Abstract
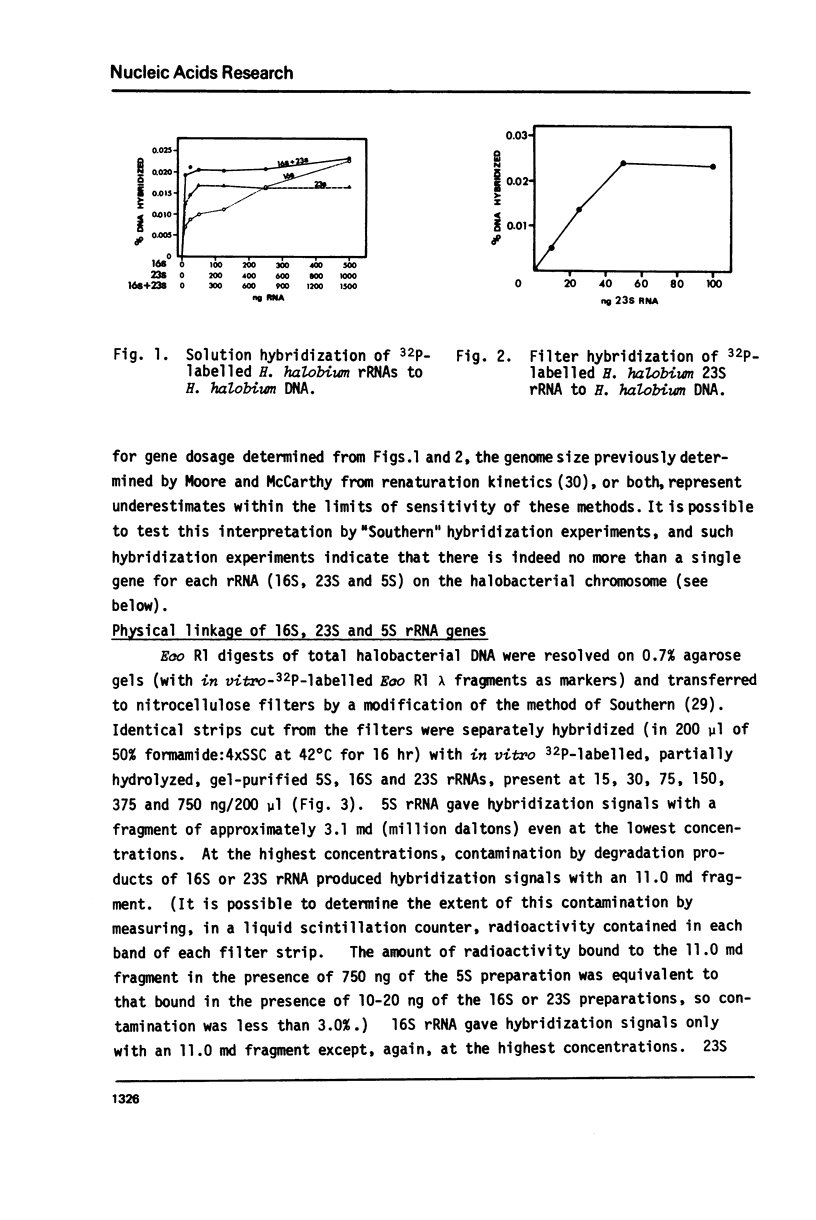
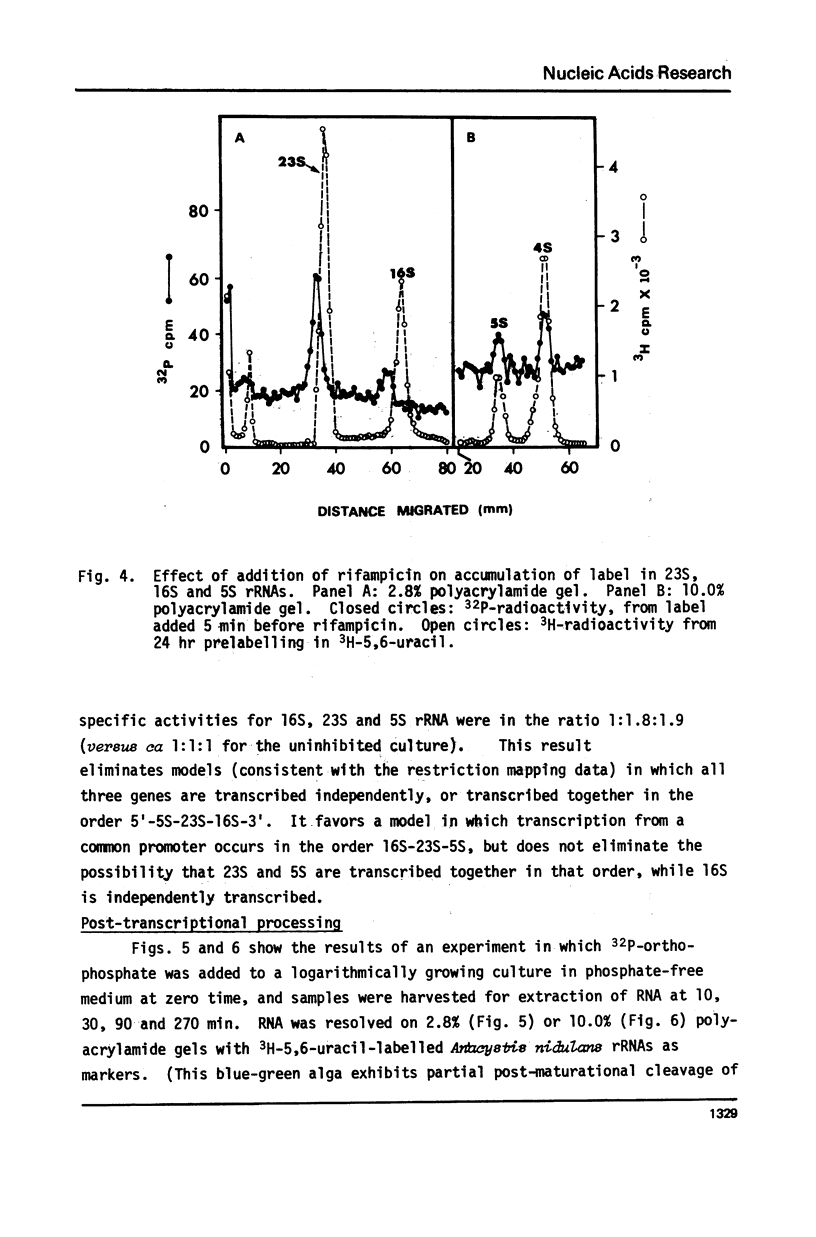
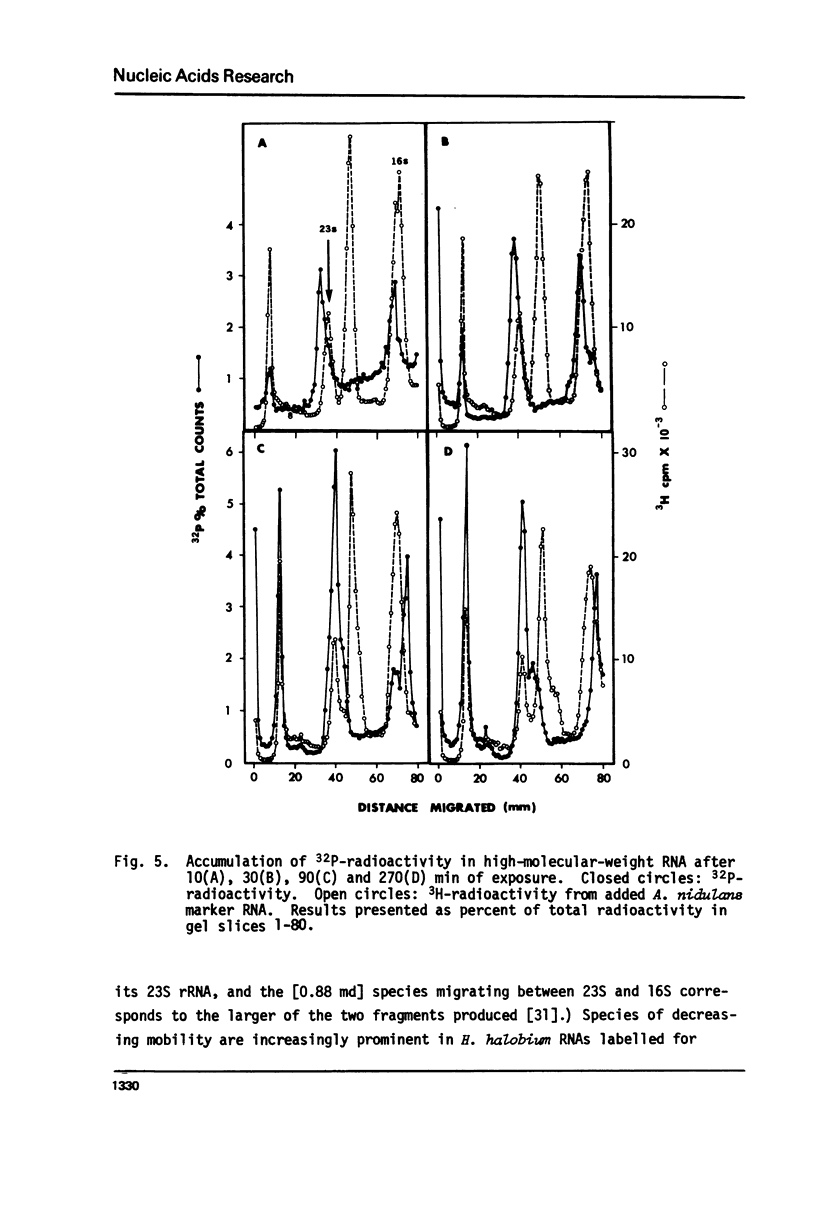
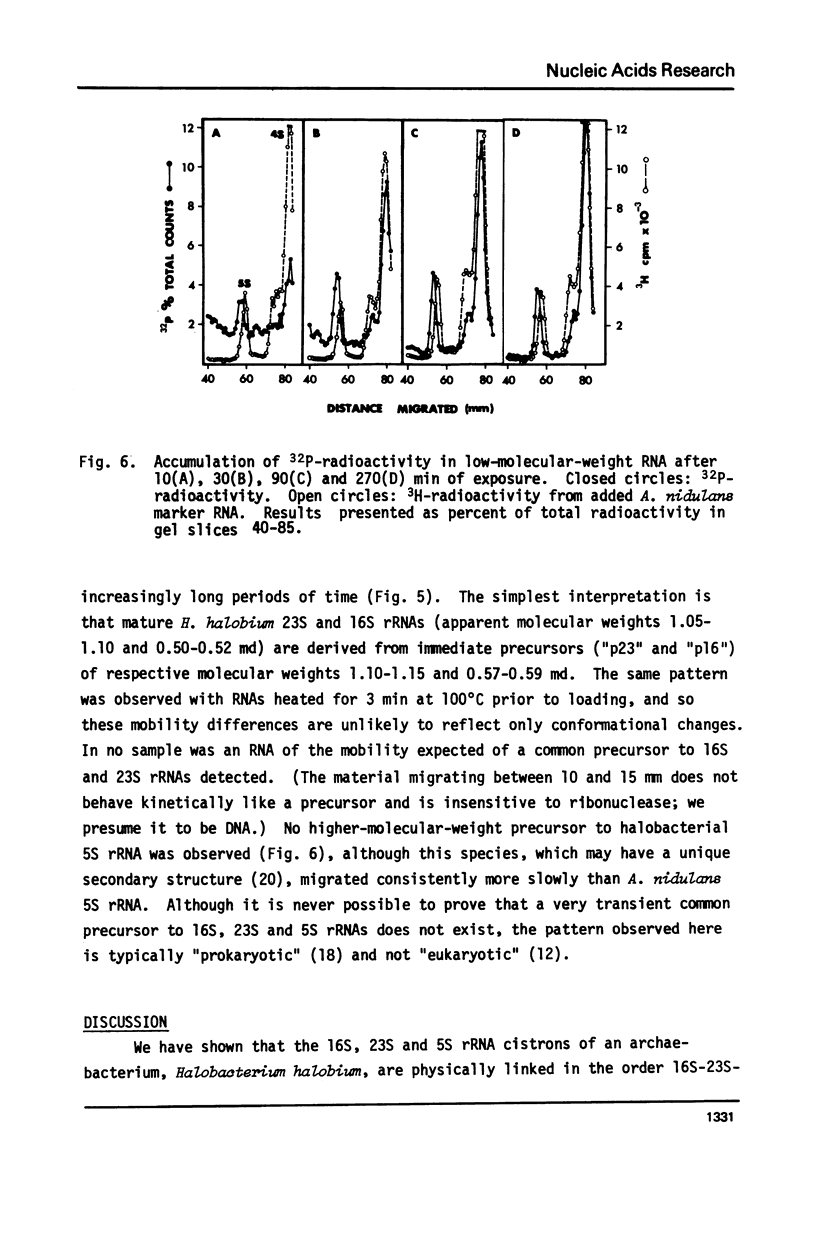
Because it is now clear that archaebacteria may be as distinct from eubacteria as either group is from eukaryotic cells, and because a specifically archaebacterial ancestry has been proposed for the nuclear-cytoplasmic component of eukaryotic cells, we undertook to characterize, for the first time, the ribosomal RNA cistrons of an archaebacterium (Halobacterium halobium). We found these cistrons to be physically linked in the order 16S-23S-5S, and obtained evidence that they are also transcribed from a common promoter(s) in the order 5'-16S-23S-5S-3'. We showed that, although slightly larger immediate precursors of 16S and 23S are readily seen, no common precursor of both 16S and 23S can be easily detected in vivo. In all these respects the archaebacterium H. halobium is like a eubacterium and unlike the nuclear-cytoplasmic component of eukaryotic cells. We found, however, that it differs from eubacteria of comparable (large) genome size in having only one copy of the rRNA gene cluster per genome.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batts-Young B., Lodish H. F. Triphosphate residues at the 5' ends of rRNA precursor and 5S RNA from Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1978 Feb;75(2):740–744. doi: 10.1073/pnas.75.2.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley S. T., Morton R. A. Recent developments in the molecular biology of extremely halophilic bacteria. CRC Crit Rev Microbiol. 1978;6(2):151–205. doi: 10.3109/10408417809090622. [DOI] [PubMed] [Google Scholar]
- Bell G. I., DeGennaro L. J., Gelfand D. H., Bishop R. J., Valenzuela P., Rutter W. J. Ribosomal RNA genes of Saccharomyces cerevisiae. I. Physical map of the repeating unit and location of the regions coding for 5 S, 5.8 S, 18 S, and 25 S ribosomal RNAs. J Biol Chem. 1977 Nov 25;252(22):8118–8125. [PubMed] [Google Scholar]
- Berkner K. L., Folk W. R. Polynucleotide kinase exchange reaction: quantitave assay for restriction endonuclease-generated 5'-phosphoroyl termini in DNA. J Biol Chem. 1977 May 25;252(10):3176–3184. [PubMed] [Google Scholar]
- Bonen L., Cunningham R. S., Gray M. W., Doolittle W. F. Wheat embryo mitochondrial 18S ribosomal RNA: evidence for its prokaryotic nature. Nucleic Acids Res. 1977 Mar;4(3):663–671. doi: 10.1093/nar/4.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonen L., Doolittle W. F., Fox G. E. Cyanobacterial evolution: results of 16S ribosomal ribonucleic acid sequence analyses. Can J Biochem. 1979 Jun;57(6):879–888. doi: 10.1139/o79-108. [DOI] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A. Structure and function of prokaryotic and eukaryotic ribosomes. Prog Biophys Mol Biol. 1977;32(3):193–231. [PubMed] [Google Scholar]
- Cunningham R. S., Gray M. W. Isolation and characterization of 32P-labeled mitochondrial and cytosol ribosomal RNA from germinating wheat embryos. Biochim Biophys Acta. 1977 Apr 4;475(3):476–491. doi: 10.1016/0005-2787(77)90063-6. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F., Pace N. R. Transcriptional organization of the ribosomal RNA cistrons in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1786–1790. doi: 10.1073/pnas.68.8.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F. Postmaturational cleavage of 23s ribosomal ribonucleic acid and its metabolic control in the blue-green alga Anacystis nidulans. J Bacteriol. 1973 Mar;113(3):1256–1263. doi: 10.1128/jb.113.3.1256-1263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff N. V. On spacers. Cell. 1979 Apr;16(4):697–710. doi: 10.1016/0092-8674(79)90086-2. [DOI] [PubMed] [Google Scholar]
- Magrum L. J., Luehrsen K. R., Woese C. R. Are extreme halophiles actually "bacteria"? J Mol Evol. 1978 May 12;11(1):1–8. doi: 10.1007/BF01768019. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. L., McCarthy B. J. Base sequence homology and renaturation studies of the deoxyribonucleic acid of extremely halophilic bacteria. J Bacteriol. 1969 Jul;99(1):255–262. doi: 10.1128/jb.99.1.255-262.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazar R. N., Matheson A. T., Bellemare G. Nucleotide sequence of Halobacterium cutirubrum ribosomal 5 S ribonucleic acid. An altered secondary structure in halophilic organisms. J Biol Chem. 1978 Aug 10;253(15):5464–5469. [PubMed] [Google Scholar]
- Pace N. R. Structure and synthesis of the ribosomal ribonucleic acid of prokaryotes. Bacteriol Rev. 1973 Dec;37(4):562–603. doi: 10.1128/br.37.4.562-603.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. L., Morowitz H. J. Partial purification of native rRNA and tRNA cistrons from mycoplasma sp. (Kid). Proc Natl Acad Sci U S A. 1969 Aug;63(4):1282–1289. doi: 10.1073/pnas.63.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searcy D. G., Stein D. B., Green G. R. Phylogenetic affinities between eukaryotic cells and a thermophilic mycoplasma. Biosystems. 1978 Apr;10(1-2):19–28. doi: 10.1016/0303-2647(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tornabene T. G., Langworthy T. A., Holzer G., Oró J. Squalenes, phytanes and other isoprenoids as major neutral lipids of methanogenic and thermoacidophilic "archaebacteria". J Mol Evol. 1979 Jun 8;13(1):73–83. doi: 10.1007/BF01732755. [DOI] [PubMed] [Google Scholar]
- Walseth T. F., Johnson R. A. The enzymatic preparation of [alpha-(32)P]nucleoside triphosphates, cyclic [32P] AMP, and cyclic [32P] GMP. Biochim Biophys Acta. 1979 Mar 28;562(1):11–31. doi: 10.1016/0005-2787(79)90122-9. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Fox G. E. Phylogenetic structure of the prokaryotic domain: the primary kingdoms. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5088–5090. doi: 10.1073/pnas.74.11.5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrede P., Erdmann V. A. Escherichia coli 5S RNA binding proteins L18 and L25 interact with 5.8S RNA but not with 5S RNA from yeast ribosomes. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2706–2709. doi: 10.1073/pnas.74.7.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M., Ginder G. D., Felsenfeld G. A new method for the purification and identification of covalently closed circular DNA molcules. Nucleic Acids Res. 1978 Apr;5(4):1139–1152. doi: 10.1093/nar/5.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingales B., Colli W. Ribosomal RNA genes in Bacillus subtilis. Evidence for a cotranscription mechanism. Biochim Biophys Acta. 1977 Feb 16;474(4):562–577. doi: 10.1016/0005-2787(77)90076-4. [DOI] [PubMed] [Google Scholar]



