Abstract
Background
Thrombin-dependent platelet activation is heightened in the setting of percutaneous coronary intervention (PCI) and may cause arterial thrombosis with consequent myocardial necrosis. Given the high incidence of adverse effects in patients with acute coronary syndromes (ACS), there remains an unmet need for the development of new therapeutics that target platelet activation without unduly affecting hemostasis. The thrombin receptor, PAR1, has recently emerged as a promising new target for therapeutic intervention in ACS patients.
Methods and Results
We report the development of a first-in-class intracellular PAR1 inhibitor with optimized pharmacokinetic properties for use during PCI in ACS patients. PZ-128 is a cell-penetrating ‘pepducin’ inhibitor of PAR1 which targets the receptor-G protein interface on the inside surface of platelets. The structure of PZ-128 closely resembles the predicted off-state of the corresponding juxtamembrane region of the third intracellular loop of PAR1. The onset of action of PZ-128 was rapid and suppressed PAR1 aggregation and arterial thrombosis in guinea pigs and baboons and strongly synergized with oral clopidogrel. There was full recovery of platelet function by 24 h. Importantly, PZ-128 had no effect on bleeding or coagulation parameters in primates or in blood from PCI patients.
Conclusions
Based on the efficacy data in non-human primates with no noted adverse effects on hemostasis, we anticipate that the rapid onset of platelet inhibition and reversible properties of PZ-128 are well suited to the acute interventional setting of PCI and may provide an alternative to long-acting small molecule inhibitors of PAR1.
Keywords: antiplatelet therapy, drug delivery system, platelets, thrombosis, PAR1
The occurrence of life-threatening arterial thrombotic events during acute coronary syndromes (ACS) and percutaneous coronary interventions (PCI) are critically dependent on reactive platelets.1 Antiplatelet therapy thus plays a central role in preventing stent thrombosis and periprocedural myocardial infarction (MI) in the high risk group of ACS and PCI patients.2, 3 Platelets are also essential for maintaining normal hemostasis and preventing hemorrhage following vascular injury.4 Platelet activation is initiated and perpetuated by binding of multiple agonists to specific G–protein coupled receptors (GPCRs).5 Reinforcement of the adhesive contacts by activating G protein-dependent shape change, granule release, and integrins permits growth of a stable thrombus that is resistant to the high shear stress of arterial blood flow.6 Drugs that target the secondary thromboxane and ADP autocrine mediators of platelet thrombus formation such as aspirin and thienopyridines have proven to be beneficial, however, many patients taking these drugs still sustain thrombotic events, and therefore, might benefit from new therapeutics that inhibit platelet function.7-9
The high affinity thrombin receptor, PAR1, has emerged as a new candidate for therapeutic intervention in patients with acute coronary syndromes and chronic atherothrombotic disease.10-13 Thrombin cleaves and activates both the high affinity PAR1 and lower affinity PAR4 receptor.10, 14-17 Thrombin inhibitors such as bivalirudin effectively suppress PAR1-dependent platelet activation in PCI patients18, however, direct inhibition of thrombin may potentially facilitate bleeding in PCI patients as it also interferes with activation of the PAR4 thrombin receptor and fibrinogen-dependent hemostasis.19 Currently, there are no approved PAR1 inhibitors for the treatment of ACS or other cardiovascular indications. Two PAR1 small molecule inhibitors, vorapaxar (SCH530348)20, 21 and atopaxar (E5555)22 have been evaluated in phase II trials and have been associated with a reduction in ischemic event occurrence. In the TRA-PCI trial with non-urgent PCI patients20 and an accompanying study in NSTE-ACS patients21, vorapaxar reduced the occurrence of periprocedural MI when added to dual antiplatelet therapy. Similarly, atopaxar significantly reduced early ischemia on Holter monitoring in the LANCELOT-ACS phase II study.23 In the recently completed TRACER and TRA-2P Phase III trials, vorapaxar was found to significantly reduce the composite endpoint of death from cardiovascular causes, MI or stroke in ACS patients12 and in patients treated chronically for secondary prevention of atherothrombotic events.13 However, the limitations of vorapaxar include an extremely long pharmacodynamic (PD) half life of up to 3 weeks and oral administration leading to a slower onset of PD effects during PCI, and an elevated risk of bleeding.{Becker, 2009 #2454; Kosoglou, 2012 #2596; Tricoci, 2012 #2601; Morrow, 2012 #2877} The ability to rapidly and reversibly inhibit PAR1 signaling by a parenteral strategy would be an attractive option in high risk patients undergoing PCI.
To block thrombin activation of platelets without interfering with the normal hemostatic functions of thrombin, we report a first-in-class intracellular inhibitor of PAR1. PZ-128 is a lipidated ‘pepducin’ which targets the cytoplasmic surface of PAR1 and interrupts signaling to internally-located G proteins.25-29 The structure of PZ-128 was found to mimic the off-state of the corresponding intracellular region of PAR1 which is critical for coupling to G proteins. PZ-128 rapidly and reversibly inhibits PAR1 platelet activation and arterial thrombosis in guinea pigs and primates without affecting bleeding or other coagulation parameters. These data provide support for the further development of PZ-128 as a novel intervention of PAR1-driven arterial thrombosis in patients undergoing PCI.
Methods
NMR Structural Determination of PZ-128
PZ-128 (palmitate-KKSRALF-NH2) pepducin was synthesized by standard fmoc solid phase methods and purified to 99.1% by reverse-phase high-performance liquid chromatography. NMR samples were prepared by dissolving lyophilized PZ-128 in a buffer comprising 5% glucose-d7, 6.8 mM PZ-128 (final concentration), pH 7.1 with 10% D2O. Samples at acidic pH were prepared by adding perdeuterated acetic acid to 10 mM and adjusting the pH to 4.9. Spectra were collected at 25 °C on Bruker Avance-600 and AMX-500 spectrometers. 2D NOESY sand TOCSY spectra were collected using mixing times of 100 ms and 31 ms, respectively. Spectra were assigned using standard methodology and the distances corresponding to 210 NOE measurements were calculated as previously described using CNS.30
Human Platelet Aggregation
In accordance with informed consent procedures approved by the Tufts Medical Center Institutional Review Board, whole blood from healthy donors was collected into a 30 ml syringe containing sodium citrate (0.4% vol/vol final). Platelets were isolated from platelet rich plasma (PRP) using Sepharose 2B columns in modified PIPES buffer as described previously.17
Human ACT Evaluation
Adult outpatients with angina referred for coronary angiography or PCI were enrolled in the Tufts Medical Center Adult Cardiac Catheterization Laboratory. All patients provided written informed consent before the initiation of the study. The study protocol was approved by the Tufts Medical Center Institutional Review Board. Blood was collected prior to PCI or angiography and PZ-128 was spiked into 1 ml samples of whole blood at a range of final concentrations (0-150 μmol/L). ACT was measured immediately in duplicate. To serve as a positive control for elevated ACT, blood was also collected from patients at the end of the PCI procedure, who received a weight-adjusted dosage of bivalirudin administered intravenously as a 0.75 mg/kg bolus followed by continuous infusion of 1.75 mg /kg/hr during the procedure. Bivalirudin concentrations in plasma were measured by LC/MS/MS as previously described.18
Guinea Pig Arterial Thrombosis and Platelet Aggregation
All guinea pig experiments were carried out in accordance with the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee (IACUC) of Tufts University School of Medicine. Male Hartley guinea pigs (150~220 g) were purchased from Charles River Laboratories. A 0.61 mm-diameter catheter was inserted into the left jugular vein of anesthetized animals for administration of infusions of 5% USP dextrose vehicle or PZ-128. A 0.5 V-Doppler probe (Transonic Systems, Ithaca, NY) was placed around the right carotid artery to record blood flow. A range of doses of PZ-128 from 0.05 to 1.6 mg/kg in 0.9 ml volumes was delivered at an injection rate of 0.09 ml/min by a Harvard syringe pump. Five minutes after the infusion ended, arterial thrombosis was induced by placing a 5×5 mm2 piece of filter paper soaked in freshly made 20% FeCl3 solution on the right carotid artery 5 mm distal to the probe for 20 minutes. If vessel occlusion did not occur within 60 minutes of injury, the experiment was stopped and time to occlusion was assigned a value of 60 minutes. To examine possible synergistic effects of PZ-128 and P2Y12-ADP receptor inhibition, 1 mg/kg clopidogrel was administered by oral gavage 4 hours prior to FeCl3 injury. In these synergy experiments, the maximum endpoint was set at 90 minutes for occlusion time.
Guinea pigs weighing 600-650 g were used for platelet aggregation experiments. PZ-128 (3 or 6 mg/kg) was administered by a 10 min intravenous infusion, and blood was collected by cardiac puncture into sodium citrate (0.4% vol/vol final) 5 minutes after cessation of the infusion. PRP was prepared and PPACK added to a final concentration of 100 μg/ml. PRP was calcified with 2.5 mM CaCl2 and aggregation was performed as described above.
Baboon Arterial-Venous Shunt Thrombosis and Platelet Aggregation
Non-terminal thrombosis and platelet aggregation studies were performed on 12 healthy male baboons (Papio anubis) weighing 9-12 kg at the Oregon National Primate Research Center (ONPRC). Protocols were approved by the IACUC at Oregon Health and Sciences University. All animals had a chronic exteriorized silicone rubber shunt (A-V shunt) placed between the femoral artery and vein, and arterial thrombosis on Dacron grafts (4 mm diameter) quantified as previously described.31 Whole blood (10 ml) was collected into PPACK at a final concentration of 100 μg/ml just prior to infusion (baseline) and 15 min to 24 h after the PZ-128 infusion was terminated. Platelet counts and hematocrit were measured immediately. PRP was prepared from whole blood and platelet aggregation performed as described above. Bleeding time (BT) measurements were performed on the shaved volar surface of the forearm using the standard template method as previously described.31
Quantification of PZ-128 in Baboon Plasma
Various doses of PZ-128 were infused intravenously for 45 min to baboons. At sequential time points, whole blood was drawn into 3.2% citrate buffer and immediately centrifuged at 3000 rpm for 10 min. Platelet-poor plasma (PPP) samples were harvested and stored at -80 °C. PZ-128 drug levels in PPP samples were determined was using an API 4000 LC/MS/MS system (Agilux Laboratories, Worcester, MA).
PT and aPTT Measurements in Cynomolgus Monkeys
PZ-128 (0, 3, 10 or 30 mg/kg) was administered intravenously to 2.5-4.5 kg male and female cynomolgus monkeys by infusion over 1 h at MPI Laboratories (Mattawan, MI). Peripheral venous blood was collected from cynomolgus monkeys into K3EDTA anticoagulant at baseline (Day -8) and at two time points (Day 1 and Day 5) after daily 1-h intravenous PZ-128 infusions on days 1-4. PT and aPTT were analyzed immediately on a MLA-800 coagulation analyzer.
Data Analysis
Statistical analyses were performed using GraphPad Prism Software, STATA or SAS. Significance of anti-platelet effects in guinea pigs and baboons were assessed using the non-parametric Kruskal-Wallis test with the Dunn’s multiple pairwise comparisons or by ANOVA with Bonferroni post-test correction. Baboon arterial thrombosis experiments were analysed using a repeated measures mixed effects model. Coagulation and hemostasis parameters in baboons and cynomolgus monkeys were compared by Wilcoxon matched pairs signed rank test or by linear mixed effects modeling.32 The null hypothesis was rejected at P<0.05.
Results
Structure and Anti-platelet Activity of PZ-128
From a screen of 57 pepducins derived from the i1, i2, i3 and i4 intracellular loops of PAR1, we identified PZ-128 as a highly-efficacious inhibitor of PAR1-dependent platelet aggregation. PZ-128 is a cell-penetrating lipopeptide derived from the juxtamembrane region of the i3 loop and N-terminus of transmembrane domain 6 (TM6) of PAR1 (Figure 1A). This region has been shown to be essential for coupling of PAR1 with associated G proteins.25 Incorporation of the N-terminal palmitate lipid facilitates rapid and highly efficient translocation of the pepducin across the plasma membrane to the inner leaflet of the lipid bilayer.25, 26, 33 The solution structure of PZ-128 was determined by NMR (Figure 1B) and the peptide was found to form a well-defined α-helix extending from the palmitate lipid. We generated structural models of full-length PAR1 in the off- and on-states using the refined x-ray structures of rhodopsin (1HZX) and opsin bound to the Gα C-terminal peptide (3DQB)34 as templates, respectively, for comparison with the NMR-derived structure of the PZ-128 peptide. PZ-128 was found to form a highly similar structure as the corresponding region of PAR1 (residues 307-313) in the off-state with a RMSD of 1.4 Å (Figure 1C).
Figure 1.

Structure and anti-platelet effects of the cell-penetrating PAR1 pepducin, PZ-128. A, Depiction of the mechanism of action of the cell-penetrating PZ-128 pepducin targeting the third intracellular loop (red) of PAR1. B, The NMR structure of PZ-128 was determined by simulated annealing methods using 210 distance restraints and included restraints to the proximal 3 hydrocarbons of the lipid. C, PZ-128 (green) had an RMSD of 1.4 Å with the corresponding peptide backbone region of PAR1 (red) residues 307-313 modeled on the 2.8 Å x-ray structure of rhodopsin in the off-state. D, PZ-128 inhibits PAR1-dependent platelet aggregation. Gel filtered human platelets were treated with various concentrations of PZ-128 and then challenged with the PAR1 agonist SFLLRN (2.5 μM), 20 μM ADP, 200 μM AYPGKF or 1 mg/ml Ristocetin. E, Human platelets were treated with 3 μM PZ-128, 3 μM RWJ-56110, or 5% dextrose vehicle before the addition of various concentrations of thrombin (n=3-5).
PZ-128 completely inhibited human platelet aggregation in response to the PAR1 agonist SFLLRN with an IC50 value of 0.5 μmol/L, but had no inhibitory activity against PAR4 (AYPGKF), ADP or ristocetin agonists (Figure 1D). PZ-128 also markedly right-shifted thrombin-induced aggregation by 5-fold. By comparison, the small molecule RWJ-56110 which antagonizes PAR1 at the extracellular ligand-binding site14 gave a 2-fold right shift in the thrombin activation curve of human platelets.
PZ-128 Inhibits Platelet Aggregation and Arterial Thrombosis in Guinea Pigs
Aside from humans and other primates, the only other animal species known to harbor PAR1 on their platelets are guinea pigs.14 The PAR1 agonist, SFLLRN, was confirmed to activate guinea pig platelets with an EC50 value of 2.5 μmol/L (Figure 2A). PZ-128 was delivered by internal jugular vein infusions over 10 min. At the 15 min time point, 3 and 6 mg/kg PZ-128 provided significant, dose-dependent inhibition of ex vivo platelet aggregation to SFLLRN (Figure 2B). PZ-128 had no effect on aggregation to ADP or the thromboxane mimetic, U46119 (Figure 2C).
Figure 2.

Effects of PZ-128 on platelet aggregation and arterial thrombosis in guinea pig. PZ-128 or 5% dextrose USP vehicle was infused for 10 min into the jugular vein of male and female guinea pigs (0.55-0.65 kg). A-C, At the 15 min time point, whole blood was collected by cardiac puncture in 100 μg/ml PPACK/4% Na-citrate (final) anti-coagulant and platelet rich plasma (PRP) prepared and aggregation measurements were performed. (A) PRP from vehicle-treated animals (n=3) was challenged with SFLLRN to obtain an EC50 of 2.5 μM. B-C, PRP obtained at the 15 min time point after infusion with vehicle, 3 mg/kg PZ-128 or 6 mg/kg PZ128 was challenged with 2.5 μM SFLLRN, 20 μM ADP or 20 μM thromboxane mimetic, U46119. Individual data points (n=3) are overlayed on bar graphs depicting mean ± SD. D, PZ-128 was delivered by 10 min infusion, 5 min prior to initiation of FeCl injury. The time at which the blood-flow decreased to less than 0.01 volts was recorded as occlusion time of vessels. E, Observed synergistic effect of co-administration of low dose of PZ-128 (0.05 mg/kg) and clopidogrel (1 mg/kg PO 4 h prior to start of infusion) on the mean increase of occlusion time over a 90 min period (n=5). Data in B-D were analyzed by the non-parametric Kruskal-Wallis test with the Dunn’s multiple pairwise comparison post-test. Data in E were analysed by two-way ANOVA. *P<0.05, **P<0.01. Global P values were 0.044 for B, 0.33 for C, 0.018 for D, and 0.047 for E.
We used a carotid artery FeCl3 injury model in guinea pigs to assess the anti-thrombotic efficacy of PZ-128 within 15 min of initiation of drug administration. FeCl3 denudes the artery and exposes type I collagen and other subendothelial matrix proteins to initiate platelet-dependent thrombosis.35, 36 Guinea pigs received 10 min intravenous infusions of PZ-128, 5 min prior to carotid artery injury. There was a significant dose-dependent protection against arterial occlusion with an EC50 of 0.075 mg/kg in guinea pig (Figure 2D). Mean occlusion times increased by 4-fold to 40 min at doses above 0.05 mg/kg PZ-128.
The anti-thrombotic effects of PZ-128 when used in combination with clopidogrel were next assessed in order to explore the possibility that dual inhibition of PAR1 and the P2Y12 ADP receptor may synergistically protect against arterial thrombosis. We selected sub-therapeutic doses of each drug that provided non-significant protection when used alone in the guinea pigs. As shown in Figure 2E, treatment of animals with clopidogrel and PZ-128 together significantly extended the carotid artery occlusion time by at least 7-fold as compared to vehicle-treated animals. These data indicate that dual inhibition of PAR1 and P2Y12 provides strong synergistic effects in preventing carotid artery thrombosis.
PZ-128 Inhibits Platelet Aggregation in Baboons
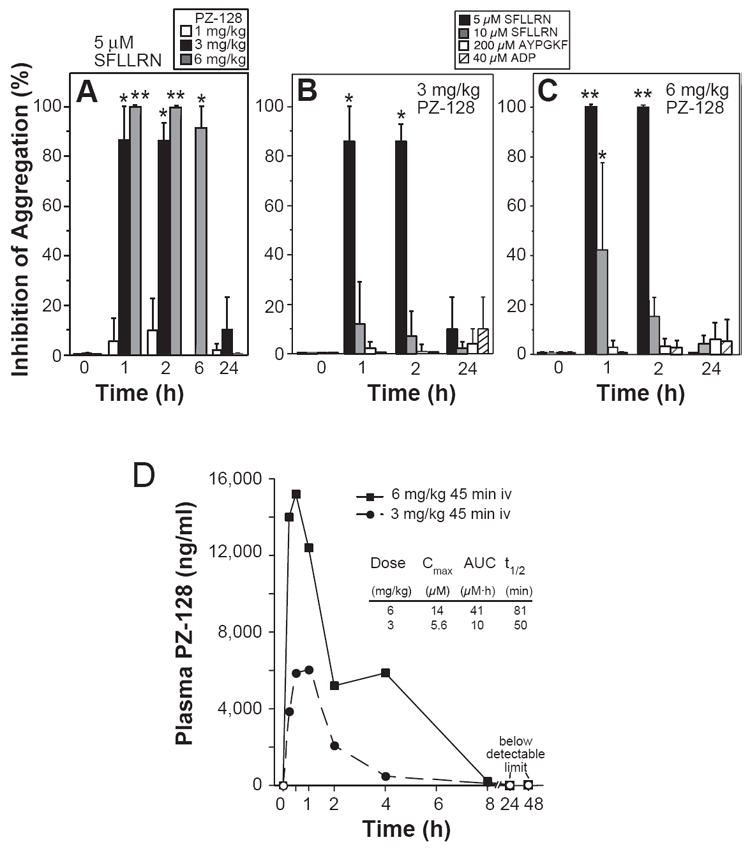
The anti-platelet effects of PZ-128 were next examined in baboons at various time points after receiving different doses of intravenous infusions of PZ-128. Data from baboons showed excellent pharmacodynamic correlations with dose and time-dependent inhibition of PAR1-induced ex vivo platelet aggregation (Figure 3). At the lowest dose tested, 1 mg/kg PZ-128 (30 min infusion), PAR1-dependent aggregation (5 μM SFLLRN) was inhibited by only 5-10% at the 1-2 h time points (Figure 3A). At the 3 mg/kg dose (30 min infusion), PAR1-dependent aggregation was inhibited by 85% at the 1 h and 2 h time points, but was not appreciably inhibited at the 24 h time point (Figure 3B). At the 6 mg/kg dose (45 min infusion), PAR1-dependent aggregation was inhibited by 100% at 1-2 h time points, 90% at 6 h, but was completely recovered by 24 h (Figure 3A). Inhibition of PAR1 by PZ-128 was reversible, as evidenced by loss of inhibition with higher concentrations of SFLLRN agonist (10 μM) at both the 3 mg/kg and 6 mg/kg doses (Figure 3B-C). As a further assessment of in vivo specificity, PZ-128 gave no inhibition at any dose of either the ADP or AYPGKF (PAR4) responses at any time point.
Figure 3.

Inhibition of PAR1-dependent platelet aggregation in baboons. A-C, Male baboons (10-12 kg) were administered 1, 3 or 6 mg/kg PZ-128, or 5% dextrose USP vehicle by iv infusion and blood collected into 100 μg/ml PPACK anticoagulant at 0, 1, 2, 6, or 24 h time points. Light transmission platelet aggregometry was performed with platelet rich plasma with the indicated agonists (SFLLRN for PAR1, AYPGKF for PAR4 and ADP for P2Y12 and P2Y1). Data are reported as mean ± SD (n=3-7) relative to time 0 controls (0%) and were analysed by repeated measures one-way ANOVA with Bonferroni post-test correction; *P<0.05, **P<0.01 relative to time 0. Global P values were >0.05 for 1 mg/kg, 0.004 for 3 mg/kg, <0.0001 for 6 mg/kg. D, Pharmacokinetics of 3 and 6 mg/kg 45-min iv infusions of PZ-128 in male baboons. Plasma PZ-128 levels were measured by LC/MS/MS at 9 sequential time points: baseline, 15 min, 30 min, 1 h, 2 h, 4 h, 8 h, 24 h and 48 h after the start of infusion. Open symbols indicated plasma concentrations that were under the measurement threshold (5 ng/ml).
Peak plasma levels of PZ-128 in baboons were reached at 30 min-1 h after the start of intravenous infusions at both 3 and 6 mg/kg doses. The maximal plasma concentration of PZ-128 was 14 μmol/L at the 6 mg/kg dose and 5.6 μmol/L for the 3 mg/kg dose. PZ-128 was nearly completely cleared from plasma by 8 h with a half life of 50-81 min. PZ-128 was not detectable in plasma at 24-48 h time points. The pharmacokinetic and anti-platelet pharmacodynamic properties of PZ-128 indicate that this lipopeptide reaches maximal activity during and immediately after intravenous infusion and is completely eliminated by the next day.
Effect of PZ-128 on Baboon Arterial Thrombosis
Baboon arterial thrombosis experiments were conducted to determine whether the PZ-128 pepducin had the potential to inhibit arterial thrombosis in primates. An arterial-venous shunt equipped with a dacron vascular graft with an internal lumen diameter of 4 mm at a high flow rate of 100 ml/min was used. Thrombogenesis was assessed by measuring platelet content of the head and tail regions of the developing thrombus (Figure 4A) and quantified by 111Indium-labeled platelet imaging over 60 min. PZ-128 at a dose of 1 mg/kg had no effect on platelet-thrombus deposition in the baboon (data not shown). As shown in Figure 4B, the 6 mg/kg iv infusion dose of PZ-128 gave a significant protective effect against platelet arterial thrombus formation as compared to vehicle (P=0.0028). The effects of the 3 mg/kg dose were not significant but showed a tendency to be protective against arterial thrombosis. These data indicate that PZ-128 can inhibit platelet-dependent thrombus formation in non-human primates under conditions of high arterial flow.
Figure 4.

Inhibition of arterial thrombosis in baboons by PZ-128. 10-14 kg baboons were administered 3 mg/kg or 6 mg/kg PZ-128 by a 45 min iv infusion versus 5% dextrose vehicle (n=2-6). A, Net platelet accumulation was measured during the first 60 minutes of thrombus growth on a femoral arterio-venous Dacron graft (4 mm ID) inserted between silicone rubber tubing segments comprising a high-flow shunt. Blood flow was maintained at 100 ml/min by distal clamping the shunt. Autologous platelets were radiolabeled with indium-111 (1 mCi), and reinjected into the animals before thrombosis experiments. B, Deposition of platelets (mean ± SD) was quantified in the head plus tail regions of the thrombus by 111Indium-labeled platelet imaging with 5-minute data acquisition periods starting at 60 min after initiation of the infusion. Statistical significance was determined using a variance stabilizing LN (natural log) transformation and a repeated measures mixed effects model with an autoregressive covariance structure. Subjects (individual baboons) were included in the model as a random effect. P=0.606 for 3 mg/kg vs vehicle and P=0.0028 for 6 mg/kg vs vehicle.
Effect of PZ-128 on Hemostatic Parameters in Primates and Blood from PCI Patients
We evaluated whether PZ-128 had any adverse effects on hemostasis or coagulation indices in baboons and monkeys. At all doses tested (1-6 mg/kg), PZ-128 had no effect on bleeding time, platelet counts or hematocrit in baboons (Table 1). PZ-128 was also administered daily for 4 days to adult male and female cynomolgus monkeys with 1 h iv infusions of 3 mg/kg, 10 mg/kg and 30 mg/kg PZ-128. Coagulation parameters prothrombin time (PT) and activated partial thromboplastin time (aPTT) were unaffected in all monkeys at 3-30 mg/kg PZ-128 at either day 1 and day 5 as compared to baseline or vehicle-treated animals (Table 2). No spontaneous, venous access, or retinal bleeding was observed in any monkey (n=38) even at PZ-128 plasma levels (Cmax) exceeding 200 μM.
Table 1.
PZ-128 Does not Enhance Bleeding Time in Baboons
| PZ-128 Dose | Baseline | 1-2 h | P value |
|---|---|---|---|
| Platelets (k/μL) | |||
| 1 mg/kg, n=3 | 270 ± 39 | 258 ± 44 | 0.75 |
| 3 mg/kg, n=5 | 339 ± 74 | 334 ± 99 | 0.88 |
| 6 mg/kg, n=4 | 286 ± 96 | 294 ± 80 | 0.63 |
| Hematocrit (%) | |||
| 1 mg/kg, n=3 | 39 ± 3 | 41 ± 3 | 0.25 |
| 3 mg/kg, n=4 | 36 ± 1 | 39 ± 2 | 0.13 |
| 6 mg/kg, n=4 | 36 ± 4 | 40 ± 4 | 0.13 |
| Bleeding time (min) | |||
| ASA+Clopidogrel, n=1 | 5.5 | >20 | - |
| 1 mg/kg, n=3 | 2.8 ± 1.3 | 3.3 ± 1.2 | 0.50 |
| 3 mg/kg, n=5 | 4.4 ± 1.9 | 4.6± 1.5 | 1.0 |
| 6 mg/kg, n=3 | 4.0 ± 2.3 | 3.7 ± 1.6 | 0.59 |
Venous blood was collected from male baboons into PPACK anticoagulant (final concentration 100 μg/ml) at baseline and at 1-2 h after the start of the intravenous PZ-128 infusion (1 mg/kg over 15 min, 3 mg/kg over 30 min, or 6 mg/kg over 45 min). Platelet counts and hematocrit were measured using a micro-60 automated cell counter (Horiba ABX, Diagnostics, Montpellier, France). Template bleeding time (BT) (Surgicutt; ITC, Edison, NJ) was measured at baseline at either 1 or 2 h after the start of the PZ-128 infusion. Data are reported as Mean ± SD and P values were determined by Wilcoxon matched pairs signed rank test.
Table 2.
PZ128 Does Not Affect Coagulation Parameters in Cynomolgus Monkeys
| PZ-128 Dose | Day -8 | Day 1 | Day 5 |
|---|---|---|---|
| APTT (sec) | |||
| Female | |||
| 0 mg/kg (n=5) | 26.9 ± 2.8 | 27.1 ± 2.7 | 25.5 ± 2.8 |
| 3 mg/kg (n=3) | 25.7 ± 2.2 | 27.2 ± 2.7 | 26.8 ± 2.6 |
| 10 mg/kg (n=3) | 27.6 ± 3.6 | 27.3 ± 2.3 | 27.3 ± 3.7 |
| 30 mg/kg (n=5) | 26.5 ± 2.8 | 26.2 ± 2.8 | 27.1 ± 1.8 |
| Male | |||
| 0 mg/kg (n=5) | 25.9 ± 1.4 | 25 ± 1.7 | 23.5 ± 1.1 |
| 3 mg/kg (n=3) | 30.1 ± 2.0 | 30.5 ± 1.1 | 28.1 ± 1.4 |
| 10 mg/kg (n=3) | 30.6 ± 2.4 | 21.3 ± 2.3 | 29.6 ± 2.9 |
| 30 mg/kg (n=4-5) | 32.2 ± 2.9 | 30.9 ± 5.7 | 33.7 ± 6.5 |
| PT (sec) | |||
| Female | |||
| 0 mg/kg (n=5) | 11.5 ± 0.3 | 11.9 ± 0.5 | 11.6 ± 0.4 |
| 3 mg/kg (n=3) | 11.6 ± 0.5 | 11.8 ± 0.5 | 11.6 ± 0.2 |
| 10 mg/kg (n=3) | 10.9 ± 0.7 | 11.0 ± 0.3 | 11.4 ± 0.6 |
| 30 mg/kg (n=5) | 11.6 ± 0.1 | 11.7 ± 0.3 | 11.8 ± 0.6 |
| Male | |||
| 0 mg/kg (n=5) | 11.1 ± 0.5 | 11.3 ± 0.5 | 11.5 ± 0.6 |
| 3 mg/kg (n=3) | 11.7 ± 0.3 | 11.8 ± 0.4 | 11.7 ± 0.2 |
| 10 mg/kg (n=3) | 11.7 ± 0.3 | 12.2 ± 0.6 | 12.2 ± 0.3 |
| 30 mg/kg (n=4-5) | 11.9 ± 0.4 | 12.0 ± 0.6 | 12.5 ± 0.6 |
Venous blood was collected from cynomolgus monkeys into K3EDTA anticoagulant at baseline (Day -8) and at two time points (Day 1 and Day 5) after daily 1-h intravenous PZ-128 infusions on days 1-4. PT and aPTT was measured by using a MLA-800 coagulation analyzer. Data are reported as Mean ± SD. P values were not significant as determined by repeated measures linear mixed effects modeling.
Lastly, we measured the effects of PZ-128 on activated clotting time (ACT) in human blood samples freshly obtained from adult patients undergoing PCI. At concentrations of PZ-128 up to 150 μM, there were no effects on ACT in the human PCI blood samples (Figure 5). By comparison, the ACT was highly elevated at the 30 min time period in all PCI patients who received intravenous infusions of the direct thrombin inhibitor, bivalirudin. Together, these data indicate that downstream inhibition of the platelet thrombin receptor with PZ-128 does not adversely affect hemostasis or coagulation parameters in primates as compared to direct inhibition of thrombin.
Figure 5.
PZ-128 does not affect activated clotting time of blood from PCI patients. PZ-128 (□) was spiked at various concentrations (0-150 μM) into fresh whole blood obtained from patients just prior to PCI. By comparison, blood was obtained at the 30 min time point from PCI patients (n=22) after a weight-adjusted dosage of bivalirudin (●) administered intravenously as a 0.75 mg/kg bolus followed by continuous infusion of 1.75 mg /kg/hr during the procedure. ACT assays were performed immediately using a Hemochron 801 with FTCA510-4 ACT cartridges containing silica, phospholipids, and diatomaceous earth (kaolin). The open circle represents the mean (± SD) ACT and mean bivalirudin concentration at the 30 min time point in the 22 PCI patients.
Discussion
Disruption of atherosclerotic plaques and formation of occlusive platelet thrombi remains a leading cause of morbidity and mortality in the United States.2 Antiplatelet therapy thus plays a critical role in preventing arterial thrombosis and myocardial infarction in high risk patients with acute coronary syndromes, atherothrombotic disease and in patients who have undergone PCI.3-5 PZ-128 is a first-in-class cell-penetrating pepducin inhibitor targeted against the PAR1 receptor-G protein interface being developed as an anti-platelet agent to be used during coronary interventions. We demonstrated that PZ-128 is a rapid-acting and specific inhibitor of PAR1-dependent platelet aggregation and does not suppress ADP, thromboxane or PAR4 responses. PZ-128 attenuated PAR1 aggregation and arterial thrombosis in guinea pigs within 15 min, and effectively inhibited PAR1 platelet activity and arterial thrombosis in baboons with full recovery of platelet function by 24 h. The inhibitory effects of the pepducin were fully reversible and overcome by high concentrations of PAR1 agonist even at early time points. PZ-128 had no effect on bleeding or coagulation parameters in baboons and monkeys, or in blood samples from PCI patients.
Current antiplatelet therapy for secondary prevention of vascular events mainly consists of oral administration of aspirin and blockade of the P2Y12 ADP receptor with thienopyridines.9 Patients with a higher risk of thrombosis while undergoing coronary interventions are also often treated with intravenous GP IIb/IIIa antagonists in addition to aspirin, thienopyridine and heparin.4 Although dual antiplatelet therapy has been shown to attenuate ischemic event occurrence during ACS and PCI, drug response variability, the persistent occurrence of ischemic events, and the increased risk of bleeding events remain major concerns. Notably, treatment with the most potent P2Y12 receptor blockers are associated with only a 16-19% relative risk reduction compared to clopidogrel and approximately 10% of patients still suffer from recurrent ischemic events within one year of treatment.37, 38 The latter observations indicate that there may be a ceiling effect in a strategy solely employing aspirin and P2Y12 receptor inhibition in the attenuation of platelet-mediated ischemic event occurrence. A current hypothesis is that persistent ischemic events in the presence of P2Y12 blockade and aspirin are due to thrombin and collagen-mediated platelet activation under high arterial shear that is unchecked by currently available agents.9 PZ-128 was found to be effective at inhibiting both thrombin-induced PAR1 activation and collagen-initiated arterial thrombosis35, 36 in guinea pigs triggered by FeCl3 injury. Moreover, the PAR1 pepducin PZ-128 acts in synergy with the P2Y12 antagonist, clopidogrel, to significantly inhibit arterial thrombosis in guinea pigs.
Contrary to potent thrombin (e.g. bivalirudin, hirudin, argatroban, dabigatran), or factor Xa inhibitors (rivaroxaban, apixaban)39, fully reversible PAR1 inhibitors do not directly affect coagulation and increased bleeding should not accompany their use40 — an observation that is consistent with our studies in non-human primates. As thrombin-dependent fibrin generation is unaffected by inhibition of PAR1 and reversible PAR1 antagonists can be overcome by robust hemostatic thrombin generation, a thrombin-receptor antagonist may provide a safer therapeutic index than a thrombin or Xa inhibitor in preventing arterial thrombosis.41, 42 Likewise, the PZ-128 pepducin had no adverse effects on bleeding, coagulation, or clotting time in non-human primates and human blood samples. In recent studies35, PZ-128 did not impact initial platelet adhesion to exposed collagen surfaces, but prevented large occlusive thrombi from forming. Taken together, these findings support the notion that PAR1 inhibitors such as PZ-128 can permit the formation of an initial platelet-fibrin monolayer necessary for control of hemostasis, but still block pathological thrombus propagation that occurs at the site of endothelial denudation.43
It was notable that the highly potent PAR1 small molecule inhibitor, vorapaxar, was recently shown to significantly increase the rate of moderate and severe bleeding in both ACS patients and in patients being treated for secondary prevention of atherothrombotic events.12, 13 Two possible explanations for the elevated bleeding include: 1) the extremely long pharmacodynamic effect of vorapaxar which significantly inhibits platelet function for up to 3 weeks (plasma half-life of 5-11 days){Kosoglou, 2012 #2596} with a single loading dose; 2) vorapaxar was administered daily for a median time of 1-2.5 years in combination with both aspirin and a P2Y12 inhibitor.12, 13 In a subgroup analysis of TRACER, it was found that Vorapaxar did not increase the hazard of GUSTO moderate or severe bleeding in the patients who did not receive a thienopyridine.12 Therefore, it is likely that concomitant blockade of P2Y12 and thromboxane receptors along with PAR1 may also contribute to the observed bleeding risk in the ACS patients. A much shorter-acting and reversible PAR1 antagonist such as PZ-128 (plasma half-life of 50-80 min) is expected to help mitigate any untoward periprocedural bleeding in the context of dual anti-platelet therapy. Moreover, small molecule inhibitors such as vorapaxar and atopaxar interact with the ligand binding site on the extracellular surface of the receptor. By comparison, PZ-128 works by an entirely different mechanism of action on the inner surface of the lipid bilayer where it modulates the interactions of PAR1 with intracellular G proteins.44, 45 The structure of PZ-128 was found to closely resemble the predicted off-state of the corresponding juxtamembrane region of the third intracellular loop and helix 6 region of PAR1, consistent with a mechanism whereby PZ-128 may stabilize or mimic the off-state of PAR1. Intervention of PAR1-dependent platelet activation with the PZ-128 pepducin thus represents an entirely new therapeutic strategy for suppressing arterial thrombosis, which could potentially benefit PCI patients being treated for severe atherothrombotic heart disease.
Clinical Perspective.
Antiplatelet therapy is of paramount importance in the effective treatment of acute coronary syndrome (ACS) patients and those undergoing coronary intervention (PCI). Thrombin is the most potent platelet activator. The thrombin receptor PAR1 has emerged as an important new therapeutic target to inhibit platelet function in ACS patients undergoing PCI. We describe the development of PZ-128, a first-in-class PAR1 inhibitor which targets the cytoplasmic loops of G protein-coupled receptors. PZ-128 rapidly suppressed PAR1-induced platelet aggregation and arterial thrombosis in guinea pigs and baboons and was synergistic to oral clopidogrel. PZ-128 did not affect bleeding or coagulation in non-human primates or in blood from PCI patients. Platelet function returned to baseline 24 hours following PZ-128 infusion. PAR1 inhibition by PZ-128 appears to be a novel promising therapy for ACS patients. Planned clinical trials will establish where this novel class of medication fits into our therapeutic armamentarium.
Acknowledgments
Funding Sources: This work was supported by grants from the National Heart Lung and Blood Institute (RC2 HL101783, R01 HL64701) to Dr Kuliopulos.
Footnotes
Conflict of Interest Disclosures: Drs. Kuliopulos and Covic are scientific founders of Anchor Therapeutics.
References
- 1.Gurbel PA, Bliden KP, Saucedo JF, Suarez TA, DiChiara J, Antonino MJ, Mahla E, Singla A, Herzog WR, Bassi AK, Hennebry TA, Gesheff TB, Tantry US. Bivalirudin and clopidogrel with and without eptifibatide for elective stenting: effects on platelet function, thrombelastographic indexes, and their relation to periprocedural infarction results of the CLEAR PLATELETS-2 (Clopidogrel with Eptifibatide to Arrest the Reactivity of Platelets) study. J Am Coll Cardiol. 2009;53:648–657. doi: 10.1016/j.jacc.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 2.Lansky AJ, Stone GW. Periprocedural myocardial infarction: prevalence, prognosis, and prevention. Circ Cardiovasc Interv. 2010;3:602–610. doi: 10.1161/CIRCINTERVENTIONS.110.959080. [DOI] [PubMed] [Google Scholar]
- 3.Brar SS, ten Berg J, Marcucci R, Price MJ, Valgimigli M, Kim HS, Patti G, Breet NJ, DiSciascio G, Cuisset T, Dangas G. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. A collaborative meta-analysis of individual participant data. J Am Coll Cardiol. 2011;58:1945–1954. doi: 10.1016/j.jacc.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 4.Coller BS. Historical perspective and future directions in platelet research. J Thromb Haemost. 2011;9(Suppl 1):374–395. doi: 10.1111/j.1538-7836.2011.04356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smyth SS, Woulfe DS, Weitz JI, Gachet C, Conley PB, Goodman SG, Roe MT, Kuliopulos A, Moliterno DJ, French PA, Steinhubl SR, Becker RC. G-protein-coupled receptors as signaling targets for antiplatelet therapy. Arterioscler Thromb Vasc Biol. 2009;29:449–457. doi: 10.1161/ATVBAHA.108.176388. [DOI] [PubMed] [Google Scholar]
- 6.Jackson SP, Nesbitt WS, Kulkarni S. Signaling events underlying thrombus formation. J Thromb Haemost. 2003;1:1602–1612. doi: 10.1046/j.1538-7836.2003.00267.x. [DOI] [PubMed] [Google Scholar]
- 7.Steinhubl SR, Schneider DJ, Berger PB, Becker RC. Determining the efficacy of antiplatelet therapies for the individual: lessons from clinical trials. J Thromb Thrombolysis. 2008;26:8–13. doi: 10.1007/s11239-007-0160-3. [DOI] [PubMed] [Google Scholar]
- 8.Faxon DP, Freedman JE. Facts and controversies of aspirin and clopidogrel therapy. Am Heart J. 2009;157:412–422. doi: 10.1016/j.ahj.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Gurbel PA, Tantry US. Combination antithrombotic therapies. Circulation. 2010;121:569–583. doi: 10.1161/CIRCULATIONAHA.109.853085. [DOI] [PubMed] [Google Scholar]
- 10.Leger AJ, Covic L, Kuliopulos A. Protease-Activated Receptors and Cardiovascular Diseases. Circulation. 2006;113:1070–1077. doi: 10.1161/CIRCULATIONAHA.105.574830. [DOI] [PubMed] [Google Scholar]
- 11.Antoniak S, Pawlinski R, Mackman N. Protease-activated receptors and myocardial infarction. IUBMB Life. 2011;63:383–389. doi: 10.1002/iub.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, White HD, Aylward PE, Wallentin L, Chen E, Lokhnygina Y, Pei J, Leonardi S, Rorick TL, Kilian AM, Jennings LH, Ambrosio G, Bode C, Cequier A, Cornel JH, Diaz R, Erkan A, Huber K, Hudson MP, Jiang L, Jukema JW, Lewis BS, Lincoff AM, Montalescot G, Nicolau JC, Ogawa H, Pfisterer M, Prieto JC, Ruzyllo W, Sinnaeve PR, Storey RF, Valgimigli M, Whellan DJ, Widimsky P, Strony J, Harrington RA, Mahaffey KW. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366:20–33. doi: 10.1056/NEJMoa1109719. [DOI] [PubMed] [Google Scholar]
- 13.Morrow DA, Braunwald E, Bonaca MP, Ameriso SF, Dalby AJ, Mary Polly Fish BA, Fox KAA, Lipka LJ, Liu X, Nicolau JC, Ophuis AJO, Paolasso E, Scirica BM, Spinar J, Theroux P, Wiviott SD, Strony J, Murphy SA. for the TRA 2P–TIMI 50 Steering Committee and Investigators. Vorapaxar in the Secondary Prevention of Atherothrombotic Events. N Engl J Med. 2012;366:1404–13. doi: 10.1056/NEJMoa1200933. [DOI] [PubMed] [Google Scholar]
- 14.Leger AJ, Jacques SL, Badar J, Kaneider NC, Derian CK, Andrade-Gordon P, Covic L, Kuliopulos A. Blocking the protease-activated receptor 1-4 heterodimer in platelet-mediated thrombosis. Circulation. 2006;113:1244–1254. doi: 10.1161/CIRCULATIONAHA.105.587758. [DOI] [PubMed] [Google Scholar]
- 15.Jacques S, LeMasurier M, Sheridan PJ, Seeley SK, Kuliopulos A. Substrate-Assisted Catalysis of the PAR1 Thrombin Receptor: Enhancement of Macromolecular Association and Cleavage. J Biol Chem. 2000;275:40671–40678. doi: 10.1074/jbc.M004544200. [DOI] [PubMed] [Google Scholar]
- 16.Jacques SL, Kuliopulos A. Protease-activated receptor-4 uses dual prolines and an anionic recognition motif for thrombin recognition and cleavage. Biochem J. 2003;376:733–740. doi: 10.1042/BJ20030954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Covic L, Gresser AL, Kuliopulos A. Biphasic Kinetics of Activation and Signaling for PAR1 and PAR4 Thrombin Receptors in Platelets. Biochemistry. 2000;39:5458–5467. doi: 10.1021/bi9927078. [DOI] [PubMed] [Google Scholar]
- 18.Kimmelstiel C, Zhang P, Kapur NK, Weintraub A, Krishnamurthy B, Castaneda V, Covic L, Kuliopulos A. Bivalirudin is a dual inhibitor of thrombin and collagen-dependent platelet activation in patients undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2011;4:171–179. doi: 10.1161/CIRCINTERVENTIONS.110.959098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Covic L, Singh C, Smith H, Kuliopulos A. Role of the PAR4 Thrombin Receptor in Stabilizing Platelet-Platelet Aggregates as revealed by a Patient with Hermansky-Pudlak Syndrome. Thromb Haemost. 2002;87:722–727. [PubMed] [Google Scholar]
- 20.Becker RC, Moliterno DJ, Jennings LK, Pieper KS, Pei J, Niederman A, Ziada KM, Berman G, Strony J, Joseph D, Mahaffey KW, Van de Werf F, Veltri E, Harrington RA. Safety and tolerability of SCH 530348 in patients undergoing non-urgent percutaneous coronary intervention: a randomised, double-blind, placebo-controlled phase II study. Lancet. 2009;373:919–928. doi: 10.1016/S0140-6736(09)60230-0. [DOI] [PubMed] [Google Scholar]
- 21.Goto S, Yamaguchi T, Ikeda Y, Kato K, Yamaguchi H, Jensen P. Safety and exploratory efficacy of the novel thrombin receptor (PAR-1) antagonist SCH530348 for non-ST-segment elevation acute coronary syndrome. J Atheroscler Thromb. 2010;17:156–164. doi: 10.5551/jat.3038. [DOI] [PubMed] [Google Scholar]
- 22.Goto S, Ogawa H, Takeuchi M, Flather MD, Bhatt DL. Double-blind, placebo-controlled Phase II studies of the protease-activated receptor 1 antagonist E5555 (atopaxar) in Japanese patients with acute coronary syndrome or high-risk coronary artery disease. Eur Heart J. 2010;31:2601–2613. doi: 10.1093/eurheartj/ehq320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Donoghue ML, Bhatt DL, Wiviott SD, Goodman SG, Fitzgerald DJ, Angiolillo DJ, Goto S, Montalescot G, Zeymer U, Aylward PE, Guetta V, Dudek D, Ziecina R, Contant CF, Flather MD. Safety and tolerability of atopaxar in the treatment of patients with acute coronary syndromes: the lessons from antagonizing the cellular effects of Thrombin-Acute Coronary Syndromes Trial. Circulation. 2011;123:1843–1853. doi: 10.1161/CIRCULATIONAHA.110.000786. [DOI] [PubMed] [Google Scholar]
- 24.Kosoglou T, Reyderman L, Tiessen RG, van Vliet AA, Fales RR, Keller R, Yang B, Cutler DL. Pharmacodynamics and pharmacokinetics of the novel PAR-1 antagonist vorapaxar (formerly SCH 530348) in healthy subjects. Eur J Clin Pharmacol. 2012;68:249–58. doi: 10.1007/s00228-011-1120-6. [DOI] [PubMed] [Google Scholar]
- 25.Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc Natl Acad Sci U S A. 2002;99:643–648. doi: 10.1073/pnas.022460899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Covic L, Tchernychev B, Jacques S, Kuliopulos A. Pharmacology and in vivo efficacy of pepducins in hemostasis and arterial thrombosis. In: Langel U, editor. Handbook of Cell-Penetrating Peptides. 2. New York: Taylor & Francis; 2007. pp. 245–257. [Google Scholar]
- 27.Covic L, Misra M, Badar J, Singh C, Kuliopulos A. Pepducin-based intervention of thrombin receptor signaling and systemic platelet activation. Nature Med. 2002;8:1161–1165. doi: 10.1038/nm760. [DOI] [PubMed] [Google Scholar]
- 28.Sevigny LM, Zhang P, Bohm A, Lazarides K, Perides G, Covic L, Kuliopulos A. Interdicting protease-activated receptor-2-driven inflammation with cell-penetrating pepducins. Proc Natl Acad Sci U S A. 2011;108:8491–8496. doi: 10.1073/pnas.1017091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuliopulos A, Covic L. Blocking receptors on the inside: pepducin-based intervention of PAR signaling and thrombosis. Life Sciences. 2003;74:255–262. doi: 10.1016/j.lfs.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Liu Z, Androphy E, Chen J, Baleja JD. Design and characterization of helical peptides that inhibit the E6 protein of papillomavirus. Biochemistry. 2004;43:7421–7431. doi: 10.1021/bi049552a. [DOI] [PubMed] [Google Scholar]
- 31.Hanson SR, Kotze HF, Savage B, Harker LA. Platelet interactions with Dacron vascular grafts. A model of acute thrombosis in baboons. Arteriosclerosis. 1985;5:595–603. doi: 10.1161/01.atv.5.6.595. [DOI] [PubMed] [Google Scholar]
- 32.Pinheiro JC, Bates DM. Mixed-effects Models in S and S-plus. New York: Springer; 2000. [Google Scholar]
- 33.Wielders SJ, Bennaghmouch A, Reutelingsperger CP, Bevers EM, Lindhout T. Anticoagulant and antithrombotic properties of intracellular protease-activated receptor antagonists. J Thromb Haemost. 2007;5:571–576. doi: 10.1111/j.1538-7836.2007.02364.x. [DOI] [PubMed] [Google Scholar]
- 34.Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 35.Trivedi V, Boire A, Tchernychev B, Kaneider NC, Leger AJ, O’Callaghan K, Covic L, Kuliopulos A. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137:332–343. doi: 10.1016/j.cell.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckly A, Hechler B, Freund M, Zerr M, Cazenave JP, Lanza F, Mangin PH, Gachet C. Mechanisms underlying FeCl3-induced arterial thrombosis. J Thromb Haemost. 2011;9:779–789. doi: 10.1111/j.1538-7836.2011.04218.x. [DOI] [PubMed] [Google Scholar]
- 37.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 38.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 39.Roe MT, Ohman EM. A new era in secondary prevention after acute coronary syndrome. N Engl J Med. 2011;366:85–87. doi: 10.1056/NEJMe1112770. [DOI] [PubMed] [Google Scholar]
- 40.Chintala M, Shimizu K, Ogawa M, Yamaguchi H, Doi M, Jensen P. Basic and translational research on proteinase-activated receptors: antagonism of the proteinase-activated receptor 1 for thrombin, a novel approach to antiplatelet therapy for atherothrombotic disease. J Pharmacol Sci. 2008;108:433–438. doi: 10.1254/jphs.08r06fm. [DOI] [PubMed] [Google Scholar]
- 41.Derian CK, Damiano BP, Addo MF, Darrow AL, D’Andrea MR, Nedelman M, Zhang HC, Maryanoff BE, Andrade-Gordon P. Blockade of the thrombin receptor protease-activated receptor-1 with a small-molecule antagonist prevents thrombus formation and vascular occlusion in nonhuman primates. J Pharmacol Exp Ther. 2003;304:855–861. doi: 10.1124/jpet.102.042663. [DOI] [PubMed] [Google Scholar]
- 42.Kato Y, Kita Y, Hirasawa-Taniyama Y, Nishio M, Mihara K, Ito K, Yamanaka T, Seki J, Miyata S, Mutoh S. Inhibition of arterial thrombosis by a protease-activated receptor 1 antagonist, FR171113, in the guinea pig. Eur J Pharmacol. 2003;473:163–169. doi: 10.1016/s0014-2999(03)01973-3. [DOI] [PubMed] [Google Scholar]
- 43.Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thromb Haemost. 2009;102:248–257. doi: 10.1160/TH09-03-0192. [DOI] [PubMed] [Google Scholar]
- 44.Cisowski J, O’Callaghan K, Kuliopulos A, Yang J, Nguyen N, Deng Q, Yang E, Fogel M, Tressel S, Foley C, Agarwal A, Hunt SW, 3rd, McMurry T, Brinckerhoff L, Covic L. Targeting protease-activated receptor-1 with cell-penetrating pepducins in lung cancer. Am J Pathol. 2011;179:513–523. doi: 10.1016/j.ajpath.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dimond P, Carlson K, Bouvier M, Gerard C, Xu L, Covic L, Agarwal A, Ernst OP, Janz JM, Schwartz TW, Gardella TJ, Milligan G, Kuliopulos A, Sakmar TP, Hunt SW., 3rd G protein-coupled receptor modulation with pepducins: moving closer to the clinic. Ann N Y Acad Sci. 2011;1226:34–49. doi: 10.1111/j.1749-6632.2011.06039.x. [DOI] [PubMed] [Google Scholar]


