4-Ipomeanol (IPO1; 1-(3-Furyl)-4-hydroxy-1-pentanone) is a furanoterpenoid toxin produced by sweet potatoes (Ipomoea batatas) in response to infection by the mold Fusariam solani (Doster et al., 1978). Cattle that ingest infected sweet potatoes can develop interstitial pneumonia that is characterized by pulmonary edema and emphysema. Moldy sweet potatoes have been linked to the death of livestock since 1952 (Monlux et al., 1953), including recent cases in Wisconsin where 200 steer died (Spoto, 2011) and England, where six cows died in what is believed to be the first case of IPO toxicity in the UK (Steckel and Rhodes, 2007). IPO is lethal to cattle in doses as low as 7.5 mg/kg which can be achieved by ingesting as little as 6 kg of spoiled sweet potatoes (Doster et al., 1978).
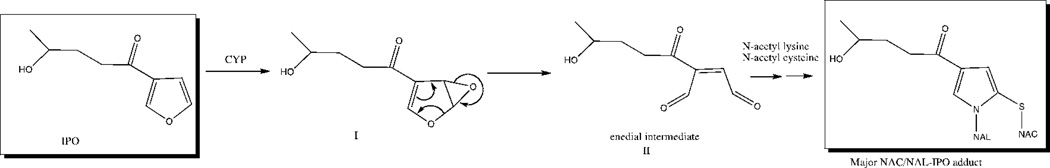
IPO is a pulmonary pro-toxin in both rabbits and rats which bioactivate the compound in a reaction that has been shown to be mediated readily by pulmonary cytochrome P450 4B1 (CYP4B1) (Verschoyle et al., 1993). The present study was undertaken to determine the ability of bovine lung to bioactivate IPO and to assess involvement of bovine lung CYP4B1 in the activation of IPO to potentially pneumotoxic species. Previous studies to assess in vitro activation of IPO have relied on the use of radiolabeled substrate and the detection of protein-bound adducts (Devereux et al., 1982). Here, we use a recently developed in vitro bioactivation assay for IPO which employs the nucleophilic amino acids N-acetyl-cysteine (NAC) and N-acetyl-lysine (NAL) to trap a reactive ene-dial intermediate (Figure 1) and generate a stable IPO adduct which can be detected by LC/MS (Baer et al., 2004).
Figure 1.
P450-mediated 4-ipomeanol activation to the putative reactive ene-dial intermediate and subsequent reaction with nucleophilic trapping agents NAC and NAL to yield a stable NAC/NAL-IPO adduct. Formation of this adduct can be monitored by LC/MS and used as an in vitro marker of 4-ipomeanol bioactivation.
Microsomes were prepared by differential centrifugation according to previously published protocols (Guengerich, 1994). Microsomal experiments were conducted in triplicate with lung and liver microsomes prepared from frozen tissue from single animals as obtained from the vendors; Pel-Freez Biologicals (Rogers, Arkansas USA) and R&R Research (Stanwood, WA USA). In vitro bioactivation was assessed by incubating microsomal preparations in triplicate with IPO (50 µM), potassium phosphate buffer (100 mM pH 7.4), NADPH (1 mM) and NAC and NAL (20 mM), and subsequently monitoring formation of the adduct shown in Figure 1 at m/z 353 by LC/MS/MS on a Micromass Quattro II tandem quadrupole mass spectrometer coupled to a Shimadzu LC system. All metabolic incubations were allowed to proceed for 30 minutes at 37°C in a shaking water bath. Reactions were terminated by the addition of an equal volume of ice-cold methanol containing the internal standard, furafylline. CYP4 involvement in bioactivation was evaluated with the selective CYP4 ligand, HET0016 (Miyata et al., 2001), an N-aryl formamidoxime that is known to be a potent inhibitor of CYP4A (Seki et al., 2005) CYP4F (Wang et al., 2006), CYP4V (Nakano et al., 2009) and CYP4B1 (vide infra) enzymes. To further determine the contribution of CYP4B1 specifically, towards the activation of IPO, microsomal preparations were conducted in the presence of goat Anti-CYP4B1 IgG (raised against rabbit CYP4B1) or goat IgG control as previously described (Serabjit-Singh et al., 1979). The antibody was also used to visualize the presence of CYP4B protein(s) in pulmonary microsomes by western blotting at a dilution of 1:5000 and visualized using a donkey Anti-goat IRDye® 800CW secondary antibody from LI-COR Biosciences (Lincoln, Nebraska USA).
P450 spectral content in cow lung microsomes was 0.09 nmol/mg protein, similar to the levels observed in rabbit lung of 0.11 nmol/mg. To help put these values in context and to assess the viability of frozen, stored tissue, we also measured P450 specific contents in our bovine and rabbit liver microsomes. These latter values, of 0.34 nmol/mg and 0.98 nmol/mg, respectively, were <50% lower than literature data for freshly prepared liver microsomes from cattle (Giantin et al., 2008) and rabbits (Robertson et al., 1983). Despite the relatively low lung P450 contents, cow and rabbit lung microsomes formed the NAC/NAL-IPO adduct at higher rates than the respective liver microsomes (Figure 2).
Figure 2.
Rates of formation of the NAC/NAL-IPO adduct in bovine and rabbit lung and liver microsomes. The rate of adduct formation in liver microsomes is significantly lower than the rate of formation in lung microsomes in both bovine and rabbit (p < 0.005; Students t test).
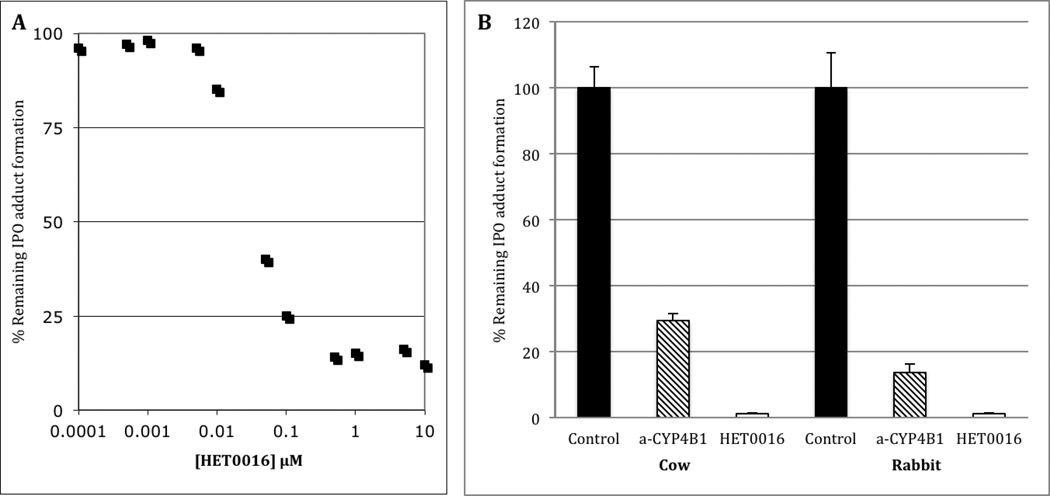
Formation of the IPO adduct was NADPH-dependent in all microsomal preparations (not shown), which is consistent with P450 involvement in the bioactivation process. Figure 3A depicts inhibition by HET0016 of the NAC/NAL-IPO adduct generated from reconstituted, purified rabbit CYP4B1. HET0016 was found to be a potent inhibitor of IPO activation with IC50 values of 37 nM and 23 nM in purified rabbit CYP4B1 and bovine lung microsomes, respectively. NAC/NAL-IPO adduct formation in both bovine lung and rabbit lung microsomes was decreased by more than 90% when metabolic reactions contained 300 nM HET0016 (Figure 3B). This concentration of HET0016 was chosen to maintain CYP4 selectivity, because it is approximately ten times the IC50 value in bovine or rabbit lung, but is still ten-fold lower than the IC50 towards any mammalian non-CYP4 family enzyme (Miyata et al., 2001).
Figure 3.
In vitro bioactivation of 4-ipomeanol. (A) HET0016 inhibition of IPO-adduct formation by purified rabbit CYP4B1 with an IC50 of 37 nM. (B) The effect of α-CYP4B1 and 300 nM HET0016 on the formation of 4-ipomeanol adducts from cow or rabbit lung microsomes. Activity in the presence of the chemical inhibitor and antibody were significantly lower than controls from both species (p < 0.005; Students t test).
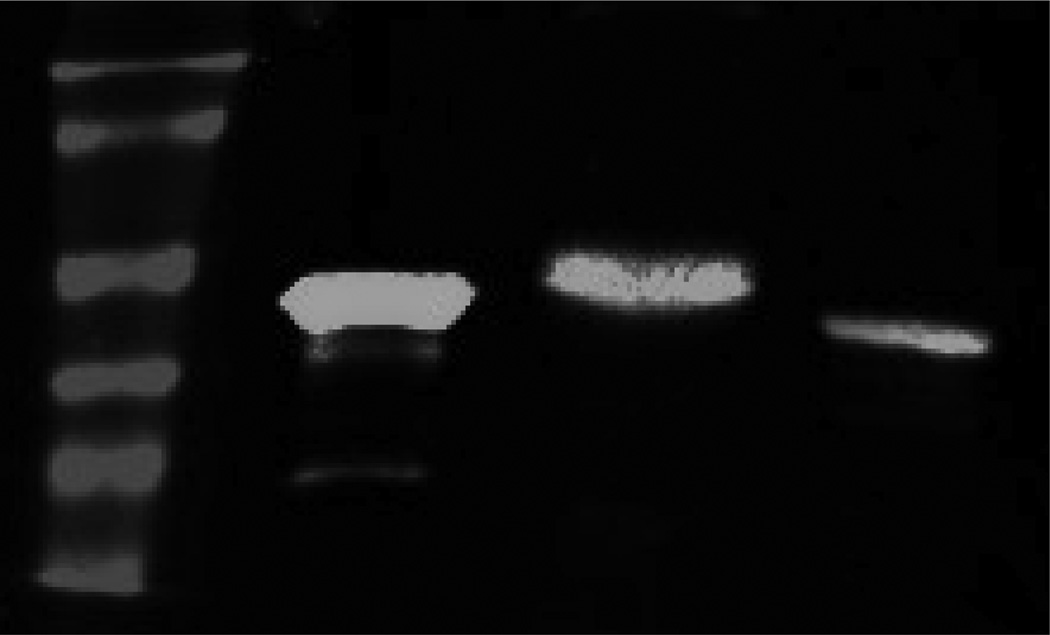
To specifically probe for bovine CYP4B involvement in IPO bioactivation, we employed a polyclonal antibody raised against rabbit CYP4B1. This antibody is monospecific for rabbit lung CYP4B1 and maximally inhibits lung microsomal CYP4B1-dependent catalysis at a concentration of 4 mg IgG/mg microsomal protein (Rettie et al., 1995; Serabjit-Singh et al., 1979). Moreover, the antibody is known to cross-react with pulmonary CYP4B1 from numerous other animal species including rats, hamsters, mice, guinea pigs and monkeys (Vanderslice et al., 1987). The protein sequences of bovine CYP4B1 and rabbit CYP4B1 are also highly related (>80% identical); therefore the antibody raised against rabbit CYP4B1 would be expected to immunochemically cross-react with the bovine ortholog.. NAC/NAL-IPO adduct formation was reduced by 70% and 85%, respectively in bovine and rabbit lung microsomes when reactions were pre-incubated with Anti-CYP4B1 IgG compared to control IgG (Figure 3B). The lower degree of immunoinhibition in bovine lung presumably reflects the fact that the antibody was raised specifically against the rabbit enzyme. Residual activity in both rabbit and bovine lung may be due to other pulmonary P450 enzymes, such as CYP2B4 (Rettie et al., 1995). Indeed, a CYP2B4 ortholog has been detected immunochemically in bovine lung (Arinc et al., 1995). Importantly, the Anti-CYP4B1 antibody used here detects only a single intense protein band in cow lung microsomes (Figure 4). Cow lung CYP4B migrates on SDS-PAGE with a higher apparent molecular weight than rabbit CYP4B1 and appears to be an even more prevalent component of pulmonary microsomal P450 than the rabbit ortholog. In conclusion, the data presented here are the first to; i) identify a CYP4B protein in cow lung microsomes, ii) demonstrate a role for bovine CYP4B in the bioactivation of IPO, and iii) demonstrate that HET0016 is a potent, nanomolar inhibitor of the CYP4B1-mediated bioactivation process.
Figure 4.
Western blot analysis of CYP4B1 content. Lane 1: Ladder, Lane 2: purified rabbit CYP4B1, Lane 3: Bovine lung microsomes, Lane 4: Rabbit lung microsomes.
In marked contrast to cow liver microsomes, which were devoid of IPO bioactivation, rabbit liver microsomal incubations produced substantial levels of the NAC/NAL-IPO adduct (Figure 2B). However, IPO is not recognized as a liver toxin in either cows or rabbits. This suggests that rabbit liver microsomes possess either efficient detoxifying mechanisms for the reactive intermediate and/or Phase II enzyme(s) that effectively compete for the pro-toxin. Further work is needed to determine what species-selective protective mechanisms are operative in rabbit liver.
IPO is one of several deadly plant-derived toxins known to lead to acute bovine pulmonary emphysema and edema. Perilla ketone (1-(3-furyl)-4-methyl-1-pentanone), which is derived from perilla mint (Perilla frutescens), causes an identical spectrum of symptoms to IPO and has been called the most dangerous to livestock plant toxin in the southeastern US (Steckel and Rhodes, 2007; Wilson et al., 1977). The high structural similarity between the two pulmonary toxins (both are furans substituted at the 3-position with a pentanone moiety) strongly suggests that perilla mint also may be activated by cow lung CYP4B to form a reactive ene-dial, as illustrated by the activation pathway for IPO (Figure 1).
Finally, CYP4B1 chemical inhibitors would not be expected to provide an effective therapeutic approach for treatment of cattle post-ingestion of IPO or perilla ketone, but may conceivably have promise as prophylactics. IPO poisoning typically occurs when cows are fed spoiled sweet potatoes, as cases of poisoning arising from ingestion of wild sweet potatoes are rare. Perilla mint poisoning peaks in late summer/early fall when the availability of grasses and other forages is decreased. Targeted CYP4B1 inhibition may be desirable to ranchers who rely on sweet potatoes as part of the herd’s diet or in the southeastern states at times when grass is in short supply. HET0016 is not ideal in this regard because it is a broad-spectrum CYP4 inhibitor whose ingestion, if it were to survive first pass metabolism in vivo and attain significant circulating concentrations in cattle, might be expected to disrupt important endogenous CYP4A and CYP4F-dependent reactions such as the generation of 20-HETE (Miyata et al., 2001). Nonetheless, the findings presented here may prompt additional efforts to determine if HET0016-derived analogs have promise as veterinary agents for mitigating the susceptibility of at-risk cattle populations to IPO and perilla ketone toxicosis.
ACKNOWLEDGEMENTS
This investigation was supported by NIH grant GM49054 from the National Institutes of Health (AER).
Footnotes
IPO, 4-Ipomeanol; NAC, N-acetyl-cysteine; NAL, N-acetyl-lysine; IgG, Immunoglobulin G; LC/MS/MS, Liquid-chromatography tandem mass-spectrometry.
REFERENCES
- Arinc E, Hanukoglu I, Sen A, Adali O. Tissue- and Species- Dependent Expression of Sheep Lung Microsomal Cytochrome P4502B(LgM2) Biochemistry and Molecular Biology International. 1995;37:1121–1126. [PubMed] [Google Scholar]
- Baer BR, Rettie AE, Henne KR. Bioactivation of 4-Ipomeanol by CYP4B1: Adduct Characterization and Evidence for an Enedial Intermediate. Chemical Research in Toxicology. 2004;18:855–864. doi: 10.1021/tx0496993. [DOI] [PubMed] [Google Scholar]
- Devereux TR, Jones KG, Bend JR, Fouts JR, Statham CN, Boyd MR. in Vitro Metabolic Activation of the Pulmonary Toxin, 4-Ipomeanol, in Nonciliated Bronchiolar Epithelial (Clara) and Alveolar Type II Cells Isolated from Rabbit Lung. The Journal of Pharmacology and Experimental Therapeutics. 1982;220:223–227. [PubMed] [Google Scholar]
- Doster AR, Mitchell FE, Farrell RL, Wilson BJ. Effects of 4-Ipomeanol, a Product from Mold-Damaged Sweet Potatoes, on the Bovine Lung. Veterinary Pathology. 1978;15:367–375. doi: 10.1177/030098587801500312. [DOI] [PubMed] [Google Scholar]
- Giantin M, Carlettie M, Capolongo F, Pegolo S, Lopparelli RM, Gusson F, nebbia C, Cantiello M, Martin P, Pineau T, Dacasto M. Effect of Breed upon Cytochromes P450 and Phase II Enzyme Expression in Cattle Liver. Drug Metab Dispos. 2008;36:885–893. doi: 10.1124/dmd.107.019042. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Analysis and Characterization of Enzymes. In: Hayes AW, editor. Principles and Methods of Toxicology. New York: Raven Press; 1994. pp. 1259–1313. [Google Scholar]
- Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda YM, M. Doi SK, Tomishima Y, Ueki T, Sato M, Kameo K. HET0016, a Potent and Selective Inhibitor of 20-HETE Synthesizing Enzyme. British Journal of Pharmacology. 2001;133:325–329. doi: 10.1038/sj.bjp.0704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monlux W, Fitte J, Kendrick G, Dubuisson H. Progressive Pulmonary Adenomatosis in Cattle. The Southwestern Veterinarian. 1953:6. [Google Scholar]
- Nakano M, Kelly EJ, Rettie AE. Expression and characterization of CYP4V2 as a fatty acid omega-hydroxylase. Drug Metab Dispos. 2009;37:2119–2122. doi: 10.1124/dmd.109.028530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettie AE, Sheffels PR, Korzekwa KR, Gonzalez FJ, Philpot RM, Baillie TA. CYP4 Isozyme Specificiy and the Relationship between w-Hydroxylation and Terminal Desaturation of Valproic Acid. Biochemistry. 1995;34:7889–7895. doi: 10.1021/bi00024a013. [DOI] [PubMed] [Google Scholar]
- Robertson IG, Serabjit-Singh CS, Croft JE, Philpot RM. The Relationship between Increases in the Hepatic Content of Cytochrome P-450, Form 5, and in the Metabolism of Aromatic Amines to Mutagenic Products following Treatment of Rabbits with Phenobarbital. Molecular Pharmacology. 1983;24:156–162. [PubMed] [Google Scholar]
- Seki T, Wang MH, Miyata N, Laniado-Schwartzman M. Cytochrome P450 4A isoform inhibitory profile of N-hydroxy-N'-(4-butyl-2-methylphenyl)-formamidine (HET0016), a selective inhibitor of 20-HETE synthesis. Biol Pharm Bull. 2005;28:1651–1654. doi: 10.1248/bpb.28.1651. [DOI] [PubMed] [Google Scholar]
- Serabjit-Singh CS, Wolf CR, Philpot RM. The Rabbit Pulmonary Monooxygenase System. The Journal of Biological Chemistry. 1979;254:9901–9907. [PubMed] [Google Scholar]
- Spoto C. Moldy Sweet Potatoes Caused 200 Steer Deaths. In Wausau Daily Herald (Wausau Wisconsin) 2011. [Google Scholar]
- Steckel L, Rhodes N. University of Tennessee Agricultural Extension Publication w135. 2007. Perilla Mint. [Google Scholar]
- Vanderslice RR, Domin BA, Carver GT, Philpot RM. Species-dependent expression and induction of homologues of rabbit cytochrome P-450 isozyme 5 in liver and lung. Mol Pharmacol. 1987;31:320–325. [PubMed] [Google Scholar]
- Verschoyle RD, Philpot RM, Wolf CR, Dinsdale D. CYP4B1 Activates 4-Ipomeanol in Rat Lung. Toxicology and Applied Pharmacology. 1993;123:193–198. doi: 10.1006/taap.1993.1237. [DOI] [PubMed] [Google Scholar]
- Wang MZ, Saulter JY, Usuki E, Cheung YL, Hall M, Bridges AS, Loewen G, Parkinson OT, Stephens CE, Allen JL, Zeldin DC, Boykin DW, Tidwell RR, Parkinson A, Paine MF, Hall JE. CYP4F enzymes are the major enzymes in human liver microsomes that catalyze the O-demethylation of the antiparasitic prodrug DB289 [2,5-bis(4-amidinophenyl)furan-bis-O-methylamidoxime] Drug Metab Dispos. 2006;34:1985–1994. doi: 10.1124/dmd.106.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J, Grant JE, Linnabary RD, Channell RB. Perilla Ketone: A Potent Lung Toxin from the Mint Plant, Perilla Frutescens Britton. Science. 1977;197:573–574. doi: 10.1126/science.877573. [DOI] [PubMed] [Google Scholar]