Abstract
Background
The amenability of the chick embryo to a variety of manipulations has made it an ideal experimental model organism for over 100 years. The ability to manipulate gene function via in ovo electroporations has further revolutionized its value as an experimental model in the last 1½ decades. Although in ovo electroporations are simple to conduct in embryos ≥ E2, in ovo electroporations at early E1 stages have proven to be technically challenging due to the tissue damage and embryonic lethality such electroporations produce.
Results and Conclusions
Here we report our success with in vivo microelectroporations of E1 embryos as young as Hamburger-Hamilton Stage 4 (HH4). We provide evidence that such electroporations can be varied in size and can be spatially targeted. They cause minimal disruption of tissue size, 3-dimensional morphology, cell survival, proliferation and cell-fate specification. Our paradigm is easily adapted to a variety of experimental conditions since it does not depend upon the presence of a lumen to enclose the DNA solution during electroporation. It is thus compatible with the in vivo examination of E1 morphogenetic events (e.g., neural tube closure) where preservation of 3-dimensional morphology is critical.
Keywords: E1, neural plate, gene misexpression, gene manipulations, in ovo electroporation
Introduction
The introduction of in vivo electroporations to alter gene regulation has revolutionized the use of the chick as an embryonic model system (Itasaki et al., 1999; Voiculescu et al., 2008). This technique involves using small electric shocks to open up transient pores within cell membranes. DNA, RNA interference constructs or morpholinos can enter the cell through these pores and alter gene function (Sauka-Spengler and Barembaum, 2008). The most common electroporation paradigms involve relatively high voltages (10-25V) and large diameter (~400-500 μm) electrodes held at a fixed distance (Muramatsu et al., 1997; Funahashi et al., 1999; Itasaki et al., 1999; Sakamoto et al., 1998). While such “macroelectroporation” paradigms produce large swaths of transgene expression, they often lead to extensive damage, making them unsuitable for certain types of tissues and experiments (Agarwala et al., 2001).
A few years after the innovation of “macroelectroporation” techniques, “microelectroporation” techniques were introduced, which used lower voltages (~7V) and a small diameter (40 μm) negative tungsten electrode (Momose et al., 1999). These authors reported efficient and focal transgene expression along with improved tissue health and embryonic viability (Momose et al., 1999). In previous work, we adapted this technique to study the morphogenetic function of Sonic Hedgehog (SHH) and created focal sources of SHH in distinct shapes and patterns in the chick midbrain (Agarwala et al., 2001). In recent years, we have successfully microelectroporated ≥HH7 chick midbrains in ovo, although only small percentage of embryos electroporated at HH7 remained viable and suitable for analyses (Bayly et al., 2007).
In vivo electroporation paradigms targeting ages ≤HH7 have not yet been developed. This is in part due to the tissue damage, dysmorphology and embryonic lethality associated with the large currents required for such in ovo electroporations (Voiculescu et al., 2008). In addition, many tissues undergoing morphogenesis, e.g., the neural plate, do not yet possess lumina within which the DNA can be injected prior to electroporation. Thus, most experiments requiring E1 electroporations (≥HH3) have employed in vitro paradigms with varying degrees of success (Hatakeyama and Shimamura, 2008; Tanaka et al., 2010; Uchikawa, 2008; Voiculescu et al., 2008). However, many morphogenetic events that occur at E1 (.e.g, neural tube closure) involve complex 3-dimensional tissue transformations that would ideally be studied in vivo.
In this study, we report that further modifications of the microelectroporation technique permit the easy in ovo electroporation of E1 embryos as young as HH4. Such electroporations have a reasonable rate of success, and can be focally targeted to specific regions of the neural tube and do not require that the neural tube be closed (Eom et al., 2011). Furthermore, they do not significantly alter cell-fate specification, 3-dimensional tissue morphology or size. We have recently successfully used this method to study the early cellular and molecular events involved in midbrain neural tube closure (Eom et al., 2011).
Experimental Procedures and Results
General Methods
Fertilized Leghorn eggs (Ideal Poultry, Texas) were incubated at 38°C and staged according to Hamburger and Hamilton (Hamburger and Hamilton, 1951). Embryos were electroporated either early (HH4-6) or late (HH9-11) with 1 μg/μl of DNA using previously described expression vectors {EFX-EGFP, EFX-m (membrane-targeted) EGFP, EFX-RFP}, which utilize the elongation factor 1α promoter (Bayly et al., 2007; Eom et al., 2011; Johnson and Krieg, 1994).
Control (un-electroporated) and electroporated embryos were stage-matched both at the time of manipulation and at harvesting, and remained outside the incubator for identical times during manipulations. Controls were windowed, opened, irrigated and in general processed exactly as the electroporated embryos, except that they were not injected with DNA and did not receive current pulses. Embryos were harvested and targeted for the immunohistochemical detection of phosphorylated histone H3 (pHH3; 1:2000; Upstate Biotechnology, MA), LMX1A/B (1:200; DHSB, IA), FOXA2 (1:250, DHSB, IA) and activated caspase3 (1:200; Cell Signaling) as previously described (Eom et al., 2011).
Quantitative Methods
All quantification was conducted using ImageJ software as described in previous studies (Eom et al., 2011). Data within control and experimental groups were pooled and displayed as the mean ± s.e.m. Differences between groups were evaluated using a Student’s t-test.
Rostrocaudal Length
Whole embryos were photographed in sagittal view at the same magnification. Rostrocaudal length was measured as the distance between the rostral and caudal tips of each embryo. Mean differences between the rostrocaudal lengths of un-electroporated (n=3) and electroporated embryos (n=3) were compared using a t-test. Midbrain Circumference: Three 14 μm sections at the rostrocaudal midpoint of electroporated (n=3) and un-electroporated (n=3) midbrains were photographed and their inner perimeters measured using ImageJ. Differences were evaluated using a t-test.
Tissue Thickness
ImageJ was used to measure the ventricular-pial thickness of control (n=3) and electroporated midbrains (n=3) in 3 cross-sections taken from the rostrocaudal midpoint of each brain. Measurements were made in both dorsal and ventral midbrain at a distance of 200 μm from the dorsal and ventral midline, respectively. Mean differences between controls and electroporated midbrains were evaluated as before.
Cell Proliferation
Mitotic cells were labeled with pHH3 in un-electroporated and electroporated midbrain sections. As described above, 3 sections from the rostrocaudal midpoint of each midbrain (n=3 midbrains/group) were photographed and a sampling box subtending 200μm x 200μm was dropped within an electroporated region in both the dorsal and ventral region of each section. All pHH3+ cells within this box were counted and compared with the corresponding data from un-electroporated controls using a t-test.
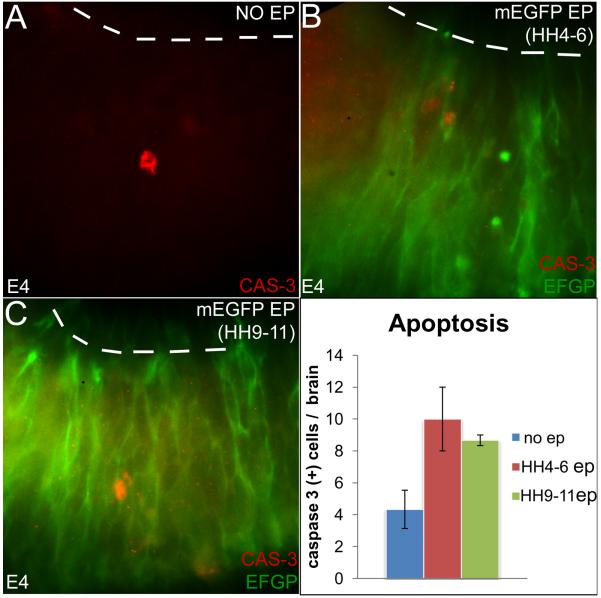
Apoptosis
Cell death in un-electroporated-, early (HH4-6)- and late (HH9-11)-electroporated midbrains was evaluated using activated-caspase3 staining. Since the number of apoptotic cells/section in each group was small and highly variable, all activated caspase3+ cells were counted from six 14 μm thick sections taken from the rostrocaudal midpoint of each midbrain (n=3 midbrains/group). Differences between the groups were evaluated by pair-wise comparisons using a t-test.
Electrode Assembly
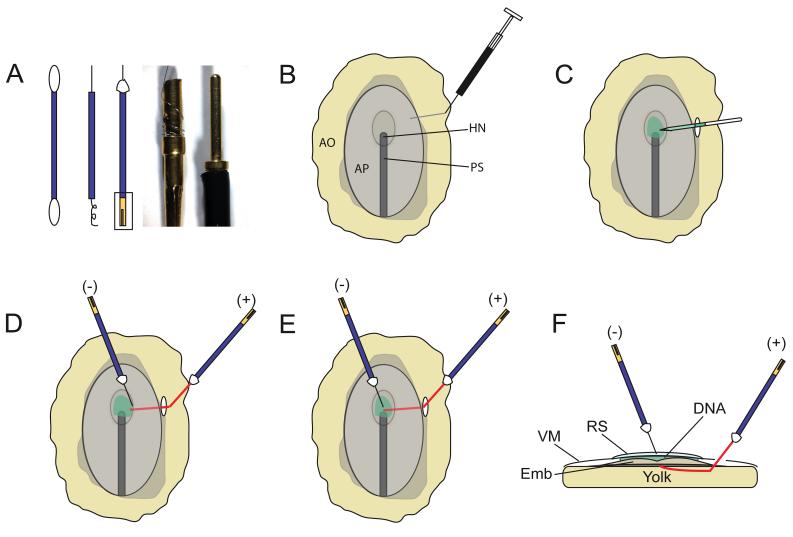
A cheap and simple electrode for microelectroporation purposes was devised by Dr. R. Parnaik in Dr. Clifton Ragsdale’s laboratory at the University of Chicago and was modified for use in our early electroporations (Parnaik and Ragsdale, unpublished methods). This involved cutting the top and bottom of a cotton swab and using its hollow plastic “sleeve” for insulating the electrode (Fig 1A). The negative electrode was made from a 25μm diameter platinum wire (AM Systems, WA, USA; Table 1) wrapped around a “female/socket” gold pin connector (Newark Electronics, NJ, USA), and then threaded through the plastic sleeve of the cotton swab (Fig. 1A). Approximately 2 mm wire protruded from the plastic sleeve at the electroporating end and was fixed to the sleeve with tacky wax (available at hardware stores; Fig. 1A). The positive electrode was similarly constructed from 50μm diameter platinum wire (AM Systems, WA, USA), with approximately 1 cm wire protruding from its front/electroporating end. The protruding length of wire was gently curved at an ~ 130° angle at its approximate midpoint (Fig. 1D-F). This curve allowed for easy insertion underneath the embryo during electroporations, and for reducing the embryo’s exposure to an applied electric current. The cables connecting the electrodes to the electroporator (ECM 830; Harvard Apparatus; Holliston, MA via VWR) were modified by replacing the “micrograbber” alligator clip terminals with “male” gold pin connectors (panels 3-5; Fig. 1A; See Table 1 for catalog numbers). The male pins were inserted into the female sockets attached to each electrode to complete the electric circuit. These modifications made the connecting cables lighter and more ergonomic, allowing us to hold the electrodes by hand during long bouts of electroporation.
Figure 1. The experimental setup for early electroporations.
(A) Electrode assembly. Electrodes were made from platinum wire threaded through the hollow plastic sleeve of a cotton swab (panels 1, 2). The wire was fixed to the top of the plastic sleeve with tacky wax (white triangle, panel 3). The bottom end of the wire was wrapped around a “female” socket pin (box, Panel 3). Panel 4 shows a magnified view of the female socket pin, (boxed area in panel 3). A “male” gold pin (panel 5) soldered to the connecting cables (not shown) is inserted into the female pin to complete the circuit. (B-E) Top-down view of HH5 embryo demonstrating the electroporation procedure. (B) India ink injection for visualizing the embryo. (C) Injection of the DNA and Fast Green solution between the vitelline membrane and the region (e.g. presumptive midbrain) of the embryo targeted for electroporation. (D-F) Electrode positions for dorsal (D) ventral (E, F) electroporations. A cross-sectional view of E is shown in F. The paler segments of the capillary tube (C) or the positive (+) electrode (D, E) depict the segments of each that lie subjacent to the vitelline membrane and are thus partially obscured from view.
Table 1.
A list of critical equipment and catalog numbers required for E1 electroporations. More detailed information is available upon request.
| Reagent | Company | Catalog # |
|---|---|---|
| Pelikan drawing ink A, black | Pelikan (Hannover, Germany) | 201665 |
| PV830 Pneumatic Picopump | World Precision Instruments, Inc. (Sarasota, FL) |
SYS-PV830 |
| Microcapillary tube size 1-5μL | Sigma-Aldrich (Milwaukee, WI) | P0549 |
| Fast Green FCF | Sigma-Aldrich (Milwaukee) | F7258 |
| Connectors, male, pin, 18AWG |
Newark Electronics (Palatine, IL) | 96F7924 |
| Connectors, female, socket, 18AWG |
Newark Electronics (Palatine, IL) | 92F5795 |
| Platinum wire, .001” bare, 10ft | A-M Systems, Inc. (Sequim, WA) | 764500 |
| Platinum wire, .002” bare, 10ft | A-M Systems, Inc. (Sequim, WA) | 765000 |
| ECM830 Electro square porator |
BTX (Holliston, MA) via VWR | 47745-928 |
| Connector cable, banana plug to micrograbber |
BTX (Holliston, MA) via VWR | 82019-192 |
| CUY21 in vivo electroporator | Bex Co. Ltd. (Tokyo, Japan) | CUY21EX |
| Transparent polyethylene tape | 3M (Austin, TX) | 70-0060-3404-8 |
Since the diameter of each electrode was small, no etching or grinding of the tip was necessary (Momose et al., 1999; Agarwala et al., 2001). However, we repeatedly cut off the tips of our negative electrodes (~once/12 electroporations) as they became coated with albumen and less able to conduct current. In addition, the exposed portion of the positive electrode was cleaned frequently by gently scraping the wire with forceps to remove any albumen or yolk deposited on its surface. This ensured that the conductivity of the electrodes remained the same over successive electroporations.
In ovo electroporation
Egg preparation and the standard electroporation reagents and techniques can be obtained from published protocols and are not described here (Gammill and Krull, 2011; Voiculescu et al., 2008). Eggs were windowed either 4-5 hours after the start of incubation at E0, or just prior to electroporation at E1 using previously established protocols (Agarwala et al., 2001). For electroporations, embryos were irrigated with 2-3 drops of Ringer’s solution. A 1 ml tuberculin syringe fitted with a 30-gauge hypodermic needle was filled with 1% India ink (Pelikan, Hamburg, Germany) dissolved in Chick Ringer’s solution. The needle was bent at a 130° angle, inserted through area opaca, and positioned underneath the embryonic area targeted for electroporation (Fig. 1B). Since India ink injections were a factor in the embryonic lethality and deformation, only the smallest volume (~ 20 μl) required for visualization was injected, with care taken to prevent any disruption of the yolk (Voiculescu et al., 2008).
Approximately 2 μl of circular plasmid DNA (1μg/μl) in a 0.1% Fast Green (Sigma, MO, USA) solution in double distilled H2O was front-filled into a glass capillary tube (Sigma, MO, USA) using a vacuum source attached to a picospritzer (WPI Instruments, FL, USA). This volume of DNA was sufficient to electroporate up to 24 HH4-6 embryos, with each embryo receiving approximately 50-300 nl of the DNA/Fast Green solution.
Prior to electroporation, HH4-6 embryos were re-irrigated with ~10 drops of Ringers’ solution. A small incision was made in the vitelline membrane at the area opaca, area pellucida interface with a pair of #55 forceps. The DNA-filled capillary tube was inserted via this incision into the space between the vitelline membrane and the target tissue, in our case, the presumptive midbrain neural plate (Fig. 1C; Eom et al., 2011). DNA solution sufficient to cover the entire target region was then expelled from the capillary tube using a picospritzer attached to a foot pedal (Fig. 1C).
Although the incision was made at a distance from the injection site to prevent DNA diffusion, the DNA was not confined within an enclosed space and dispersed rapidly within 0.5-1min. This required the speedy execution of the subsequent steps. The negative electrode was positioned so that < 1mm of its tip was submerged in the Ringer’s solution previously applied to the vitelline membrane surface. The positive electrode was positioned subjacent to the region to be electroporated, via the incision previously used to introduce DNA. The tip of the positive electrode was directly underneath the target region, while the remaining length of the electrode curved away from this region (Fig. 1D-F). When properly positioned, the 2 electrodes subtended a ~100-110° angle with each other (Fig. 1D-F). Electrodes were held by hand for optimal positioning and care was taken to avoid damage and dysmorphology by preventing the electrodes from coming in contact with one another or the embryo. A foot pedal attached to the electroporator was employed to transfect the DNA using three-50 msec, 5V pulses, separated by 100 msec interpulse intervals. Following the procedure, the egg was sealed with polyethylene tape (3M; Austin, Texas) and returned to the incubator until required for further analyses.
Efficacy of Electroporations
Unlike in vitro HH3 manipulations, very few embryos survived vivo electroporations at HH3 and were not analyzed further (Tanaka et al., 2010). 55.55% embryos (n=40/72) survived until E4 when electroporated at HH4-6. 10% of the surviving embryos (n=4/40) were deformed and were excluded from further analyses. 80% of the remaining embryos (n=32/40) displayed robust GFP/RFP expression between 3hr-4 days of electroporation (Fig. 2; Eom et al., 2011).
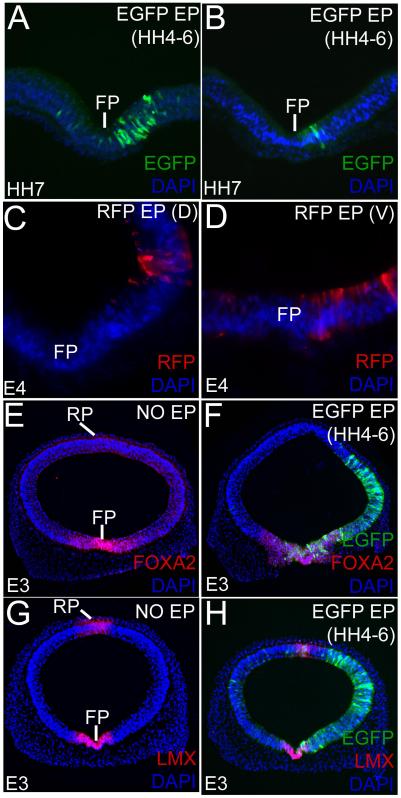
Figure 2. Early electroporations can be varied in size and location and do not affect cell fate specification.
(A, B) Broad (A) and focal (B) ventral EGFP expression in HH7 midbrains electroporated at HH5. All sections (A-H) are counterstained with DAPI (blue). (C, D) E4 brains showing targeted dorsolateral (C) and ventral (D) RFP electroporations. (E-H) Un-electroporated (E, G) and early-electroporated (F, H) midbrains showing that broad, unilateral EGFP electroporations do not affect FOXA2 and LMX1A/B expression in the floor plate (FP) and roof plate (RP) at E3.
Controlling the size and location of the electroporation
The electroporations could be controlled to produce unilateral or bilateral, broad or focal, dorsal or ventral midbrain transgene misexpression (Fig. 2A-H). The size of the electroporation was controlled by altering the distance between the two electrodes, usually accomplished by regulating the extent to which the negative electrode was submerged in Ringer’s solution (Compare Fig. 2A, B and F). To a smaller extent, this could also be accomplished by moving the positive electrode in depth, toward the yolk and away from the embryo. In the latter case, care was taken to prevent disruption of the yolk, as this resulted in reduced embryonic viability. In addition to reducing the size of the electroporation, this also helped minimize damage. Moving the electrodes laterally, away from the axial midline produced dorsal or dorsolateral electroporations (Fig. 1D; 2C, 4B). By contrast, moving the electrodes toward the axial midline generally produced ventral electroporations (Fig. 1E, F; 2A, B, D).
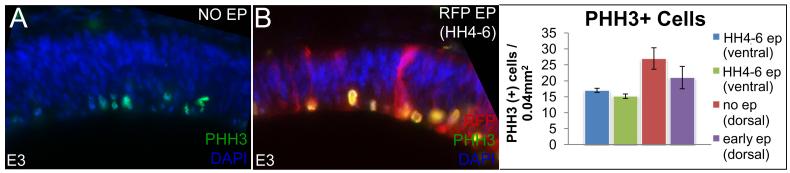
Figure 4. Early electroporations do not affect cell proliferation.
(A-B) Comparable pHH3 staining in dorsal midbrain in control (A) and early-electroporated (B) embryos. Note that pial is up and ventricular is down in this dorsal section and that PHH3+ cells occur along the ventricular/apical surface lining the lumen of the midbrain. (C) Quantitation of cell proliferation in dorsal and ventral midbrain. (Number of mitotic cells/ 0.04 mm2: un-electroporated ventral midbrains: 17.00 +/− 0.65 cells; early-electroporated ventral midbrain: 15.16 +/− 0.70 cells; p= 0.168; un-electroporated dorsal midbrains: 27.00 +/− 3.36 cells; early-electroporated dorsal midbrains: 21 +/− 3.51 cells; p= 0.270).
Cell fate specification is not affected in E1 electroporations
To determine whether early electroporations affected midbrain patterning, we compared the FOXA2 and LMX1A/B expression in the control and early-electroporated midbrains at E3 (Fig. 2E-H). No differences in FOXA2 (floor plate) and LMX1A/B (roof plate and floor plate) expression patterns were noted between the un-electroporated controls and the EGFP/RFP electroporated embryos (Fig. 2E-H). Thus, as seen with late-stage electroporations in many studies, early electroporations did not affect cell-fate specification (Bayly et al., 2007; Eom et al., 2011).
Early electroporations do not affect midbrain size or morphology
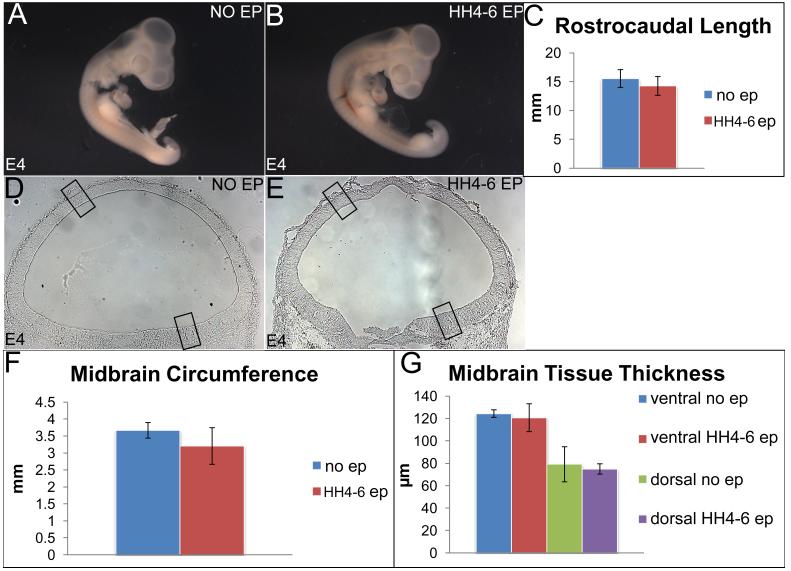
We compared the gross morphology of E4 embryos electroporated at HH 4-6 with that of un-electroporated embryos (Fig. 2, 3). In general, the early-electroporated embryos appeared to be slightly smaller, although the differences in their rostrocaudal lengths were not significant (Fig. 3A-C; rostrocaudal length, un-electroporated embryos: 15.55 mm; early-electroporated embryos: 14.27 mm; p=0.525). The cross-sectional morphology and circumference of the HH4-6 electroporated midbrains were also comparable to the un-electroporated controls (Fig. 3D-F; circumference, un-electroporated midbrains: 3.67 mm; early-electroporated midbrains: 3.20mm; p= 0.495). The ventral and dorsal thickness of midbrain neurectoderm also did not differ between un-electroporated controls and early-electroporated embryos at E4 (Fig. 3D, E, G; ventral thickness, controls: 124.38 μm; electroporated: 120.68 μm; p=0.798; dorsal thickness, controls: 79.11 μm; electroporated 79.94 μm; p=0.819). We conclude that early-electroporations do not significantly affect tissue size and morphology.
Figure 3. Early electroporations do not affect the size of the embryo, midbrain size and morphology.
(A-C) Sagittal view of un-electroporated (A) and early-electroporated (B) embryos showing no significant differences in their rostrocaudal length. (C) Quantitation of A, B (rostrocaudal length, un-electroporated: 15.55 +/− 0.525mm; early-electroporated 14.27 +/− 0.945 mm; p=0.525. (D, E) Un-electroporated (D) and early-electroporated midbrains (E) showing similar sizes and tissue morphology. (F, G) Quantitative data demonstrating that midbrain circumference (F) and ventricular-pial thickness (G) do not differ in un-electroporated and electroporated embryos. (Circumference: un-electroporated: 3.67 +/− 0.229 mm; early electroporation: 3.20 +/− 0.540 mm; p= 0.495; ventral thickness: controls 124.38 +/− 3.35 μm; electroporated: 120.68 +/− 12.44 μm; p=0.798; dorsal thickness: controls: 79.11 +/− 15.66 μm; electroporated 79.94 +/− 4.60 μm; p=0.819).
Cell proliferation is not affected by early electroporations
We next compared cell proliferation in ventral and dorsal midbrains at E3 in control and early electroporated embryos and found that the number of pHH3+ cells did not differ between the two groups (Fig. 4A-C; number of pHH3+ mitotic cells / 0.04 mm2, un-electroporated ventral midbrain: 17.00 cells; early-electroporated ventral midbrain: 15.16 cells; p= 0.168; un-electroporated dorsal midbrain: 27.00 cells; early-electroporated dorsal midbrain: 21 cells; p= 0.270).
Cell death and early electroporations
Few and variable numbers of activated caspase3+ apoptotic cells were noted in control and electroporated embryos examined at E4 (Fig 5A-C). Compared to controls, the number of apoptotic cells more than doubled in the early electroporated midbrains (average number of caspase3+ cells/ control brain, 4.3; early electroporations, n=10; p=0.086). However, possibly due their small numbers and the high degree of within-group variability, differences in the numbers of apoptotic cells between un-electroporated and early electroporated were not significant. Similar comparisons of apoptosis between early vs. late electroporations were also not significantly different (average number of caspase3+ cells/midbrain early, n=10; late, n=8.67; p=0.575) Notably, the total number of apoptotic cell/brain in each group was small (< 2cells/14μm section). As a result, electroporation-induced apoptosis did not result in altered cell fate specification, cell-proliferation or in significant differences in midbrain size and morphology (Fig 2-4).
Figure 5. The rate of apoptosis does not differ between control, early and late electroporated midbrains.
(A-C) Activated cas-3 staining demonstrating apoptosis in control (A), early-(B) and late-electroporated (C) midbrains. (D) Quantitation of apoptosis, showing that cell death does not differ between control and early-electroporated midbrains or between early and late-electroporated midbrains. (Un-electroporated controls: n=4.3 +/− 1.20 cells/midbrain; early electroporations: n=10 +/− 2 cells/midbrain; p=0.086. early electroporations: 10 +/− 2 cells/midbrain; late electroporations: 8.67 cells +/− 0.33 cells/brain; p=0.575).
Discussion
Our study shows that embryos ≥ HH4 can be electroporated in ovo without perturbing overall 3-dimensional morphology, tissue size, cell proliferation and cell-fate specification. Furthermore, by trapping the DNA between the vitelline membrane and the target tissue, our paradigm obviates the need for confining DNA within an enclosed lumen and can thus be adapted for a wide variety of tissues at many ages. In our hands, the critical parameters involved in successfully electroporating HH4-6 embryos were low voltage and electrode design, diameter and placement. Together, these modifications enabled us to bring the electrodes closer together, giving us the ability to focally electroporate targeted regions of the neural plate without causing damage. While 5V was the lowest voltage that could be applied with the ECM830 electroporator, a smaller voltage/current can potentially be elicited by including a set of resistors in the electric circuit, or utilizing an electroporator capable of applying a lower voltage (e.g., CUY21EX electroporator; Bex Co. Ltd, Tokyo, Japan; Table 1).
As noted by previous studies, an important variable in embryonic viability was the type and amount of India ink used. We had the best viability with the smallest volume (~20μl/embryo) and lowest concentration (1%) of India ink that permitted good visualization of the embryo (Voiculescu et al., 2008). In addition, the India ink became particulate with age and affected embryonic viability. In our hands, it was best used within 6 months after it was opened and discarded if it became particulate before that.
It is not fully clear to us why HH3 embryos did not survive our electroporations, while a significant number of HH4 embryos did. The survival at HH5-6 was higher still. One possible explanation for this is the progressive epithelialization of the chick neurectoderm over this time period, as also noted in the zebrafish (Dominic and Agarwala, unpublished observations; Eom et al., 2011; Hong and Brewster, 2006). The idea is that as the neural plate progresses from a partially to a fully polarized epithelium, it may better withstand the electrical insult imposed by electroporation (Voiculescu et al., 2008). Thus, in vitro paradigms need to be employed until further innovation makes ≤HH3 in vivo electroporations feasible (Uchikawa, 2008; Tanaka et al., 2010).
Bullets.
> In vivo gene manipulations in E1 chick embryos are technically challenging and currently not feasible. > Most studies therefore employ in vitro electroporations of cultured embryos at E1.>We describe a novel technique for in vivo electroporations of E1 chick embryos as young as Hamburger-Hamilton stage 4.>Our technique permits the in vivo examination of early morphogenetic events (e.g. neural tube closure) that occur at E1 and where proper three-dimensional tissue morphology is critical.
Acknowledgements
We thank Drs. R. Parnaik and C. Ragsdale for sharing their unpublished electrode design with us. This research was supported by a grant from NIH-NINDS to SA.
Abbreviations
- (+)
positive electrode
- (−)
negative electrode
- AO
area opaca
- AP
area pellucida
- cas-3
activated caspase3
- D
dorsal
- EP
electroporated
- Emb
embryo
- RS
Ringer’s solution
- FP
floor plate
- HH
Hamburger and Hamilton
- HN
Henson’s Node
- pHH3
phosphorylated histone H3
- PS
primitive streak
- RP
roof plate
- V
ventral
- VM
vitelline membrane.
References
- Agarwala S, Sanders TA, Ragsdale CW. Sonic hedgehog control of size and shape in midbrain pattern formation. Science. 2001;291:2147–2150. doi: 10.1126/science.1058624. [DOI] [PubMed] [Google Scholar]
- Bayly RD, Ngo M, Aglyamova GV, Agarwala S. Regulation of ventral midbrain patterning by Hedgehog signaling. Development. 2007;134:2115–21124. doi: 10.1242/dev.02850. [DOI] [PubMed] [Google Scholar]
- Eom D, Amarnath S, Fogel JL, Agarwala S. Bone morphogenetic proteins regulate neural tube closure by interacting with the apicobasal polarity pathway. Development. 2011;138:3179–3188. doi: 10.1242/dev.058602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi J-i, Okafuji T, Ohuchi H, Noji S, Tanaka H, Nakamura H. Role of Pax-D in the regulation of a mid-hindbrain organizer’s activity. Develop. Growth Differ. 1999;41:59–72. doi: 10.1046/j.1440-169x.1999.00401.x. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Krull CE. Embryological and genetic manipulation of chick development. In: Pelegri FJ, editor. Vertebrate Embryogenesis, Methods. Mol. Biol. Vol. 770. Springer Science; Dordrecht, Netherlands: 2011. pp. 119–37. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal tages in the development of the chick embryo. Journal of Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- Hatakeyama J, Shimamura K. Method for electroporation for the early chick embryo. Develop. Growth Differ. 2008;50:449–452. doi: 10.1111/j.1440-169X.2008.01040.x. [DOI] [PubMed] [Google Scholar]
- Hong E, Brewster R. N-cadherin is required for the polarized cell behaviors that drive neurulation in the zebrafish. Development. 2006;133:3895–9905. doi: 10.1242/dev.02560. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Bel-Vialar S, Krumlauf R. ‘Shocking’ developments in chick embryology: electroporation and in ovo gene expression. Nature Cell Biology. 1999;1:203–207. doi: 10.1038/70231. [DOI] [PubMed] [Google Scholar]
- Johnson AD, Krieg PA. pXeX, a vector for efficient expression of cloned sequences in Xenopus embryos. Gene. 1994;147:223–226. doi: 10.1016/0378-1119(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Momose T, Tonegawa A, Takeuchi J, Ogawa H, Umesono K, Yasuda K. Efficient targeting of gene expression in chick embryos by microelectroporation. Develop. Growth Differ. 1999;41:335–344. doi: 10.1046/j.1440-169x.1999.413437.x. [DOI] [PubMed] [Google Scholar]
- Muramatsu T, Mizutani Y, Ohmori Y, Okumura J-i. Comparison of three nonviral transfection methods for foreign gene expression in early chicken embryos in ovo. Biochemical and Biophysical Research Communications. 1997;230:376–380. doi: 10.1006/bbrc.1996.5882. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Nakamura H, Takagi M, Takeda S, Katsube K-i. Ectopic expression of lunatic Fringe leads to downregulation of Serrate-1 in the developing chick neural tube; analysis using in ovo electroportation transfection technique. FEBS Letters. 1998;426:337–341. doi: 10.1016/s0014-5793(98)00369-x. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Barembaum M. Gain- and loss-of-function approaches in the chick embryo. Methods Cell Biology. 2008;87:237–256. doi: 10.1016/S0091-679X(08)00212-4. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Harada H, Ito K, Ogura T, Nakamura H. Gene manipulation of chick embryos in vitro, early chick culture, and long survival in transplanted eggs. Develop. Growth Differ. 2010;52:629–634. doi: 10.1111/j.1440-169X.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- Uchikawa M. Enhancer analysis by chicken embryo electroporation with the aid of genome comparison. Develop. Growth Differ. 2008;50:467–474. doi: 10.1111/j.1440-169X.2008.01028.x. [DOI] [PubMed] [Google Scholar]
- Vioculescu O, Papanayotou C, Stern CD. Spatially and temporally controlled electroporation of early chick embryos. Nature Protocols. 2008;3:419–426. doi: 10.1038/nprot.2008.10. [DOI] [PubMed] [Google Scholar]