacitative Ca2+ entry. The regulated trafficking of SK3 channels may additionally modulate cytosolic Ca2+ levels by acting as either feedforward (increase in surface SK3 channels) or feedback (decrease in surface SK3 channels) loop with NO production.
In conclusion, murine aortic ECs express two members of the SK channel family, IK1 and SK3. Selective subcellular localization and differential association with caveolae engender distinct functions that likely influence NO and EDHF pathways and vessel tone. In light of this, SK3 channel activity is modulated not only by changes in [Ca2+]i to directly activate the channels but also by dynamic surface expression via Ca2+-dependent caveolar trafficking. On the other hand, IK1 channel expression remains constant and the relative contribution of IK1 channels is only secondarily affected by the change in membrane surface area and thus current density. Depending on metabolic conditions, SK3 channel trafficking may modulate the number of SK3 channels on the endothelial plasma membrane and affect endothelium-mediated responses. Their trafficking may also act as autonomic regulator for regional blood flow and pressure. Thus fine-tuning SK3 channel activity, trafficking, and surface expression may endow ECs the capability to fine-tune vascular tone in different vascular beds.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant K99-HL-102056 (to M. T. Lin).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.T.L. and J.P.A. conception and design of research; M.T.L. performed experiments; M.T.L. and J.M. analyzed data; M.T.L., J.P.A., and J.M. interpreted results of experiments; M.T.L. prepared figures; M.T.L. drafted manuscript; M.T.L., J.P.A., and J.M. edited and revised manuscript; M.T.L., J.P.A., and J.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Hui-ya Hsieh for help on animal surgery and experimental preparation.
REFERENCES
- 1. Absi M, Burnham MP, Weston AH, Harno E, Rogers M, Edwards G. Effects of methyl beta-cyclodextrin on EDHF responses in pig and rat arteries; association between SK(Ca) channels and caveolin-rich domains. Br J Pharmacol 151: 332–340, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balut CM, Gao Y, Murray SA, Thibodeau PH, Devor DC. ESCRT-dependent targeting of plasma membrane localized KCa3.1 to the lysosomes. Am J Physiol Cell Physiol 299: C1015–C1027, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balut CM, Loch CM, Devor DC. Role of ubiquitylation and USP8-dependent deubiquitylation in the endocytosis and lysosomal targeting of plasma membrane KCa3.1. FASEB J 25: 3938–3948, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bolton TB, Lang RJ, Takewaki T. Mechanisms of action of noradrenaline and carbachol on smooth muscle of guinea-pig anterior mesenteric artery. J Physiol 351: 549–572, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cayouette S, Boulay G. Intracellular trafficking of TRP channels. Cell Calcium 42: 225–232, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res 102: 1247–1255, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dora KA, Sandow SL, Gallagher NT, Takano H, Rummery NM, Hill CE, Garland CJ. Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J Vasc Res 40: 480–490, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, caveolae, and endothelial cell function. Arterioscler Thromb Vasc Biol 23: 1161–1168, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- 10. Gao Y, Balut CM, Bailey MA, Patino-Lopez G, Shaw S, Devor DC. Recycling of the Ca2+-activated K+ channel, KCa2.3, is dependent upon RME-1, Rab35/EPI64C, and an N-terminal domain. J Biol Chem 285: 17938–17953, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hansen CG, Nichols BJ. Exploring the caves: cavins, caveolins and caveolae. Trends Cell Biol 20: 177–186, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res 100: 246–254, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci USA 94: 11651–11656, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 273: 1709–1714, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Köhler R, Ruth P. Endothelial dysfunction and blood pressure alterations in K+-channel transgenic mice. Pflügers Arch 459: 969–976, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Ledoux J, Bonev AD, Nelson MT. Ca2+-activated K+ channels in murine endothelial cells: block by intracellular calcium and magnesium. J Gen Physiol 131: 125–135, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA 105: 9627–9632, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin MT, Hessinger DA, Pearce WJ, Longo LD. Modulation of BK channel calcium affinity by differential phosphorylation in developing ovine basilar artery myocytes. Am J Physiol Heart Circ Physiol 291: H732–H740, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Lin MT, Luján R, Watanabe M, Frerking M, Maylie J, Adelman JP. Coupled activity-dependent trafficking of synaptic SK2 channels and AMPA receptors. J Neurosci 30: 11726–11734, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maylie J, Bond CT, Herson PS, Lee WS, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin. J Physiol 554: 255–261, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol 8: 185–194, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Predescu SA, Predescu DN, Malik AB. Molecular determinants of endothelial transcytosis and their role in endothelial permeability. Am J Physiol Lung Cell Mol Physiol 293: L823–L842, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res 86: 341–346, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Scherer PE, Lewis RY, Volonte D, Engelman JA, Galbiati F, Couet J, Kohtz DS, van Donselaar E, Peters P, Lisanti MP. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem 272: 29337–29346, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Shmygol A, Noble K, Wray S. Depletion of membrane cholesterol eliminates the Ca2+-activated component of outward potassium current and decreases membrane capacitance in rat uterine myocytes. J Physiol 581: 445–456, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh BB, Lockwich TP, Bandyopadhyay BC, Liu X, Bollimuntha S, Brazer SC, Combs C, Das S, Leenders AGM, Sheng ZH, Knepper MA, Ambudkar SV, Ambudkar IS. VAMP2-dependent exocytosis regulates plasma membrane insertion of TRPC3 channels and contributes to agonist-stimulated Ca2+ influx. Mol Cell 15: 635–646, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Stocker M. Ca2+-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci 5: 758–770, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Strobaek D, Jorgensen TD, Christophersen P, Ahring PK, Olesen SP. Pharmacological characterization of small-conductance Ca2+-activated K+ channels stably expressed in HEK 293 cells. Br J Pharmacol 129: 991–999, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun RJ, Muller S, Stoltz JF, Wang X. Shear stress induces caveolin-1 translocation in cultured endothelial cells. Eur Biophys J 30: 605–611, 2002 [DOI] [PubMed] [Google Scholar]
- 30. Taylor MS, Bonev AD, Gross TP, Eckman DM, Brayden JE, Bond CT, Adelman JP, Nelson MT. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ Res 93: 124–131, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Wang LH, Sudhof TC, Anderson RG. The appendage domain of alpha-adaptin is a high affinity binding site for dynamin. J Biol Chem 270: 10079–10083, 1995 [DOI] [PubMed] [Google Scholar]
- 32. Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci USA 97: 8151–8156, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu T, Ashery U, Burgoyne RD, Neher E. Early requirement for alpha-SNAP and NSF in the secretory cascade in chromaffin cells. EMBO J 18: 3293–3304, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]