Summary
Background
The ability to objectively measure lung function in children is critical in the assessment and treatment of asthma in this age group. We thus determined the effectiveness of impulse oscillometry (IOS) as a non-invasive technique to assess lung function in children and in comparison to spirometry for sensitivity and specificity, testing variability, and the order effect of sequential testing of IOS and spirometry.
Methods
One hundred seventeen children sequentially evaluated in a pediatric clinic and under medical care for disease, were asked to perform IOS and spirometry. The utility of IOS and spirometry in differentiating children that had asthma versus those children who did not was then analyzed.
Results
In the primary analysis (n = 117), bronchodilator response using IOS distinguished asthmatics from non-asthmatics, P = 0.0008 for R10. Receiver–operator characteristic curve (ROC) analysis of R10 bronchodilator response at the best cut-off (–8.6% change) correctly identified 77% of patients with asthma and excluded 76% of non-asthmatics. Amongst those children able to perform spirometry (asthmatics, n = 66; non-asthmatics, n = 16), FEV1 did not reveal a difference between these two groups, while area of reactance (AX) did distinguish these groups (P = 0.0092). Sequential testing of IOS and then spirometry (n = 47) showed a significant decrement in lung function as determined by IOS following performance of spirometry (P = 0.0309).
Conclusion
In the diagnosis and management of children with lung disease, IOS is a non-invasive approach that easily and objectively measures lung impedance and should be considered as both an adjunct, and in some situations, an alternative to standard spirometry.
Keywords: pediatric asthma, lung function, bronchodilator response, spirometry, impulse oscillometry
INTRODUCTION
Asthma is among the most common chronic diseases of childhood.1 The ability to objectively diagnose and assess asthma severity is crucial to the skilled provider's selection of appropriate therapy in the management of pediatric asthma. Current guidelines recommended by the National Asthma Education and Prevention Program's Expert Panel Report include the use of spirometry in children ≥5 years old to determine airway obstruction and reversibility.2 While the use of spirometry to assess lung function is of great value, one recent survey indicated that only 21% of medical practitioners use spirometry in the diagnosis of asthma in children.3 This may be due to a number of factors including lack of access to spirometry, the difficulty in interpretation of results in a younger age group, and that young and handicapped children may be unable technically to perform spirometry, as it requires the performance of effort dependant lung maneuvers.4 In this setting, impulse oscillometry (IOS) has been introduced as an alternative technique to assess lung function with a particular application to younger children with asthma. This is because IOS is non-invasive, easy to perform and requires only minimal patient cooperation. IOS is based on the production of small pressure oscillations that are applied at the mouth and transmitted into the lungs, which in turn permits the measurement of the resistance and reactance to the impedance of the respiratory system during spontaneous quiet breathing; and thus provides an indirect analysis of lung function.5
The purpose of the present study was to prospectively compare the utility of IOS and spirometry in the evaluation of children with well-controlled asthma in a pediatric allergy clinic in comparison to children who did not have asthma. This was accomplished by assessing lung function by IOS and comparing the findings to measurement of lung function by spirometry. Response to bronchodilator was also assessed, using standard cutoff values for reversibility and when considering trial-to-trial coefficient of variability (CV). When performing both IOS and spirometry sequentially, an analysis of the effects of test order was also performed. We hypothesized that IOS is superior to spirometry in identifying those with asthma in the pediatric age group. As will be shown, we found that when independently analyzing IOS lung parameters and then comparing them to FEV1, IOS was more specific and sensitive than spirometry in differentiating those with asthma from those who did not have asthma, validating the above hypothesis. Other findings relating to IOS will also be discussed.
MATERIALS AND METHODS
Subjects
All children that were referred to the Pediatric Allergy Clinic at the National Institutes of Health, Bethesda, MD, between March 2007 and December 2009 and were able to sit for IOS on their initial evaluation were entered into the study. One hundred seventeen children met this criterion and were enrolled on an NIAID IRB approved protocol following informed consent.
All patients that were evaluated were presented at their first visit with an allergic symptom questionnaire (see Supplementary Appendix) and underwent a history, physical examination, skin prick testing (when indicated by suspicion of an allergic disease by history); and both IOS and spirometry (when capable). Laboratory studies included measurement of total IgE and serum tryptase. In 88 of these patients, a diagnosis of asthma was confirmed or established by staff physicians following NHBLI guidelines for asthma (which includes a history and physical findings suggestive of recurrent episodes of airway obstruction and the use of objective measures of lung function such as spirometry to determine airway obstruction reversibility, ≥12% improvement above baseline).2 Of 117 patients entered into the study, 82 (66 with asthma; 16 who did not asthma) were able to perform spirometry in addition to IOS. When a patient had more than one analysis of lung function performed, one visit during which IOS and spirometry was performed was selected at random for analysis. An analysis for order effect was performed on 47 patients who were able to return to clinic specifically for this study.
Patients were classified as atopic based on history and questionnaire; and a serum IgE >90 IU/ml (NIH, Bethesda, MD) and/or specific IgE for a specific allergen by skin prick testing or by ImmunoCap (0.35 kU/L for aeroallergens or in accordance with standards for clinical reactivity as defined for foods6). Skin testing was done using the Greerpick®. Reactions >3 mm in diameter above the negative control recorded at 15 min were considered positive. In patients unable to undergo skin testing, specific IgE testing based on history using ImmunoCAP was employed.
IOS
The IOS system (MasterScreen Impulse Oscillometry by CareFusion, Yorba Linda, CA) was calibrated through several full strokes of a single volume (3 L) of air at different flow rates, which were verified with a reference resistance device (2.0 cmH2O/L/sec) supplied by the manufacturer. Patients withheld the use of both short and long-acting bronchodilators starting the night prior to testing, which was performed and analyzed in accordance with ERS/ATS guidelines.7,8 For performance of IOS, the child was placed in a sitting position and instructed to place their lips around the mouthpiece of the IOS pneumotachometer and to breathe normally. A nasal clip was used and the hands of the child or parent were placed on the cheeks to limit their expansion. Pulmonary impedance was measured and reported as resistance (R), the energy required to propagate the pressure wave through the airways and reactance (X), which reflects the viscoelastic properties of the respiratory system. A 30-sec (minimum) interval of testing was performed from which the mean values of reactance and resistance were calculated at frequencies from 5 to 20 Hz, specifically R5, R10, R20, and X5. In addition, the area of reactance (AX), which represents a summation of reactance values below resonance frequency, was measured. Assuming the coherence, which is a measure of testing reliability, was acceptable (>0.80 at 10 Hz)9,10 and there was no evidence of coughing, swallowing, vocalization, or breath holding, the trial was saved. An average of three adequate measurements was analyzed and graphically displayed. When airway reversibility was being assessed, a bronchodilator was administered (two inhalations of albuterol, 90 mcg) using a spacer. After 15 min, IOS was repeated. The IOS apparatus calculates the CV, which is an indicator of trial-to-trial variability and served as an index of test repeatability. The bronchodilator response was then compared to the pre- and post-test CV to determine the reliability of the response. Predicted values for R and X were based on gender and height according to the equipment's default normal references values as recommended by the manufacturer based on existing reference values.11,12
Spirometry
Flow volume curves were measured using Vmax Encore Model 20C, Care Fusion (Yorba Linda, CA). The system was calibrated according to manufacture standards and testing procedures were in accordance with ATS guidelines for determination of expiratory flow volume measurements.7,13,14 Spirometry was performed with the child standing with nose clips in place. The child was encouraged to produce the greatest expiratory flow. FEV1 was reported from the best of three to six attempts. If the operator determined that the effort was suboptimal or expiration could not be maintained until close to residual volume, the test was discarded. Children below age 6 were in general able to perform IOS. Fewer were able to perform spirometry. Where applicable a standard cut-off of 12% for reversibility was employed.15 Spirometry and IOS measurements were performed by one research nurse that was trained in both procedures. All results were stored within their associated computer program, abstracted into spread sheets by three individuals and presented to the statistician who analyzed data amongst and within diagnostic criteria for statistical differences without bias.
Statistical Analysis
Three groups of patient were analyzed consisting of the 117 children who performed IOS, 82 children who completed IOS and spirometry, and 42 children that underwent sequential IOS and spirometry. Wilcoxon sign rank tests of order effect data were computed in GraphPad Prism 5. CV normalized the IOS parameters as defined by the X or R percent change divided by twice the higher (pre or post) CV. An unpaired Student's t-test was used to compare CV between the patients with asthma and healthy (non-asthmatic) patients.
Receiver–operator characteristic (ROC) curve analyses were performed using a custom algorithm in R [www.r-project.org]. Patients with multiple visits had a single visit randomly sampled for analysis, otherwise the initial visit was used. Sequences of 5,000 possible cut-off values were created for each diagnostic metric (e.g., FEV1, R5, etc.) using the minimum and maximum levels from each patient. False positive and false negative rates were calculated for each of the 5,000 possible cut-off values, using each patient's pulmonary function readings (e.g., FEV1, R5, etc.) to generate predicted outcomes (e.g., asthma or no asthma, drug increase or decrease) and using recorded patient data as the true outcomes (e.g., asthma diagnosis). Area under the curve (AUC) was computed for each ROC curve using the trapezoid method and the best cut-off values were estimated as the point on each curve with the minimal distance to sensitivity = 100% and 1 – specificity = 0%. Best cut-off values and AUC were determined for each curve.
Bootstrap resampling tests were used to determine if any differences in ROC AUC were statistically signifi-cant. Data were resampled 1,000 times with replacement and ROC AUC was computed for each resample, then two-sided P-values were computed from the true differences in ROC AUC in the real data using a histogram of the resampled differences in ROC AUC as the null distribution. No differences in ROC AUC were statistically significant.
RESULTS
Demographics
Within the total group of 117 patients there were no significant differences in age, gender, height, weight, and ethnicity between the asthmatic and non-asthmatic groups. The age range for those with asthma was 3–18 years, and those without asthma was 3–17 years, with gender equally represented. The percentage of children with atopy was >85% in both groups, consistent with elevated mean total serum IgE, 1430.9 (SD 3519.3) IU/ml in asthmatic versus 1090.1 (SD 4149.0) IU/ml in non-asthmatics. Mean serum tryptase levels were within normal limits in both groups [3.6 (1.8) ng/ml vs. 3.8 (1.4) ng/ml] (Table 1).
TABLE 1.
Patient Demographics
| Parameter | Asthma (n = 88) | Non-asthma (n = 29) | P-value |
|---|---|---|---|
| Age mean (SD) | 7.7 (3.6) | 7.3 (4.2) | 0.7091 |
| Male gender, n (%) | 50 (58.8%) | 16 (59.3%) | 1.0000 |
| Height (cm) | 126.4 (19.9) | 126.8 (25.5) | 0.9480 |
| Weight (kg) | 33.9 (27.2) | 30.4 (16.9) | 0.4311 |
| Ethic origin | |||
| Caucasian | 46 (56.1%) | 16 (61.5%) | 0.6566 |
| Black | 16 (19.2%) | 6(23.1%) | 0.7809 |
| Hispanic | 6 (7.3%) | 0 (0.0%) | 0.3322 |
| Asian | 14(17.1%) | 4 (15.4%) | 1.0000 |
| Atopy (%) | 75 (89.3%) | 24 (88.9%) | 1.0000 |
| Total IgE, IU/ml (SD) | 1430.9 (3519.3) | 1090.1 (4149.0) | 0.7090 |
| Tryptase (ng/ml) | 3.6 (1.8) | 3.8 (1.4) | 0.5716 |
| Asthma based on parent assessment (%) | 67 (77.9%) | 1 (3.7%) | 0.0001 |
| Use of Monteleukast | 18 (20.5%) | 0 (0.0%) | 0.0060 |
| Use of inhaled corticosteroids | 32 (36.4%) | 0 (0.0%) | 0.0001 |
| Baseline measurements | |||
| R5 (cmH2O/L/sec) | 102.8 (23.8) | 105.2 (21.7) | 0.6274 |
| R10 (cmH2O/L/sec) | 95.3 (21.6) | 102.1 (15.9) | 0.0898 |
| R20 (cmH2O/L/sec) | 95.2 (21.9) | 98.2 (17.3) | 0.4809 |
| R5-R20 (cmH2O/L/sec) | 29.0 (10.6) | 30.1 (11.4) | 0.6536 |
| X5 (cmH2O/L/sec) | 108.7 (49.7) | 127.1 (75.2) | 0.2483 |
| AX (cmH2O/L) | 115.4 (71.2) | 132.0 (48.7) | 0.2399 |
| Bronchodilator response | |||
| ΔR5 (%) | –16.0(11.1) | –9.0 (12.0) | 0.0082* |
| ΔR10(%) | –15.5 (9.7) | –8.0 (9.9) | 0.0008* |
| ΔR20(%) | –9.3 (11.7) | –5.9 (11.9) | 0.2079 |
| ΔR5–R20 (%) | 23.8 (10.2) | 27.6 (10.7) | 0.1024 |
| ΔX5 (%) | –17.4 (23.7) | –6.8 (21.0) | 0.0269* |
| ΔAX (%) | –38.4 (20.5) | –19.7 (25.5) | 0.0009* |
P < 0.05.
Based on intake questionnaires from the first visit, 77.9% of patients diagnosed with asthma were correctly assessed to have asthma by parental assessment, while only one non-asthmatics was assessed to have asthma (P = 0.0001). Amongst those with asthma, the use of controller medicine at the initial visit was as follows: leukotriene inhibitor (20.5%), inhaled corticosteroid (36.6%), and short or long acting β2 agonist (64.1%).
IOS
For the primary analysis, patients with multiple visits were randomized to select one visit for study. Pre-bronchodilator measurements for all 117 patients using normal reference values for IOS did not provide evidence of airway obstruction and did not differ significantly between those with and without asthma, remembering that those with asthma were under therapy. However, when comparing percent change in pre- and post-bronchodilator response, there was a statistically significant difference in the percent change of R5, R10, X5, and AX, with R10 most noteworthy, P = 0.0008 (Table 1) in those with asthma compared with those without asthma.
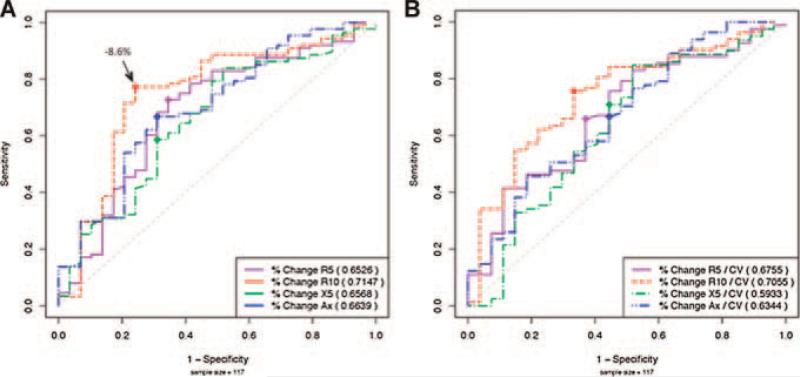
Amongst the IOS parameters statistically significant for bronchodilator response, the best profile for detection of clinical asthma using ROC was found in R10 which had the highest AUC (0.7147), followed by AX (AUC = 0.6639) (Fig. 1A). The best cut-off point, where the sum of the sensitivity and specificity was greatest (Table 2) for R10, was found to be –8.6%. At this point the sensitivity and specificity of using IOS to correctly diagnose those with asthma versus those without asthma was 77% and 76%, respectively. For AX the cut-off point was –29.1%, corresponding to a sensitivity of 67% and specificity of 69%.
Fig 1.
ROC curves for IOS. A: Receiver operator characteristic curves for R5, R10, X5, and AX are displayed (n = 117) comparing asthmatics and non-asthmatics. Area-under-curve (AUC) is calculated to indicate the profile of sensitivity and specificity for each test parameter. Best cut-off value for R10 in noted. B: ROC for CV index (bronchodilator response/2CV) CV index ≥1 indicates positive response.
TABLE 2.
ROC Cut-Off Points
| Metric | Cut-off | Sensitivity | 1 – Specificity |
|---|---|---|---|
| FEV1 | 6.23 | 0.54 | 0.40 |
| R5 | –11.24 | 0.73 | 0.34 |
| R10 | –8.58 | 0.77 | 0.24 |
| R20 | –5.91 | 0.62 | 0.35 |
| X5 | –18.15 | 0.59 | 0.31 |
| AX | –29.11 | 0.67 | 0.31 |
| R5.CV | –0.59 | 0.66 | 0.37 |
| R10.CV | –0.53 | 0.76 | 0.33 |
| R20.CV | –0.32 | 0.63 | 0.33 |
| X5.CV | –0.21 | 0.71 | 0.44 |
| Ax.CV | –0.66 | 0.67 | 0.44 |
Coefficient of Variability
We next determined if the CV, a measure of trial-to-trial reproducibility, could be used to improve the profile of objective cut-off points for the detection of clinical asthma. When the CV is high, it may indicate that the cut-off for the response following bronchodilator should be increased. For a low CV, a more conservative change may indicate clinical reactivity. For all patients (n = 117) the mean CV ranged from 6.6% for R10 (baseline without asthma) to 21.4% for X5 (post-bronchodilator with asthma), and was generally lower in those without asthma although this did not reach significance (Table 3). The effect of factoring CV in the ROC profiles (dividing the percent change by twice the CV) did not improve the sensitivity and specificity of the baseline curve (Fig. 1B).
TABLE 3.
Coefficient of Variability (CV)
| Parameter | All patients (n = 117), mean (std dev) | Asthma (n = 88), mean (std dev) | Non-asthma (n = 29), mean (std dev) | Unpaired t-test, P-value |
|---|---|---|---|---|
| Pre-bronchodilator | ||||
| R5 | 8.2 (6.3) | 8.2 (6.1) | 8.6 (7.2) | 0.7919 |
| R10 | 7.1 (5.1) | 7.3 (5.1) | 6.8 (5.0) | 0.6469 |
| R20 | 8.2 (5.9) | 8.3 (5.9) | 7.9 (6.0) | 0.7674 |
| AX | 18.0 (13.0) | 19.2 (12.8) | 15.0 (13.1) | 0.1597 |
| X5 | 16.9 (12.3) | 17.3 (12.6) | 16.2 (11.5) | 0.6700 |
| Post-bronchodilator | ||||
| R5 | 7.9 (6.2) | 7.9 (6.8) | 8.16 (3.8) | 0.7902 |
| R10 | 6.6 (4.9) | 6.7 (5.1) | 6.72 (4.1) | 0.9444 |
| R20 | 7.7 (5.9) | 7.6 (5.7) | 8.3 (6.5) | 0.6471 |
| AX | 20.2 (12.2) | 19.8 (10.4) | 21.8 (16.8) | 0.5787 |
| X5 | 20.4 (40.6) | 21.4 (46.1) | 18.1 (13.4) | 0.5752 |
ROC analysis of the most symptomatic visit revealed the same conclusions for X, R with the highest AUC for R10 (=0.692) and the lowest for FEV1, and no significant change when dividing by CV (data not shown). Thus, although it seems prudent to note the extent of CV when interpreting the objective responses to bronchodilator, direct factoring of CV into this result did not substantially improve the ability to distinguish between those with and without asthma.
Comparison of IOS to Spirometry
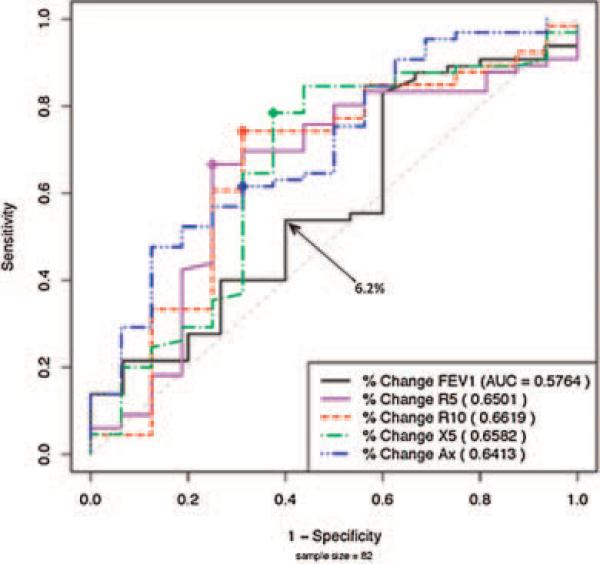
In patients able to perform both spirometry and IOS (asthmatics, n = 66; non-asthmatics, n = 16), baseline measurements using spirometry or IOS did not reveal a difference between these two groups. Change in response to bronchodilator was significant for AX (P = 0.0092) but not for FEV1 (P = 0.3766) nor other spirometric indices (Table 4). Comparison using ROC also indicated that IOS is better at identifying asthmatics than non-asthmatics (Fig. 2). R10 and AX had the best profile of sensitivity and specificity. When plotted in comparison to FEV1, the cut-off point for FEV1 was 6.2% at which point ROC analysis detected only 54% of asthmatics and excluded 40% of non-asthmatics (Fig. 2). Thus, based on ROC analysis of IOS and spirometry, lung parameters in IOS were superior in identifying asthmatics.
TABLE 4.
Baseline Measurements and Bronchodilator Response for Patients Performing Both IOS and Spirometry
| Parameter | Asthma (n = 66) | Non-asthma (n = 16) | Test statistic | P-value |
|---|---|---|---|---|
| Baseline measurements | ||||
| FEV1% predicted | 91.3 (23.9) | 88.9 (15.2) | T = –0.4824 | 0.6325 |
| FVC | 2.3 (0.9) | 2.3 (1.0) | T = –0.037 | 0.9711 |
| FEV1/FVC | 80.6 (9.9) | 87.4 (8.1) | T = –2.866 | 0.0080 |
| PEF | 4.0 (1.5) | 4.1 (2.0) | T = –0.362 | 0.7214 |
| R5 (cmH2O/L/sec) | 104.1 (26.0) | 100.3 (18.6) | T = –0.641 | 0.5274 |
| R10 (cmH2O/L/sec) | 96.0 (23.8) | 95.5 (15.1) | T = –0.088 | 0.9301 |
| R20 (cmH2O/L/sec) | 94.7 (23.5) | 90.0 (18.0) | T = –0.833 | 0.4133 |
| R5–R20 (cmH2O/L/sec) | 29.0 (10.6) | 30.1 (11.4) | T = –0.452 | 0.6536 |
| X5 (cmH2O/L/sec) | 117.6 (53.0) | 123.6 (41.1) | T = 0.463 | 0.6477 |
| AX (cmH2O/L) | 126.8 (80.0) | 131.7 (44.2) | T = 0.259 | 0.7981 |
| Bronchodilator response | ||||
| ΔFEV1 (%) | 6.1 (10.2) | 4.2 (6.9) | T = –0.897 | 0.3766 |
| ΔFVC (%) | 2.7 (7.3) | 3.2 (5.8) | T = –0.266 | 0.7926 |
| ΔFEV1/FVC (%) | 4.9 (8.0) | 1.7 (8.2) | T = 1.343 | 0.1941 |
| ΔPEF (%) | 6.0 (16.2) | 9.1 (11.3) | T = –0.863 | 0.3952 |
| ΔR5 (%) | –15.6(12.1) | –10.6 (11.1) | T = 1.589 | 0.1249 |
| ΔR10 (%) | –15.4(10.4) | –10.7 (11.2) | T = 1.553 | 0.1349 |
| ΔR20 (%) | –9.6 (12.5) | –10.0(14.0) | T = –0.106 | 0.9171 |
| ΔR5–R20 (%) | 23.8 (10.2) | 27.6 (10.7) | T = –1.667 | 0.1024 |
| ΔX5 (%) | –16.5 (24.6) | –7.4 (20.5) | T = 1.519 | 0.1405 |
| ΔAX (%) | –37.0 (20.9) | –18.5 (23.5) | T = 2.867 | 0.0092* |
P < 0.05.
Fig 2.
ROC curves IOS versus FEV1. ROC curves in patients able to perform both IOS and spirometry (n = 82—asthmatics; n = 66 non-asthmatics; n = 16).
Order Effect
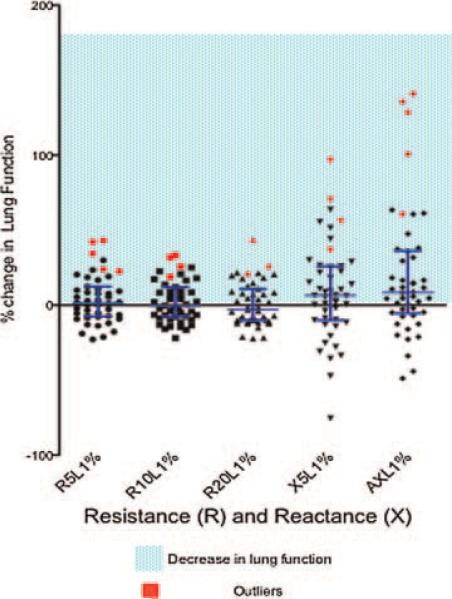
In our clinic, we routinely perform IOS followed by spirometry due to a concern that performing spirometry may cause transient bronchospasm. To determine if this is a valid concern, a subset of patients (47) were able to return to clinic for this study involving testing with IOS and then spirometry, immediately followed by repeat IOS. The IOS measurements prior and following spirometry were compared. Median IOS impedance values (post-spirometry vs. pre-spirometry) changed in the direction of worsening lung function in all parameters except for R20. The change in AX reached statistical significance (P = 0.0309) (Table 5). The percent changes as measured by R and X as indicated are displayed in Figure 3. Median and interquartile ranges are skewed toward a decrease in lung function (increase in R and X) (Table 6). A determination of outliers (Fig. 3) identified five patients to have the greatest decrease in lung function based on significant increases in R and X. An analysis of these patients (Table 7) indicates they were atopic, had reversible obstruction, active chest symptoms, and were on controller anti-inflammatory medication. This data suggest that performance of spirometry may cause bronchospasm in certain patients, as detected by IOS.
TABLE 5.
Comparison of IOS Pre- and Post-Spirometry
| Patients (n = 47) |
||
|---|---|---|
| Median of paired differences | One tailed Wilcoxon signed rank test (P-value) | |
| R5 | 7.66 | 0.2046 |
| R10 | 6.29 | 0.2947 |
| R20 | 5.39 | 0.3754 |
| X5 | –2.65 | 0.1201 |
| AX | 18.45 | 0.0309* |
P < 0.05.
Fig 3.
Order effect of IOS, spirometry and IOS. A total of 47 patients evaluated with baseline IOS, spirometry and immediate repeat IOS with mean and interquartile ranges for reactance and resistance. Outliers are indicated in red (n = 5).
TABLE 6.
Percent Change of IOS Pre- and Post-Spirometry
| Patients (n = 47) |
|||
|---|---|---|---|
| Median | Interquartile range, 25% | Interquartile range, 75% | |
| ΔR5 | 1.41 | –7.44 | 12.36 |
| ΔR10 | –0.28 | –7.78 | 12.08 |
| ΔR20 | –3.13 | –10.11 | 10.95 |
| ΔX5 | 6.45 | –10.11 | 25.91 |
| ΔAX | 8.50 | –5.43 | 36.21 |
TABLE 7.
Outliers, Greatest Decrease in Lung Function
| Pt. no. | FEV1% | AX% | Chest symptoms | ICS | Atopy SPT | IgE (Ul/ml) |
|---|---|---|---|---|---|---|
| 1 | 3 | –45 | Cough | ICS in recent past | (+) SPT | 8 |
| 2 | 8 | –16 | Recent URI wheezing | Singulair | (+) SPT | 956 |
| 3 | 13 | –41 | Cough, wheezing | None | (+) SPT | 11,100 |
| 4 | 11 | –77 | Cough, wheezing, SOB, chest tightness | Advair | (+) SPT | 283 |
| 5 | 8 | 31 | None | None | (+) SPT | 80 |
DISCUSSION
The general purpose of this study was to determine the utility of IOS and spirometry in the evaluation of children in a general pediatric allergy clinic with well-controlled asthma in comparison to children who did not have asthma. This study showed overall that IOS was more sensitive and specific in identifying children with reversible obstruction than spirometry. When analyzing independent IOS lung parameters and comparing them to FEV1, R10 best differentiated those with asthma and non-asthmatics. Using objective cut-off points for lung parameter responses to bronchodilator, the consideration of CV did not contribute significantly to interpreting the results of the response. Additionally, the action of performing forced expiratory maneuvers in spirometry may cause a decrement in lung function detected by IOS. Thus, when both tests are performed sequentially, the data support the recommendation to perform IOS before spirometry.
The demographic data (Table 1) indicate, based on a questionnaire, that parental assessment of asthma correctly identified 78% of patients diagnosed with asthma. This finding is remarkably consistent with an International Study of Asthma and Allergies in Childhood (ISAAC)-based questionnaire administered to parents of 6,295 children aged 1–6 whereupon 77% of the children parentally assessed to have asthma were diagnosed as such in their medical record,16 thus highlighting the important consideration of parental appraisal of childhood asthma.
When comparing pre- and post-bronchodilator responses in all 117 patients we found a statistically significant difference in R5, R10, X5, and AX, with R10 most remarkable (P = 0.0008). This is consistent with other studies of IOS bronchodilator responses that found R5 and R10 to best differentiate between asthmatic and non-asthmatic children.10,17 Although others have found R5–R20, frequency dependance of resistance, to be highly correlated with AX and a sensitive measure of bronchomotor tone,18,19 our analysis did not reveal this correlation, nor did this parameter significantly distinguish between the asthmatics and nonasthmatics at baseline, or following administration of bronchodilator (Tables 1 and 4). This may be due to the greater variability in age, larger number of subjects or differences in severity of asthma in our disease population in comparison to other studies. ROC curve comparisons in Figure 1A also displays R10 with the best profile of sensitivity (77%) and specificity (76%) at a cut-off point of –8.6%. This suggests that an 8.6% or greater decrease in post-bronchodilator airway resistance at 10 Hz distinguishes between those with and without asthma. Similarly, cut-off points for R5 were –11.2%, and –29.1% for AX (Table 2). Previous studies in children and adults have suggested higher cut-off values for a “positive bronchodilator response,” such as 20–40%, 15–20%, and 50% for R5, R10, and AX, respectively.14,20,21 The lower cut-off values determined herein may be a reflection of the methodology used to determine these cut-offs, the age of our patients and/or study selection of well-maintained asthmatic children.
We used the CV to assist in the interpretation of response to bronchodilator. The mean CV in our total study group (n = 117) ranged from 6.6% for R10 to 21.4% for X5 and was generally lower in those without asthma, although the difference between groups did not reach significance (Table 3). This range is consistent with one study that found day-to-day measurements of CV using forced oscillation technique in healthy adults to be 7%.22 To determine the relevance of using the CV in the interpretation of a response to bronchodilator we employed the criteria as others have suggested8 to require a percentage improvement in IOS lung parameters following bronchodilator that is at least twice the preor post-CV, whichever is higher. This is formulated by the CV index (percent change in R or X following bronchodialator/2CV). A CV index ≥1 indicates a positive response to bronchodilator and may include patients with lower bronchodilator responses and lower variability, whereas a CV index <1 suggests too much variability and may exclude patients that met objective criteria (i.e., 20% for R10) for a positive response. Although the consideration of using the CV index to refine the interpretation of response to bronchodilator is reasonable, when factored into the ROC curves and replotted there was no significant improvement in sensitivity and specificity profiles (Fig. 1B).
Based on the bronchodilator response in those patients that were able to perform spirometry (asthmatics, n = 66; non-asthmatics, n = 16) and IOS, we have shown that IOS is better than spirometry at identifying children with asthma (Table 4) and the ROC comparison of FEV1 to other IOS parameters suggests it is a more sensitive and specific tool for distinguishing between these groups (Fig. 2). The advantages of IOS over spirometry gleaned from this study are supported by others.10,17,19,23–26 Our data are consistent with an earlier report which showed that significant differences in IOS-assessed bronchodilator responses distinguished between young Korean asthmatics and non-asthmatics, which was not detected by spirometry.17 In another study, when 24 clinically stable adolescent asthmatics were assessed with pulmonary function testing over 3 consecutive days, significant differences in reactance and resistance was detected by IOS but not by spirometry.19 Others have reported that in long-term studies of lung function in children treated with inhaled corticosteroids, IOS (specifically AX) may be more informative in determining lung function than spirometry.27
Other limitations of spirometry include effort dependency, which may preclude accurate testing in younger children and those mentally or physically compromised. Due to these factors, the failure rate in children under 4 years old was 100% (11/11) and overall, 25% (33/117) of our patients were unable to perform spirometry. A 17-year-old subject in our study with recurrent pneumothoraces and pulmonary fibrosis secondary to chemotherapy was prohibited from performing spirometry because of the risk inducing a pneumothorax. However, his pulmonary status was assessed repeatedly and un-eventfully over years using IOS. We successfully performed IOS down to 2 years old, while we found that spirometry was not useful for those ≤5. Others have shown that IOS can be performed successfully in 3- to 6-year-old asthmatics, and showed reproducible and sensitive indices of lung function.10,17,24,26,28,29 In a 2-year study to determine the effects of inhaled corticosteroids on the development of asthma in pre-school children, IOS was exclusively used to assess lung function because only 56% of the participants were able to perform spirometry.30
Our data support the conclusion that when IOS and spirometry are performed sequentially, IOS should be performed first (Table 5). Performing forced expiration during spirometry for multiple trials may cause bronchospasm detected by IOS (Fig. 3). As our data suggest, patients with atopic asthma on controller medications with active chest symptoms are more likely to experience a decrease in lung function following spirometry.
In summary, this study supports the objective utility of IOS in the evaluation of children with asthma. Current guidelines for pediatric asthma in terms of the establishment of a diagnosis, classification of severity, assessment of impairment and response to therapy recommend lung function testing with spirometry. The greater sensitivity and specificity of IOS shown in this study in addition to its broader application in younger or physically compromised children merits the consideration to incorporate IOS into future standard guidelines for the treatment of children with asthma.
REFERENCES
- 1.Foliaki S, Annesi-Maesano I, Daniel R, Fakakovikaetau T, Magatongia M, Tuuau-Potoi N, Waqatakirewa L, Cheng SK, Pearce N. Prevalence of symptoms of childhood asthma, allergic rhino-conjunctivitis and eczema in the Pacific: the International Study of Asthma and Allergies in Childhood (ISAAC). Allergy. 2007;62:259–264. doi: 10.1111/j.1398-9995.2007.01343.x. [DOI] [PubMed] [Google Scholar]
- 2.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein JA, Lozano P, Shulruff R, Inui TS, Soumerai SB, Ng M, Weiss KB. Self-reported physician practices for children with asthma: are national guidelines followed? Pediatrics. 2000;106:886–896. [PubMed] [Google Scholar]
- 4.Stanojevic S, Wade A, Cole TJ, Lum S, Custovic A, Silverman M, Hall GL, Welsh L, Kirkby J, Nystad W, Badier M, Davis S, Turner S, Piccioni P, Vilozni D, Eigen H, Vlachos-Mayer H, Zheng J, Tomalak W, Jones M, Hankinson JL, Stocks J. Spirometry centile charts for young Caucasian children: the Asthma UK Collaborative Initiative. Am J Respir Crit Care Med. 2009;180:547–552. doi: 10.1164/rccm.200903-0323OC. [DOI] [PubMed] [Google Scholar]
- 5.Goldman MD. Clinical application of forced oscillation. Pulm Pharmacol Ther. 2001;14:341–350. doi: 10.1006/pupt.2001.0310. [DOI] [PubMed] [Google Scholar]
- 6.Sampson HA. Food allergy accurately—identifying clinical reactivity. Allergy. 2005;60:19–24. doi: 10.1111/j.1398-9995.2005.00853.x. [DOI] [PubMed] [Google Scholar]
- 7.Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, Bisgaard H, Davis GM, Ducharme FM, Eigen H, Gappa M, Gaultier C, Gustafsson PM, Hall GL, Hantos Z, Healy MJ, Jones MH, Klug B, Lodrup Carlsen KC, McKenzie SA, Marchal F, Mayer OH, Merkus PJ, Morris MG, Oostveen E, Pillow JJ, Seddon PC, Silverman M, Sly PD, Stocks J, Tepper RS, Vilozni D, Wilson NM. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175:1304–1345. doi: 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- 8.Oostveen E, MacLeod D, Lorino H, Farre R, Hantos Z, Desager K, Marchal F. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22:1026–1041. doi: 10.1183/09031936.03.00089403. [DOI] [PubMed] [Google Scholar]
- 9.Frei J, Jutla J, Kramer G, Hatzakis GE, Ducharme FM, Davis GM. Impulse oscillometry: reference values in children 100 to 150cm in height and 3 to 10 years of age. Chest. 2005;128:1266–1273. doi: 10.1378/chest.128.3.1266. [DOI] [PubMed] [Google Scholar]
- 10.Marotta A, Klinnert MD, Price MR, Larsen GL, Liu AH. Impulse oscillometry provides an effective measure of lung dys-function in 4-year-old children at risk for persistent asthma. J Allergy Clin Immunol. 2003;112:317–322. doi: 10.1067/mai.2003.1627. [DOI] [PubMed] [Google Scholar]
- 11.Dencker M, Malmberg LP, Valind S, Thorsson O, Karlsson MK, Pelkonen A, Pohjanpalo A, Haahtela T, Turpeinen M, Wollmer P. Reference values for respiratory system impedance by using impulse oscillometry in children aged 2–11 years. Clin Physiol Funct Imaging. 2006;26:247–250. doi: 10.1111/j.1475-097X.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 12.Nowowiejska B, Tomalak W, Radlinski J, Siergiejko G, Latawiec W, Kaczmarski M. Transient reference values for impulse oscillometry for children aged 3–18 years. Pediatr Pulmonol. 2008;43:1193–1197. doi: 10.1002/ppul.20926. [DOI] [PubMed] [Google Scholar]
- 13.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 14.Standardization of Spirometry. 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 15.Dundas I, Chan EY, Bridge PD, McKenzie SA. Diagnostic accuracy of bronchodilator responsiveness in wheezy children. Thorax. 2005;60:13–16. doi: 10.1136/thx.2004.029934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hederos CA, Hasselgren M, Hedlin G, Bornehag CG. Comparison of clinically diagnosed asthma with parental assessment of children's asthma in a questionnaire. Pediatr Allergy Immunol. 2007;18:135–141. doi: 10.1111/j.1399-3038.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- 17.Song TW, Kim KW, Kim ES, Park JW, Sohn MH, Kim KE. Utility of impulse oscillometry in young children with asthma. Pediatr Allergy Immunol. 2008;19:763–768. doi: 10.1111/j.1399-3038.2008.00734.x. [DOI] [PubMed] [Google Scholar]
- 18.Meraz EG, Nazeran H, Ramos CD, Nava P, Diong B, Goldman MD. Analysis of impulse oscillometric measures of lung function and respiratory system model parameters in small airway-impaired and healthy children over a 2-year period. Biomed Eng Online. 2011;10:21. doi: 10.1186/1475-925X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldman MD, Carter R, Klein R, Fritz G, Carter B, Pachucki P. Within- and between-day variability of respiratory impedance, using impulse oscillometry in adolescent asthmatics. Pediatr Pulmonol. 2002;34:312–319. doi: 10.1002/ppul.10168. [DOI] [PubMed] [Google Scholar]
- 20.Hellinckx J, De Boeck K, Bande-Knops J, van der Poel M, Demedts M. Bronchodilator response in 3–6. 5 years old healthy and stable asthmatic children. Eur Respir J. 1998;12:438–443. doi: 10.1183/09031936.98.12020438. [DOI] [PubMed] [Google Scholar]
- 21.Smith HJ, Reinhold P, Goldman MD. In: Forced oscillation technique and impulse oscillometry. Journals E, editor. ERS Journals LTD; 2005. [Google Scholar]
- 22.Aronsson H, Solymar L, Dempsey J, Bjure J, Olsson T, Bake B. A modified forced oscillation technique for measurements of respiratory resistance. J Appl Physiol. 1977;42:650–655. doi: 10.1152/jappl.1977.42.4.650. [DOI] [PubMed] [Google Scholar]
- 23.Al-Mutairi SS, Sharma PN, Al-Alawi A, Al-Deen JS. Impulse oscillometry: an alternative modality to the conventional pulmonary function test to categorise obstructive pulmonary disorders. Clin Exp Med. 2007;7:56–64. doi: 10.1007/s10238-007-0126-y. [DOI] [PubMed] [Google Scholar]
- 24.Olaguibel JM, Alvarez-Puebla MJ, Anda M, Gomez B, Garcia BE, Tabar AI, Arroabarren E. Comparative analysis of the bronchodilator response measured by impulse oscillometry (IOS), spirometry and body plethysmography in asthmatic children. J Investig Allergol Clin Immunol. 2005;15:102–106. [PubMed] [Google Scholar]
- 25.Song TW, Kim KW, Kim ES, Kim KE, Sohn MH. Correlation between spirometry and impulse oscillometry in children with asthma. Acta Paediatr. 2008;97:51–54. doi: 10.1111/j.1651-2227.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- 26.Bisgaard H, Klug B. Lung function measurement in awake young children. Eur Respir J. 1995;8:2067–2075. doi: 10.1183/09031936.95.08122067. [DOI] [PubMed] [Google Scholar]
- 27.Larsen GL, Morgan W, Heldt GP, Mauger DT, Boehmer SJ, Chinchilli VM, Lemanske RF, Jr., Martinez F, Strunk RC, Szefler SJ, Zeiger RS, Taussig LM, Bacharier LB, Guilbert TW, Radford S, Sorkness CA. Impulse oscillometry versus spirometry in a long-term study of controller therapy for pediatric asthma. J Allergy Clin Immunol. 2009;123:861–867. e861. doi: 10.1016/j.jaci.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duiverman EJ, Neijens HJ, Van der Snee-van Smaalen M, Kerrebijn KF. Comparison of forced oscillometry and forced expirations for measuring dose-related responses to inhaled methacholine in asthmatic children. Bull Eur Physiopathol Respir. 1986;22:433–436. [PubMed] [Google Scholar]
- 29.Lebecque P, Spier S, Lapierre JG, Lamarre A, Zinman R, Coates AL. Histamine challenge test in children using forced oscillation to measure total respiratory resistance. Chest. 1987;92:313–318. doi: 10.1378/chest.92.2.313. [DOI] [PubMed] [Google Scholar]
- 30.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, Bacharier LB, Lemanske RF, Jr., Strunk RC, Allen DB, Bloomberg GR, Heldt G, Krawiec M, Larsen G, Liu AH, Chinchilli VM, Sorkness CA, Taussig LM, Martinez FD. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]