Abstract
cAMP induces both active Cl− and active K+ secretion in mammalian colon. It is generally assumed that a mechanism for K+ exit is essential to maintain cells in the hyperpolarized state, thus favoring a sustained Cl− secretion. Both Kcnn4c and Kcnma1 channels are located in colon, and this study addressed the questions of whether Kcnn4c and/or Kcnma1 channels mediate cAMP-induced K+ secretion and whether cAMP-induced K+ secretion provides the driving force for Cl− secretion. Forskolin (FSK)-enhanced short-circuit current (indicator of net electrogenic ion transport) and K+ fluxes were measured simultaneously in colonic mucosa under voltage-clamp conditions. Mucosal Na+ orthovanadate (P-type ATPase inhibitor) inhibited active K+ absorption normally present in rat distal colon. In the presence of mucosal Na+ orthovanadate, serosal FSK induced both K+ and Cl− secretion. FSK-induced K+ secretion was 1) not inhibited by either mucosal or serosal 1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole (TRAM-34; a Kcnn4 channel blocker), 2) inhibited (92%) by mucosal iberiotoxin (Kcnma1 channel blocker), and 3) not affected by mucosal cystic fibrosis transmembrane conductance regulator inhibitor (CFTRinh-172). By contrast, FSK-induced Cl− secretion was 1) completely inhibited by serosal TRAM-34, 2) not inhibited by either mucosal or serosal iberiotoxin, and 3) completely inhibited by mucosal CFTRinh-172. These results indicate that cAMP-induced colonic K+ secretion is mediated via Kcnma1 channels located in the apical membrane and most likely contributes to stool K+ losses in secretory diarrhea. On the other hand, cAMP-induced colonic Cl− secretion requires the activity of Kcnn4b channels located in the basolateral membrane and is not dependent on the concurrent activation of apical Kcnma1 channels.
Keywords: fluid secretion; short-circuit current; potassium fluxes; adenosine 3′,5′-cyclic monophosphate
active chlorine secretion is the primary driving force for fluid secretion in infectious diarrheas such as cholera (4, 5). Active Cl− secretion requires both basolateral Cl− uptake and apical Cl− exit mechanisms and a K+ extrusion pathway that maintains cells in a hyperpolarized state as well as delivering K+ ions to the Na-K-2Cl cotransporter (NKCC) and Na,K-ATPase located in the basolateral membrane. NKCC mediates Cl− uptake across the basolateral membrane, whereas cystic fibrosis transmembrane regulator (CFTR) mediates active Cl− secretion across the apical membrane. Intermediate-conductance, Ca2+-activated K+ channels localized to the basolateral membrane have been shown to provide the driving force for agonist (Ca2+, cAMP)-induced Cl− secretion in human and mouse colon (22, 23). Molecular studies have identified intermediate conductance K+ channels in different species, which are variously referred to as intermediate conductance K+ channel, mouse intermediate conductance K+ channel, small conductance K+ channel 4, Ca2+-activated K+ channel 3.1, and Kcnn4 (14, 17, 30). Immunofluorescence studies have localized Kcnn4-like proteins to both the apical and basolateral membranes of human, guinea pig, and rat colon (3, 10, 11). The splice variants Kcnn4c and Kcnn4b have been shown to encode apical and basolateral Kcnn4 channels in rat distal colon (3). Activation of Kcnn4c with 5,6-dichloro-1-ethyl-1,3-dihydro-2H-benzimidazole-2-one (DC-EBIO; a Kcnn4 channel opener and CFTR activator) has been shown, in part, to provide the driving force for Cl− secretion in rat distal colon (16).
Electrophysiological and immunofluorescence studies have identified Kcnma1 channels in the apical membranes of mammalian (including human) colon (3, 15). cAMP has been shown to activate apical Kcnma1 channels in rat colon, as well as stimulating K+ secretion via Kcnma1 channels in mouse colon (21, 26). The aims of the present study were to determine whether Kcnma1 and/or Kcnn4c channels mediate cAMP-induced K+ secretion and whether cAMP-induced K+ secretion provides the driving force for Cl− secretion in rat distal colon. The results indicate that apical Kcnma1 channels (but not Kcnn4c channels) mediate cAMP-induced K+ secretion, whereas basolateral Kcnn4b channels (but not apical Kcnma1 or Kcnn4c channels) provide the driving force for cAMP-induced Cl− secretion. We conclude that cAMP-induced K+ and Cl− secretion occurs independently and that the cAMP-induced K+ secretion via Kcnma1 channels may contribute to high fecal K+ losses in some cases of secretory diarrhea.
METHODS
Animals.
Nonfasting normal male Sprague-Dawley rats (201–225 g) were maintained on standard rat chow. Animals were given food and water ad libitum. The experimental protocols used in these studies were approved by the West Virginia University Institutional Animal Care and Use Committee.
Ion flux studies.
86Rb (K+ surrogate; PerkinElmer, Billerica, MA) fluxes, short-circuit current (Isc; a measure of net electrogenic ion transport), and trans-epithelial conductance (G) were measured across colonic mucosa bathed in symmetrical Ringer solution (see below) and mounted under voltage-clamp conditions at 0 mV in EasyMount Ussing chambers (Physiological Instruments, San Diego, CA), as previously described (16). Thus, by removing the transepithelial electrical potential difference that would be present under open-circuit (mimicking in vivo) conditions, the net flux of the ionic species under study reflected its active (i.e., potential independent) transport. In brief, distal colons excised from euthanized rats were flushed with ice-cold saline. The distal colon was opened along the mesenteric border, and mucosal sheets were gently separated from serosal muscular layers. Two distal (1 cm proximal to rectum) segments obtained from each animal were mounted on to snap wells with an opening of 1.12 cm2. The snap wells placed in the sliders were inserted into the chambers, and both sides were bathed with equal volumes (5 ml) of Ringer solution containing (in mM) 115 NaCl, 25 NaHCO3, 2.4 K2HPO4, 0.4 KH2PO4, 1.2 CaCl2, 1.2 MgCl2, and 10 glucose, titrated to pH 7.4. Bathing solutions were maintained at 37°C and gassed with 5% CO2-95% O2. The Isc and G were recorded every 20 s using an automated multichannel voltage/current clamp instrument (Physiological Instruments). Positive Isc represented active Cl− secretion, whereas negative Isc represented active K+ secretion.
For K+ flux studies, a trace of 86RbCl (1 μCi/chamber) was added to either the mucosal (m) or serosal (s) bath solutions. After a 45-min equilibration period, mucosal-to-serosal (m-s) and serosal-to-mucosal (s-m) 86Rb+ fluxes were measured under voltage-clamp conditions in different tissues. Net fluxes were calculated from the difference between m-s and s-m fluxes in tissue pairs that were matched based on differences in basal conductance of <10%. Positive and negative values represent active absorption and active secretion, respectively.
The K+ fluxes were measured over 15-min periods. At the end of each period, 0.5-ml samples were withdrawn from the bath opposite to the “hot” side (i.e., isotope-containing side). Following sample removal, 0.5 ml regular Ringer was added to maintain the bath volume. Basal fluxes were measured immediately following an equilibrium period. Following the basal flux period, forskolin (FSK; 10 μM), an adenylate cyclase inhibitor that increases intracellular cAMP levels, was added to the serosal bath. In additional experiments, the effects of mucosal 1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole (TRAM-34; a Kcnn4 channel blocker; 10 μM), serosal TRAM-34 (500 nM), iberiotoxin (IbTX; a Kcnma1 channel blocker; 100 nM), chromanol 293B (Chrom-293B; a KCNQ1 channel blocker; 100 μM), BMS-191011 (BMS; a Kcnma1 channel opener; 10 μM), and CFTR inhibitor (CFTRinh-172; 100 μM) were examined on the changes in Isc, G, and K+ fluxes induced by FSK. The K+ fluxes are presented as μeq/h·cm2, whereas Isc are presented as μA/cm2. G values are presented as milliSiemens.
Statistics.
Values presented are means ± SE of six tissue pairs obtained from six different rat distal colons. Statistical analyses were performed using unpaired or paired Student's t-test or Bonferroni's one-way ANOVA post-hoc test using Originpro 8.0 (OriginLab, Northampton, MA). P < 0.05 was considered to be statistically significant.
RESULTS
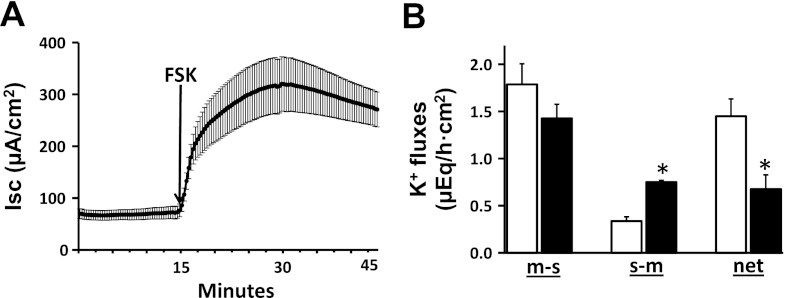
In initial studies, the effect of serosal addition of 10 μM FSK (an adenylate cyclase inhibitor that increases intracellular cAMP levels) on Isc and K+ fluxes was examined in rat distal colon (Fig. 1). FSK stimulated Isc, indicating an increase in Cl− secretion (Fig. 1A). As shown previously, net K+ absorption was present under basal conditions (Fig. 1B). FSK significantly decreased net K+ absorption from 1.5 ± 0.2 to 0.7 ± 0.2 μeq/h·cm2 (P < 0.001) (Fig. 1B) by increasing the s-m K+ flux from 0.3 ± 0.04 to 0.8 ± 0.02 μeq/h·cm2 (P < 0.001) without changing the m-s K+ flux (Fig. 1B).
Fig. 1.
Effect of forskolin (FSK) on short-circuit current (Isc) and K+ fluxes in rat distal colon. A: time course of Isc in the absence and in the presence of FSK. FSK (10 μM) was added to serosal bath (arrow). B: mucosal to serosal (m-s), serosal to mucosal (s-m), and net K+ fluxes (net) were measured in the absence (white bars) and in the presence (black bars) of FSK. Both Isc and K+ fluxes were measured under voltage-clamp conditions. *P < 0.001, compared with respective fluxes in the absence of FSK.
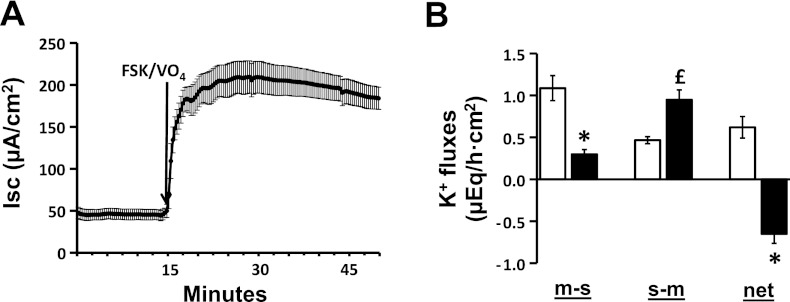
Although FSK enhanced the s-m K+ flux, it did not elicit active K+ secretion in the distal colon (Fig. 1B). It is possible that H+,K+-ATPase located in the apical membrane mediated a K+ absorptive (m-s) flux that masked FSK-induced K+ secretion (8, 19). Experiments were therefore performed to evaluate K+ fluxes in the absence of active K+ absorption. These involved adding 1 mM Na+ orthovanadate (VO4; a P-type ATPase inhibitor) to the mucosal bath solution to inhibit apical H+,K+-ATPase. In the same way that FSK stimulated Cl− secretion in the absence of VO4 (Fig. 1A), the simultaneous addition of FSK to the serosal bath solution and VO4 to the mucosal bath solution (FSK/VO4) also enhanced Cl− secretion (Fig. 2A). However, in contrast to the persistence of net K+ absorption in absence of VO4 (Fig. 1B), adding the FSK/VO4 combination elicited net K+ secretion (basal vs. FSK/VO4: 0.6 ± 0.1 vs. −0.7 ± 0.1 μeq/h.cm2; P < 0.001) (Fig. 2B). Active K+ secretion induced by FSK/VO4 reflected both a decrease in the m-s K+ flux (basal vs. FSK/VO4: 1.1 ± 0.2 vs. 0.3 ± 0.1; P < 0.001) and an increase in the s-m K+ flux (basal vs. FSK/VO4: 0.5 ± 0.04 vs. 0.9 ± 0.1; P < 0.001) (Fig. 2B). These results indicate that FSK stimulates both active Cl− secretion and active K+ secretion in rat distal colon. All further studies to characterize the FSK-induced active K+ secretory process were therefore performed in the presence of VO4.
Fig. 2.
Effect of FSK in the presence of mucosal Na+ orthovanadate (VO4) on Isc and K+ fluxes in rat distal colon. A: time course of Isc in the absence and in the presence of FSK and VO4. VO4 (1 mM) was added to mucosal bath, whereas 10 μM FSK was added to serosal bath (FSK/VO4). B: m-s, s-m, and net K+ fluxes were measured in the absence (white bars) and in the presence (black bars) of FSK. Both Isc and K+ fluxes were measured under voltage-clamp conditions. *P < 0.001, compared with respective fluxes in the absence of FSK/VO4. £P < 0.05, compared with in the absence of FSK/VO4.
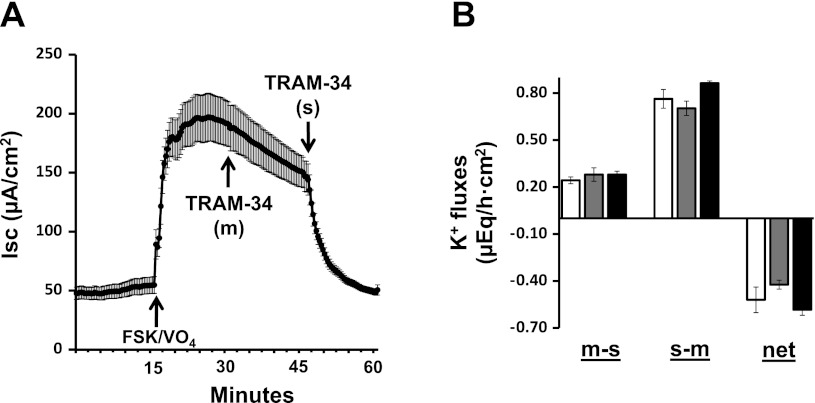
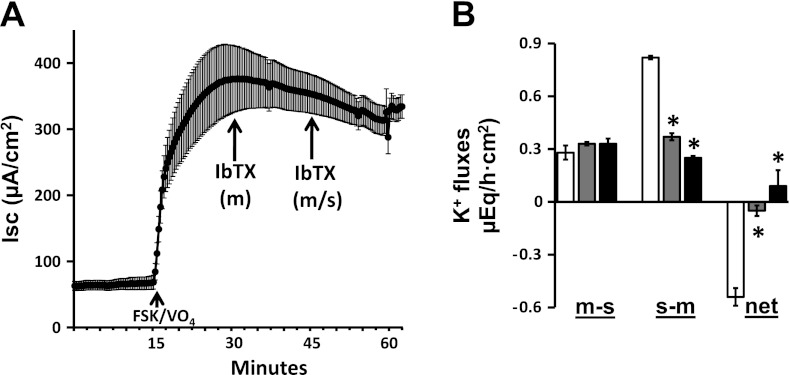
Additional studies were performed to identify the K+ channels involved in FSK-induced K+ secretion and to determine whether FSK-induced K+ secretion provides the driving force for FSK-induced Cl− secretion. To this end, we used TRAM-34 (a Kcnn4 channel blocker) and IbTX (a Kcnma1 channel blocker). The mucosal addition of 50 μM TRAM-34 did not significantly inhibit either FSK-induced Cl− secretion (Fig. 3A) or FSK-induced K+ secretion (Fig. 3B). However, in the presence of mucosal TRAM-34, a serosal addition of 500 nM TRAM-34 completely inhibited FSK-induced Cl− secretion (Fig. 3A) but had no effect on FSK-induced K+ secretion (Fig. 3B). In contrast to TRAM-34, neither the mucosal nor the serosal addition of 100 nM IbTX inhibited FSK-induced Cl− secretion (Fig. 4A), whereas mucosal IbTX completely inhibited FSK-induced K+ secretion (Fig. 4B). These observations indicate that 1) K+ efflux via basolateral Kcnn4 (Kcnn4b) channels, but not via apical Kcnma1 channels, provide the driving force for FSK-induced Cl− secretion; 2) the apical Kcnma1 channels, but not Kcnn4c channels, mediate FSK-induced K+ secretion; and 3) FSK may activate basolateral Kcnn4b, but not apical Kcnn4c K+ channels in rat distal colon.
Fig. 3.
Effect of 1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole (TRAM-34) on FSK-induced Isc and active K+ secretion in rat distal colon. A: time course of Isc in the absence and in the presence of FSK and VO4. VO4 (1 mM) was added to mucosal bath, whereas 10 μM FSK was added to serosal bath (FSK/VO4). Isc in the presence of FSK/VO4 was also measured in presence of 50 μM mucosal [TRAM-34 (m)] and 500 nM serosal [TRAM-34 (s)] Arrows indicate the time of addition of respective drug. B: m-s, s-m, and net K+ fluxes were measured in the presence of FSK/VO4 (white bars). The K+ fluxes were also measured in the presence of mucosal [TRAM-34 (m); gray bars] and serosal [TRAM-34 (m/s); black bars] TRAM-34. Both Isc and K+ fluxes were measured under voltage-clamp conditions.
Fig. 4.
Effect of iberiotoxin (IbTX) on FSK-induced Isc and active K+ secretion in rat distal colon. A: time course of Isc in the absence and in the presence of FSK and VO4. VO4 (1 mM) was added to mucosal bath, whereas 10 μM FSK was added to serosal bath (FSK/VO4). Isc in the presence of FSK/VO4 was also measured in additional presence of 100 nM mucosal [IbTX (m)] and serosal [IbTX (m/s)] IbTX. B: m-s, s-m, and net K+ fluxes were measured in the presence of FSK/VO4 (white bars). The K+ fluxes were also measured in the presence of mucosal [IbTX (m); gray bars] and serosal [IbTX (m/s); black bars] IbTX. Both Isc and K+ fluxes were measured under voltage-clamp conditions. *P < 0.001, compared with respective fluxes in the absence of IbTX.
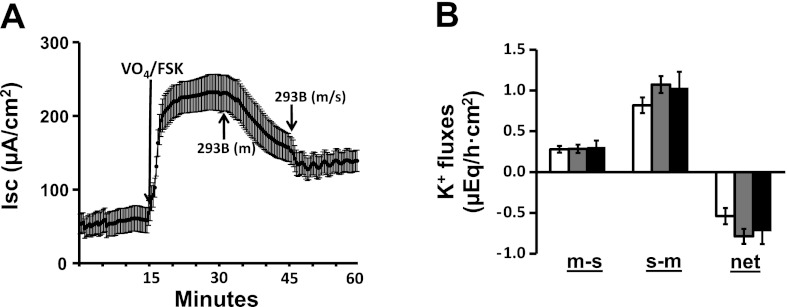
Since Chrom-293B-sensitive cAMP-activated K+ channels (KCNQ1) have been identified in the basolateral membranes of colonic crypts (31), we examined the effect of Chrom-293B on FSK-induced Cl− and K+ secretion (Fig. 5). Mucosal Chrom-293B progressively inhibited FSK-induced Cl− secretion to 52%. In the continued presence of mucosal Chrom-293B, serosal Chrom-293B had no further effect on FSK-induced Cl− secretion (Fig. 5A). Serosal Chrom-293B alone (i.e., in the absence of mucosal Chrom-293B) inhibited FSK-induced Cl− secretion by ∼40% (data not shown). The fact that mucosal plus serosal Chrom-293B or serosal Chrom-293B alone inhibited Cl− secretion to similar extents suggests that Chrom-293 freely permeated both the mucosal and the serosal membranes. In contrast to Cl− secretion, FSK-induced K+ secretion was not significantly altered by Chrom-293B (Fig. 5B). The observations that complete and partial inhibition of FSK-enhanced Cl− secretion by TRAM-34 (Fig. 3A) and Chrom-293B (Fig. 5A), respectively, suggest that it is likely that either the Chrom-293B-sensitive KCNQ1 expressed in basolateral membrane is also sensitive to TRAM-34 or that more than one Kcnn4 isoform may be expressed in basolateral membranes, one of which may be sensitive to Chrom-293B.
Fig. 5.
Effect of chromanol 293B (293B) on FSK-induced Isc and active K+ secretion in the presence of mucosal VO4 in rat distal colon. A: time course of Isc in the absence and in the presence of FSK and VO4. VO4 (1 mM) was added to mucosal bath, whereas 10 μM FSK was added to serosal bath (FSK/VO4). Isc in the presence of FSK/VO4 was also measured in additional presence of 100 μM mucosal [239B (m)] and serosal [293B (m/s)] 293. B: m-s, s-m, and net K+ fluxes were measured in the presence of FSK/VO4 (white bars). The K+ fluxes were also measured in the presence of mucosal [293B (m); gray bars] and serosal [293B (m/s); black bars] 293B. Both Isc and K+ fluxes were measured under voltage-clamp conditions.
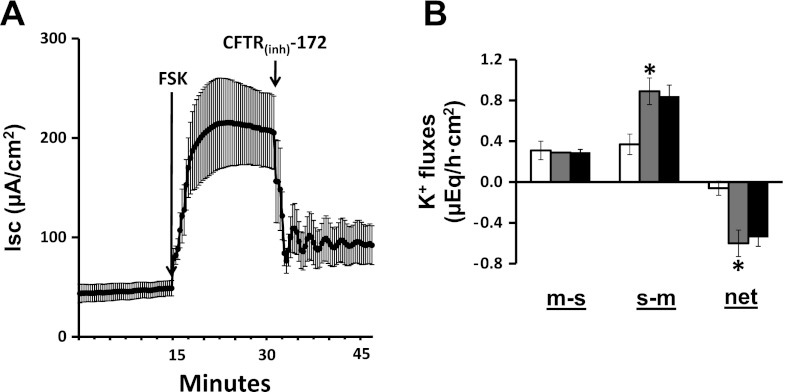
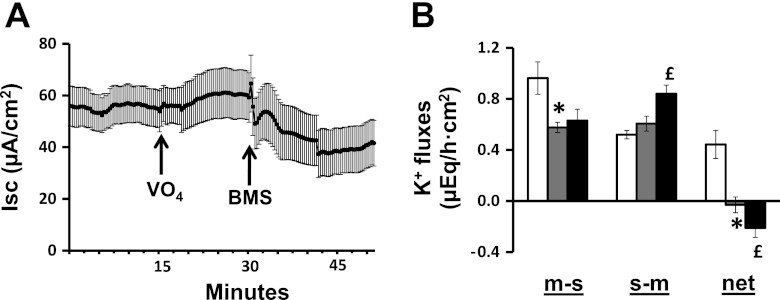
Our next step was to explore the possibility that Cl− and K+ secretion might be interdependent by examining the effects of a Cl− channel blocker on K+ secretion and a K+ channel opener on Cl− secretion. Mucosal addition of 100 μM CFTRinh-172 (a CFTR channel blocker) completely inhibited the FSK-enhanced Cl− secretion (Fig. 6A) but had no effect on FSK-induced K+ secretion (Fig. 6B). In additional experiments, in which 1 mM VO4 was present in the mucosal bath solution, which had no effect on Isc (Fig. 7A), the mucosal addition of 10 μM BMS (a Kcnma1 channel opener) decreased Isc, consistent with the activation of electrogenic K+ secretion (Fig. 7A). Mucosal VO4 having completely inhibited net K+ absorption (Fig. 7B), the subsequent mucosal addition of BMS induced net K+ secretion. These results provide strong evidence that FSK-induced Cl− secretion mediated via apical CFTR proceeds independently of K+ secretion mediated via apical Kcnma1 channels. As a corollary, K+ secretion via apical Kcnma1 channels do not provide the driving force for FSK-induced Cl− secretion in rat distal colon.
Fig. 6.
Effect of cystic fibrosis transmembrane conductance regulator inhibitor (CFTRinh-172) on FSK-induced Isc and active K+ secretion in the presence of mucosal VO4 in rat distal colon. A: time course of Isc in the absence and in the presence of 10 μM serosal FSK (FSK). Isc in the presence of FSK was also measured in additional presence of 100 μM mucosal CFTRinh-172. VO4 (1 mM) was added to mucosal bath at the beginning of the experiment. B: m-s, s-m, and net K+ fluxes were measured in the absence (white bars) and in the presence of FSK/VO4 (gray bars). The K+ fluxes in the presence of FSK/VO4 were also measured in the presence of mucosal [CFTRinh-172; black bars]. Both Isc and K+ fluxes were measured under voltage-clamp conditions. *P < 0.001, compared with respective fluxes in the absence of IbTX.
Fig. 7.
Effect of BMS-191011 (BMS) on Isc and K+ fluxes in rat distal colon. A: time course of Isc in the absence and in the presence of 1 mM mucosal VO4. Isc in the presence of VO4 also measured in additional presence of mucosal BMS (10 μM). B: m-s, s-m, and net K+ fluxes were measured in the absence (white bars) and in the presence of 1 mM mucosal VO4 (gray bars). K+ fluxes in the presence of VO4 were also measured in the additional presence of mucosal BMS (black bars). Both Isc and K+ fluxes were measured under voltage-clamp conditions. *P < 0.001, compared with respective fluxes in the absence of VO4; £P < 0.001, compared with respective fluxes in the presence of VO4 and absence of BMS.
DISCUSSION
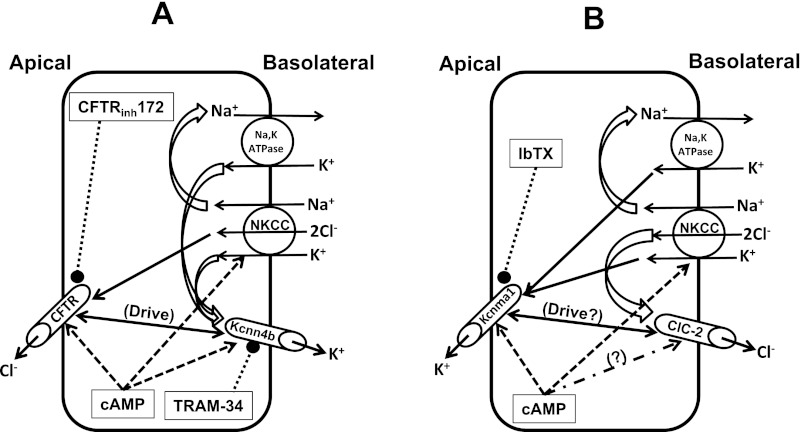
In the present study we have shown that cAMP-induced K+ secretion via apical Kcnma1 channels and cAMP-induced Cl− secretion via apical CFTR are regulated independently (Fig. 8). Furthermore, basolateral Kcnn4 (Kcnn4b) channels, but not apical Kcnn4 (Kcnn4c) or Kcnma1 channels, provide the driving force for the cAMP-induced Cl− secretion in rat distal colon.
Fig. 8.
Cellular models of cAMP-induced electrogenic Cl− and K+ secretion localized in different cell types in rat distal colon. A: coordinated activation of apical CFTR channels and basolateral Na-K-2Cl cotransporter (NKCC) and Kcnn4b channels regulates the cAMP-induced electrogenic Cl− secretion. Kcnn4b channels that mediate K+ exit provide the driving force (Drive) for cAMP-induced Cl− secretion, which is inhibited (see text) by blocking either Kcnn4b channels (using TRAM-34) or CFTR (using CFTRinh-172) but not by blocking Kcnma1 channels blocker (using IbTX). B: coordinated activation of apical Kcnma1 channels and basolateral NKCC and chloride channel 2 (ClC-2) (?) regulates cAMP-induced K+ secretion, which is inhibited by IbTX but not by CFTRinh-172 or TRAM-34 (see text). ClC2 has been shown to present in basolateral membranes of mammalian colon (20). Direction of transcellular electrolyte movements (solid arrows), cAMP-activation (dashed arrows), channel blockers (dotted bullets), electrolyte recycling (2-dimensional curvy arrow), and driving force (double-headed arrows) are indicated.
In infectious diarrheas such as cholera, movement of water into the gut lumen occurs secondary to active Cl− secretion. Active Cl− secretion requires the coordinated regulation of apical Cl− channels and the NKCC, Na,K-ATPase, and K+ channels located in the basolateral membrane. Chloride ions entering cells via basolateral NKCC exits via apical CFTR. Sustained Cl− secretion also requires the activity of K+ channels, which keep cells hyperpolarized, thus providing the driving force for apical Cl− exit (4, 5). Previous studies have implicated a role for basolateral Kcnn4 channels in providing the driving force for active Cl− secretion (22, 23), but apical Kcnn4c and Kcnma1 channels have been identified in mouse, rat and human colon (3, 10, 11, 16, 21, 24–26), and apical Kcnn4c channel activity appears to be involved in electrogenic Cl− secretion (16). However, it has been unclear whether active K+ secretion mediated via apical Kcnma1 channels also contributes to the driving force required for Cl− secretion, as well as to the excessive stool K+ losses seen in certain types of secretory diarrhea.
The observation that the mucosal addition of BMS (a Kcnma1 channel opener) induced active K+ secretion points to the presence of apical Kcnma1 channels in rat distal colon (Fig. 7B), as shown by previous patch-clamp studies in this epithelium (6, 24). Although Kcnma1 channels are localized to the apical membrane, active K+ secretion is not present under basal condition in rat distal colon (Fig. 7B). This situation differs from that in mouse distal colon in which apical Kcnma1 channels are present along the crypt axis and mediate both basal and Ca2+-activated K+ secretion (26). Based on the different patterns of apical Kcnma1 channel distribution along the surface-crypt axis, distinct patterns of cAMP-activated colonic K+ secretion have been identified in crypt cells in different mouse strains (9) and in surface cells in rat colon (21). Therefore, it is likely that the absence or presence of basal K+ secretion may reflect different expression patterns of Kcnma1 channels in rat colon and mouse colon, respectively.
Although both Kcnn4c and Kcnma1 channels are present at the apical membrane of rat distal colon, cAMP-induced active K+ secretion only via Kcnma1 channels (Fig. 1B). Phosphorylation of apical Kcnma1 channels by the protein kinase A-mediated signaling pathway most likely underlies cAMP-activated colonic K+ secretion in rat colon (21), whereas Ba2+- and IbTX-sensitive K+ secretion in wild-type (Kcnma1+/+) but not in Kcnma1-deficient (Kcnma1−/−) animals has been proposed as evidence for the involvement of Kcnma1 channels in cAMP-stimulated K+ secretion in mouse distal colon (27). Thus, although K+ secretion is absent in rat distal colon under basal condition, cAMP-stimulated IbTX-inhibitable K+ secretion is entirely mediated by apical Kcnma1 channels.
The present study in rat distal colon demonstrates that although the process of active K+ secretion most likely maintains cells in a hyperpolarized state and contributes to the driving force for Cl− secretion, the activation of apical Kcnma1 channels is not in itself required for cAMP-enhanced Cl− secretion. This conclusion is supported by several observations. First, the Kcnma1 channel blocker IbTX inhibited cAMP-stimulated K+ secretion, but not cAMP-stimulated Cl− secretion (Fig. 4, A and B). Second, the CFTR blocker CFTRinh-172 inhibited cAMP-stimulated Cl− secretion but not cAMP-stimulated K+ secretion (Fig. 6, A and B). Third, serosal TRAM-34 (a Kcnn4 channel blocker) inhibited cAMP-stimulated Cl− secretion, indicating that basolateral Kcnn4 channels provide the driving force for cAMP-stimulated Cl− secretion (Fig. 3A). It is possible that apical Kcnma1 and CFTR channels are located in different cell types, since patch-clamp studies have identified apical Kcnma1 channels in surface cells of rat and human colon (6, 25), whereas apical CFTR is present in crypt cells in these species (1, 18, 28).
The importance of apical Kcnma1 channels in determining stool K+ losses has recently been highlighted by several clinical studies. For example, patients with end-stage renal disease exhibit enhanced expression of apical Kcnma1 channels in colonic surface cells (15), which are likely to play an important role in their ability to increase colonic K+ losses to maintain K+ homeostasis (15). An increase in apical Kcnma1 channel expression also occurs along the entire surface cell-crypt cell axis in active ulcerative colitis (25), which may explain, at least in part, the excessive K+ losses and hypokalemia that sometimes occurs in these patients (2, 12–13). Overexpression of apical Kcnma1 channels is likely to underlie massive stool K+ losses and profound hypokalemia, which occur in some cases of colonic pseudo-obstruction (29). It also seems likely that hypokalemia complicating the chronic abuse of cAMP-mediated laxatives may reflect excessive stool K+ losses via cAMP-stimulated apical Kcnma1 channels (7).
In summary, this study indicates that in rat distal colon, 1) cAMP stimulates Kcnma1 channel-mediated K+ secretion and CFTR-mediated Cl− secretion, 2) cAMP-stimulated K+ secretion does not provide the driving force for the cAMP-stimulated Cl− secretion, and 3) basolateral Kcnn4b channels provide the driving force for cAMP-stimulated Cl− secretion. Our results also raise the possibility that the K+ and Cl− secretory processes regulated by cAMP may be localized in different cell types. Finally, we propose that cAMP-regulated apical Kcnma1 channels may play a critical role in determining stool K+ losses in some types of secretory diarrhea.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-018777.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
G.I.S. and V.M.R. conception and design of research; G.I.S. and V.M.R. analyzed data; G.I.S. and V.M.R. interpreted results of experiments; G.I.S. and V.M.R. edited and revised manuscript; G.I.S. and V.M.R. approved final version of manuscript; V.M.R. performed experiments; V.M.R. prepared figures; V.M.R. drafted manuscript.
REFERENCES
- 1. Ameen NA, van Donselaar E, Posthuma G, de Jonge H, McLaughlin G, Geuze HJ, Marino C, Peters PJ. Subcellular distribution of CFTR in rat intestine supports a physiologic role for CFTR regulation by vesicle traffic. Histochem Cell Biol 114: 219–228, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Archampong EQ, Harris J, Clark CG. The absorption and secretion of water and electrolytes across the healthy and the diseased human colonic mucosa measured in vitro. Gut 13: 880–886, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barmeyer C, Rahner C, Yang Y, Sigworth FJ, Binder HJ, Rajendran VM. Cloning and identification of tissue-specific expression of KCNN4 splice variants in rat colon. Am J Physiol Cell Physiol 299: C251–C263, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol 62: 535–572, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Binder HJ, Sandle GI, Rajendran VM. Colonic fluid and electrolyte transport in health and disease. In: The Large Intestine, edited by Phillips SF, Shorter R, Pemberton W. New York: Raven, 1991, p. 141–168 [Google Scholar]
- 6. Butterfield I, Warhurst G, Jones MN, Sandle GI. Characterization of apical potassium channels induced in rat distal colon during potassium adaptation. J Physiol 501: 537–547, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cummings JH. Laxative abuse. Gut 15: 758–766, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Del Castillo JR, Rajendran VM, Binder HJ. Apical membrane localization of ouabain-sensitive K+-activated ATPase activities in rat distal colon. Am J Physiol Gastrointest Liver Physiol 261: G1005–G1011, 1991 [DOI] [PubMed] [Google Scholar]
- 9. Flores CA, Cid LP, Sepulveda FV. Strain-dependent differences in electrogenic secretion of electrolytes across mouse colon epithelium. Exp Physiol 95: 686–698, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Furness JB, Robbins HL, Selmer IS, Hunne B, Chen MX, Hicks GA, Moore S, Neylon CB. Expression of intermediate conductance potassium channel immunoreactivity in neurons and epithelial cells of the rat gastrointestinal tract. Cell Tissue Res 314: 179–189, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Halm ST, Liao T, Halm DR. Distinct K+ conductive pathways are required for Cl− and K+ secretion across distal colonic epithelium. Am J Physiol Cell Physiol 291: C636–C648, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Harris J, Shields R. Absorption and secretion of water and electrolytes by the intact human colon in diffuse untreated proctocolitis. Gut 11: 27–33, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hawker PC, McKay JS, Turnberg LA. Electrolyte transport across colonic mucosa from patients with inflammatory bowel disease. Gastroenterology 79: 508–511, 1980 [PubMed] [Google Scholar]
- 14. Joiner WJ, Basavappa S, Vidyasagar S, Nehrke K, Krishnan S, Binder HJ, Boulpaep EL, Rajendran VM. Active K+ secretion through multiple KCa-type channels and regulation by IKCa channels in rat proximal colon. Am J Physiol Gastrointest Liver Physiol 285: G185–G196, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Mathialahan T, Maclennan KA, Sandle LN, Verbeke C, Sandle GI. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol 206: 46–51, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Nanda Kumar NS, Singh SK, Rajendran VM. Mucosal potassium efflux mediated via Kcnn4 channels provides the driving force for electrogenic anion secretion in colon. Am J Physiol Gastrointest Liver Physiol 299: G707–G714, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neylon CB, Lang RJ, Fu Y, Bobik A, Reinhart PH. Molecular cloning and characterization of the intermediate-conductance Ca2+-activated K+ channel in vascular smooth muscle: relationship between KCa channel diversity and smooth muscle cell function. Circ Res 85: e33–e43, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Novaira HJ, Ornellas DS, Ortiga-Carvalho TM, Zhang XM, Souza-Menezes J, Guggino SE, Guggino WB, Morales MM. Atrial natriuretic peptide modulates cystic fibrosis transmembrane conductance regulator chloride channel expression in rat proximal colon and human intestinal epithelial cells. J Endocrinol 189: 155–165, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Pandiyan V, Rajendran VM, Binder HJ. Mucosal ouabain and Na+ inhibit active Rb+(K+) absorption in normal and sodium-depleted rat distal colon. Gastroenterology 102: 1846–1853, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Pena-Munzenmayer G, Catalan M, Cornejo I, Figueroa CD, Melvin JE, Niemeyer MI, Cid LP, Sepulveda FV. Basolateral localization of native ClC-2 chloride channels in absorptive intestinal epithelial cells and basolateral sorting encoded by a CBS-2 domain di-leucine motif. J Cell Sci 118: 4243–4252, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Perry MD, Sandle GI. Regulation of colonic apical potassium (BK) channels by cAMP and somatostatin. Am J Physiol Gastrointest Liver Physiol 297: G159–G167, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rufo PA, Jiang L, Moe SJ, Brugnara C, Alper SL, Lencer WI. The antifungal antibiotic, clotrimazole, inhibits Cl− secretion by polarized monolayers of human colonic epithelial cells. J Clin Invest 98: 2066–2075, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rufo PA, Merlin D, Riegler M, Ferguson-Maltzman MH, Dickinson BL, Brugnara C, Alper SL, Lencer WI. The antifungal antibiotic, clotrimazole, inhibits chloride secretion by human intestinal T84 cells via blockade of distinct basolateral K+ conductances. Demonstration of efficacy in intact rabbit colon and in an in vivo mouse model of cholera. J Clin Invest 100: 3111–3120, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sandle GI, Butterfield I. Potassium secretion in rat distal colon during dietary potassium loading: role of pH regulated apical potassium channels. Gut 44: 40–46, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sandle GI, Perry MD, Mathialahan T, Linley JE, Robinson P, Hunter M, MacLennan KA. Altered cryptal expression of luminal potassium (BK) channels in ulcerative colitis. J Pathol 212: 66–73, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Sausbier M, Matos JE, Sausbier U, Beranek G, Arntz C, Neuhuber W, Ruth P, Leipziger J. Distal colonic K+ secretion occurs via BK channels. J Am Soc Nephrol 17: 1275–1282, 2006 [DOI] [PubMed] [Google Scholar]
- 27. Sorensen MV, Matos JE, Sausbier M, Sausbier U, Ruth P, Praetorius HA, Leipziger J. Aldosterone increases KCa1.1 (BK) channel-mediated colonic K+ secretion. J Physiol 586: 4251–4264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strong TV, Boehm K, Collins FS. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest 93: 347–354, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Dinter TG, Jr, Fuerst FC, Richardson CT, Ana CA, Polter DE, Fordtran JS, Binder HJ. Stimulated active potassium secretion in a patient with colonic pseudo-obstruction: a new mechanism of secretory diarrhea. Gastroenterology 129: 1268–1273, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Vandorpe DH, Shmukler BE, Jiang L, Lim B, Maylie J, Adelman JP, de Franceschi L, Cappellini MD, Brugnara C, Alper SL. cDNA cloning and functional characterization of the mouse Ca2+-gated K+ channel, mIK1. Roles in regulatory volume decrease and erythroid differentiation. J Biol Chem 273: 21542–21553, 1998 [DOI] [PubMed] [Google Scholar]
- 31. Warth R, Riedemann N, Bleich M, Van Driessche W, Busch AE, Greger R. The cAMP-regulated and 293B-inhibited K+ conductance of rat colonic crypt base cells. Pflügers Arch 432: 81–88, 1996 [DOI] [PubMed] [Google Scholar]