Abstract
Background
Vegetables of the genus Allium are widely consumed but remain poorly understood genetically. Genetic mapping has been conducted in intraspecific crosses of onion (Allium cepa L.), A. fistulosum and interspecific crosses between A. roylei and these two species, but it has not been possible to access genetic maps and underlying data from these studies easily.
Description
An online comparative genomics database, AlliumMap, has been developed based on the GMOD CMap tool at http://alliumgenetics.org. It has been populated with curated data linking genetic maps with underlying markers and sequence data from multiple studies. It includes data from multiple onion mapping populations as well as the most closely related species A. roylei and A. fistulosum. Further onion EST-derived markers were evaluated in the A. cepa x A. roylei interspecific population, enabling merging of the AFLP-based maps. In addition, data concerning markers assigned in multiple studies to the Allium physical map using A. cepa-A. fistulosum alien monosomic addition lines have been compiled. The compiled data reveal extensive synteny between onion and A. fistulosum.
Conclusions
The database provides the first online resource providing genetic map and marker data from multiple Allium species and populations. The additional markers placed on the interspecific Allium map confirm the value of A. roylei as a valuable bridge between the genetics of onion and A. fistulosum and as a means to conduct efficient mapping of expressed sequence markers in Allium. The data presented suggest that comparative approaches will be valuable for genetic and genomic studies of onion and A. fistulosum. This online resource will provide a valuable means to integrate genetic and sequence-based explorations of Allium genomes.
Background
The large monocot genus Allium comprises hundreds of species and includes several with great economic, culinary and health value. Onion and shallot (Allium cepa L.; 2n = 2X = 16) are among the most economically significant monocot species outside the commelinoid grasses [1]. A. fistulosum (Japanese Bunching or Welsh Onion; 2n = 2X = 16), leek (A. porrum; (2n = 4X = 32) and garlic (A. sativum; 2n = 2X = 16) are widely grown and traded, with many other species being locally significant as spices and flavorings. Allium species are notable for their very large genomes, typically in the range 10–20 Gbp [2], which have complicated genomic studies and precluded genome sequencing to date. Genetic map development in onion and other Allium has been limited by difficulty in developing, maintaining and exchanging genetic stocks, high degrees of heterozygosity, and a dearth of sequence data [3].
The first published genetic map of an Allium species was that developed by King and colleagues [4] in the intraspecific onion cross 'BYG15-23 x AC43'. Constructed initially using RFLP markers, this map was subsequently augmented with SNP and SSR markers derived from EST sequencing [5,6]. These more portable markers enabled partial map construction in other intraspecific onion crosses to enable map-based genetic analysis of fertility restoration [7], color [8] and other bulb traits [9,10].
The breeding systems of A. fistulosum have facilitated development of several larger mapping pedigrees and detailed genetic maps based initially on SSR and AFLP markers [11,12]. These maps were used to conduct QTL analysis for seedling vigor [13]. More recently Tsukazaki and colleagues [14] reported a further A. fistulosum map based on A. fistulosum genomic SSR markers and onion EST-derived SNP and SSR markers, providing further scope for comparative studies between onion and A. fistulosum genomes. The only Allium relative known to readily produce fertile hybrids with onion is A. roylei[15], which has been used to develop an interspecific map [16] and backcross progenies with valuable disease resistance [17,18]. Since A. roylei also crosses with A. fistulosum, this has enabled development of bridge crosses containing all three genomes [19], thus enabling a potential path for introgression of A. fistulosum genetics into onion.
The key resource that has enabled alignment of Allium genetic maps to physical chromosomes and facilitated comparison among species is the sets of A. fistulosumA. cepa alien monosomic addition lines (AMALs) developed by Shigyo and colleagues [20]. These were initially applied to anchor AFLP-based maps in the interspecific A. cepa x A. roylei cross [21] and subsequently to anchor the 'BYG15-23 x AC43' map [6]. Subsequently they were used to anchor SSR-based maps in A. fistulosum[12] to physical chromosomes, and more recently to assign many more onion EST-derived anchor markers used in A. fistulosum maps [14].
In other studies, a large number of phenotypic and molecular markers, including many candidate genes relating to economic traits, have also been assigned to chromosomes [6,22-26], providing a valuable guide for functional and QTL studies. These findings have been reported in diverse publications but have not to date been available in an accessible or integrated manner.
Genome sequence, map and marker data from Allium species have to date been limited and difficult to access. Marker assays from the 'BYG15-23 x AC43' population have been accessible through Genbank [27] and garlic EST data have been presented through a web database [28]. Recently, Bhasi and colleagues [29] presented RobustDb, a generic online genomics database most notably containing garlic map and marker data. The VegMarks database [30] contains detailed information concerning A. fistulosum markers. Neither of these databases provides comparative data. Increasing development of doubled haploid stocks [31,32] and availability of next-generation sequencing mean that Allium marker and map resources will expand rapidly in the near future. Therefore it is important to provide existing map and marker data in an accessible form with links to underlying sequence, to enable integration of new data with past studies.
Comparative genomic approaches have been widely used and proven in crop genetics, and are of growing interest as improved sequencing technologies enable ever broader and more detailed surveys of germplasm [33]. Online databases integrating genetic map, marker, sequence and germplasm data such as Gramene [34] and GDR [35] are now key tools for publishing and exploiting such data from the monocot grasses and the Rosaceae family respectively. Given their economic significance, there is a clear and pressing need for such resources in Allium.
The use of many common onion EST-derived markers and the extensive use of AMALs to anchor both onion and A. fistulosum maps provide the potential for similar comparative approaches to be used in Allium genetics and genomics. In this study we present an integrated view of genetic maps in onion and A. roylei and an online database in which these can be explored.
Construction and content
Interspecific allium map integration
The interspecific A. cepa x A. roylei interspecific map was augmented with additional genetic markers to increase correspondences among Allium maps. A total of 107 markers comprising 73 additional onion EST-SSRs, 3 A. fistulosum genomic SSRs and 31 SNP markers derived from onion ESTs were evaluated in the population previously used to construct an AFLP-based linkage map [16] using previously published methods [9]. Previously unpublished markers are shown in Table 1. Revised genetic maps were calculated using JoinMap 4.0 software [36]. Linkage groups were first formed using LOD 5 cutoff from two data sets each containing co-dominant markers plus dominant markers from one parental phase. These were then merged and linkage maps constructed using default settings and Kosambi distances.
Table 1.
Previously unpublished primer sets mapped in the interspecific Allium cepa x A. roylei population
| Primer Set | Genbank Accession | FORWARD PRIMER | REVERSE PRIMER |
|---|---|---|---|
| ACABE58 |
CF447676 |
TCTTCGAGAACTATCCCGACAT |
ACTCAACCGCTGTTACAAGGAT |
| ACI017 |
AY585678 |
CCGACTACATGTAAGTTGCATTAAC |
TCTTGCATAATTTCACTGCACA |
| ACM005 |
BI095610 |
CGCTTCAGCAGTGAGTTGTT |
TGTTGTCCGATACAGAGTTGCT |
| ACM021 |
CF448154 |
AAAACCCTCAACATCTCACTCC |
TCTCTTCTTCCTCGTCCTGC |
| ACM037 |
CF438925 |
GACCGACTCCAAAGCCATA |
CTCTCCCGTTCTCAAAATGC |
| ACM049 |
CF447728 |
TAACGACATCCCTACCGC |
GCTTCTTCTTCCACTTTCGG |
| ACM050 |
CF447828 |
GGTTCTCTGTTTGGGACA |
CCGTTTCGGCTACCTTGTAT |
| ACM052 |
CF441811 |
CAGCAGCAACAAAGAATGC |
CTGGGGAGAATGAGAAGCAC |
| ACM053 |
CF437211 |
CTGGGCTCTTTTGTTCATCC |
ATGGTGGAGGTATGTGAGGG |
| ACM058 |
CF435771 |
GGAGTCACACAACAGAAACACAA |
AAGAAGGAATAGAGATGTAGCCGA |
| ACM060 |
CF435985 |
ATCAGCAGCCTTCCCAGTAA |
ATCACACCCGCAAAAGAAT |
| ACM065 |
CF449328 |
GCTCTGATGGAGGATGGTTC |
CTTGCCATCTTTGTCGGT |
| ACM072 |
CF441584 |
TGAATTCAGGCCAAACATGA |
GAGGAAAGCCTGAAGAGTAGCA |
| ACM076 |
CF449018 |
ATTAGAAACATCCATCGCCG |
CGCGATCATCATTTTCCATA |
| ACM080 |
CF449761 |
GCATTATGCAGTAACGGGCT |
GCAGCAGCATTTGATTGAAC |
| ACM081 |
CF447998 |
CTGAAAAGAAACCCGCAGAG |
TCAGGATGCACTTGCTTCAG |
| ACM082 |
CF436620 |
CACCGTTCCTCAGCTCACTT |
AGAGGGACGAAATGAAAGCA |
| ACM092 |
CF451134 |
GTGATTTGAAGCCACCACCT |
TGAATGGTGGTTATTCGGGT |
| ACM096 |
CF446191 |
TGTGGGCAATTCACGTTATG |
AAAAGTTGTGAACGGCATCC |
| ACM105 |
CF441894 |
CAAGTGGAGCGGGTATTTGT |
GAGGCACAACTTCCTCTTCG |
| ACM107 |
CF449837 |
CCTTCATTCCCAAAGCACAT |
GCGATAAAGAGGGACAGCAG |
| ACM114 |
CF436720 |
TAAGTTTTGCCTCCACCACC |
GCTCCACTTCAAGGCTGTTT |
| ACM129 |
CF442903 |
CTAGGTTTCCGTGCTCCAAG |
CAGTTGGAGATCAACAGGCA |
| ACM140 |
CF442000 |
TTGAAGCTATTCTCCGCAGC |
AGGGGGTCATTGATCCTAGC |
| ACM144 |
CF441789 |
GCAACGGTAGAAGAACCTGC |
AACCTCTTTTGGTGCCTCCT |
| ACM149 |
CF440830 |
GAAGATGGGTTTGAGTGGGA |
CAAGCCTGCCCTTACTCTTG |
| ACM174 |
CF451831 |
TGCCCAATTATCGTTTCCAT |
GATGAGGCGAGTTTAGAGCG |
| ACM183 |
CF443106 |
GATGATGGTGATGGCATTGA |
GTTTGCAGGCTCCATTGATT |
| ACM231 |
CF441488 |
AAAGCTTCTACCCTGGCGAT |
TCCCTACGAACTCGTCATCC |
| ACM238 |
CF443464 |
TGATAGCCAGTTGATTGCGA |
TTCCCCAGTACACACCTTCC |
| ACM240 |
CF444554 |
GTGCAACTCCAAGAGAAGGG |
AATATAAAGGCGTTGGCCTG |
| ACM245 |
CF445289 |
GGATCTGATCGGAGATTGGA |
GCGCACCTCTCTGCTAGACT |
| ACM255 |
CF449065 |
AAATTCCCAAAACGAAACCC |
GGGTTTCAGGAACAGTCAGC |
| ACM295 |
CF445600 |
AGATCCGTCCCATGAAACT |
GATCCGCTTCTGAAATCTCG |
| ACM304 |
DQ273270 |
GAATTTAGGCCCATTTCAAGG |
TGATTTGCCTAATGTTTTTACG |
| ACM322 |
ES449660 |
TTCTTCTCCTATCCAGCTATCG |
GTGATTTGGGAGGGGATTTT |
| ACM340 |
CF437547 |
AAGTCTGGTGGTTGGTCCAG |
GGTGCCCAAGAAGTTGGTTA |
| ACP002 |
AA451591 |
AAGCTTCTTTCGATCCTTTGTG |
GCTTCGATTCCATTTCAAGTTC |
| ACP003 |
BE205590 |
AAGCTCTTAAAGCTGCTGATGG |
ATGCACGATAGCACAAGACATC |
| ACP034 |
BQ580357 |
CAGTCTTGTGGTCATTGGTCA |
AACCCATGCGTATTTGAAGG |
| ACP052 |
CF445805 |
TTCCCTCCTCACTCCCTACA |
CGACCACAAACACAAGCAAC |
| APSR |
AF212155 |
CAGCTGCAGACTTTTCCTAC |
CCACGTGATCGAGTAGATCGT |
| GGCS |
AF401621 |
CTGGAGTCACACCTGCAGAG |
TCGCCTTCGGAACTGTTATT |
| GGT |
AF401622 |
TGTTGCTACCGATGATGGTC |
ATGCAACCCTGCAATTTCTC |
| SPS3'UTR |
EU164758 |
AAAGGGAGATACAGACCAT |
ATTATACATCTCATCATGTCACT |
| SUCS | CF440928 | TTTGAAGTGTGGCCTTACCTTGAG | TGATGAAGTCTGTTCGATCATGGC |
Database configuration and curation
Map and marker data provided by authors of previously published linkage mapping studies [4,8,12,14,16,37] were compiled in a MySQL relational database and reformatted in a form suitable for import into CMAP [38]. Marker data from the `BYG15-23 x AC43' cross [6] were reformatted in cross-pollinator format for JoinMap 4 and linkage maps were recalculated using default settings. Correspondences between loci with identical names were added using the cmap_admin.pl utility provided in CMap, or manually added based on use of common underlying sequences, as identified through information provided by authors and/or identified in the MySQL database. Further correspondences were identified by cross-checking primer sets against the Onion Gene Index [39] using the primersearch tool from the EMBOSS suite [40] and creating correspondences for any marker pairs amplifying the same sequence. AMAL data were compiled into a Google® spreadsheet and published in searchable form using Simile Widgets http://www.simile-widgets.org[41]. Sequences used for marker design were re-formatted to include marker names in fasta header lines and formatted to provide a BLAST [42] database. Information concerning PCR primer sets used to reveal SNP and SSR markers is provided via custom SQL queries to an external database included in modifications to the distributed CMAP feature information templates. QTL information was compiled from published data and manually added as map features using the CMAP administrator interface, or bulk uploads with the cmap_admin.pl tool.
Utility
The resources provided at http://alliumgenetics.org may be browsed through direct links to maps organized by species and publication, or through the standard CMAP interface. Markers or any other features may be searched using the built-in feature search option in CMAP, or through a simple form interface provided to enable searching for details of specific markers or primer sets. A BLAST facility is provided to enable querying any sequences of interest against targets of existing markers.
The markers assigned using AMALs may be browsed and filtered through a web page and the RDF data source may be used as input for other Web2.0 mashups [43].
AlliumMap currently contains 1,776 markers from 10 Allium maps and 512 correspondences between markers. Genetic maps may be browsed through a standard CMAP interface, and marker hyperlinks provide access to marker information including links to GenBank sequences and other marker assay details.
Discussion
Integration of the interspecific allium map
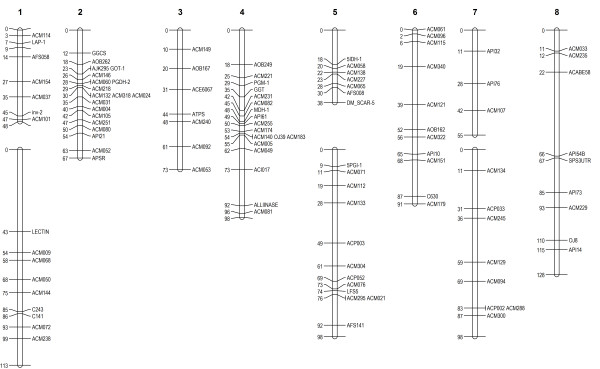
The addition of 74 co-dominant markers to the A. cepa x A. roylei interspecific map has enabled integration of male and female maps previously constructed primarily with dominant AFLP markers. The map comprises 11 linkage groups spanning 1 Morgan (Figure 1) compared with a length of 660 cM (Kosambi) reported observed for the original AFLP-based map [16]. This is the expected map length for onion based on chiasma frequency [44] and suggests that this map spans most of the genome. The combination of anchor loci assigned using AMALs and mapped in the interspecific cross has provided many additional landmarks for aligning genetic linkage maps in A. cepa and A. fistulosum. Alignment of linkage groups in this cross with the 'BYG15-23 x AC43' onion map [6] reveals useful synteny, as reported previously in studies of onion chromosome 8 [9].
Figure 1.
Isozyme and PCR-based marker anchor loci on integrated A. cepa ‘Jumbo’ x A. roylei interspecific map. Numerals at top denote chromosome number based on AMAL assignments. Scale denotes distance in Haldane units (cM). AFLP markers are omitted for clarity.
Approximately 30 % of onion EST-derived PCR-based markers do not amplify in A. roylei, but may nevertheless be mapped as dominant markers in the A. cepa x A. roylei cross. This high degree of polymorphism means that this cross is extremely useful for developing detailed genetic maps. Development of additional crosses of this type for mapping with new SNP and other marker resources developed with next-generation sequencing in onion would be desirable to provide highly informative stocks for researchers mapping new genes of interest.
Consensus maps in allium
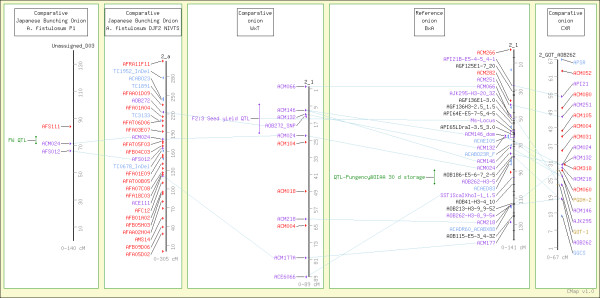
The present database contains 512 correspondences between markers on different Allium maps. Map comparison reveals useful degrees of expressed marker portability and suggests considerable potential for comparative methods to resolve common questions of crop evolution, biological function and economic trait regulation across these major cultivated Allium species. A comparative view of Allium chromosome 2 is shown in Figure 2. The Ms locus conditioning restoration of male-fertility in S cytoplasm is the basis for most F1 hybrid production in onion, and has been mapped to this chromosome [7], and we observed association of markers in this region with seed yield from selfed F2, due to segregation at Ms, plants (McCallum et al., unpublished observations) in the 'W202A x Texas Grano 438' family used to map bulb composition QTL [9,10]. QTL have been reported in an adjacent chromosomal region for onion bulb composition [45] and A. fistulosum seedling vigor [13]. This comparative view allows ready comparison between the QTL locations and linked markers from these studies and provides potential markers for more detailed studies of these regions in these or other genetic backgrounds. Comparison of the onion and interspecific maps for this linkage group illustrates the typically good agreement between marker order and map length in these maps. The relatively small population sizes used to date in these studies do not yet allow conclusive identification of inversions or other major rearrangements in Allium maps.
Figure 2.
AlliumMap comparative view of Allium chromosome 2, based on genetic maps from onion [6],[8],[10] and A. fistulosum[12].
Conclusions
Previous comparative studies have shown no microsynteny of asparagus with rice or onion [46], suggesting that comparative genomic studies must focus within the genus Allium. AlliumMap provides an integrated point to access details of the genetic markers and sequence resources employed across multiple studies in cultivated Allium. New denser linkage maps and underlying marker resources currently under development using next-generation transcriptome sequencing will be deposited in AlliumMap in the near future and ongoing curation will ensure integration with past studies. Despite the rapid advances in sequencing technologies, the enormous size of Allium nuclear genomes will preclude full sequencing in the short term. However, reduced representation approaches are already practical and the data contained in AlliumMap will be valuable for aligning contigs from such studies with genetic and physical maps.
The resource will enable comparative genomics approaches, particularly for basic studies of plant physiology, metabolism and bioprotection in onion and A. fistulosum. Current transcriptome sequencing initiatives in onion will provide a rapidly expanding resource of anchor loci to expand the correspondences reported in this paper.
Availability and requirements
The database and associated tools may be freely accessed at http://alliumgenetics.org. Data concerning AMAL assignments can be accessed as an RDF data sources at http://spreadsheets.google.com/pub?key=pUofr7CKURDMvUcUlAecgPQ&hl=en
Abbreviations
AFLP, Amplified Fragment Length Polymorphism; AMAL, Alien monosomic addition line; EST, Expressed Sequence Tags; QTL, Quantitative Trait Loci; RFLP, Restriction fragment length polymorphism; SNP, Single-nucleotide polymorphism; SSR, Simple Sequence Repeat.
Authors’ contributions
JM conceived the database and developed web interface. SB and JM curated mapping data and prepared manuscript YD configured CMAP and underlying database. MS curated data concerning monosomic alien addition lines. SvH constructed the interspecific A. cepa x A. roylei map. MPJ and FK conducted genetic marker evaluations in the A. cepa x A. roylei population.
Contributor Information
John McCallum, Email: john.mccallum@plantandfood.co.nz.
Samantha Baldwin, Email: samantha.baldwin@plantandfood.co.nz.
Masayoshi Shigyo, Email: shigyo@yamaguchi-u.ac.jp.
Yanbo Deng, Email: yanbo.deng@gmail.com.
Sjaak van Heusden, Email: sjaak.vanheusden@wur.nl.
Meeghan Pither-Joyce, Email: Meeghan.Pither-Joyce@plantandfood.co.nz.
Fernand Kenel, Email: Fernand.Kenel@plantandfood.co.nz.
Acknowledgements
AlliumMap development was funded by the New Zealand Foundation for Research Science and Technology contracts C02X0203 and C02X0803. Travel funding to support collaborations was provided by the International Science and Technology (ISAT) Linkages Fund and the Japanese Society for the Promotion of Science. We thank Michael Havey (USDA-ARS) for providing marker data from onion mapping populations, Hikaru Tsukazaki and Tadayuki Wako (NIVTS, NARO, Japan) for providing marker and sequence data from A. fistulosum mapping populations and Akiko Kamoi (House Foods Ltd, Japan) for technical assistance.
References
- Shigyo M, Kik C. In: Vegetables II, Volume 2 of Handbook of Plant Breeding. Prohens J, Nuez F, editor. Springer New York, ; 2008. Onion; pp. 121–159. [Google Scholar]
- Ricroch A, Yockteng R, Brown S, Nadot S. Evolution of genome size across some cultivated Allium species. Genome. 2005;48(3):511–520. doi: 10.1139/g05-017. [DOI] [PubMed] [Google Scholar]
- McCallum J. In: Vegetables, Volume 5 of Genome Mapping and Molecular Breeding in Plants. Kole C, Kole C, editor. Springer, Berlin Heidelberg; 2007. Onion; pp. 331–347. [Google Scholar]
- King J, Bradeen J, Bark O, McCallum J, Havey M. A low-density genetic map of onion reveals a role for tandem duplication in the evolution of an extremely large diploid genome. Theor Appl Genet. 1998;96:52–62. doi: 10.1007/s001220050708. [DOI] [Google Scholar]
- Kuhl J, Cheung F, Yuan Q, Martin W, Zewdie Y, McCallum J, Catanach A, Rutherford P, Sink K, Jenderek M. et al. A unique set of 11,008 onion expressed sequence tags reveals expressed sequence and genomic differences between the monocot orders Asparagales and Poales. Plant Cell. 2004;16:114. doi: 10.1105/tpc.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W, McCallum J, Shigyo M, Jakse J, Kuhl J, Yamane N, Pither-Joyce M, Gokce A, Sink K, Town C. et al. Genetic mapping of expressed sequences in onion and in silico comparisons with rice show scant colinearity. Mol Genet Genom. 2005;274(3):197–204. doi: 10.1007/s00438-005-0007-6. [DOI] [PubMed] [Google Scholar]
- Gokce A, McCallum J, Sato Y, Havey M. Molecular tagging of the Ms locus in onion. J Am Soc Hortic Sci. 2002;127(4):576–582. [Google Scholar]
- Khar A, Jakse J, Havey M. Segregations for Onion-Bulb Colors Reveal that Red is Controlled by at Least Three Loci. J Am Soc Hortic Sci. 2008;133:42–47. [Google Scholar]
- McCallum J, Clarke A, Pither-Joyce M, Shaw M, Butler R, Brash D, Scheffer J, Sims I, van Heusden S, Shigyo M. et al. Genetic mapping of a major gene affecting onion bulb fructan content. Theor Appl Genet. 2006;112(5):958–967. doi: 10.1007/s00122-005-0199-5. [DOI] [PubMed] [Google Scholar]
- McCallum J, Pither-Joyce M, Shaw M, Kenel F, Davis S, Butler R, Scheffer J, Jakse J, Havey M. Genetic mapping of sulfur assimilation genes reveals a QTL for onion bulb pungency. Theor Appl Genet. 2007;114(5):815–822. doi: 10.1007/s00122-006-0479-8. [DOI] [PubMed] [Google Scholar]
- Ohara T, Song Y, Tsukazaki H, Wako T, Nunome T, Kojima A. Genetic mapping of AFLP markers in Japanese bunching onion (Allium fistulosum) Euphytica. 2005;144(3):255–263. doi: 10.1007/s10681-005-6768-5. [DOI] [Google Scholar]
- Tsukazaki H, Yamashita K, Yaguchi S, Masuzaki S, Fukuoka H, Yonemaru J, Kanamori H, Kono I, Hang T, Shigyo M. et al. Construction of SSR-based chromosome map in bunching onion (Allium fistulosum) Theor Appl Genet. 2008;117(8):1213–1223. doi: 10.1007/s00122-008-0849-5. [DOI] [PubMed] [Google Scholar]
- Ohara T, Tsukazaki H, YeonSang S, Wako T, Yamashita K, Kojima A. Mapping of quantitative trait loci controlling seedling growth in bunching onion (Allium fistulosum L.) J Jpn Soc Horticultural Sci. 2009;78(4):436–442. doi: 10.2503/jjshs1.78.436. [DOI] [Google Scholar]
- Tsukazaki H, Yamashita K, Yaguchi S, Yamashita K, Hagihara T, Shigyo M, Kojima A, Wako T. Direct determination of the chromosomal location of bunching onion and bulb onion markers using bunching onion-shallot monosomic additions and allotriploid-bunching onion single alien deletions. Theor Appl Genet. 2010;122(3):501–510. doi: 10.1007/s00122-010-1464-9. [DOI] [PubMed] [Google Scholar]
- Meer Q, Vries J. An interspecific cross between Allium roylei Stearn and Allium cepa L., and its backcross to A. cepa. Euphytica. 1990;47:29–31. doi: 10.1007/BF00040359. [DOI] [Google Scholar]
- Van Heusden A, Van Ooijen J, Vrielink-van Ginkel R, Verbeek W, Wietsma W, Kik C. A genetic map of an interspecic cross in Allium based on amplified fragment length polymorphism (AFLP ™) markers. Theor Appl Genet. 2000;100:118–126. doi: 10.1007/s001220050017. [DOI] [Google Scholar]
- Scholten O, Van Heusden A, Khrustaleva L, Burger-Meijer K, Mank R, Antonise R, Harrewijn J, Van Haecke W, Oost E, Peters R. et al. The long and winding road leading to the successful introgression of downy mildew resistance into onion. Euphytica. 2007;156(3):345–353. doi: 10.1007/s10681-007-9383-9. [DOI] [Google Scholar]
- Goldschmied P. Improvement of onion traits: the creation of Botrytis leaf blight resistant and synthetic onion varieties through alternate breeding approaches. PhD thesis. Cornell University, Department of Horticulture, ; 2006. [Google Scholar]
- Khrustaleva L, Kik C. Cytogenetical studies in the bridge cross Allium cepa (A. fistulosum A. roylei) Theor Appl Genet. 1998;96:8–14. doi: 10.1007/s001220050702. [DOI] [Google Scholar]
- Shigyo M, Tashiro Y, Isshiki S, Miyazaki S. Establishment of a series of alien monosomic addition lines of Japanese bunching onion (Allium fistulosum L.) with extra chromosomes from shallot (A. cepa L. Aggregatum group) Gene Genet Syst. 1996;71(6):363–371. doi: 10.1266/ggs.71.363. [DOI] [PubMed] [Google Scholar]
- Heusden AV, Shigyo M, Tashiro Y, van Ginkel RV, Kik C. AFLP linkage group assignment to the chromosomes of Allium cepa L. via monosomic addition lines. Theor Appl Genet. 2000;100(3):480–486. doi: 10.1007/s001220050062. [DOI] [Google Scholar]
- Masuzaki S, Shigyo M, Yamauchi N. Complete assignment of structural genes involved in flavonoid biosynthesis influencing bulb color to individual chromosomes of the shallot (Allium cepa L.) Gene Genet Syst. 2006;81(4):255–263. doi: 10.1266/ggs.81.255. [DOI] [PubMed] [Google Scholar]
- Masuzaki S, Araki N, Yamauchi N, Yamane N, Wako T, Kojima A, Shigyo M. Chromosomal locations of microsatellites in onion. HortSci. 2006;41(2):315–318. [Google Scholar]
- Yaguchi S, Yamauchi N, Shigyo M. Single alien chromosome additions from shallot (Allium cepa L. Aggregatum group) increase endogenous polyphenol contents in Japanese bunching onion. J Jpn Soc Horticultural Sci. 2009;78(4):431–435. doi: 10.2503/jjshs1.78.431. [DOI] [Google Scholar]
- Yaguchi S, Nakajima T, Sumi T, Yamauchi N, Shigyo M. Profling of nondigestible carbohydrate products in a complete set of alien monosomic addition lines explains genetic controls of its metabolisms in Allium cepa. J Am Soc Hortic Sci. 2009;134(5):521–528. [Google Scholar]
- Yaguchi S, McCallum J, Shaw M, Pither-Joyce M, Onodera S, Shiomi N, Yamauchi N, Shigyo M. Biochemical and genetic analysis of carbohydrate accumulation in Allium cepa L. Plant Cell Physiol. 2008;49(5):730. doi: 10.1093/pcp/pcn048. [DOI] [PubMed] [Google Scholar]
- Havey M. NCBIAlliumcepa Genome: Build 0 version 1. 2008. http://www.ncbi.nlm.nih.gov/mapview/mapnsearch.cgi?taxid=4679
- Kim D, Jung T, Nam S, Kwon H, Kim A, Chae S, Choi S, Kim D, Kim R, Park H. GarlicESTdb: an online database and mining tool for garlic EST sequences. BMC Plant Biol. 2009;9:61. doi: 10.1186/1471-2229-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasi A, Simon P, Senalik D, Kumar B, Manikandan V, Philip P, Senapathy P. RoBuST: An Integrated Resource of Genomics Information for Plants in the Root and Bulb Crop Families Apiaceae and Alliaceae. BMC Plant Biol. 2010;10:161. doi: 10.1186/1471-2229-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VegMarks, a DNA database for vegetables. http://vegmarks.nivot.affrc.go.jp/
- Alan A, Brants A, Cobb E, Goldschmied P, Mutschler M, Earle E. Fecund gynogenic lines from onion (Allium cepa L.) breeding materials. Plant Sci. 2004;167(5):1055–1066. doi: 10.1016/j.plantsci.2004.06.007. [DOI] [Google Scholar]
- Duangjit J, Bohanec B, Havey MJ. Plant and Animal Genomes XIX Conference, January 15–19, 2011. Town and Country Center, San Diego, CA; 2011. Development Of A Haploid Mapping Family For Onion. [Google Scholar]
- Paterson A. Comparative Genomics in Crop Plants. Mol Tech Crop Improv. 2009;1:23–61. [Google Scholar]
- Liang C, Jaiswal P, Hebbard C, Avraham S, Buckler E, Casstevens T, Hurwitz B, McCouch S, Ni J, Pujar A. et al. Gramene: a growing plant comparative genomics resource. Nucleic Acids Res. 2008;36:D947. doi: 10.1093/nar/gkm968. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Staton M, Lee T, Blenda A, Svancara R, Abbott A, Main D. GDR (Genome Database for Rosaceae): integrated web-database for Rosaceae genomics and genetics data. Nucleic Acids Res. 2007;36(Database issue):D1034–D1040. doi: 10.1093/nar/gkm803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ooijen J. In: Kyazma BV, editor. Wageningen, Netherlands; 2006. JoinMap 4.0, Software for the calculation of genetic linkage maps in experimental populations. [Google Scholar]
- Zewdie Y, Havey M, Prince J, Jenderek M. The first genetic linkages among expressed regions of the garlic genome. J Am Soc Hortic Sci. 2005;130(4):569. [Google Scholar]
- Youens-Clark K, Faga B, Yap I, Stein L, Ware D. CMap 1.01: a comparative mapping application for the Internet. Bioinformatics. 2009;25(22):3040. doi: 10.1093/bioinformatics/btp458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onion Gene Index Release 2.0. http://compbio.dfci.harvard.edu/tgi/cgi-bin/tgi/gimain.pl?gudb=onion
- Rice P, Longden I, Bleasby A. et al. EMBOSS: the European molecular biology open software suite. Trends Genet. 2000;16(6):276–277. doi: 10.1016/S0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- Huynh D, Karger D, Miller R. Proceedings of the 16th international conference on World Wide Web. ACM; 2007. Exhibit: lightweight structured data publishing; pp. 737–746. [Google Scholar]
- Altschul S, Gish W, Miller W, Myers E, Lipman D. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Belleau F, Nolin M, Tourigny N, Rigault P, Morissette J. Bio2RDF: Towards a mashup to build bioinformatics knowledge systems. J Biomed Informat. 2008;41(5):706–716. doi: 10.1016/j.jbi.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Labani R, Elkington T. Nuclear DNA variation in the genus Allium L.(Liliaceae) Heredity. 1987;59:119–128. doi: 10.1038/hdy.1987.103. [DOI] [Google Scholar]
- Galmarini C, Goldman I, Havey M. Genetic analyses of correlated solids, flavor, and health-enhancing traits in onion (Allium cepa L.) Mol Genet Genom. 2001;265(3):543–551. doi: 10.1007/s004380100445. [DOI] [PubMed] [Google Scholar]
- Jakse J, Telgmann A, Jung C, Khar A, Melgar S, Cheung F, Town C, Havey M. Comparative sequence and genetic analyses of asparagus BACs reveal no microsynteny with onion or rice. Theor Appl Genet. 2006;114:31–39. doi: 10.1007/s00122-006-0407-y. [DOI] [PubMed] [Google Scholar]