Abstract
Leptin receptor (LepRb) signaling in the hindbrain is required for energy balance control. Yet the specific hindbrain neurons and the behavioral processes mediating energy balance control by hindbrain leptin signaling are unknown. Studies here employ genetic [adeno-associated virally mediated RNA interference (AAV-RNAi)] and pharmacological methodologies to specify the neurons and the mechanisms through which hindbrain LepRb signaling contributes to the control of food intake. Results show that AAV-RNAi-mediated LepRb knockdown targeting a region encompassing the mNTS and area postrema (AP) (mNTS/AP LepRbKD) increases overall cumulative food intake by increasing the size of spontaneous meals. Other results show that pharmacological hindbrain leptin delivery and RNAi-mediated mNTS/AP LepRb knockdown increased and decreased the intake-suppressive effects of intraduodenal nutrient infusion, respectively. These meal size and intestinally derived signal amplification effects are likely mediated by LepRb signaling in the mNTS and not the AP, since 4th icv and mNTS parenchymal leptin (0.5 μg) administration reduced food intake, whereas this dose did not influence food intake when injected into the AP. Overall, these findings deepen the understanding of the distributed neuronal systems and behavioral mechanisms that mediate the effects of leptin receptor signaling on the control of food intake.
Keywords: area postrema, adeno-associated viral-short hairpin RNA-interference, food intake, intraduodenal, vagus
the adipose tissue-derived hormone leptin acts on neurons in multiple brain regions to regulate energy balance (13, 14, 36). Within the dorsal vagal complex (DVC) of the hindbrain, the area postrema (AP) and the nucleus tractus solitarius (NTS) express the leptin receptor (LepRb) in rats (3, 7, 23, 24, 28, 37, 41). An endogenous role for AP/NTS LepRb signaling in energy balance is supported by recent findings (19) showing that knockdown of leptin receptors (LepRbKD) targeted to the NTS and AP using adeno-associated viral-short hairpin RNA interference (AAV-shRNAi) increases food intake and body weight in rats without affecting energy expenditure parameters (physical activity and core temperature). LepRb signaling in the medial NTS (mNTS) may influence food intake by amplifying the intake-suppressive effects of gastrointestinally derived satiation signals. Indeed, forebrain intracerebroventricular (icv) leptin administration, which provides ligand to many structures, including potentially the hindbrain, enhanced the intake-suppressive effect of cholecystokinin (CCK) (9, 29, 30) and gastric nutrient infusion (8). Previous findings highlight the mNTS as a likely critical site of integration between leptin and gastrointestinal (GI) satiation signals, since hindbrain-delivered leptin amplifies the food intake-suppressive effect of gastric distention (25). Complementing these finding are results showing that mNTS/AP-targeted LepRbKD reduced the potent food intake-suppressive effects of exogenous CCK (19).
Further support for the hypothesis that hindbrain leptin signaling reduces food intake by amplifying the intake-inhibitory effects of GI satiation signals will require examining the effects of physiological satiation signaling, such as that produced by intestinal nutrient infusion. In addition, given the essential role of NTS processing of gastrointestinally derived, vagally mediated signals in meal size control (12), it is important to determine whether endogenous LepRb signaling in the mNTS controls food intake by a specific meal size regulatory effect. Both questions are addressed here using pharmacological and RNAi/AAV-mediated LepRbKD methodologies.
RNAi-mediated LepRb knockdown targeted a region encompassing both the AP and the medial subnucleus of the NTS (mNTS) in previous work (19) and in the present experiments. Thus, it is necessary to evaluate the respective contribution of each of these distinct LepRb-expressing populations to food intake control. Leptin-induced activation of signal transducer and activator of transcription 3 (pSTAT3), a transcription factor critical in the LepRb-mediated control of energy balance (1), is substantially more robust in the mNTS than in the AP (25, 35, 39). Furthermore, the number of neurons coactivated by gastric distention (c-Fos) and by exogenous leptin (pSTAT3) observed in the mNTS was considerably greater than that seen in the AP (25). Thus, we hypothesize that LepRb signaling in the mNTS and not the AP contributes critically to the control of food intake. This hypothesis is assessed by measuring the effects of selective mNTS- and AP-targeted delivery of leptin on food intake and body weight.
MATERIALS AND METHODS
Subjects
Adult male Sprague-Dawley rats (250–300 g upon arrival; Charles River Laboratories, Wilmington, MA), housed individually in hanging metal cages and maintained on a 12:12-h light-dark cycle, had ad libitum access to rodent chow (5001 Rodent diet; Lab Diets, St. Louis, MO) and water unless otherwise noted. All protocols and procedures conformed to the institutional standards of the University of Pennsylvania Animal Care and Use Committee and were approved by the committee.
Surgery
Rats were injected with ketamine (90 mg/kg; Bulter Animal Health Supply, Dublin, OH), xylazine (2.7 mg/kg; Anased, Shenandoah, IA), and acepromazine (0.64 mg/kg; Bulter Animal Health Supply) anesthesia and analgesia (2 mg/kg Metacam; Boehringer Ingelheim Vetmedica, St. Joseph, MO) for all surgeries.
Cannula implantation and placement verification.
Guide cannulae (26-gauge; Plastics One, Roanoke, VA) were implanted with the tip stereotaxically positioned 2.0 mm above the target injection site at the following coordinates: 1) fourth ventricle: on midline, 2.5 mm anterior to occipital suture, 5.2 mm ventral from skull surface; 2) bilateral caudal mNTS: ± 0.75 mm lateral to midline, 1.0 mm posterior to occipital crest, 6.7 mm ventral from skull surface; 3) AP: on midline, 1.0 mm posterior to occipital crest, 6.4 mm ventral from skull surface. Intended anatomic positions of fourth icv and mNTS injection sites were evaluated 1 wk postsurgery by measurement of the sympathoadrenal-mediated glycemic response to an injection of 5-thio-d-glucose (210 μg/2 μl 4th icv or 24 μg/100 nl unilateral mNTS injection) (38). A postinjection elevation in baseline plasma glucose level of ≥100% was required for subject inclusion. All AP injection sites were functionally verified by examining the food intake-suppressive response following parenchymal AP 0.4 μg of salmon calcitonin (sCT) injections, as described previously (18, 32). Parenchymal injection sites were also verified histologically through postmortem verification of the position of 100-nl pontamine sky blue injections. Only animals passing verifications were included in final statistical analyses.
Chronic duodenal catheter implantation.
As described previously (15, 16, 44), rats were deprived of food but not water overnight before duodenal catheter implantation surgery, which was carried out immediately prior to bilateral mNTS cannula implantation. A 21-cm silicone rubber catheter (0.025 in. inner diameter, 0.047 in. outer diameter; VWR, Bridgeport, NJ) was inserted 2 cm distal to the pylorus and advanced 5 cm within the duodenal lumen in an aborad direction. The exposed end of the catheter exited subcutaneously through a skin incision between scapulas and was occluded with a stainless-steel obturator, which was removed only for daily flushing of the catheter (0.3 ml 0.9% NaCl) and for experimental infusions. A minimum of 7 days was allowed for recovery prior to experimental infusions. The placement of the infusion site was verified after euthanization by measuring the length of catheter inside the intestine lumen.
Design and Construction of shRNA and Viral Production, Purification, and Delivery
Hairpin RNA was designed to target specific regions of LepRb mRNA, as described (22). Briefly, viral production was accomplished using a triple-transfection, helper-free method and purified as described (21, 22). The virus was purified via iodixanol gradients and titered by infection of camptothecin-treated HT1080 cells. Enhanced green fluorescent protein (eGFP) is coexpressed by the AAVs and is used as a marker for successful infection.
Approximately 2 wk following cannula implantation surgery, rats were lightly anesthetized via ketamine (9.0 mg/kg), xylazine (0.27 mg/kg), and acepromazine (0.064 mg/kg) for viral delivery. As described previously for bilateral mNTS targeting (19), a total of 0.5 μl of purified virus (1–2 × 1011 infectious particles/ml) was delivered over a 5-min period per hemisphere via a Hamilton syringe connected to an injector (Plastics One) that extended 2.0 mm below the tip of the bilateral mNTS guide cannula.
Tissue Collection
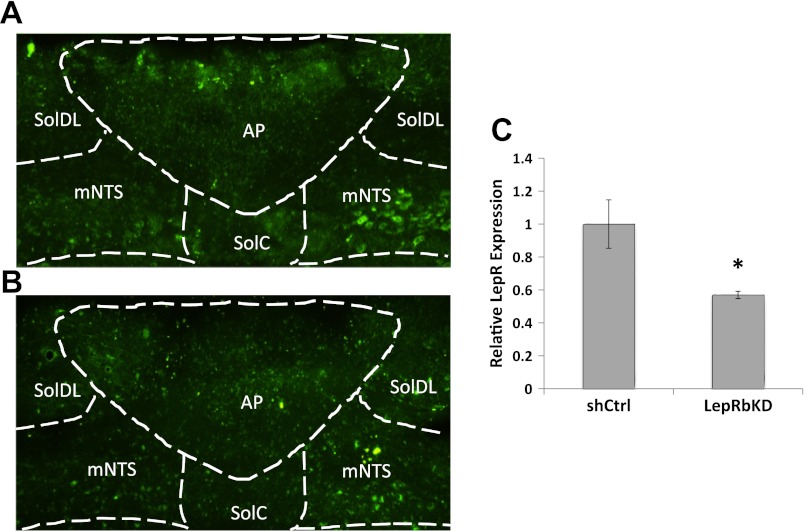
mNTS/AP LepRbKD and short hairpin control (shCtrl) rats were euthanized by decapitation. Brains were rapidly removed, flash-frozen in isopentane, and stored at −80°C until processing. As described previously (19), serial coronal sections (30 μm) of the caudal brain stem were mounted on a slide and viewed immediately under a fluorescent microscope (Eclipse 80i; Nikon) for histological verification of injection site by visualization of eGFP under ×10 magnification (representative coronal sections for visualization of eGFP are shown in Fig. 1, A and B). Once eGFP was visualized in the target site, separate micropunches of the AAV-shLepRb/shCtrl-infected mNTS or AP tissue were taken separately from two consecutive 80-μm frozen coronal sections for subsequent real-time PCR analysis of LepRb. The next coronal section (30 μm) was again mounted for visualization of eGFP presence. This process continued until micropunches were harvested for the entire mNTS (at the AP level) and until visualization of eGFP-expressing neurons was absent. Only data from rats with confirmed eGFP-expressing neurons in the mNTS/AP region were used in the final statistical analysis.
Fig. 1.
Representative coroncal section illustrating enhanced green fluorescent protein expression in the medial nucleus tractus solitarius (mNTS)/area postrema (AP) following short hairpin control-adeno-associated viral (shCntrl-AAV; A) and LepRb-AAV delivery (B). C: quantitative real-time PCR reveals significant suppression in leptin receptor (LepRb) mRNA expression in mNTS micropunched tissue from mNTS-directed AAV-LepRb-treated rats compared with AAV-shCtrl rats. *P < 0.05. SolDL, dorsolateral solitary nucleus; SolC, commissural solitary nucleus.
Inguinal, retroperitoneal, epididymal, and perirenal white adipose tissue (WAT) depots were removed and weighed postmortem from mNTS/AP-LepRbKD and -shCtrl rats.
RNA Isolation and Real-Time PCR
The efficacy of LepRb-AAV was confirmed using a representative subset of rats from the meal pattern group: shCtrl (n = 5) and LepRbKD (n = 4). Total RNA was extracted from mNTS tissue using Trizol (Invitrogen), and for further purification, the RNeasy kit (Qiagen) was used. cDNA was synthesized from 1 μg of total RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Quantitative real-time PCR was done in duplicate using TaqMan Universal qPCR Master Mix (Applied Biosystems), and samples were run using the Eppendorf Mastercycler eprealplex. The primer/probe sets for LepRb and GAPDH (internal control) were from Applied Biosystems (LEPR-Rn00664624_m1 and GAPD-435293 2E, respectively). Relative mRNA expression was calculated using the comparative CT method as described (2).
Procedures
mNTS-directed LepRbKD: food intake, body weight, adiposity, and meal pattern analysis.
Daily food intake and body weight measurements were made in mNTS/AP LepRbKD (n = 5) and shCtrl (n = 6) rats beginning 5 days prior to AAV administration. Meal pattern parameters were recorded in wire-bottom cages with an access hole to powdered rodent chow (5001 Rodent diet; Lab Diets) resting on an electronic scale. The food cup weight was monitored continuously by computer software (LabView), and meal pattern parameters were calculated objectively via a custom macro program in Microsoft Excel. A meal was defined as ingestion amounting to ≥0.25 g, with a minimum 10-min ingestion-free period between meals.
Rats were habituated to the automated feeding chambers and the powdered rodent chow for two 24-h periods 7–8 days prior to LepRb and shCtrl AAV delivery. We demonstrated previously that food intake and body weight differences emerge between mNTS/AP LepRbKD and shCtrl rats approximately 2 wk after AAV delivery (19). Thus, the rats were placed in the automated feeding chambers for evaluation of meal pattern parameters during the feeding (dark) cycle every 3rd day starting on day 14 and ending on day 42 after AAV administration. Rats were returned to the home cages for the 2 intervening days; daily consumption of chow was recorded in the home cages. On day 43 after AAV administration, the rats were switched to high-fat diet maintenance and returned to the home cage for the remainder of the study (days 43–72).
Food intake suppression by intestinal nutrient infusion and hindbrain leptin administration.
Prior to the start of experimental testing, rats (n = 11) were given a duodenal infusion of saline to habituate to the infusion procedures. On experimental infusion test days, overnight (16 h) food-deprived rats with 4th icv cannula received icv injections of either leptin (5 μg/2 μl) or its vehicle (NaHCO3) 25 min prior to intestinal infusion. The infusate (0.33 kcal/ml concentration of Intralipid, pH 7.35–7.4; Baxter Healthcare, Deerfield, NJ) was prepared immediately prior to testing and delivered using a syringe infusion pump (Harvard Apparatus, South Natick, MA) at a rate of 0.4 ml/min for 20 min with a total volume of 8 ml, which has been shown to be within the physiological range of gastric distension and emptying (11, 17, 31, 43). Rats were given duodenal infusion with either Intralipid or physiological saline (0.9% sodium chloride; Sigma-Aldrich, St. Louis, MO). Chow was returned 5 min after the termination of infusion, and subsequent chow intake was measured at 90 min. The conditions were counterbalanced, and each experimental trial was separated by 48 h.
mNTS/AP LepRbKD: food intake suppression by intestinal nutrient infusion.
mNTS/AP LepRbKD (n = 8) and shCtrl rats (n = 6) were habituated to the intestinal nutrient infusion procedure, as described above. Counterbalanced experimental duodenal infusions of 10 ml of a complete liquid meal, vanilla Ensure (diluted in saline; Abbott Laboratories, Columbus, OH), or saline were infused at a rate of 0.45 ml/min following overnight (16 h) food deprivation. Chow was returned 7 min after the termination of infusion, and subsequent chow intake was measured at 30 and 60 min. Intraduodenal nutrient infusion was selected as the method of choice to examine the effects of LepRbKD on sensitivity to GI satiation signals because it produces a broad range of nutrient-induced, intestinally derived satiation signals; testing was conducted twice/wk (1 Ensure and 1 saline treatment separated by 3 days) for 4 wk using a descending concentration order (100, 66, 58, and 40% Ensure containing 14.8, 9.8, 8.6, and 5.9 kcal, respectively).
Food intake following 4th icv, mNTS, and AP leptin administration.
Ad libitum-fed rats (n = 8, ∼350–450 g) implanted with 4th icv cannula were injected with either leptin (0.5 μg/1 μl; Harbor-UCLA Research and Education Institute, Torrance, CA) or vehicle (NaHCO3) in a counterbalanced fashion immediately before dark onset. Subsequent chow intake (spillage accounted for) and body weight were recorded at 24 h. All of the experiment conditions were separated by 48–72 h.
A separate group of rats (n = 9, ∼358–436 g) implanted with mNTS cannula received injections of leptin (0.5 μg/100 nl) or vehicle in a counterbalanced fashion with the use of a syringe infusion pump (Harvard Apparatus, Holliston, MA). Food was returned immediately before dark onset. Chow intake and body weight were assessed at 24 h.
A separate group of rats (n = 8, ∼330–400 g) implanted with AP cannula were injected with leptin (0.5 μg/100 nl) or vehicle in a counterbalanced fashion using a syringe infusion pump (Harvard Apparatus). Food intake and body weight were recorded at 24 h. One week following the last leptin treatment, AP cannulation placement was verified functionally by examining food intake suppression following parenchymal AP injection of sCT (0.4 μg/100 nl; Bachem, Torrance, CA) or its vehicle (artificial cerebrospinal fluid) following overnight food deprivation. Injections occurred immediately before dark onset, and chow was returned for assessment of 24-h chow intake.
Data and Statistical Analysis
All data are expressed as means ± SE and were analyzed by one-way or repeated-measures ANOVA, followed by Newman-Keuls post hoc tests when main effects or interactions were significant. All statistical analysis was performed using Statistica Software (Statsoft, Tulsa, OK). P < 0.05 was interpreted as a significant difference.
RESULTS
mNTS/AP LepRbKD Increases Daily Food Intake, Meal Size, Body Weight, and Adiposity
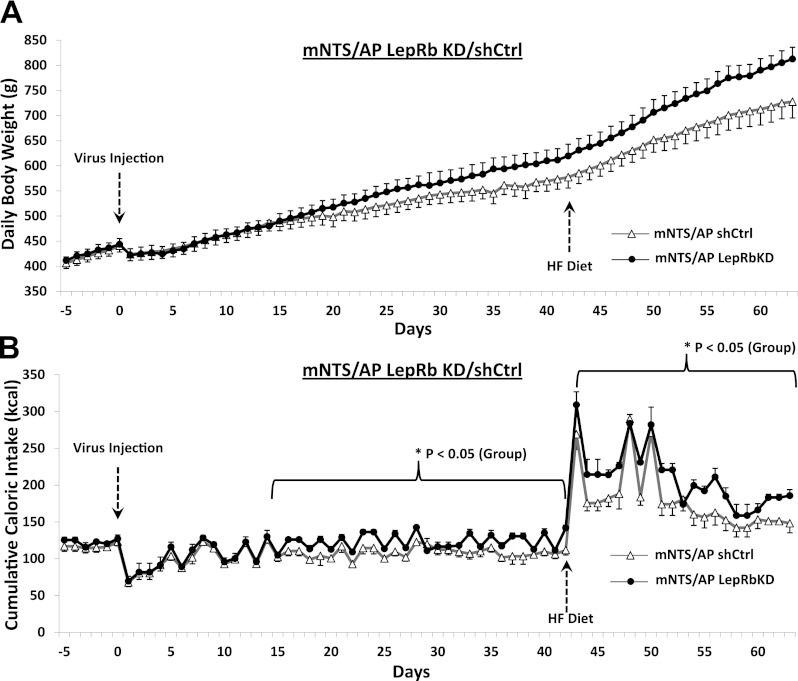
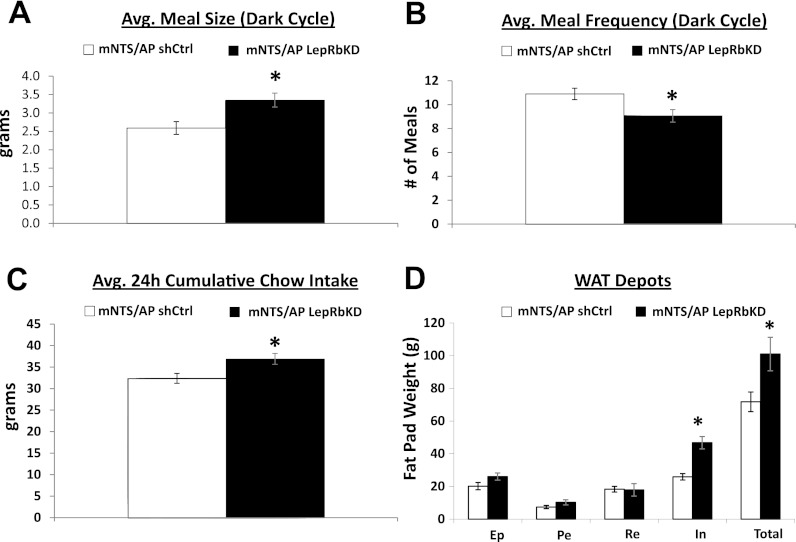
Consistent with results from a previous report from our laboratory using the same AAV-LepRbKD and -shCtrl (19), we observed an ∼40% reduction in relative LepRb expression in mNTS lysates for rats injected intra-mNTS/AP with the LepRb AAV relative to rats injected with the shCtrl AAV (Fig. 1C). This previous report showed that mNTS/AP LepRbKD increases daily food intake, body weight gain, and adiposity compared with shCtrl rats (19). Here, we replicate and extend these findings by showing that the increased food intake (Fig. 2B) and body weight (Fig. 2A) observed in mNTS/AP LepRbKD rats are based in part on increased average meal size during the feeding (dark) cycle (P < 0.05) (Fig. 3A). This elevated meal size effect was accompanied by a significant compensatory decrease in average dark cycle meal frequency (P < 0.05; Fig. 3B); however, the decreased meal frequency did not fully compensate for elevated meal size because overall 24-h cumulative food intake was also increased for LepRbKD rats compared with shCtrl rats during the period in which meal pattern analysis occurred (days 14–42, group main effect, P < 0.05; Figs. 2B and 3C) and during the high-fat diet maintenance period (days 43–63, group main effect, P < 0.05; Fig. 2B). Consistent with our previous findings, the elevation in body weight for LepRbKD rats was accompanied by a significant increase in inguinal and total fat pad weights (P < 0.01), whereas retroperitoneal, epididymal, and perirenal WAT pad mass did not differ by group (Fig. 3D).
Fig. 2.
A: mNTS-directed LepRb knockdown significantly increased daily body weight gain relative to shCtrl rats; group × day interaction = P < 0.01. B: mNTS-directed knockdown of leptin receptors (LepRbKD) produced significant increase on daily caloric intake compared with shCtrl. HF, high fat.
Fig. 3.
Compared with shCtrl rats, mNTS-directed LepRb knockdown significantly increased average meal size (A) and decreased average meal frequency (B) during the dark cycle; however, decreased meal frequency only partially compensated for increased meal size, which resulted in a significant increase in 24-h cumulative food intake (C). D: inguinal (In) and total fat pad weights were elevated in mNTS-directed LepRbKD rats relative to shCtrl rats, whereas epididymal (Ep), perirenal (Pe), and retroperitoneal (Re) fat pad mass did not differ by group. *P < 0.05.
Hindbrain Leptin Delivery and Duodenal Nutrient Infusion Interact to Suppress Food Intake in a Synergistic Fashion
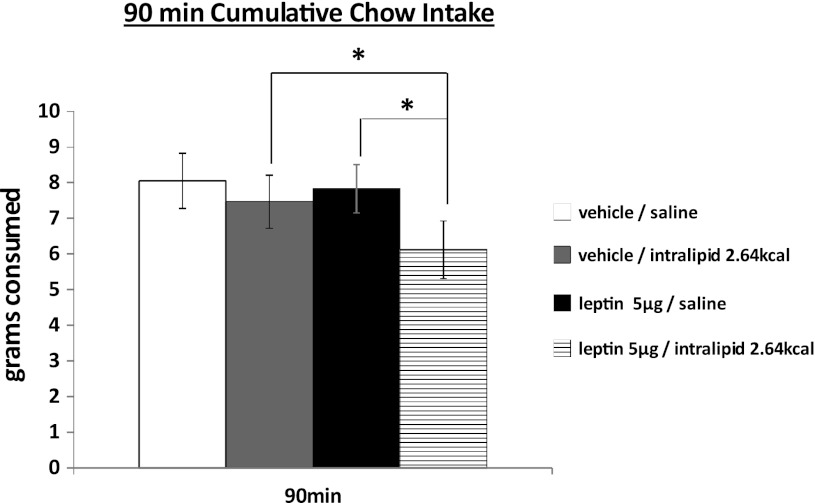
To investigate the possible interaction of endogenous GI satiation signals and hindbrain leptin signaling, duodenal infusion of a fat emulsion, Intralipid (0.33 kcal/ml), was combined with hindbrain (4th icv, 5 μg) leptin delivery to intact rats, using a design similar to what we have described previously (25). Neither duodenal infusion of Intralipid nor 4th icv leptin delivery influenced 90-min chow intake alone in overnight food-deprived rats. However, when combined, significant intake suppression was observed at 90 min relative to vehicle/vehicle treatment (P < 0.05; Fig. 4).
Fig. 4.
Neither duodenal Intralipid infusion (0.33 kcal/ml infused at a rate of 0.4 ml/min for 8 ml) nor leptin [5 μg, 4th intracerebroventricular (icv)] alone had influence on 90-min cumulative chow intake in overnight food-deprived rats. When combined together, a significant suppressive effect was observed on 90-min chow intake compared with control group. *P < 0.05.
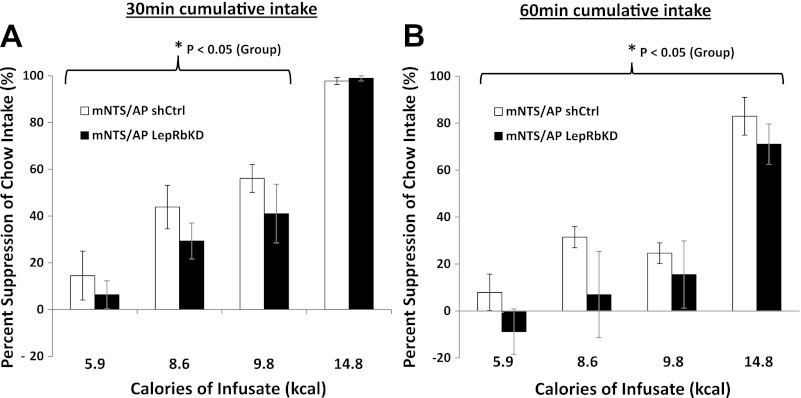
mNTS/AP LepRbKD Reduces Sensitivity to Food Intake Suppression by Intestinal Nutrient Infusion
To investigate the contribution of endogenous mNTS/AP LepRb signaling in processing GI satiation signals, the effect of duodenal infusion of Ensure at varying concentrations on chow intake was measured in mNTS/AP LepRbKD and shCtrl rats. LepRbKD rats were less sensitive to the food intake-suppressive effect of duodenal infusion of Ensure (Fig. 5). When analyzed as percent suppression of chow intake following Ensure relative to saline infusion across the four different concentrations, the group main effect did not achieve significance at 30 min (P < 0.08). However, because a floor effect was observed for the 14.8 kcal concentration (100% Ensure infusion produced ∼100% suppression of intake), the same analysis applied across the lower three concentrations (5.9, 8.6, and 9.8 kcal) revealed a significant main effect of group (P < 0.05) for 30-min chow intake (Fig. 5A). LepRbKD rats also showed reduced sensitivity to food intake suppression by duodenal Ensure infusion at the 60-min time point. A significant main effect of group was observed for 60-min chow intake (P < 0.05) when percent suppression was analyzed across all four concentrations (Fig. 5B).
Fig. 5.
A: duodenal infusion of Ensure (0.45 ml/min for 10 ml) reduced 30-min chow intake for both mNTS-directed LepRbKD and shCtrl rats, with a maximal suppressive effect achieved at 14.8 kcal. However, infusion produced a greater magnitude of suppression in mNTS-directed LepRbKD rats than it did in shCtrl rats, producing a significant main effect of group (P < 0.05) on 30-min chow intake across the other 3 concentrations (5.9, 8.6, and 9.8 kcal). B: 60-min chow intake was reduced for both groups at each infusion concentration. There was a significant main effect of group (P < 0.05) throughout all of the concentrations.
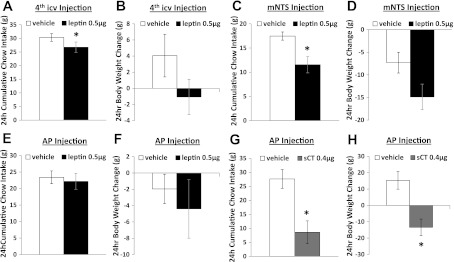
Leptin Administered to the mNTS but Not to the AP Suppresses Food Intake and Body Weight
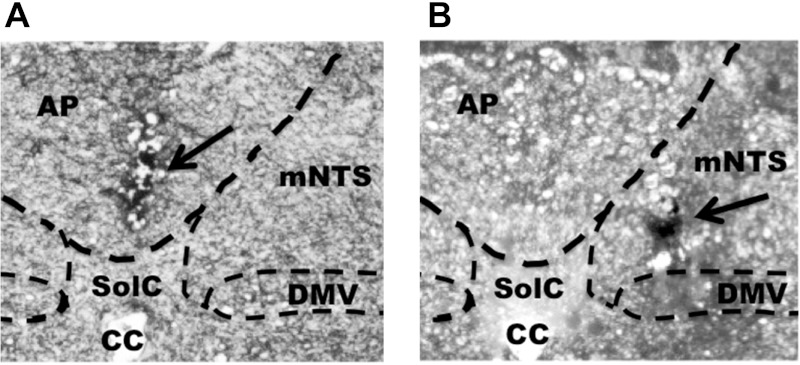
Hindbrain ventricular delivery of leptin (0.5 μg, 4th icv) significantly suppressed 24-h chow intake (P < 0.05; Fig. 6A) and produced a moderate but nonsignificant decrease in 24-h body weight (P = 0.16; Fig. 6B). Unilateral delivery of the same dose of leptin into the mNTS produced a significant decrease in 24-h chow intake (P < 0.05; Fig. 6C) and a nonsignificant decrease of 24-h body weight (P = 0.12; Fig. 6D). However, 0.5 μg of leptin delivered directly to the AP was without effects on 24-h food intake and body weight (Fig. 6, E and F). Functional confirmation of AP injection site was provided by examining the effect of parenchymal AP delivery of the amylin receptor agonist sCT (0.4 μg), which produced significant suppression of 24-h chow intake and body weight (P < 0.01; Fig. 6, G and H). Histological confirmation was also provided by localization of 100 nl of pontamine sky blue ink injected directly to the AP (Fig. 7A) or to the mNTS (Fig. 7B).
Fig. 6.
A and B: leptin, 0.5 μg 4th icv, produced significant suppression on 24-h chow intake and a moderate decrease in 24-h body weight. C and D: 0.5 μg of leptin directly delivered into unilateral mNTS induced a significant suppression of 24-h chow intake and a nonsignificant decrease in 24-h body weight. E and F: 0.5 μg of leptin directly delivered into AP had no effect on food intake or body weight. G and H: functional verification of AP injection site by delivery of amylin agonist salmon calcitonin (sCT) into AP; 0.4 μg of sCT significantly suppressed 24-h chow intake and 24-h body weight gain. *P < 0.05 relative to vehicle.
Fig. 7.
Representative injection site following unilateral 100-nl injection of pontamine sky blue ink into the AP (A) and mNTS (B). CC, central canal; DMV, dorsal motor nucleus of the vagus.
DISCUSSION
Central leptin signaling is essential to the normal control of energy balance (1, 5, 10, 19, 27, 40). Previous findings show that one mechanism through which central nervous system leptin signaling reduces food intake and body weight is by amplifying the neural processing of GI-derived, vagally mediated satiation signals (6, 8, 9, 13, 19, 29, 30). The anatomic basis for this interaction between LepRb and GI-derived satiation signaling has begun to be elucidated by experiments that target hindbrain sites of integration and employ pharmacological as well as genetic strategies (19, 25). The results of the present experiments show that endogenous LepRb signaling in the mNTS is required for the normal control of food intake and body weight and for meal size regulation via the processing of physiological satiation signals.
Previous work shows that AAV-mediated knockdown of LepRb targeted to the mNTS/AP region in rats (19) and selective deletion of LepRb on a subset of hindbrain and spinal cord neurons expressing the transcription factor paired-like homeobox 2b (42) in mice increase caloric intake and body weight gain. Here, we extend and refine these findings in several ways. Results showed that the increased food intake and body weight gain effects observed in mNTS/AP LepRbKD rats are likely mediated by neuronal processing in mNTS, and not the AP, since mNTS parenchymal delivery of leptin suppressed food intake and body weight in ad libitum-fed rats, whereas AP leptin delivery was without effect. Experiments also show that the hyperphagia induced by mNTS/AP LepRbKD resulted from an increase in meal size, which was accompanied by a compensatory decrease in meal frequency that only partially counteracted the elevated meal size effect on increased daily caloric intake. This outcome is consistent with other reports showing that global LepRb deficiency in rats [Koletsky (fak/fak)] (4, 34) and leptin deficiency in mice (ob/ob) (20) result in an obese phenotype driven in part by hyperphagia associated with increased meal size. Here, we identify LepRb processing in the mNTS/AP region as a critical mediator of this meal size regulatory effect. Whether meal size control by endogenous mNTS LepRb signaling involves endemic processing in the hindbrain or involves ascending communication to hypothalamic and other forebrain and/or midbrain regions requires further study.
Results of pharmacological and AAV-mediated knockdown studies were complementary and showed that the endogenous role of mNTS LepRb signaling in food intake control is based, at least in part, on enhancing the intake inhibitory effect of endogenous intestinally derived satiation signaling resulting from duodenal nutrient infusion. When a dose of hindbrain-delivered leptin was combined with a concentration of intestinal infusion of the fat emulsion Intralipid, significant food intake suppression was observed at 90 min, whereas neither treatment alone was effective in suppressing intake during this time frame. The interaction between hindbrain leptin and intestinal satiation signaling appears to be one that is physiologically relevant to food intake control, since mNTS-directed LepRb knockdown reduced the magnitude of food intake suppression produced across a range of concentrations of intestinal infusion of a mixed nutrient meal (Ensure). These findings build on previous work from our group in which the combination of hindbrain-delivered leptin and gastric distention produced food intake suppression, whereas neither treatment alone had any effect on feeding (25). Furthermore, we reported recently that LepRb knockdown in the mNTS/AP region reduced the magnitude of food intake suppression by exogenous administration of the satiation gut peptide CCK (19). Collectively, these findings show that LepRb signaling in the mNTS modulates the effectiveness of a variety of gastric and intestinally derived satiation signals, and this appears to contribute to the normal control of food intake.
In conclusion, the present findings strongly support the hypothesis that endogenous neuronal LepRb signaling in the mNTS contributes to the control of meal-related satiation signaling and overall food intake and body weight regulation. Virally mediated reduction of LepRb signaling in the mNTS/AP increased food intake and body weight gain relative to control rats with mNTS LepRb signaling intact. This increased food intake resulted from an increase in spontaneous meal size. The endogenous role for mNTS LepRb signaling in food intake control appears to be based in part on its amplifying effect on the intake-suppressive efficacy of endogenous satiation signals by a variety of GI stimuli, including intraduodenal nutrient infusion, exogenous CCK delivery, and gastric distention (19, 24, 25). These results and several other recent reports (19, 22, 26, 33, 34) support the perspective that the energy balance effects of leptin are mediated by an anatomically distributed network that includes endogenous neuronal LepRb signaling in the mNTS (13, 14).
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-21397 (H. J. Grill), DK-085435 (M. R. Hayes), and DK-089752 (S. E. Kanoski), the China Scholarship Council (S. Zhao), and National Natural Science Foundation of China Grants 30970973 and 31000518 (J. Yan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.E.K., S.Z., D.J.G., R.J.D., B.C.D.J., M.R.H., and H.J.G. did the conception and design of the research; S.E.K., S.Z., and M.R.H. performed the experiments; S.E.K., S.Z., and B.C.D.J. analyzed the data; S.E.K., S.Z., B.C.D.J., K.K.B., M.R.H., and H.J.G. interpreted the results of the experiments; S.E.K. and S.Z. prepared the figures; S.E.K. and S.Z. drafted the manuscript; S.E.K., S.Z., D.J.G., K.K.B., M.R.H., and H.J.G. edited and revised the manuscript; S.E.K., S.Z., J.Y., M.R.H., and H.J.G. approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank the following for their substantial contributions to this work: Samantha Fortin, Amber Alhadeff, Jennifer Gilbert, Jeffrey Chen, Derek Zimmer, and Kimberly Rak.
REFERENCES
- 1. Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature 421: 856–859, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med 12: 917–924, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Caron E, Sachot C, Prevot V, Bouret SG. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol 518: 459–476, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Castonguay TW, Upton DE, Leung PM, Stern JS. Meal patterns in the genetically obese Zucker rat: a reexamination. Physiol Behav 28: 911–916, 1982 [DOI] [PubMed] [Google Scholar]
- 5. Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Eckel LA, Langhans W, Kahler A, Campfield LA, Smith FJ, Geary N. Chronic administration of OB protein decreases food intake by selectively reducing meal size in female rats. Am J Physiol Regul Integr Comp Physiol 275: R186–R193, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Elias CF, Kelly JF, Lee CE, Ahima RS, Drucker DJ, Saper CB, Elmquist JK. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol 423: 261–281, 2000 [PubMed] [Google Scholar]
- 8. Emond M, Ladenheim EE, Schwartz GJ, Moran TH. Leptin amplifies the feeding inhibition and neural activation arising from a gastric nutrient preload. Physiol Behav 72: 123–128, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol Regul Integr Comp Physiol 276: R1545–R1549, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Farooqi IS, O'Rahilly S. Mutations in ligands and receptors of the leptin-melanocortin pathway that lead to obesity. Nat Clin Pract Endocrinol Metab 4: 569–577, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Fraser KA, Raizada E, Davison JS. Oral-pharyngeal-esophageal and gastric cues contribute to meal-induced c-fos expression. Am J Physiol Regul Integr Comp Physiol 268: R223–R230, 1995 [DOI] [PubMed] [Google Scholar]
- 12. Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 14, Suppl 5: 216S–221S, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Grill HJ. Leptin and the systems neuroscience of meal size control. Front Neuroendocrinol 31: 61–78, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol 23: 2–40, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Hayes MR, Bradley L, Grill HJ. Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150: 2654–2659, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hayes MR, Chory FM, Gallagher CA, Covasa M. Serotonin type-3 receptors mediate cholecystokinin-induced satiation through gastric distension. Am J Physiol Regul Integr Comp Physiol 291: R115–R123, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Hayes MR, Covasa M. Gastric distension enhances CCK-induced Fos-like immunoreactivity in the dorsal hindbrain by activating 5-HT3 receptors. Brain Res 1088: 120–130, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D, De Jonghe BC, Kanoski SE, Grill HJ, Bence KK. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metab 13: 320–330, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11: 77–83, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ho A, Chin A. Circadian feeding and drinking patterns of genetically obese mice fed solid chow diet. Physiol Behav 43: 651–656, 1988 [DOI] [PubMed] [Google Scholar]
- 21. Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med 9: 1539–1544, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51: 801–810, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Hosoi T, Kawagishi T, Okuma Y, Tanaka J, Nomura Y. Brain stem is a direct target for leptin's action in the central nervous system. Endocrinology 143: 3498–3504, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Huo L, Gamber KM, Grill HJ, Bjørbaek C. Divergent leptin signaling in proglucagon neurons of the nucleus of the solitary tract in mice and rats. Endocrinology 149: 492–497, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huo L, Maeng L, Bjørbaek C, Grill HJ. Leptin and the control of food intake: neurons in the nucleus of the solitary tract are activated by both gastric distension and leptin. Endocrinology 148: 2189–2197, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Kanoski SE, Hayes MR, Greenwald HS, Fortin SM, Gianessi CA, Gilbert JR, Grill HJ. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology 36: 1859–1870, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, Jones JC, Rhodes CJ, Chua S, Jr, Diano S, Horvath TL, Seeley RJ, Becker JB, Münzberg H, Myers MG., Jr Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab 10: 89–98, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li HY, Wang LL, Yeh RS. Leptin immunoreactivity in the central nervous system in normal and diabetic rats. Neuroreport 10: 437–442, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Matson CA, Reid DF, Cannon TA, Ritter RC. Cholecystokinin and leptin act synergistically to reduce body weight. Am J Physiol Regul Integr Comp Physiol 278: R882–R890, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Matson CA, Ritter RC. Long-term CCK-leptin synergy suggests a role for CCK in the regulation of body weight. Am J Physiol Regul Integr Comp Physiol 276: R1038–R1045, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Mazda T, Yamamoto H, Fujimura M, Fujimiya M. Gastric distension-induced release of 5-HT stimulates c-fos expression in specific brain nuclei via 5-HT3 receptors in conscious rats. Am J Physiol Gastrointest Liver Physiol 287: G228–G235, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Mollet A, Gilg S, Riediger T, Lutz TA. Infusion of the amylin antagonist AC 187 into the area postrema increases food intake in rats. Physiol Behav 81: 149–155, 2004 [DOI] [PubMed] [Google Scholar]
- 33. Morton GJ, Blevins JE, Kim F, Matsen M, Figlewicz DP. The action of leptin in the ventral tegmental area to decrease food intake is dependent on Jak-2 signaling. Am J Physiol Endocrinol Metab 297: E202–E210, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Morton GJ, Blevins JE, Williams DL, Niswender KD, Gelling RW, Rhodes CJ, Baskin DG, Schwartz MW. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 115: 703–710, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145: 4880–4889, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Myers MG, Jr, Münzberg H, Leinninger GM, Leshan RL. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab 9: 117–123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Patterson CM, Leshan RL, Jones JC, Myers MG., Jr Molecular mapping of mouse brain regions innervated by leptin receptor-expressing cells. Brain Res 1378: 18–28, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213: 451–453, 1981 [DOI] [PubMed] [Google Scholar]
- 39. Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, Anderson CM, Parkes DG, Baron AD. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA 105: 7257–7262, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest 98: 1101–1106, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol 514: 518–532, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scott MM, Williams KW, Rossi J, Lee CE, Elmquist JK. Leptin receptor expression in hindbrain Glp-1 neurons regulates food intake and energy balance in mice. J Clin Invest 121: 2413–2421, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Willing AE, Berthoud HR. Gastric distension-induced c-fos expression in catecholaminergic neurons of rat dorsal vagal complex. Am J Physiol Regul Integr Comp Physiol 272: R59–R67, 1997 [DOI] [PubMed] [Google Scholar]
- 44. Yox DP, Brenner L, Ritter RC. CCK-receptor antagonists attenuate suppression of sham feeding by intestinal nutrients. Am J Physiol Regul Integr Comp Physiol 262: R554–R561, 1992 [DOI] [PubMed] [Google Scholar]