Abstract
Ca2+ signals are commonly measured using fluorescent Ca2+ indicators and microscopy techniques, but manual analysis of Ca2+ measurements is time consuming and subject to bias. Automated region of interest (ROI) detection algorithms have been employed for identification of Ca2+ signals in one-dimensional line scan images, but currently there is no process to integrate acquisition and analysis of ROIs within two-dimensional time lapse image sequences. Therefore we devised a novel algorithm for rapid ROI identification and measurement based on the analysis of best-fit ellipses assigned to signals within noise-filtered image sequences. This algorithm was implemented as a plugin for ImageJ software (National Institutes of Health, Bethesda, MD). We evaluated the ability of our algorithm to detect synthetic Gaussian signal pulses embedded in background noise. The algorithm placed ROIs very near to the center of a range of signal pulses, resulting in mean signal amplitude measurements of 99.06 ± 4.11% of true amplitude values. As a practical application, we evaluated both agonist-induced Ca2+ responses in cultured endothelial cell monolayers, and subtle basal endothelial Ca2+ dynamics in opened artery preparations. Our algorithm enabled comprehensive measurement of individual and localized cellular responses within cultured cell monolayers. It also accurately identified characteristic Ca2+ transients, or Ca2+ pulsars, within the endothelium of intact mouse mesenteric arteries and revealed the distribution of this basal Ca2+ signal modality to be non-Gaussian with respect to amplitude, duration, and spatial spread. We propose that large-scale statistical evaluations made possible by our algorithm will lead to a more efficient and complete characterization of physiologic Ca2+-dependent signaling.
Keywords: signaling, ImageJ, detection, microscopy, algorithm
regulation of intracellular Ca2+ is both spatially and temporally complex and includes signals that form transients, oscillations, and cell-wide waves (3, 4, 6, 7). This array of Ca2+ signal modalities is thought to underlie regulation of physiological processes through effector specificity (3, 4, 6, 7). Recently, localized basal Ca2+ transients, or Ca2+ pulsars, were identified in mouse mesenteric artery endothelial cells (17). These signals were implicated in the regulation of resting arterial tone and may form the basis for a wide range of signal modification patterns including frequency modulation (13, 17). These findings underscore the physiological importance of the spatial and temporal diversity of Ca2+ signaling and reveal the need for statistical analysis tools that encompass this diversity.1
Relative cellular Ca2+ levels are commonly measured as time lapse image sequences using fluorescent Ca2+ indicators, such as Fluo-4 (2, 10, 12, 15–16, 21–22, 26–27, 29–31). These image sequences are often analyzed with user-defined regions of interest (ROIs) at sites determined to have fluctuations in fluorescence intensity, and the mean intensity within each ROI is measured as a function of time (2, 8–11, 15, 29–31). Current ROI-based Ca2+ measurements are both time consuming and labor intensive because they require the user to select many ROIs individually and perform repetitive computations (18–20, 25). Manual ROI placement may also be prone to considerable user error, including the introduction of artificial signal modes and the loss of signal modes due to the exclusion of low amplitude or diffuse signals (20, 32).
Automated ROI detection and measurement algorithms have been implemented using a variety of statistical approaches, but they have generally been limited to analysis of line scan images, restricting data evaluation to fluorescence intensity changes along a single spatial dimension in time (5, 16, 18, 23, 25, 32). In addition, existing algorithms tend to focus on characterization of single, well-defined Ca2+ release events instead of the comprehensive evaluation of diverse periodic, localized, or wave-like events known to occur within many cells, which likely represent the true range of physiologic signaling (24, 32). No available analytical method currently encompasses this diversity. Furthermore, comprehensive evaluation is complicated by the presence of significant image artifact that confounds signal-to-noise discrimination in many experimental systems. There is a clear need for an analysis tool that quickly, accurately, and automatically defines ROIs and measures signal parameters relating to amplitude, time course, and spatial spread (32). A discriminating automated ROI detection solution should provide the crucial reduction in time and user bias required for large-scale analysis of intracellular Ca2+ activity in cell culture and tissue preparations commonly studied in biomedical research. Here we propose a novel ROI measurement algorithm, implemented as a plugin within ImageJ software (National Institutes of Health, Bethesda, MD) (1), designed to identify and analyze ROIs encompassing statistically significant fluorescent signal dynamics. We tested the detection ability of the algorithm with computer-generated data by comparing algorithm output to known parameters. We also applied the algorithm to cell culture and vascular preparations to demonstrate its ability to analyze multiple, disparate fluorescence-based Ca2+ events in live cell and tissue experiments.
MATERIALS AND METHODS
Generation of synthetic data set.
We used MATLAB to generate 512 × 512 pixel gray scale image sequences, each containing a signal embedded in simulated image noise. The signals were encoded as Gaussian pulses, varying in both amplitude (0.062–10 F/F0) and duration (0.062–5 s). Image noise was approximated with random Gaussian values (mean 1 F/F0). To define the ideal amplitude and duration values, a circular ROI with a diameter of 15 pixels was centered on the signal pulse, and the mean intensity within the ROI was computed for each time point. The algorithm was then employed to detect the same embedded signal and measure the maximum value of mean fluorescence intensity within a 15 pixel diameter ROI for comparison to ideal values.
Ca2+ imaging in cultured cell monolayers.
For acquisition and automated analysis of Ca2+-dependent fluorescence in cultured cells, a confluent monolayer of rat pulmonary microvascular endothelial cells (RPMVECs) was obtained from the University of South Alabama Center for Lung Biology Cell Culture Core. Cells were loaded with a physiological saline solution (in mM: 134 NaCl, 6 KCl, 1 MgCl, 10 HEPES, 10 glucose) containing Fluo-4 fluorescent Ca2+ indicator dye (10 μM) and Pluronic (0.03%) for 35 min at 25°C. After a 5-min wash and 20-min equilibration period, cells were mounted in a custom chamber and viewed on a Perkin Elmer RS-3 spinning disk inverted confocal microscope. Excitation and emission wavelengths were 488 nm and 510 nm, respectively. Ca2+ elevation was stimulated by application of 10 μM 4αPDD, a phorbol ester agonist of the transient vanilloid receptor potential ion channel TRPV4, at 25°C. Fluorescence intensity recordings were captured at ×20 magnification, and data were acquired with Perkin Elmer Ultraview software at 1 frame per second. Evoked Ca2+ events were analyzed offline with our automated ROI algorithm.
Ca2+ imaging in artery segments.
For automated detection of basal endothelial Ca2+ transients in intact artery segments, image sequences of Ca2+ recordings acquired from mesenteric resistance arteries of GCaMP2-expressing mice were analyzed. The circularly permutated Ca2+ sensor, GCaMP2, is under the control of the vascular endothelium connexin40 promoter and is derived by bacterial artificial chromosome transgenesis (28). Briefly, mice were euthanized by intraperitoneal injection of pentobarbital sodium (150 mg/kg) followed by a thoracotomy. Animal procedures in this study were conducted in accordance with institutional guidelines and were approved by the Institutional Animal Care guidelines for the Institut de Cardiologie de Montréal, Canada. Third-order mesenteric arteries were dissected from surrounding connective tissue, cut longitudinally, and mounted in a chamber containing a physiological saline solution (in mM: 119 NaCl, 4.7 KCl, 23 NaHCO3, 1.2 KH2PO4, 1.2 MgCl2, 11 glucose, and 1.5 CaCl2) at 37°C (17). Imaging was performed with a Revolution Andor (Andor Technology) spinning disk confocal microscope with an Andor iXon+ (Andor Technology) electron-multiplying CCD camera on an upright Nikon microscope with a ×60 water dipping objective (numerical aperture 1.0). Images were recorded at 15 frames per second with Andor Revolution iQ acquisition software (Andor Technology). Bound Ca2+ was detected by exciting at 488 nm with a solid-state laser and collecting emitted fluorescence above 510 nm. For acetylcholine-stimulated experiments, 10 μM acetylcholine was added to the perfusate solution after 45 s of recording.
RESULTS
Implementation of custom algorithm for automated ROI identification.
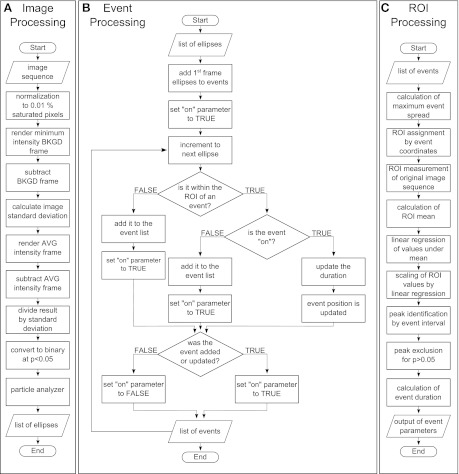
Our approach for automated ROI analysis, implemented as a plugin for ImageJ software, consisted of two procedures: 1) statistical noise filtering and 2) analysis of best-fit ellipses assigned to areas of elevated signal intensities. These processes were accomplished by three sequential subroutines implemented within the algorithm: image processing, event processing, and ROI processing (Fig. 1). The image processing subroutine (Fig. 1A) converted image sequences into a list of ellipses representing intensity values above a calculated noise threshold. Noise filtering was performed using the following process: After normalization to 0.01% saturated pixels, a background frame was rendered using the minimum intensity projection of the sequence. The background frame was then subtracted from each frame of the original image sequence. The difference between the background-subtracted image sequence and its time lapse mean intensity was computed. The image sequence standard deviation was calculated and used to generate a normalized sequence by the standard score (Eq. 1).
| (1) |
For each ROI, the time-dependent z score, z(t), was computed by subtracting the time-dependent mean intensity value, yavg, from the intensity value at each time point, y(t), and dividing by the time-dependent standard deviation (δ). A binary image sequence was obtained by thresholding the resulting image sequence using P < 0.05 for a normal distribution. We used the ImageJ Particle Analyzer Java class to assign best-fit ellipses to the loci of each image sequence frame (1).
Fig. 1.
Signal flow charts of algorithm processes. The algorithm was organized into three sections: image processing, event processing, and region of interest (ROI) processing. Image sequences are input into the flow chain, and event statistics are generated as final output. The image processing (A) subroutine of the algorithm converts the input image sequence into a list of best-fit ellipses by thresholding using the standard score and ImageJ particle analysis. Event processing (B) is a sorting subroutine used to determine optimum ROI position by organizing ellipse locations into event “sites” by time. After mean intensity measurements are taken at each ROI, statistical parameters for each event and site are then calculated by the ROI processing subroutine (C) to generate the final output. BKGD, background; AVG, average.
Optimum ROI positions were computed by the event processing subroutine (Fig. 1B), which was designed to sort the list of filtered ellipses into temporal events and to assign ROIs at the position of each event. The event processing subroutine used the Boolean “on” parameter to determine whether multiple events occurred at single ROI sites. The minimum criteria for event definition were determined both spatially and temporally. To achieve minimum event detection, ellipses in each event must have an area equal to a circle of 2 pixel radius (12.56 pixels2), and appear in at least two consecutive frames. These minimal requirements considerably reduce the probability of a false positive event detection to < 5.96 × 10-32.
After automated ROI detection, the ROI processing subroutine (Fig. 1C) measured the mean intensity over the time course of each ROI from the original image sequence using a modified version of the multimeasure plugin for ImageJ (1). F0 values for each ROI were computed by a linear regression of weighted intensity values. Intensity values greater than the sequence mean were weighted as the mean value. Thus, the weighted intensity F0 values at all points are less than or equal to the mean intensity value of the ROI, and the F0 values are determined without inclusion of superimposed Ca2+ signal transients. For image sequences including drug treatment, F0 values were computed from pretreatment intervals. Measurements were then scaled to fold-change (F/F0) and converted from frames to seconds by an acquisitioned frame rate. Peak event amplitudes were identified by computing global maxima between event intervals identified by a best-fit ellipse-sorting algorithm. Maxima were checked for significance (P < 0.05) using the population mean and standard deviation and were excluded if not significant. Event durations were computed as half-maximum peak amplitude intervals. Right-side Riemann sums were used to calculate signal area under the curve for each ROI. The spatial spread was defined as the maximum best-fit ellipse area above 95% confidence of signal threshold during the event interval.
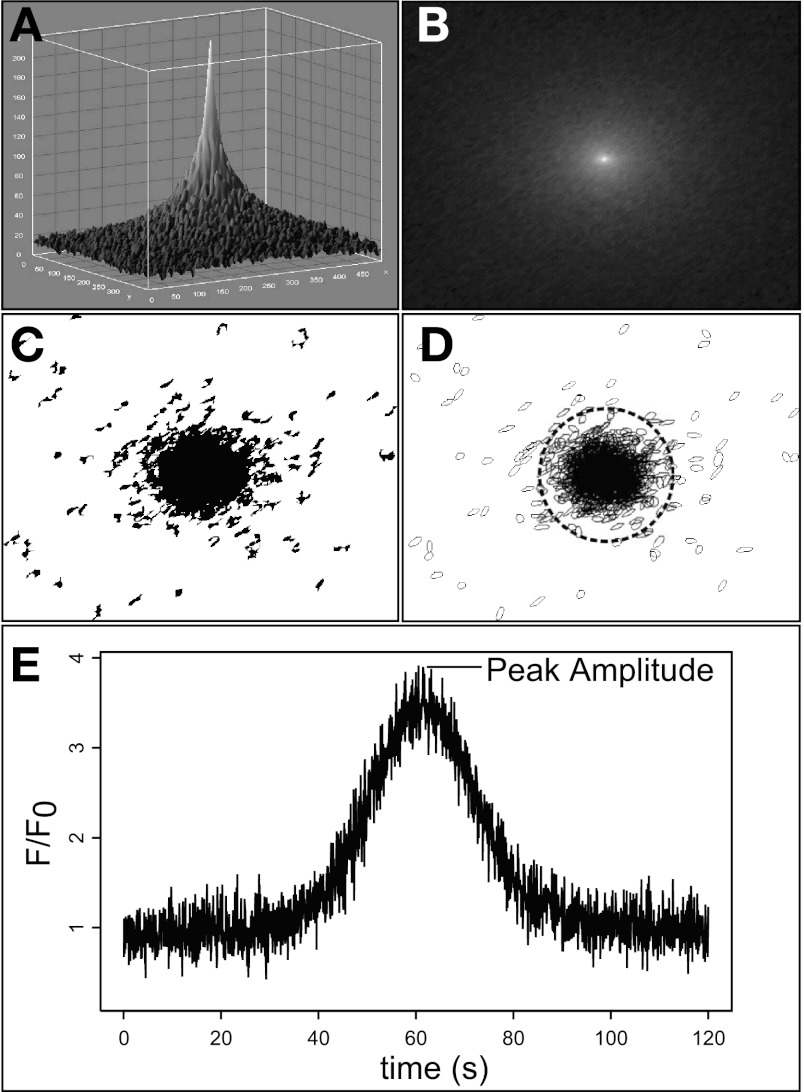
Best-fit ellipse loci in at least two consecutive frames were organized into temporal “events” by grouping centroid positions of each frame within a circular area of fixed radius. Nonoverlapping, fixed diameter ROIs were then centered at the mean event position of each loci center. Figure 2 shows automated detection of a single computer-generated Gaussian signal pulse by noise thresholding and best-fit ellipse assignment, as described above. Time-dependent mean intensity within the algorithm-placed ROI is shown in Fig. 2E.
Fig. 2.
Demonstration of automated ROI acquisition and signal detection using a computer-generated Gaussian pulse. A single signal pulse (A) was generated in MATLAB and embedded in random background noise. The gray scale image sequence was filtered to remove static background pixel values (B) and converted to binary using threshold pixel intensity values of P < 0.05 calculated by the standard score (C). ImageJ particle analysis algorithms were then applied to the image sequence to assign best-fit ellipses to pixel loci within each frame. A novel algorithm was used to group ellipses into discrete temporal “events” and determine the optimal position for each ROI based on the mean ellipse center (D). An ROI of user-defined radius is then placed at each position (dotted circle). Mean intensity value within an ROI is calculated for each frame and scaled using linear baseline approximation. Peak amplitude is identified as local maxima above P < 0.05 as defined by the standard score for a corresponding ROI tracing (E).
Evaluation of automated ROI placement in a synthetic data set.
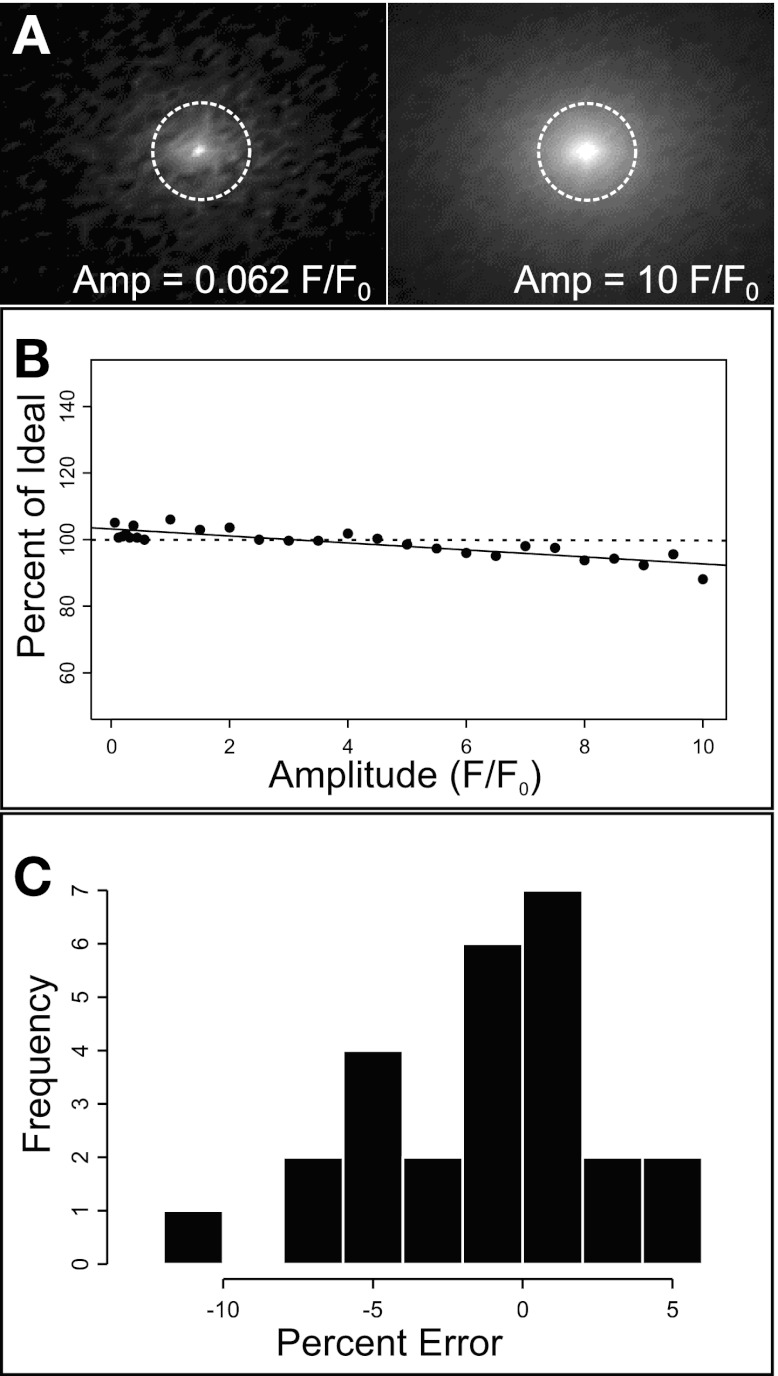
We evaluated the ability of the algorithm to consistently detect fluorescent dynamics embedded in background image noise using a variable computer-generated data set. Image sequences generated with MATLAB contained random noise of mean amplitude and standard deviation of 1 ± 1 F/F0. Each image sequence was embedded with single Gaussian signal pulses varying in both amplitude (0.062–10 F/F0) and duration (0.062–5 s) (Fig. 3A).
Fig. 3.
Evaluation of the algorithm with a synthetic data set. Automated ROI analysis was performed on a data set of sequences containing single Gaussian signal pulses (0.062–10 F/F0) embedded in random background noise (A). The dotted circles represent ROI positions where ideal intensity values were measured. To evaluate automated ROI detection over a range of signal to noise ratios, peak amplitude measurements expressed as a percentage of ideal amplitude were calculated for each image sequence (n = 20) and plotted as a scatter distribution (B). The solid line shows a linear regression for the data set (slope = −1.05, y-intercept = 103.2). The horizontal dotted line shows ideal values (100%). The distribution of percent error is shown in a histogram of error occurrence vs. percent error (C). For the data set, mean percent error was found not to be significantly different from 0 by one-sample Student's t-test. Amp, amplitude.
To measure detection performance, ideal scenarios were computed by measuring the time-dependent mean gray value within a 15 pixel diameter ROI located at the known center of the signal pulse. The image sequences were then analyzed using our automated procedure, and mean peak amplitude measurements were compared with the ideal measurements (100%). Mean amplitude detection measurements were computed as the percentage of ideal or percent error (Fig. 3, B and C, respectively). For the computer-generated data set, average detected peak amplitudes were 99.06 ± 4.11% of the known value. Mean amplitude values did not vary significantly from expected values by a one-sample Student's t-test. Notably, the algorithm slightly overestimated the lowest signal-to-noise peak amplitude value (0.062 F/F0, maximum error 11.9%), and it underestimated the highest amplitude signal-to-noise peak amplitude value (10 F/F0, maximum error 6.23%) within the 160-fold signal amplitude range tested.
Evaluation of stimulated Ca2+ events in cultured endothelial cells.
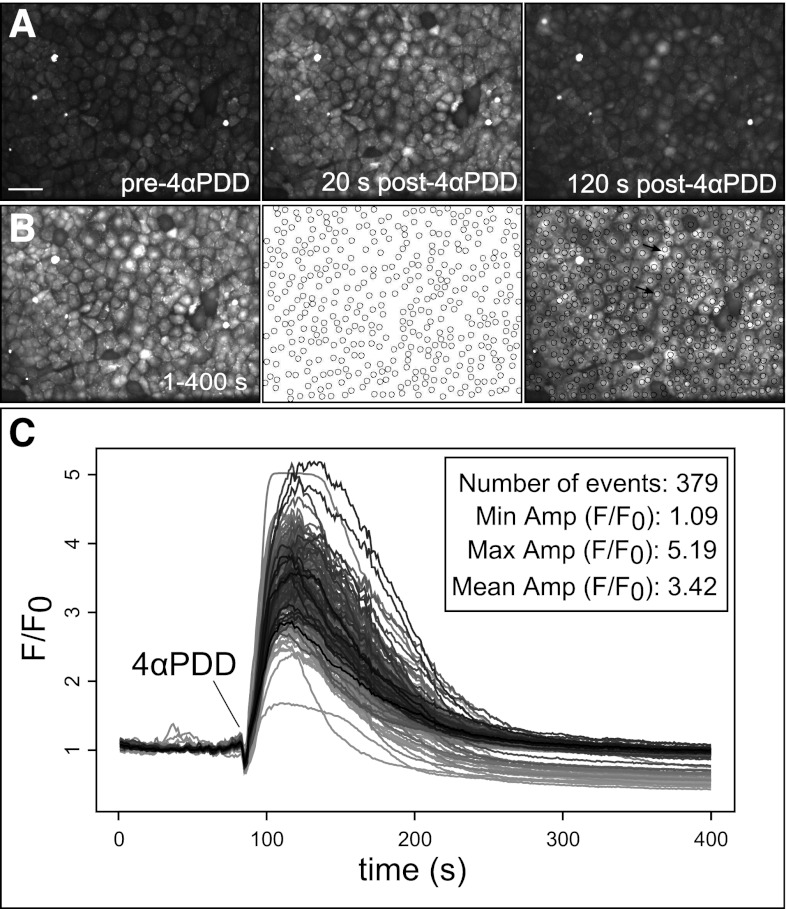
We applied the automated ROI detection algorithm to analyze stimulated cellular Ca2+ responses in cell monolayers, a common experimental system where analysis typically consists of whole field fluorescence intensity measurements or manually placed ROIs. Ca2+ events in a 224 × 171 μm field of cultured RPMVECs loaded with the Ca2+ indicator Fluo-4 were evoked by application of 10 μM 4αPDD, an agonist of the Ca2+-permeable TRPV4 channel, and recorded by confocal microscopy. A single experiment is shown in Fig. 4. Treatment with 4αPDD elicited a range of intracellular Ca2+ responses from most, but not all, cells. Overall, 379 distinct cellular Ca2+ signal sites were detected and tracked with the algorithm. Ca2+ signal parameters were normally distributed with a mean peak amplitude of 3.42 ± 0.02 F/F0 and mean duration of 120.6 ± 0.68 s.
Fig. 4.
Automated analysis of 4αPDD-evoked Ca2+ events in rat pulmonary microvascular endothelial cell (RPMVEC) monolayers. Automated ROI analysis was applied to confocal image sequences of cultured RPMVEC monolayers loaded with Fluo-4 Ca2+ indicator dye and stimulated with the phorbol ester 4αPDD. A: the addition of 4αPDD stimulated a widespread rise of intracellular Ca2+ levels at multiple cellular sites, shown at selected prestimulated, maximally responding (20 s), and poststimulated (120 s) time points. Scale bar represents 10 μm. A time lapse accumulate image (B) shows cell sites that responded to treatment within the time course (left), corresponding to circular ROIs that were automatically assigned to active sites by our algorithm (middle), and an overlay of the accumulate image and detected ROIs (right). Arrows in the overlay (right) indicate cells with single (bottom) and multiple ROIs (top). Measurements of average intensity within each ROI are shown for the time course of the image sequence (C) with the event number and mean amplitude value and range summarized in the inset.
Measurement of dynamic Ca2+ signals in endothelial cells of intact arteries.
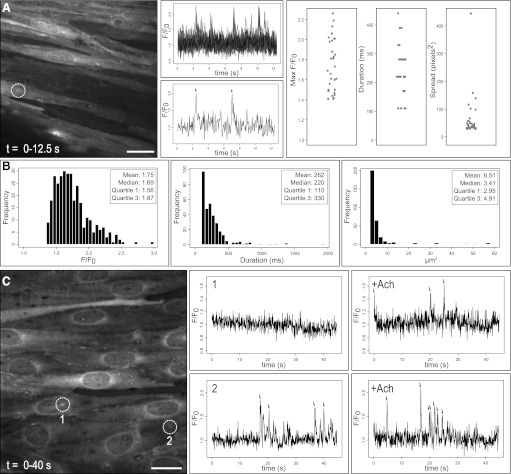
We assessed our detection procedure in an exteriorized tissue preparation where analysis is complicated by low signal-to-noise ratios, tissue autofluorescence, z-axis drift, and image artifact. Specifically, we analyzed Ca2+ pulsars, which are characteristic Ca2+ transients in the endothelium of mouse mesenteric arteries (17). We performed fluorescence intensity measurements in mesenteric arteries of the GCaMP2 mouse, which expresses an endogenous Ca2+ sensor, and recorded eight image sequences of basal Ca2+ pulsars. Figure 5A shows the time lapse (12.5 s) of a representative image sequence, along with tracings of Ca2+-dependent fluorescence measurements from multiple, automatically positioned ROIs. Signal parameters were automatically computed for each experiment, as shown by strip charts in Fig. 5A (right). Composite data for all eight experiments are shown in histograms (Fig. 5B). In all, 326 distinct events at 161 sites were detected. Mean signal peak amplitude (1.75 ± 0.15 F/F0), and half-maximum duration (262 ± 11 ms) parameter values were consistent with published values of 1.70 ± 0.02 F/F0 and 269 ± 6 ms, respectively (17). Mean spatial spread at 95% confidence was 6.51 ± 0.54 μm2. As illustrated by composite histograms, each parameter actually distributed in a distinct non-Gaussian fashion, with median values of amplitude, duration, and spread of 1.69 F/F0, 220 ms, and 3.4 μm2, respectively. Interquartile ranges for amplitude, duration, and spread were 1.56–1.87 F/F0, 110–330 ms, and 2.94–4.91 μm2, respectively.
Fig. 5.
Automated analysis of endothelial Ca2+ events (Ca2+ pulsars) in intact arterial preparations. Mesenteric resistance arteries of the GCaMP2 mouse were opened longitudinally and Ca2+-dependent fluorescence in the endothelium was measured. Distinct, low signal-to-noise ratio Ca2+ transients were detected within background noise and image artifact. A: a projected time lapse image of a 12.5-s recording from a single field of endothelial cells is shown, including average fluorescence intensity from all detected ROIs (top) and from a single ROI (bottom; corresponding to white circle in image). Strip charts summarize the parameter distributions of events detected in a single experiment, including peak amplitude, half-maximum duration, and maximum area spread. B: histograms show distributions of these parameters compiled from eight image sequences (total of 326 events from 161 sites). Parameter distributions were distinctly non-Gaussian. Summary statistics including mean, median, and interquartile ranges are shown in the inset. C: our algorithm also detected and tracked events stimulated by 10 μM acetylcholine. A projected time lapse image of a 40-s recording after acetylcholine stimulation is shown. Tracings recorded before and after acetylcholine at two detected sites (indicated by white circles) show recruitment of de novo Ca2+ pulsars at a previously inactive site (1) as well as an increase in pulsar frequency at a site with preexisting activity (2). All detected and measured events are designated by arrows. Scale bars represent 20 μm (A) and 15 μm (B).
Acetylcholine was previously reported to increase pulsar frequency and de novo recruitment of pulsar sites (17). Acetylcholine-stimulated Ca2+ pulsars from a representative experiment are shown in Fig. 5C. The leftmost panel shows a time lapse image sequence of the mesenteric artery endothelium of the GCaMP2 mouse after the addition of 10 μM acetylcholine. Two Ca2+ pulsar sites (white circles) of the 662 detected are highlighted, showing both detection of de novo events (ROI 1) and an increase in pulsar frequency (ROI 2) following stimulation.
DISCUSSION
Here we present a novel algorithm for the automated measurement and analysis of ROIs within image sequences. This algorithm advances the analysis of commonly measured fluorescent signals because its simple implementation allows for rapid and comprehensive characterization of a wide range of signals within broad sampled fields. This process improves on existing automated ROI detection algorithms by extending analysis from line scan images to two-dimensional time lapse image sequences, while minimizing user bias and error. Through the novel use of a convenient descriptor of cell shape, best-fit ellipse analysis allows simplification of the process of optimized ROI acquisition and allows a large number of ROIs to be measured rapidly and simultaneously, while computing signal parameters such as relative peak amplitude, half-maximum duration, and maximum spatial spread.
We determined the ability of the algorithm to accurately identify the spatial position of signal pulses by evaluating ROI placement within a computer-generated data set. ROIs assigned by our algorithm were well centered on signal positions as indicated by nearly identical values of measured and ideal signal amplitude (measured 99.06 ± 4.11% of ideal). Our results indicate that the algorithm detects signal pulses with high sensitivity and spatial accuracy. Interestingly, the algorithm tended to slightly overestimate the peak amplitude of low signal-to-noise ratio pulses and slightly underestimate the peak amplitude of high signal-to-noise ratio pulses. These tendencies could be accounted for by the very low signal-to-noise ratio for the lowest signal amplitudes (0.062 F/F0), and skew introduced into the signal pulse position by the level of background noise at high signal amplitudes. However, image sequence detection performance did not decrease at high noise levels, indicating the ability of the algorithm to detect signals at very low signal-to-noise ratios.
Ca2+ responses to stimulation are routinely studied in cultured cell systems. Investigators commonly analyze these responses using whole field intensity or manually placed ROI measurements. However, these analysis techniques are inadequate for characterizing the multifaceted nature of Ca2+ responses of individual cells, including heterogeneous responses within a field and identification of nonresponsive cells. Therefore, as proof of concept, we applied our automated algorithm to image sequences of cultured RPMVEC monolayers to detect agonist-evoked Ca2+ events. The algorithm effectively detected a large number of simultaneous responses of variable amplitude, duration, and spread at multiple cellular sites. ROIs mapped onto Ca2+ dynamics were centered on single and in some cases multiple intracellular sites without detecting minimal or static signal, or image artifact. This reflects the ability of the algorithm to rapidly and comprehensively analyze discrete responses within multicell systems.
Recent studies have highlighted the importance of ongoing dynamic endothelial Ca2+ signals in the physiologic regulation of vascular tone (14, 17). However, manual extraction of representative signal data from intact tissues is particularly difficult due to the disparate, often highly transient events occurring in the presence of significant image artifact resulting from autofluorescence or focal plane drift (18, 20, 25). We applied our algorithm to the analysis of basal Ca2+ dynamics in image sequences of exteriorized endothelium of mouse mesenteric arteries. In particular, we assessed Ca2+ pulsars occurring within GCaMP2 mouse mesenteric arteries as originally characterized (17). The algorithm was able to automatically detect and analyze Ca2+ pulsars with high fidelity. Indeed, mean parameter values for amplitude and duration obtained by our automated analysis were consistent with values reported by Ledoux et al. (17). Interestingly, the comprehensive histogram charts for signal parameters afforded by our algorithm revealed distinct, non-Gaussian distributions of basal Ca2+ transients. This finding underscores the necessity of large-scale analysis in the characterization of diverse signals, where an adequate description of parameter distributions is essential to allow for useful statistical evaluation of signal modalities. For instance, statistical analysis of mean value measurements from non-Gaussian distributions may lead to erroneous or inaccurate conclusions about the nature of signals within an experimental system, whereas median and interquartile ranges would provide a more meaningful assessment of signal distributions. Furthermore, large-scale automated analysis provides the means with which to statistically evaluate subtle differences among Ca2+ signals, including changes in parameter distribution in response to stimulation. Such evaluation will be essential to reveal physiological modes of spatial and temporal signal expansion.
One potential limitation of ROI analysis is the detection of false positive signals resulting from cell area or position changes, which commonly occur in contractile cells. Whereas movement of the sampled field or focal plane may be corrected by image registration or z-stack compilation, multiple changes in cell size and position cannot be easily corrected. We addressed this issue by utilizing a running average of detected event positions to determine optimum ROI placement within cell sites. As a consequence of this design, our algorithm can detect ROIs within sites subject to limited change in position and area, provided that the total movement artifact is less than the ROI radius. If the movement is greater than the radius of an ROI, an additional ROI will be assigned at a new location. Thus, under conditions of considerable movement artifact, the signal is still tracked but may resolve as two separate ROIs. An added benefit is that this design offers the ability to use the distance between ROIs and the change in ROI peak times to calculate the rate of change in site area or position for the purpose of Ca2+ wave analysis.
With our automated approach, we were able to accurately detect and measure both stimulated Ca2+ events and basal subcellular transients in cultured endothelial cells and in intact artery preparations supporting the utility of the algorithm in a wide variety of analysis applications. Notably, our algorithm also allows for designation of particular user-defined criteria (e.g., ROI diameter), providing versatility in application and empirical optimization of the analysis to specific target parameters. Also, this approach is not limited to evaluation of fluorescent Ca2+ dynamics and may be applied to multiple intensity-based measurements of fluorescent signal dynamics in image sequences. We propose that this approach may serve as a basis for a large range of future applications, including statistical analysis of Ca2+ wave speed and direction, sorting and entrainment analysis of simultaneous heterocellular recordings (e.g., distinguishing endothelial and smooth muscle Ca2+ transients in intact blood vessels), and quantification of localized and wave-like dynamics using variable, noncircular ROIs. We conclude that our novel algorithmic process represents a substantial step forward in the evaluation of biological Ca2+ signals and may serve as a template for expanded, comprehensive future analytical applications.
GRANTS
This work was supported in part by National Institutes of Health Grants HL-085887, HL-092992, S10RR027535, and MOP-93676.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.F. and M.S.T. conception and design of the research; M.F. and X.Q. analyzed the data; M.F. interpreted the results of the experiments; M.F. prepared the figures; M.F. drafted the manuscript; M.F., X.Q., J.L., J.C.P., and M.S.T. edited and revised the manuscript; C.C., J.L., and J.C.P. performed the experiments; M.S.T. approved the final version of the manuscript.
ACKNOWLEDGMENTS
Thanks to Michael McBride and Andrew McAuliffe for help with java programming, and William Gerthoffer, Mary Townsley, and Patricia Villalta for help in editing the manuscript.
Footnotes
This article is the topic of an Editorial Focus by Kay-Pong Yip and James S. K. Sham (33).
REFERENCES
- 1. Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophoton Int 11: 36–42, 2004 [Google Scholar]
- 2. Barreto-Chang OL, Dolmetsch RE. Calcium imaging of cortical neurons using Fura-2 AM. J Vis Exp 23: 1067, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berridge MJ. Inositol trisphosphate and calcium signalling. Nature 361: 315–325, 1993 [DOI] [PubMed] [Google Scholar]
- 4. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Cheng H, Song LS, Shirokova N, González A, Lakatta EG, Ríos E, Stern MD. Amplitude distribution of calcium sparks in confocal images: theory and studies with an automatic detection method. Biophys J 76: 606–617, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delisle S. The four dimensions of calcium signalling in Xenopus oocytes. Cell Calcium 12: 217–227, 1991 [DOI] [PubMed] [Google Scholar]
- 7. Dupont G, Combettes L, Leybaert L. Calcium dynamics: spatio-temporal organization from the subcellular to the organ level. Int Rev Cytol 261: 193–245, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Gaspers LD, Thomas AP. Calcium signaling in liver. Cell Calcium 38: 329–342, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Hashiatni H, Lang RJ, Suzuki H. Role of perinuclear mitochondria in the spatiotemporal dynamics of spontaneous Ca2+ waves in interstitial cells of Cajal-like cells of the rabbit urethra. Br J Pharmacol 161: 680–694, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayashi H, Miyata H. Fluorescence imaging of intracellular Ca2+. J Pharmacol Toxicol Methods 31: 1–10, 1994 [DOI] [PubMed] [Google Scholar]
- 11. Hellman B, Gylfe E, Grapengiesser E, Lund PE, Berts A. Cytoplasmic Ca2+ oscillations in pancreatic beta-cells. Biochim Biophys Acta 1113: 295–305, 1992 [DOI] [PubMed] [Google Scholar]
- 12. Hong JH, Min CH, Jeong B, Kojiya T, Morioka E, Nagai T, Ikeda M, Lee KJ. Intracellular calcium spikes in rat suprachiasmatic nucleus neurons induced by BAPTA-based calcium dyes. PLos One 5: e9634, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res 100: 246–254, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+ events in endothelial cells reflect signaling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium 44: 135–146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Krishnamoorthy G, Regehr K, Berge S, Scherer EQ, Wangemann P. Calcium sparks in the intact gerbil spiral modiolar artery. BMC Physiol 11: 15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuga N, Sasaki T, Takahara Y, Matsuki N, Ikegaya Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J Neurosci 31: 2607–2614, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA 105: 9627–9632, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lorenz JJ, Lorenz MGO, Barker JL. Pixel-based criteria-oriented analysis of time-lapse Ca2+-fluorescence images. J Neurosci Methods 127: 157–166, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Mellen NM, Tuong CM. Semi-automated region of interest generation for the analysis of optically recorded neuronal activity. Neuroimage 47: 1331–1340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mukamel EA, Nimmerjahn A, Schnitzer MJ. Automated analysis of cellular signals from large-scale calcium imaging data. Neuron 63: 747–760, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mumtaz S, Burdyga G, Borisova L, Wray S, Burdyga T. The mechanism of agonist induced Ca2+ signalling in intact endothelial cells studied confocally in in situ arteries. Cell Calcium 49: 66–77, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paredes RM, Etzler JC, Watts LT, Zheng W, Lechleiter JD. Chemical calcium indicators. Methods 46: 143–151, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Picht E, Zima AV, Blatter LA, Bers DM. SparkMaster: automated calcium spark analysis with ImageJ. Am J Physiol Cell Physiol 293: C1073–C1081, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Reidl J, Starke J, Omer DB, Grinvald A, Spors H. Independent component analysis of high-resolution imaging data identifies distinct functional domains. Neuroimage 34: 94–108, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Sebille S, Cantereau A, Vandebrouck C, Balghi H, Constantin B, Raymond G, Cognard C. Calcium sparks in muscle cells: interactive procedures for automatic detection and measurements on line-scan confocal images series. Comput Methods Programs Biomed 77: 57–70, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Silei V, Fabrizi C, Venturini G, Tagliavini F, Salmona M, Bugiani O, Lauro GM. Measurement of intracellular calcium levels by the fluorescent Ca2+ indicator Calcium-Green. Brain Res Brain Res Protoc 5: 132–134, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Simpson AW. Fluorescent measurement of [Ca2+]c: basic practical considerations. Methods Mol Biol 312: 3–36, 2006 [PubMed] [Google Scholar]
- 28. Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40BAC GCaMP2 transgenic mice. Circ Res 12: 1300–1309, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Tatsumi H, Hirai K, Katayama Y. Measurement of the intracellular calcium concentration in guinea-pig myenteric neurons by using fura-2. Brain Res 451: 371–375, 1988 [DOI] [PubMed] [Google Scholar]
- 30. Tsien RY, Rink TJ, Poenie M. Measurement of cytosolic free Ca2+ in individual small cells using fluorescence microscopy with dual excitation wavelengths. Cell Calcium 6: 145–157, 1985 [DOI] [PubMed] [Google Scholar]
- 31. Tumelty J, Hinds K, Bankhead P, McGeown NJ, Scholfield CN, Curtis TM, McGeown JG. Endothelin 1 stimulates Ca2+-sparks and oscillations in retinal arteriolar myocytes via IP3R and RyR-dependent Ca2+ release. Invest Ophthalmol Vis Sci 52: 3874–3879, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Von Wegner F, Both M, Fink RH. Automated detection of elementary calcium release events using the á trous wavelet transform. Biophys J 90: 2151–2163, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yip KP, Sham JS. Tracking stars: automated two-dimensional analysis of Ca2+ events. Focus on “Automated region of interest analysis of dynamic Ca2+ signals in image sequences.” Am J Physiol Cell Physiol (May 30, 2012). doi:10.1152/ajpcell.00178.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]