Abstract
Neuroendocrine adrenal medullary chromaffin cells receive synaptic excitation through the sympathetic splanchnic nerve to elicit catecholamine release into the circulation. Under basal sympathetic tone, splanchnic-released acetylcholine evokes chromaffin cells to fire action potentials, leading to synchronous phasic catecholamine release. Under elevated splanchnic firing, experienced under the sympathoadrenal stress response, chromaffin cells undergo desensitization to cholinergic excitation. Yet, stress evokes a persistent and elevated adrenal catecholamine release. This sustained stress-evoked release has been shown to depend on splanchnic release of a peptide transmitter, pituitary adenylate cyclase-activating peptide (PACAP). PACAP stimulates catecholamine release through a PKC-dependent pathway that is mechanistically independent of cholinergic excitation. Moreover, it has also been reported that shorter term phospho-regulation of existing gap junction channels acts to increase junctional conductance. In this study, we test if PACAP-mediated excitation upregulates cell-cell electrical coupling to enhance chromaffin cell excitability. We utilize electrophysiological recordings conducted in adrenal tissue slices to measure the effects of PACAP stimulation on cell coupling. We report that PACAP excitation increases electrical coupling and the spread of electrical excitation between adrenal chromaffin cells. Thus PACAP acts not only as a secretagogue but also evokes an electrical remodeling of the medulla, presumably to adapt to the organism's needs during acute sympathetic stress.
Keywords: acute stress, catecholamine, connexin-43, connexin-36, gap junction
adrenal medullary chromaffin cells are neural crest-derived neurosecretory cells that release catecholamine into the bloodstream (2). Chromaffin cells are innervated by the bifurcating sympathetic splanchnic nerve. Under sympathetic tone, corresponding to the “rest and digest” metabolic state, the splanchnic nerve fires at a modest rate to maintain catecholamine homeostasis. Splanchnic-released acetylcholine binds to nicotinic ionotropic receptors on the chromaffin cell membrane, leading to action potential firing and controlled, phasic catecholamine release (1, 7, 13, 18). Under the acute sympathetic stress response, the splanchnic nerve fires at a heightened rate, leading to rapid and robust cholinergic-evoked catecholamine secretion (25). Yet, the cholinergic pathway rapidly desensitizes (4, 5, 29) while elevated catecholamine secretion persists (44). Pituitary adenylate cyclase-activating peptide (PACAP) is a noncholinergic splanchnic-derived peptide transmitter released selectively during elevated splanchnic firing. PACAP has been identified as the peptide transmitter driving tonic catecholamine secretion after desensitization of cholinergic signaling in the sympathoadrenal stress response (22, 26, 47). PACAP excitation acts through a series of signaling steps that include an acute subthreshold membrane depolarization (24, 26) and parallel PKC phosphorylation-dependent recruitment of a low voltage-activated T-type calcium channel conductance to supply calcium necessary for secretion (24). In the longer term (>10 min), PACAP-mediated adrenal excitation further depolarizes the membrane and recruits an L-type Ca2+ influx (35). Ultimately, long-term PACAP excitation elicits a broader modulation of adrenal exocytic function through the activation of secretion-associated genes (38, 41, 49). More recent studies (9) have suggested a wider role for PACAP in the sympathoadrenal stress response that may include increased intercellular electrical coupling within the medulla.
Gap junctions are intercellular channels formed by head-to-head docking of connexons; hexameric assemblies of connexin (Cx) proteins (19). Connexin channels bridge the cytoplasm of two cells and allow the diffusion of ions or second messengers. The prevalence and connexin composition of gap junction coupling in the adrenal medulla are dependent on age (30, 32), species (10, 20), gender (9, 33), stress state (12), or splanchnic innervation (30, 32). The role of gap junctions in normal adrenal physiology has been studied and implicated in modulation of adrenal excitation. Martin et al. (32) showed that rat gap junctions are under tonic inhibitory control during basal sympathetic tone, while chronic cold-stress dramatically upregulates Cx36/Cx43 expression and enhances gap junction communication (12). Gap junctions also facilitate cholinergic-evoked catecholamine release, with nicotine treatment of a single chromaffin cell in situ eliciting catecholamine release from neighboring chromaffin cells (31). Thus chronic stress and cholinergic-mediated stress signaling enhance gap junction coupling.
Gap junction coupling is regulated by signaling events common to PACAP-evoked secretion. For example, PACAP binding to its high-affinity Gs-coupled PACR1 receptor elicits an elevation in cAMP, which has also been shown to regulate connexin-43 (Cx43) through PKA-mediated phosphorylation (40). Other studies (16) show that the exchange protein activated by cAMP (Epac), which signals via PLC-mediated activation of PKC and is independent of PKA action, increases junctional conductance through Cx43-composed gap junctions. Common with a role for PKC, phorbol ester treatment also elevates electrical coupling (albeit with a parallel decrease in dye coupling; Refs. 27, 43). We hypothesize that PACAP excitation elicits a signaling cascade that intersects with gap junction regulatory processes. We further hypothesize that this signaling cascade positively regulates the electrical coupling observed during acute sympathetic stress. In testing this hypothesis, we provide data demonstrating that chromaffin cell stimulation by PACAP significantly increases the degree of electrical coupling in the mouse adrenal medulla.
MATERIALS AND METHODS
Adrenal slice preparation.
Adult male and female C57BL/6 mice (4–8 wk old) from Jackson Laboratories (Bar Harbor, ME) were used in this study. Male and female mice were age matched within each experimental condition, and no significant variation in electrical coupling parameters was measured across the 4 wk age range. Anesthesia and euthanasia protocols were approved by the Case Western Reserves University Institutional Animal Care and Use Committee, a federal oversight body (Federal Welfare Assurance No. A3145–01). Mice were deeply anesthetized by isoflurane (USP; Halocarbon Products, River Ridge, NJ) inhalation and euthanized by decapitation. Adrenal glands were excised and submerged in ice-cold low-calcium bicarbonate-buffered saline (BBS) containing the following (in mM): 140 NaCl, 2 KCl, 0.1 CaCl2, 5 MgCl2, 26 NaHCO3, and 10 glucose and continuously bubbled with 95% O2-5% CO2. All BBS components were purchased from Fisher Scientific, (Fair Lawn, NJ) except MgCl2 (Sigma-Aldrich, St. Louis, MO). Osmolarity of the BBS solution was 320 mosM. Single adrenal glands were trimmed of excess fat and embedded in low melting temperature agarose (Lonza, Rockland, ME). Agarose was prepared by melting in low calcium BBS at 110°C followed by 15 min of equilibration in a 35°C water bath. Immediately after embedding, adrenal glands and agarose were placed on ice to gel. Gelled agarose was trimmed into 3- to 5-mm blocks, each containing a single adrenal gland. Agarose blocks containing the glands were glued to a sectioning stage (WPI, Sarasota, FL) with cyanoacrylate. The stage was placed in a vibrotome slicing chamber filled with ice-cold low calcium BBS that was continuously bubbled with 95% O2-5% CO2. Adrenal glands were vibrotome sectioned at 200 μm. Sections containing medulla were collected and placed in a holding chamber containing low calcium BBS bubbled with 95% O2-5% CO2 at 25°C. Experiments were carried out within 6 h of slice preparation.
Cell isolation.
Animals were deeply anesthetized and killed as described above. Adrenal glands were removed immediately and were placed in an ice-cold dissociation solution that contained the following (in mM): 80 Na glutamate, 55 NaCl, 6 KCl, 1 MgCl2, 10 HEPES, and 10 glucose. The pH was adjusted to 7.0, and the osmolarity was adjusted to 290 mosM. Glands were trimmed of fat, as above, and the adrenal cortex was removed by dissection from the medullae. The medullae were incubated in digest solution (30 U/ml papain, 1 mM DTT, and 0.5 mg/ml BSA in 1 ml of dissociation solution) for 10 min at 37°C. The tissues were transferred into a second digest solution (3 U/ml collagenase F, 0.5 mg/ml BSA, and 100 μM CaCl2 in 1 ml of dissociation solution) for 10 min at 37°C. After the second incubation, cells were transferred into a DMEM growth medium supplemented with ITS artificial serum substitute (Mediatech, Manassas, VA) and penicillin/streptomycin (20 U/ml each) and triturated with a 2-ml serological pipette. Cell-containing supernatant fluid was plated onto glass 25-mm round coverslips, and cells were allowed to settle and adhere to the glass for 10 min. DMEM was added to each tissue culture well after cell adhesion. The cells were incubated at 35°C in 10% CO2, and the experiments were performed at room temperature (∼25°C) 2–3 days after cell preparation.
Electrophysiology.
Adrenal tissue slices were constantly superfused during recordings with HEPES-buffered Ringer solution containing the following (in mM): 150 NaCl, 10 HEPES-H, 10 glucose, 2.8 CaCl2, 2.8 KCl, and 2 MgCl2. The Ringer solution osmolarity was adjusted with mannitol to 320 mosM, and pH was adjusted to 7.2 with NaOH. Tissue slices were held in place in the recording chamber by positioning a silver wire over the agarose perimeter of the adrenal slice. Patch pipettes were pulled from borosilicate glass (∼1-μm tip diameter; 4- to 5-MΩ resistance). Pipette tips were coated in molten dental wax and fire polished with a microforge (Narishige, Tokyo, Japan). All recordings were conducted in tissue slices in the perforated patch configuration, unless noted otherwise. Cells were visualized using an Olympus BW50WI fixed stage upright microscope (Center Valley, PA) with a ×40 water dipping objective (NA = 0.8) and oblique illumination contrast-enhancing optics. For voltage clamp perforated-patch experiments, pipettes were filled with an internal patch solution containing the following (in mM): 145 Cs-glutamate, 10 HEPES-H, 8 NaCl, and 0.5 TEA-Cl. Internal solution had a pH of 7.2 and osmolarity of 310 mosM. Amphotericin B (Fisher Scientific) was prepared daily in DMSO (Acros Organics) as a 100× stock solution and diluted into the internal solution to a final concentration of 0.53 mM. In Lucifer yellow (LY) dye diffusion imaging experiments, cells were held in the whole cell configuration. In these experiments, pipettes were filled with an internal patch solution containing the following (in mM): 145 Cs-glutamate, 10 HEPES-H, 8 NaCl, 2 MgATP, 1 MgCl2, 1 LY, and 0.1 EGTA. The pH was adjusted to 7.2 with CsOH, and the osmolarity was adjusted to 310 mosM with mannitol. All electrophysiological records were filtered at 10 kHz and digitized at 20 kHz using an EPC-9 amplifier controlled by “Pulse” software (v. 8.8; HEKA Electronik, Bellmore, NY). Igor Pro software (WaveMetrics Lake Oswego, OR) was used to analyze electrophysiological recordings.
The protocol used to quantify gap junction coupling was as follows: cells were held at −80 mV and delivered one depolarizing step to +50 mV, with a duration of 150 ms. The “echo” integral was measured using Igor Pro software, and an example of the analysis paradigm is shown in results (see Fig. 2). To determine membrane resistance, cells were stepped to a −130-mV potential, also 150 ms in duration. Membrane resistance was calculated under passive membrane conditions according to Ohm's law. To provide internal control conditions and to assess the effect of reagents on echo integral and input resistance, electrical protocols were delivered twice: once in normal extracellular HEPES solution and then again in a HEPES solution supplemented with 100 μm carbenoxolone (CBX), 1 μm PACAP-38, 100 nm PMA, or 100 nM Gö6983 (all from Sigma-Aldrich) as indicated in the text. A microperfusion system (Warner Instruments, Hamden, CT) was used for local rapid delivery of some pharmacological agents (see text) during recordings. Data were separated by gender of the donor animal for analysis.
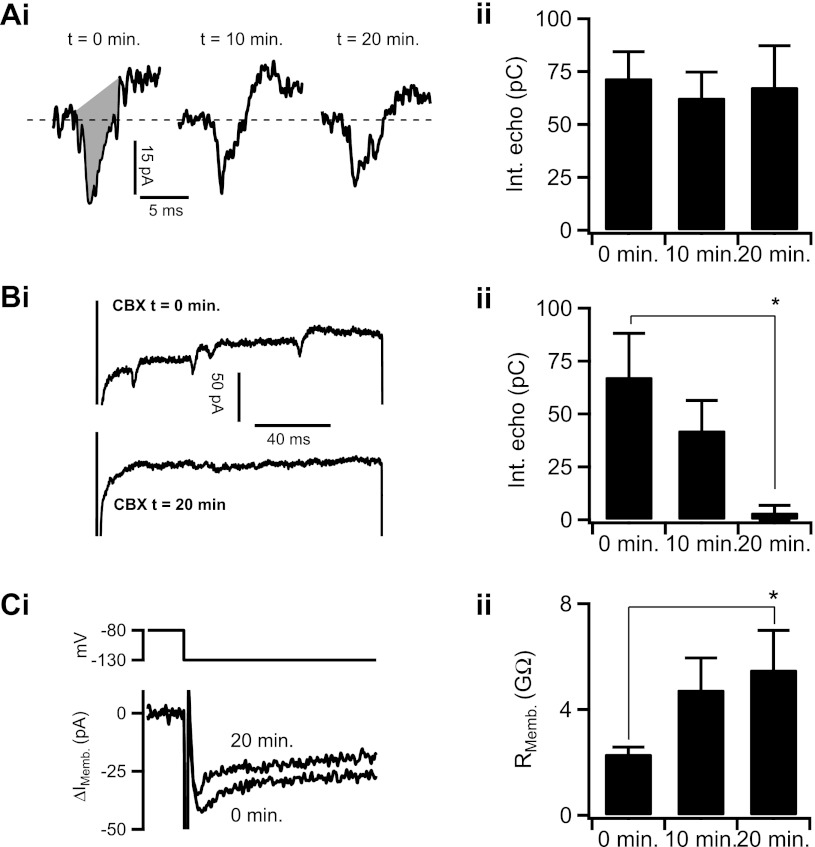
Fig. 2.
Carbenoxolone (CBX) abolishes spike-like currents and increases input resistance in chromaffin cells. Ai: electrical echoes were evoked from a voltage-clamped cell as in Fig. 1 over a 20-min time course to determine stability of the response (t = 0, 10, and 20 min). Region shaded in grey represents the integral of the echo, which is quantified in Aii and in subsequent figures. Aii: quantified data from each time point were plotted as means ± SE (n = 10 echoes/time point; P = 0.996 by ANOVA). Bi: effect of CBX on echo charge was tested. Cells were categorized as coupled if AP echoes were present at t = 0 min. An adrenal slice containing a coupled cell pair was bath-treated with 100 μm CBX. Echo charge was determined by current integration as above. Bii: echo integral (Int) data from all CBX time points (0, 10, and 20 min) were pooled, quantified, and plotted as means ± SE (n = 16 echoes/time point; *statistical significance of 0- and 20-min time points with P = 0.007 by Student's t-test). Ci: input resistance (Rmemb) was calculated using the same CBX treatment time course as in Bi. Cells were hyperpolarized to −130 mV from the holding potential of −80 mV to evoke a passive current to calculate membrane resistance (RMemb) of the cell. Representative current traces for the CBX t = 0-min and CBX t = 20-min time points are provided. Bii: this procedure was repeated at the 0-, 10-, and 20-min time points, and the RMemb. was calculated, pooled, and presented as means ± SE (n = 25, 9, and 9 cells for 0-, 10-, and 20-min time points, respectively; *statistical significance of 0- and 20-min time points at P = 0.002).
LY diffusion assay.
LY fluorescent dye (LY-dipotassium salt; Sigma-Aldrich) was introduced into pipettes, and cells were patched in the whole cell patch configuration to allow complete diffusion of LY dye into patched chromaffin cells. Dye spread was visualized with 435-nm wavelength excitation using an Olympus BW50WI fixed stage upright microscope with ×40 water dipping objective (NA = 0.8). Excitation light was shuttered between exposures to limit photobleach and phototoxicity. Images were collected with a cooled charged-coupled device camera (RetigaEX; QImaging, Surrey, BC, Canada) at >520-nm emission at a fixed exposure duration and camera gain to allow for comparison between cells. The extent of LY dye spreading was estimated by counting the number of chromaffin cells to which LY dye spread 20-min post break-in. Signals were determined to have spread if the secondary cell increased fluorescence by more than three times over background.
Western blot analysis.
Adrenal glands were extracted from male or female 6-wk-old mice. The adrenal cortex was removed, and the adrenal medulla was isolated and used for Western blot analysis. Samples were kept on ice or at 4°C. Male and female tissue were kept separate and blotted separately. When preparing samples were sampled, tissues was placed in PBS (Thermo Scientific, Logan, UT) solution containing 1% Triton X-100 (Sigma) and protease inhibitors (Roche Applied Biosciences, Indianapolis, IN). Tissue was disrupted using a pestle, and homogenized sample was passed through 22-gauge needle to completely solubilize the tissue. Samples were centrifuged at 16,000 g for 20 min at 4°C. The concentration of tissue lysate was determined by a BCA assay kit (Thermo Scientific), and 75 μg of protein were loaded in each well. Protein was resolved by SDS-PAGE on 4–20% Tris·HCl gels (Bio-Rad, Hercules, CA), transferred onto polyvinylidene difluoride membranes, and immunoblotted using the following primary antibodies: anti-Cx43 rabbit polycloncal (Cell Signaling, Danvers, MA; catalog no. 3512S), anti-Cx36 rabbit polyclonal (Invitrogen; no. 36–4600), anti-PACR1 rabbit polyclonal (Sigma-Aldrich; no. P8872), anti-β-tubulin goat polyclonal (Abcam; no. ab21057), and anti- β-actin mouse monoclonal (Sigma-Aldrich; no. A1978). Each primary antibody was diluted to 1 μg/ml final concentration. Secondary antibodies were horseradish peroxidase-conjugated anti-rabbit and anti-mouse (MP Biochemicals, Solon, OH), at 0.13 μg/ml dilution. Western blots were developed using ECL Plus reagents (GE Healthcare Biosciences, Piscataway, NJ), and signals were detected and imaged on ChemFluor E (Protein Simple, Santa Clara, CA). Tissue was processed in duplicate batches, and each of the resulting protein samples was resolved and analyzed in duplicates to reduce loading error.
Quantitative-PCR.
Medullae were dissected from 6-wk-old male and female mice, as described in the Cell isolation. RNA was isolated according to specifications determined by the amplification reagent manufacturer (Invitrogen, Grand Island, NY) using Trizol (Invitrogen) extraction. The concentration of sample RNA was determined using a NanoDrop 2000 spectrophotometer (Thermoscientific). Samples were normalized to a concentration standard of 500 ng/μl per reaction. Reverse transcription was performed using the TaqMan RNA-to-Ct 1-step kit (Invitrogen). Real-time primers were purchased from Applied Biosystems (Carlsbad, CA), including GAPDH Mm99999915_g1, Cx36 Mm00439121_m1, and Cx43 Mm00439105_m1. RT-quantitative PCR was performed according to manufacturer's specifications on the StepOnePlus Real-Time PCR system (Applied Biosystems). Comparative change in cycle threshold (ΔCt) analysis was performed for GAPDH, Cx36, and Cx43 (in triplicate), with the summary data supplied in Table 1.
Table 1.
RT-quantitative PCR detection of connexins
| Gender/Connexin | Mean ΔCt ± SE |
|---|---|
| Male Cx36 | 12.27 ± 0.28 |
| Female Cx36 | 12.76 ± 0.73 |
| Male Cx43 | 8.38 ± 0.17 |
| Female Cx43 | 7.42 ± 0.51 |
RT-quantitative PCR detection of connexin-36 and -43 (Cx36 and Cx43) message. Average change in cycle threshold (ΔCt) values are reported. No. of experiments = 3 for male and 4 for female.
Statistical analysis.
The specific analysis used for quantification of the data is included in results (see Figs. 1–5). Statistical evaluations were made using a Student's t-test (see Figs. 1–5 and Tables 1 and 2) and the one-way ANOVA test (see Fig. 2 and Table 3). Calculated P values are reported in results (see Figs. 1–5).
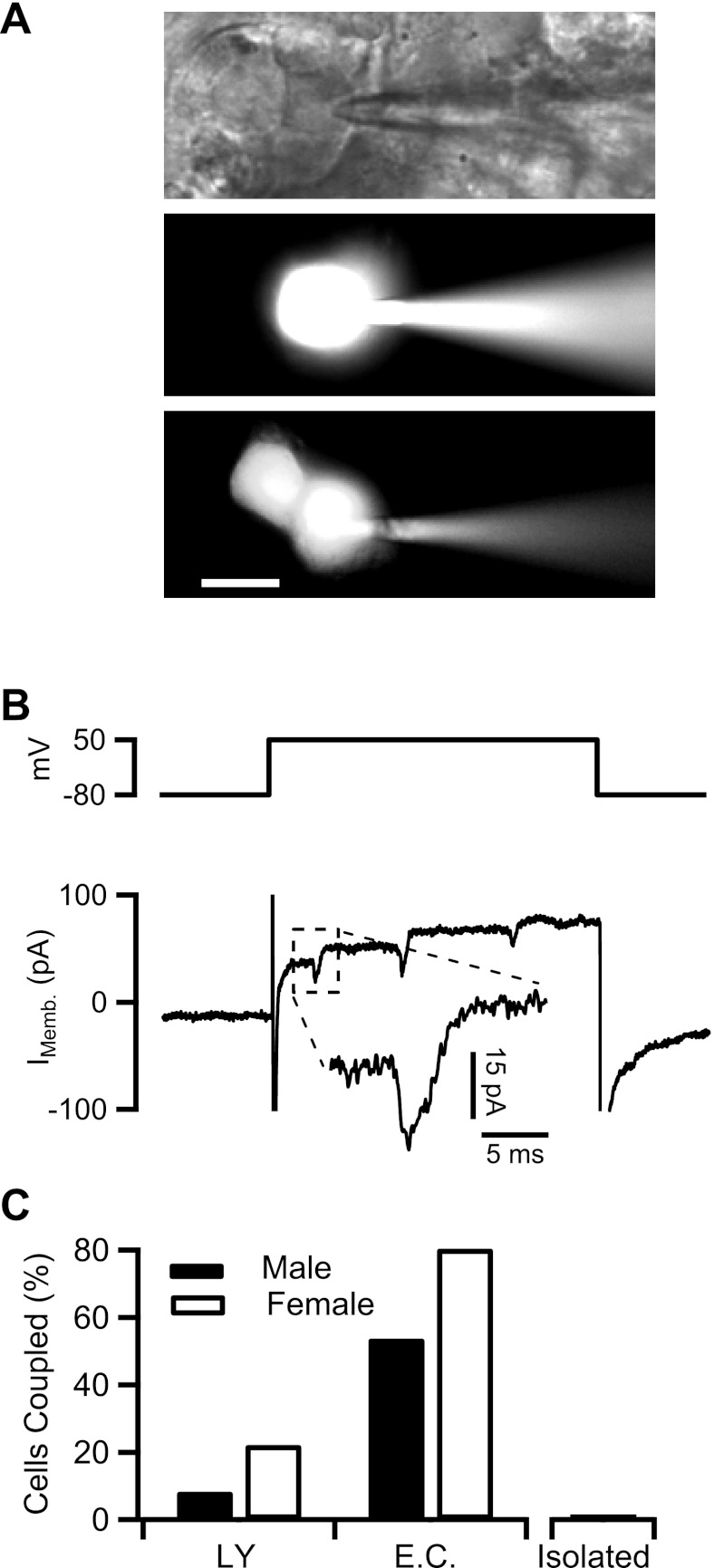
Fig. 1.
Gap junction coupling in the adrenal medulla. A, top; a bright-field image of a chromaffin cell in situ patched in whole cell configuration. Pipette solution contained 1 mM LY dye. Middle and bottom: the cells were held at −80 mV. Cells are shown 20 min after break-in to allow Lucifer yellow (LY) diffusion into the cell (scale bar = 10 μm). B, top: cell from A, middle, was held at −80 mV and delivered a 150-ms duration depolarizing step to +50 mV. Bottom: evoked membrane current is plotted. B, inset: echoing action potential (Imemb) from a neighboring unpatched cell. C: data were collected from LY dye diffusion and electrical stimulation experiments as represented above and are plotted as percentage of cells that are coupled. Sample sizes were as follows: for LY diffusion, n = 30 cells (12 male and 18 female) and for electrical coupling (EC), n = 63 cells (29 male and 34 female). Data from isolated cells are provided as a negative control (n = 10 cells).
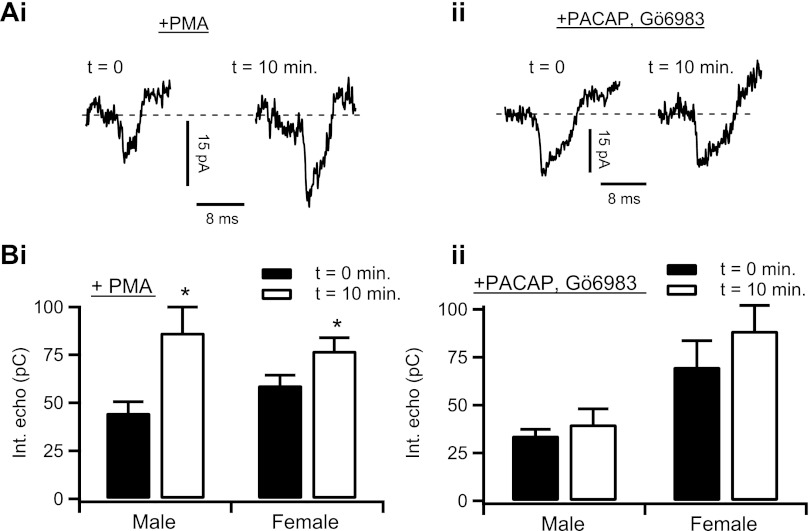
Fig. 5.
PACAP enhances electrical coupling through a PKC-dependent mechanism. Ai: electrical echoes were measured in chromaffin cells, as in Figs. 1–4, before (t = 0 min) and after (t = 10 min) focal and bulk perfusion of 100 nm PMA. Representative electrical echoes from each time point are shown. Aii: protocol was repeated in male mouse chromaffin cells (n = 10 echoes) and female mouse chromaffin cells (n = 22 echoes). Data from each PMA-stimulation time point were separated by gender and plotted as means ± SE. Male: *statistical significance at P = 0.025; female: *statistical significance at P = 0.048. Bi: an adrenal tissue slice was pretreated for 5 min with focal and bulk-perfused Gö6983 (100 nM) and then stimulated with focally perfused 1 μM PACAP for 10 min. Representative electrical echoes from the 0- and 10-min time point of PACAP stimulation are shown. Bii: protocol was repeated in male mouse chromaffin cells (n = 10 echoes) and female mouse chromaffin cells (n = 7 echoes). Data from each PACAP + Gö6983 stimulation time point were separated by gender and plotted as means ± SE. Male: P = 0.344; female: P = 0.306.
Table 2.
Western blot detection of connexins and PACR1
Values are means ± SE. Western blot detection of Cx36, Cx43, and pituitary adenylate cyclase-activating peptide (PACAP) receptor (PACR1) protein expression. β-Actin or β-tubulin normalized intensities from 3 experiments were pooled by gender and antibody type. Gene expression ratio (male:female) taken from normalized intensity values is reported.
Statistical significance was determined by unpaired Student's t-test with P value <0.001.
Table 3.
Membrane input resistance values
| Condition | Male GΩ ± SE (n) | Female GΩ ± SE (n) |
|---|---|---|
| In situ HEPES | 3.46 ± 0.31 (15) | 2.06 ± 0.28 (27)† |
| +Carbenoxolone (20 min) | 7.22 ± 2.77 (5)* | 3.81 ± 0.92 (6)*† |
| +PACAP | 1.67 ± 0.13 (5)* | 0.688 ± 0.21 (5)*† |
| +PACAP + Gö6983 | 3.31 ± 0.49 (5) | 2.15 ± 0.64 (5) |
| +PMA | 1.94 ± 0.15 (5)* | 0.514 ± 0.14 (8)*† |
| Isolated HEPES | 12.6 ± 4.85 (4)* | 12.7 ± 2.97 (6)* |
Average membrane resistance values ± SE for male and female mice are reported for all conditions. Sample size is reported in parentheses with significance for all data sets determined by an ANOVA test (P < 0.001). Paired (HEPES and treatment condition) and unpaired (between genders) Student's t-tests were performed with significance at
P < 0.05 for paired t-tests;
P < 0.05 for unpaired t-tests.
RESULTS
We investigated the possibility that acute PACAP excitation enhances electrical coupling in the adrenal medulla. Studies in other tissues report an increase in junctional conductance mediated by signaling events common to the PACAP signaling pathway, including PKC (43) and Epac (16). In this study, we report a gender dimorphism in the input resistance of chromaffin cells, in the intercellular spread of electrical excitation, and in the connexin composition of gap junctions in the adrenal medulla. Furthermore, we report that PACAP, via PKC phospho-regulation, enhances cell-cell communication in either gender through a functional increase in gap junction conductance. Experiments were performed in acute adrenal tissue slices, unless noted otherwise, and electrophysiological records were obtained in the perforated patch configuration to preserve native cytosolic composition and cell signaling processes.
Gap junctions in the mouse adrenal medulla.
LY dye diffusion assays are a commonly utilized method of studying gap junction connectivity between cells (17). Thus, to obtain an estimate of basal coupling levels in the mouse adrenal medulla, we first investigated the extent of LY diffusion between chromaffin cells in situ (Fig. 1A, top) by loading the primary patched cell with LY and measuring spread of LY signal to neighboring cells. LY diffusion was defined as spread of the fluorescence from the patched primary chromaffin cell to one or more unpatched secondary chromaffin cells to three times background at 20-min post break-in. As show in Fig. 1A, middle, LY dye does not diffuse to the neighboring chromaffin cells after 20 min. LY dye diffusion to one or more neighboring chromaffin cells (Fig. 1A, bottom) was only observed in 17.2% of patched cells. However, previous reports indicate the extent of LY dye diffusion can depend on connexin composition of gap junctions (6, 42) and may not provide a conclusive determination of electrical coupling (33). Thus we considered more sensitive electrophysiological methodologies to accurately estimate the degree of electrical coupling between chromaffin cells in situ. Moser (33) identified “echoing” action potentials from neighboring cells in electrical recordings of chromaffin cells in situ as a diagnostic determinant of cell-cell coupling. Echoing action potentials are elicited by a charge spreading from the primary voltage-clamped cell to neighboring unclamped cells. The charge injection into the secondary cell evokes an action potential that then conducts back through the gap junction to the primary, patched cell. These echoing action potentials are blocked by tetrodotoxin, confirming their dependence on voltage-activated Na+ channels and are measurable as inward current spikes on top of the primary cell current record (33). We tested if electrical echoes could be observed, even in cells that do not display LY dye diffusion. We devised an electrical protocol to depolarize the primary cell to the approximate reversal potential for inward Na+ and Ca2+ channels, and we conducted recordings in solutions that contained Cs2+ to block K+ currents in the primary cell. Together, these steps minimized active currents in the primary cell, thus maximizing the resolution of “echoes” from secondary cells. The representative record provided in Fig. 1B is from the same cell as in Fig. 1A in which LY dye spreading was not observed (middle). As seen in Fig. 1B, inset, echoing action potentials were observed on top of step-evoked membrane current. Thus, despite the lack of LY dye diffusion, the primary cell is indeed electrically coupled to at least one other cell within the medulla.
Most studies examining electrical coupling in the adrenal medulla utilized slices from female mice only (12, 31–33). Yet, males and females indeed respond differently to stress excitation (48). Thus we repeated both LY and electrical protocols in male and female mice and pooled the results, with mean percentage of coupled cell pairs reported in Fig. 1C. We report a gender dimorphism in the extent of LY dye diffusion with 22.2% of female mouse chromaffin cells exhibiting dye diffusion to one or more cells while only 8.33% of male mouse chromaffin cells exhibit dye diffusion to one or more cell. Likewise, cells were categorized as electrically coupled if action potential echoes were observed. Consistent with the LY dye diffusion data, we report a significant gender bias in the prevalence of electrical coupling. We report that 80.5% of chromaffin cells from female mice are electrically coupled, while 53.8% of chromaffin cells from male mice are electrically coupled. Evoked-current data from isolated cells did not exhibit action potential-like spikes, consistent with the conclusion that the echoes are junctional currents from neighboring cells. Thus both assays reported a gender dimorphism in coupling, but the electrical protocol was more sensitive than the LY dye spreading as previously reported (33).
Quantification of electrical coupling in the adrenal medulla.
A previous study (33) mathematically and experimentally established the relationship between amplitude of echoing action potentials and junctional conductance of neighboring cells. Similarly, the integral of echoing action potentials provides a measure of the charge passing through open gap junctions in series with the patch-clamped cell. However, accurate echo integral quantification may be complicated by factors such as a drifting or relatively high series resistance associated with the extended time frame of the perforated-patch recordings utilized here. To avoid issues relating to unstable series resistance, we eliminated cells that deviated >7 MΩ between sweeps from further analysis. Cells are treated with pharmacological agents and echoes are obtained at the 0- and 10-min time points (see Figs. 2–5). Thus, in an initial description of the system, we determined the inherent stability of echoes from single cells as a function of time. A representative electrical echo from the +50 mV step is shown in Fig. 2Ai, left (“t = 0 min”). After 10 (“t = 10 min”) and 20 min (“t = 20 min”), the patched cell was restimulated and example electrical echoes are shown again (Fig. 2Ai, middle and right). The shaded grey region indicates the region used for integral analysis. The echo integral does not significantly change between t = 0, t = 10, or t = 20 time points, thus demonstrating that cells express a stable and measureable coupling if left unperturbed (Fig. 2Aii).
CBX abolishes echo currents in chromaffin cells in situ.
Next, to test that the observed electrical coupling is indeed due to gap junctions, slices were treated with 100 μm CBX, a commonly utilized gap junction decoupling agent (14). In the representative recording shown in Fig. 2Bi, a cell was patched and held at −80 mV and stepped to +50 mV (Fig. 2Bi, “CBX t = 0 min”) in normal HEPES ringer solution. The cell was treated with 100 μm CBX for 20 min, consistent with the pharmacokinetics of CBX gap junction block (32), and stepped to +50 mV at the 10-min time point (sample data not shown) and at the completion of the 20-min (“CBX t = 20 min”) treatment period. The sample recording provided shows a loss of all electrical echoes after 20 min of CBX treatment. The integrals of echoing action potentials from t = 0 min, t = 10 min, and t = 20 min conditions were pooled and plotted as means ± SE, shown in Fig. 2Bii (cells exhibiting no echoes were assigned an integral value = 0 pC). These data demonstrate that the echo currents are CBX sensitive as expected for electrical signaling through gap junctions from secondary unclamped cells back into the primary voltage-clamped chromaffin cell. However, it is possible that some chromaffin cells are weakly electrically coupled and do not fire action potentials in response to current injection from the primary cell. Thus we turned to a complementary electrical approach based on measuring the passive cell input resistance.
Intercellular gap junction coupling directly impacts cell input resistance (Rmemb), a readily measurable parameter. If the primary cell is electrically coupled, its input resistance will be lower than that of a noncoupled cell due to a shunt circuit through the gap junction into a secondary cell. Thus we also measured input resistance of the primary cell membrane at electrically passive hyperpolarizing conditions under K+ current-block (Cs+-pipette solution, see materials and methods) and where membrane current follows Ohm's law. We hyperpolarized cells from the −80-mV holding potential to −130 mV to measure input resistance, with the result shown in Fig. 2Ci. The trace labeled 0 min was before superfusion with a HEPES Ringer solution supplemented with 100 μm CBX while the 20-min trace represents complete CBX block. Data were also collected from the CBX 10-min time point. As expected, an increase in input resistance due to the CBX block is readily apparent. We repeated this protocol as above and the pooled data from the 0-, 10-, and 20-min time points are plotted as means ± SE in Fig. 2Cii. Taken together, both the CBX-sensitive propagation of active action potential currents from neighboring cells (echoes) as well as the CBX-sensitive primary cell input resistance indicate that the electrical action potential echoes observed under +50 mV depolarization are junctional currents from coupled secondary chromaffin cells.
Gender dimorphism of adrenal medullary electrical coupling.
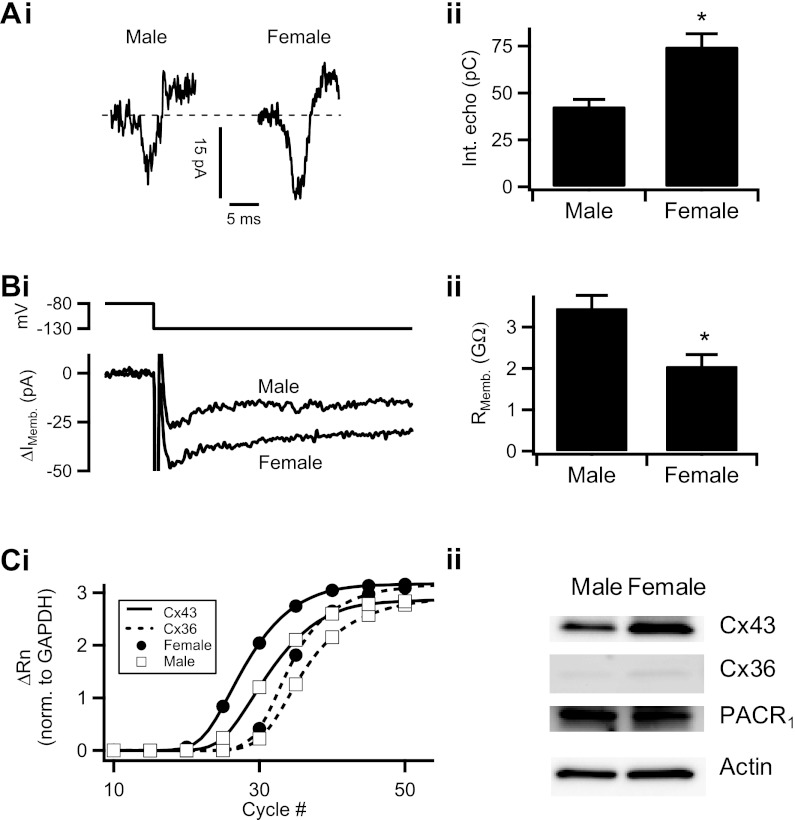
In rats, females in general express a greater increase in plasma catecholamine in response to stress (28, 48). In Fig. 1, we reported a gender dimorphism in the extent of electrical coupling in the adrenal medulla. We considered the possibility that female mouse gap junctions are able to carry more charge than male mouse chromaffin cells. To investigate this possibility, a male mouse chromaffin cell in situ was voltage-clamped and held at −80 mV. The cell was stepped to +50 mV, as in Fig. 1. A representative electrical echo (“male”) is shown in Fig. 3Ai, left. The same electrical protocol was repeated on a slice taken from a female mouse and a representative electrical echo from the resulting current trace (“female”) is shown in Fig. 3Ai, right. The electrical protocol was repeated in chromaffin cells from slices obtained from male and female mice. Echo integrals were calculated, separated by gender, and pooled. In Fig. 3Aii, the pooled data are reported as means ± SE. We report that the echo integral was significantly larger in female mice vs. male mice. Thus our data suggest that female intercellular coupling is significantly higher than in male mice.
Fig. 3.
Gender dimorphism in electrical coupling. Ai: as in Figs. 1 and 2, electrical echoes were evoked from a voltage-clamped cell and example current echoes from male and female mice are shown. Aii: protocol was repeated in male mouse chromaffin cells (n = 69 echoes) and female mouse chromaffin cells (n = 96 echoes). AP echo sizes were quantified by integration as in Fig. 2. Data were pooled and plotted as means ± SE (*statistical significance at P = 0.0003). Bi: in male and female mouse chromaffin cells, input resistance was calculated following the procedure in Fig. 2Ci. Representative traces of resulting evoked membrane currents are shown. Bii: this protocol was repeated in 15 cells from male mice and 27 cells from female mice. Pooled input resistance values are presented as mean ± SE (*statistical significance at P = 0.003). Ci: real-time quantitative PCR was performed for connexin-43 (Cx43; solid line) and connexin-36 (dotted line) in male (☐) and female mice (●). Representative amplification curves were normalized to GAPDH and plotted. Rn, normalized reporter dye fluorescence. Cii: Western blot analysis of Cx43, Cx36, and pituitary adenylate cyclase-activating peptide (PACAP) receptor (PACR1) in the male and female mouse adrenal medulla. β-Actin or β-tubulin was used as a loading control. A representative blot from one experiment is presented in Cii, with averaged actin or tubulin-normalized gene expression ratios from multiple experiments presented in Table 2.
If female mouse chromaffin cells are more highly electrically coupled than male mouse chromaffin cells, then it is expected that the input resistance will be lower in the female mouse adrenal medulla. To examine this possibility, we repeated the hyperpolarizing electrical paradigm utilized in Fig. 2C. Cells from male mice were voltage-clamped at −80 mV and delivered a single hyperpolarizing step to −130 mV. Representative current traces from male and female adrenal sections are shown in Fig. 3Bi. This hyperpolarizing protocol was repeated in male and female mouse chromaffin cells, and the input resistance was calculated as described above. Data were pooled separately for each gender and plotted as means ± SE. This analysis shows that the input resistance is significantly lower in female mice vs. male mice, consistent with the findings that the adrenal medullary cells of female mice are more highly electrically coupled.
Greater Cx43 and Cx36 expression in the female mouse adrenal medulla.
We considered that the gender dimorphism in electrical coupling may be due to a differential expression of gap junction-forming connexins. Although high-conductance Cx43 and low-conductance Cx36 have both been shown to be present in the mouse adrenal medulla (15, 34), their gender-specific expression has yet to be determined. We performed real time-quantitative PCR for Cx43 and Cx36 in age-matched males and females. Representative amplification curves internally normalized to GAPDH levels are provided for Cx36 and Cx43 in Fig. 3Ci. ΔCt scores for Cx36 and Cx43 are reported in Table 1. Message for Cx36 is similar in both genders. Although not formally statistically significant, females contained higher message for Cx43 in all PCR trials. Next, we tested protein expression levels by Western blot analysis for Cx36 and Cx43 for each gender. Representative blots for males and females are shown in Fig. 3Cii. We found that both Cx36 and Cx43 are more highly expressed in females vs. males (1.7- and 2.31-fold, respectively; actin or tubulin-normalized, see Table 2). It is important to note that circulating estrogen impacts Cx43 gene expression levels (21). Thus the biochemical data sets from female mice exhibited more variability. Moreover, we also tested if male and female mice expressed similar levels of PACAP receptor (PACR1) to determine if the females are more reactive to stress due to enhanced PACAP excitation (a point to be expanded upon below). Counter to our hypothesis, males exhibited increased expression level for PACR1 (Fig. 3Cii; Table 2), indicating that if anything, males would be more sensitive to stress if this were a factor. Take together, these data support the hypothesis that female mice express a greater message for the high conductance Cx43, as well as the low conductance Cx36, than males and that this difference may contribute to observed differences in electrical coupling behavior.
PACAP stimulation increases electrical coupling.
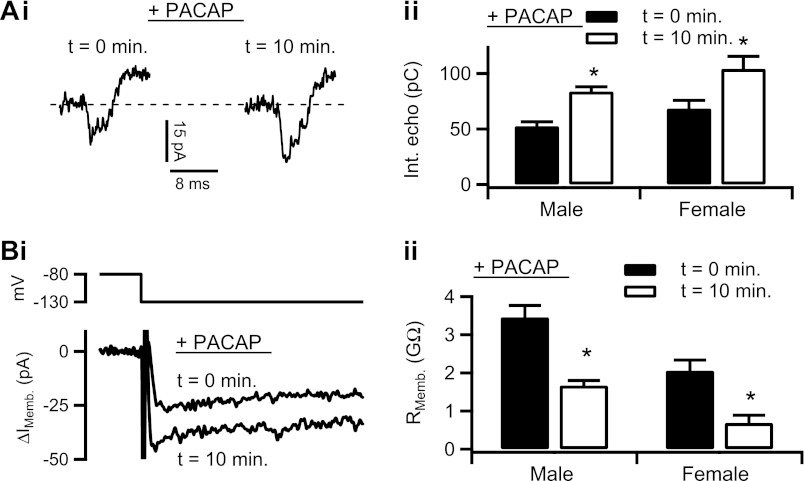
Studies (28, 48) describe gender-specific responses to acute sympathetic stress at the organismal level. Yet, little is known about the gender-specific roles of adrenal medullary gap junctions in PACAP-evoked acute stress. We investigated the possibility that PACAP stimulation leads to functional gender-specific gap junction remodeling in the adrenal medulla. We measured both echo integrals and input resistance as above, before and after cell excitation with 1 μM exogenous PACAP delivered by focal perfusion. Sample raw records for each protocol are provided in Fig. 4, Ai and Bi, and show an increased echo amplitude and decreased input resistance with 10-min PACAP excitation, respectively. The electrical protocols were repeated in male and female mouse chromaffin cells, with resulting control echo integral data quantified and pooled in Fig. 4Aii as means ± SE. PACAP excitation significantly increased echo integrals in both genders. Input resistance values were also pooled and are presented in Fig. 4Bii for both male and female mouse chromaffin and plotted as means ± SE. In both genders, PACAP treatment significantly decreased input resistance values. Taken together, both protocols report that acute PACAP excitation increases junctional communication between in situ chromaffin cells. Cells from female mice express greater coupling, but both genders' electrical coupling is proportionally increased by PACAP.
Fig. 4.
PACAP increases electrical coupling. Ai: effect of PACAP stimulation on electrical coupling was tested and a representative recording is provided. Cell was treated with 1 μM PACAP by focal perfusion for 10 min, and the electrical echo protocol was repeated, showing an increase in echo magnitude. Aii: protocol was repeated in male mouse chromaffin cells (n = 21 echoes) and female mouse chromaffin cells (n = 27 echoes). Echo integral data from each PACAP-stimulation time point were separated by gender and plotted as means ± SE. Male: *statistical significance at P = 0.0001; female: *statistical significance at P = 0.011. Bi: input resistance in response to PACAP stimulation was measured, as in Fig. 2 from a chromaffin cell. Representative traces show an increase in inward IMemb after 10 min PACAP treatment at −130 mV, indicating a decreased input resistance. Bii: this protocol was repeated in 15 cells from male mice and in 27 cells from female mice. Input resistance data were separated by gender and treatment condition and plotted as means ± SE. Male: *statistical significance at P = 0.005; female: *statistical significance at P = 0.048.
PACAP acts through PKC to increase electrical coupling.
Previous studies have demonstrated acute PACAP to signal through a requisite activation of PKC-dependent signaling (26, 39, 50). We investigated the possibility that PKC activation mirrors the effect of PACAP treatment and upregulates electrical coupling in the adrenal medulla. We again turned to the integrated action potential echo analysis as well as input resistance to test the effects of the phorbol ester PMA on adrenal medullary electrical coupling in male and female mice. Sample records for the action potential echo protocol recorded from a female medulla are provided in Fig. 5Ai and show that 10 min of PMA stimulation (100 nM, focally and bulk perfused) increased action potential echo amplitude. Echo integrals were measured, pooled, and plotted as means ± SE in Fig. 5Aii. In the negative condition, we also confirmed that acute PACAP stimulation modulates gap junction connectivity through a PKC-sensitive step by pretreating adrenal slices with general PKC inhibitor, Gö6983 (100 nM) and then treating cells with PACAP. Again, sample raw records for this condition were recorded from an adrenal slice taken from female mice and pooled and are presented in Fig. 5Bi, showing that Gö6983 blocked the increased echo amplitude seen with PACAP stimulation. Pooled data in Fig. 5Bii confirm this effect. Input resistance was calculated under both PMA and PACAP + Gö6983 conditions, and these values are presented and quantified, by gender, in Table 3. Input resistance significantly decreases in both genders under PMA stimulation. These data suggest that acute PACAP excitation increases adrenal medullary electrical coupling by a signaling mechanism that shares a PKC-dependent step, just as acute PACAP-evoked catecholamine secretion.
DISCUSSION
The sympathetic nervous system fires at a low tonic level in unstressed animals, setting them into the overall homeostatic state of energy storage. In this state, modest catecholamine release is mediated through splanchnic release of acetylcholine to trigger action potential-evoked Ca2+-dependent catecholamine exocytosis from the adrenal medulla (1, 7, 13, 18). Under basal sympathetic tone, catecholamine release acts in concert with the parasympathetic nervous system to set integral physiological functions such as heart rate, insulin secretion, and shunting of blood to the viscera. Studies (32) have also reported that the cholinergic system exerts tonic inhibitory control over gap junction coupling. It seems likely that cholinergic inhibitory control of gap junctions under sympathetic tone works to fine-tune catecholamine secretion by limiting excitation during the “rest and digest” state of energy storage. Misregulation of catecholamine secretion can lead to hypertension, cardiac arrhythmia, diabetes, hyperventilation, and paroxysmal inflammation.
Activation of the sympathoadrenal stress reflex fundamentally alters splanchnic-adrenal excitation. It has been documented that, under chronic stress, the adrenal medulla undergoes functional remodeling to increase cell-cell coupling (12), which can spread excitation to evoke catecholamine release from clusters of cells (31). While specific nicotinic acetylcholine receptor activation decreases cell coupling (11, 32), studies (32, 36) have shown that general cholinergic agonists enhance gap junctional communication or promote the spread of excitation. In the mouse hemi-sectioned adrenal gland, acetylcholine decreased the input resistance of chromaffin cells. The authors (36) proposed that the actions of ACh on input resistance likely result from ACh binding to muscarinic receptors and a subsequent decrease of junctional resistance through a muscarinic signaling pathway. Thus muscarinic ACh receptors, the activation of which lasts longer than rapidly inactivating nicotinic receptors (37), may work in concert with stress-evoked PACAP stimulation to positively modulate medullary electrical communication. This is an attractive possibility in that nicotinic receptors are clustered at the synapse while muscarinic receptors are broadly distributed (45) and may only be activated under elevated or sustained stimulation causing ACh synaptic spillover, a condition that would be expected under sympathetic stress. Another possibility is that under long-term PACAP exposure connexin gene expression increases via a PKA- or PKC-dependent mechanism, effectively remodeling the adrenal medulla to support sustained secretion. Indeed, PACAP has been shown to activate a number of stress-related genes on the hour timescale (38, 41, 49). Although a significant enhancement of connexin gene transcription is unlikely to account for the substantial and rapid enhancement in electrical coupling observed in our study, it seems likely that PACAP stimulates gene expression of connexins under a longer time scale to electrically remodel the adrenal medulla.
Gap junctions have been widely implicated in the secretion process in other tissues, as well. In the other tissues such as the pancreas, Benninger et al. (3) showed that insulin secretion is minimal under basal (low glucose) conditions in the neurosecretory β-cells of the pancreatic islet of Langerhans. However, when the islet is dissociated, isolated β-cells respond to low glucose conditions with heightened insulin secretion, suggesting that gap junctions suppress insulin secretion in the intact islet. Interestingly, Head et al. (23) showed that regulation of insulin secretion occurs through Cx36 containing gap junction channels. However, it may be that in some systems gap junctional coupling does not necessarily relate to secretory function. In the lacrimal gland, Walcott et al. (46) reported a gender dimorphism in the distribution of Cx26 and Cx32, despite similar secretory responses. Our data indicate a differential expression of connexins within the male and female adrenal medullae. Despite statistically similar transcript levels, females exhibit a 2.3-fold increase in Cx43 protein expression and a 1.7-fold increase in Cx36 protein expression over males. This difference in Cx43 expression may be dictated by differences in circulating female sex hormones (21). In Fig. 1, we report that female mouse chromaffin cells exhibit more LY dye diffusion than do male mouse chromaffin cells. Several studies indicate that the connexin composition of gap junctions has a significant impact on the extent of LY dye diffusion. For example, Cx43-containing gap junctions allow the diffusion of LY much more readily than do Cx36-containing gap junctions (8). We provide data showing that gap junctions in female mouse adrenal medullae carry more electrical charge than male mouse adrenal medullae, as evidenced by higher echo integral and decreased input resistance in female mice. However, the observations that females have larger echoes, lower input resistance, and more extensive dye spreading argue that the gap junctions linking females cells are of a higher conductance form. This is consistent with our finding that female mice express a higher proportion of Cx43-containing gap junctions than do male mice, which may account for functional differences. We also show that female mice have a higher total protein level for both connexin isoforms, which likely translates to more functional gap junctions. Interestingly, echo analysis revealed that PACAP excitation significantly increases junctional communication in both male and female mice. PACAP decreased input resistance in male and female mice, suggesting that PACAP increased junctional communication effectively in both genders.
Thus studies (48) conducted at the whole animal level have shown that females secrete more epinephrine than males in response to certain stressors. This gender-dimorphic response to stress is not necessarily occurring at the level of the electrically coupled adrenal unit and may lie in differences in gland morphology (i.e., different proportion of epinephrine- vs. norepinephrine-secreting cells, differential splanchnic innervation, etc.). Nevertheless, PACAP represents a critical component of the acute sympathetic stress response. The ability of PACAP to remodel gap junction coupling in the adrenal medulla may represent a point of regulation for secretion during acute sympathetic stress for both males and females.
GRANTS
J. Hill was supported by the National Heart, Lung, and Blood Institute Grant T32-HL-07887 and American Heart Association Grant 10PRE4100002. S.-K. Lee was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant R37 DK30344 to Walter F. Boron.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.H. conception and design of research; J.H., S.-K.L., and P.S. performed experiments; J.H. and S.-K.L. analyzed data; J.H., S.-K.L., and C.S. interpreted results of experiments; J.H. and C.S. prepared figures; J.H. drafted manuscript; J.H., S.-K.L., P.S., and C.S. edited and revised manuscript; J.H. and C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Shyue-An Chan and Angelique Do for technical assistance in adrenal slice preparation and RT-quantitative PCR detection of Cxs, respectively.
REFERENCES
- 1. Albillos A, Neher E, Moser T. R-type Ca2+ channels are coupled to the rapid component of secretion in mouse adrenal slice chromaffin cells. J Neurosci 20: 8323–8330, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aunis D. Exocytosis in chromaffin cells of the adrenal medulla. Int Rev Cytol 181: 213–320, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Benninger RK, Head WS, Zhang M, Satin LS, Piston DW. Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. J Physiol 589: 5453–5466, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bevington A, Radda GK. Declining catecholamine secretion in adrenal medulla on prolonged stimulation with acetylcholine. Biochem Pharmacol 34: 1497–1500, 1985 [DOI] [PubMed] [Google Scholar]
- 5. Boksa P, Livett BG. Desensitization to nicotinic cholinergic agonists and K+, agents that stimulate catecholamine secretion, in isolated adrenal chromaffin cells. J Neurochem 42: 607–617, 1984 [DOI] [PubMed] [Google Scholar]
- 6. Cao F, Eckert R, Elfgang C, Nitsche JM, Snyder SA, Hu DF, Willecke K, Nicholson BJ. A quantitative analysis of connexin-specific permeability differences of gap junctions expressed in HeLa transfectants and Xenopus oocytes. J Cell Sci 1: 31–43, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Chan SA, Polo-Parada L, Smith C. Action potential stimulation reveals an increased role for P/Q-calcium channel-dependent exocytosis in mouse adrenal tissue slices. Arch Biochem Biophys 435: 65–73, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Charpantier E, Cancela J, Meda P. Beta cells preferentially exchange cationic molecules via connexin 36 gap junction channels. Diabetologia 50: 2332–2341, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Colomer C, Desarmenien MG, Guerineau NC. Revisiting the stimulus-secretion coupling in the adrenal medulla: role of gap junction-mediated intercellular communication. Mol Neurobiol 40: 87–100, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colomer C, Martin AO, Desarmenien MG, Guerineau NC. Gap junction-mediated intercellular communication in the adrenal medulla: An additional ingredient of stimulus-secretion coupling regulation. Biochim Biophys Acta 1818: 1937–1951, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Colomer C, Olivos-Ore LA, Vincent A, McIntosh JM, Artalejo AR, Guerineau NC. Functional characterization of alpha9-containing cholinergic nicotinic receptors in the rat adrenal medulla: implication in stress-induced functional plasticity. J Neurosci 30: 6732–6742, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colomer C, Olivos Ore LA, Coutry N, Mathieu MN, Arthaud S, Fontanaud P, Iankova I, Macari F, Thouennon E, Yon L, Anouar Y, Guerineau NC. Functional remodeling of gap junction-mediated electrical communication between adrenal chromaffin cells in stressed rats. J Neurosci 28: 6616–6626, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Diego AM. Electrophysiological and morphological features underlying neurotransmission efficacy at the splanchnic nerve-chromaffin cell synapse of bovine adrenal medulla. Am J Physiol Cell Physiol 298: C397–C405, 2010 [DOI] [PubMed] [Google Scholar]
- 14. de Groot JR, Veenstra T, Verkerk AO, Wilders R, Smits JP, Wilms-Schopman FJ, Wiegerinck RF, Bourier J, Belterman CN, Coronel R, Verheijck EE. Conduction slowing by the gap junctional uncoupler carbenoxolone. Cardiovasc Res 60: 288–297, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Degen J, Meier C, Van Der Giessen RS, Sohl G, Petrasch-Parwez E, Urschel S, Dermietzel R, Schilling K, De Zeeuw CI, Willecke K. Expression pattern of lacZ reporter gene representing connexin36 in transgenic mice. J Comp Neurol 473: 511–525, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Duquesnes N, Derangeon M, Metrich M, Lucas A, Mateo P, Li L, Morel E, Lezoualc'h F, Crozatier B. Epac stimulation induces rapid increases in connexin43 phosphorylation and function without preconditioning effect. Pflügers Arch 460: 731–741, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Elfgang C, Eckert R, Lichtenberg-Frate H, Butterweck A, Traub O, Klein RA, Hulser DF, Willecke K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J Cell Biol 129: 805–817, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garcia de Diego AM, Arnaiz JJ, Gandia L, Hernandez-Guijo JM, Garcia AG. A comparison between acetylcholine-like action potentials and square depolarizing pulses in triggering calcium entry and exocytosis in bovine chromaffin cells. J Mol Neurosci 30: 57–58, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem 65: 475–502, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Grynszpan-Wynograd O, Nicolas G. Intercellular junctions in the adrenal medulla: a comparative freeze-fracture study. Tissue Cell 12: 661–672, 1980 [DOI] [PubMed] [Google Scholar]
- 21. Gulinello M, Etgen AM. Sexually dimorphic hormonal regulation of the gap junction protein, CX43, in rats and altered female reproductive function in CX43+/- mice. Brain Res 1045: 107–115, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guo X, Wakade AR. Differential secretion of catecholamines in response to peptidergic and cholinergic transmitters in rat adrenals. J Physiol 475: 539–545, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Head WS, Orseth ML, Nunemaker CS, Satin LS, Piston DW, Benninger RK. Connexin-36 gap junctions regulate in vivo first- and second-phase insulin secretion dynamics and glucose tolerance in the conscious mouse. Diabetes 2012. April 17 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hill J, Chan SA, Kuri B, Smith C. Pituitary adenylate cyclase-activating peptide (PACAP) recruits low voltage-activated T-type calcium influx under acute sympathetic stimulation in mouse adrenal chromaffin cells. J Biol Chem 286: 42459–42469, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Klevans LR, Gebber GL. Comparison of differential secretion of adrenal catecholamines by splanchnic nerve stimulation and cholinergic agents. J Pharmacol Exp Ther 172: 69–76, 1970 [PubMed] [Google Scholar]
- 26. Kuri BA, Chan SA, Smith CB. PACAP regulates immediate catecholamine release from adrenal chromaffin cells in an activity-dependent manner through a protein kinase C-dependent pathway. J Neurochem 110: 1214–1225, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kwak BR, van Veen TA, Analbers LJ, Jongsma HJ. TPA increases conductance but decreases permeability in neonatal rat cardiomyocyte gap junction channels. Exp Cell Res 220: 456–463, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Livezey GT, Miller JM, Vogel WH. Plasma norepinephrine, epinephrine and corticosterone stress responses to restraint in individual male and female rats, and their correlations. Neurosci Lett 62: 51–56, 1985 [DOI] [PubMed] [Google Scholar]
- 29. Marley PD. Desensitization of the nicotinic secretory response of adrenal chromaffin cells. Trends Pharmacol Sci 9: 102–107, 1988 [DOI] [PubMed] [Google Scholar]
- 30. Martin AO, Alonso G, Guerineau NC. Agrin mediates a rapid switch from electrical coupling to chemical neurotransmission during synaptogenesis. J Cell Biol 169: 503–514, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martin AO, Mathieu MN, Chevillard C, Guerineau NC. Gap junctions mediate electrical signaling and ensuing cytosolic Ca2+ increases between chromaffin cells in adrenal slices: A role in catecholamine release. J Neurosci 21: 5397–5405, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martin AO, Mathieu MN, Guerineau NC. Evidence for long-lasting cholinergic control of gap junctional communication between adrenal chromaffin cells. J Neurosci 23: 3669–3678, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moser T. Low-conductance intercellular coupling between mouse chromaffin cells in situ. J Physiol 506: 195–205, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murray SA, Pharrams SY. Comparison of gap junction expression in the adrenal gland. Microsc Res Tech 36: 510–519, 1997 [DOI] [PubMed] [Google Scholar]
- 35. Mustafa T, Walsh J, Grimaldi M, Eiden LE. PAC1hop receptor activation facilitates catecholamine secretion selectively through 2-APB-sensitive Ca(2+) channels in PC12 cells. Cell Signal 22: 1420–1426, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nassar-Gentina V, Pollard HB, Rojas E. Electrical activity in chromaffin cells of intact mouse adrenal gland. Am J Physiol Cell Physiol 254: C675–C683, 1988 [DOI] [PubMed] [Google Scholar]
- 37. Sala F, Nistri A, Criado M. Nicotinic acetylcholine receptors of adrenal chromaffin cells. Acta Physiol (Oxf) 192: 203–212, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Stroth N, Eiden LE. Stress hormone synthesis in mouse hypothalamus and adrenal gland triggered by restraint is dependent on pituitary adenylate cyclase-activating polypeptide signaling. Neuroscience 165: 1025–1030, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tanaka K, Shibuya I, Harayama N, Nomura M, Kabashima N, Ueta Y, Yamashita H. Pituitary adenylate cyclase-activating polypeptide potentiation of Ca2+ entry via protein kinase C and A pathways in melanotrophs of the pituitary pars intermedia of rats. Endocrinology 138: 4086–4095, 1997 [DOI] [PubMed] [Google Scholar]
- 40. TenBroek EM, Lampe PD, Solan JL, Reynhout JK, Johnson RG. Ser364 of connexin43 and the upregulation of gap junction assembly by cAMP. J Cell Biol 155: 1307–1318, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tonshoff C, Hemmick L, Evinger MJ. Pituitary adenylate cyclase activating polypeptide (PACAP) regulates expression of catecholamine biosynthetic enzyme genes in bovine adrenal chromaffin cells. J Mol Neurosci 9: 127–140, 1997 [DOI] [PubMed] [Google Scholar]
- 42. Valiunas V, Beyer EC, Brink PR. Cardiac gap junction channels show quantitative differences in selectivity. Circ Res 91: 104–111, 2002 [DOI] [PubMed] [Google Scholar]
- 43. van Veen TA, van Rijen HV, Jongsma HJ. Electrical conductance of mouse connexin45 gap junction channels is modulated by phosphorylation. Cardiovasc Res 46: 496–510, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Wakade AR. Multiple transmitter control of catecholamine secretion in rat adrenal medulla. Adv Pharmacol 42: 595–598, 1998 [DOI] [PubMed] [Google Scholar]
- 45. Wakade AR, Wakade TD. Contribution of nicotinic and muscarinic receptors in the secretion of catecholamines evoked by endogenous and exogenous acetylcholine. Neuroscience 10: 973–978, 1983 [DOI] [PubMed] [Google Scholar]
- 46. Walcott B, Moore LC, Birzgalis A, Claros N, Valiunas V, Ott T, Willecke K, Brink PR. Role of gap junctions in fluid secretion of lacrimal glands. Am J Physiol Cell Physiol 282: C501–C507, 2002 [DOI] [PubMed] [Google Scholar]
- 47. Watanabe T, Masuo Y, Matsumoto H, Suzuki N, Ohtaki T, Masuda Y, Kitada C, Tsuda M, Fujino M. Pituitary adenylate cyclase activating polypeptide provokes cultured rat chromaffin cells to secrete adrenaline. Biochem Biophys Res Commun 182: 403–411, 1992 [DOI] [PubMed] [Google Scholar]
- 48. Weinstock M, Razin M, Schorer-Apelbaum D, Men D, McCarty R. Gender differences in sympathoadrenal activity in rats at rest and in response to footshock stress. Int J Dev Neurosci 16: 289–295, 1998 [DOI] [PubMed] [Google Scholar]
- 49. Wong DL, Anderson LJ, Tai TC. Cholinergic and peptidergic regulation of phenylethanolamine N-methyltransferase gene expression. Ann NY Acad Sci 971: 19–26, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Zhou CJ, Yada T, Kohno D, Kikuyama S, Suzuki R, Mizushima H, Shioda S. PACAP activates PKA, PKC and Ca(2+) signaling cascades in rat neuroepithelial cells. Peptides 22: 1111–1117, 2001 [DOI] [PubMed] [Google Scholar]