Abstract
Parathyroid hormone-related protein (PTHrP) increases renin release from isolated perfused kidneys and may act as an autacoid regulator of renin secretion, but its effects on renin in vivo are unknown. In vivo, PTHrP causes hypercalcemia and anorexia, which may affect renin. We hypothesized that chronically elevated PTHrP would increase plasma renin activity (PRA) indirectly via its anorexic effects, reducing sodium chloride (NaCl) intake and causing NaCl restriction. We infused male Sprague-Dawley rats with the vehicle (control) or 125 μg PTHrP/day (PTHrP) via subcutaneous osmotic minipumps for 5 days. To replenish NaCl consumption, a third group of PTHrP-infused rats received 0.3% NaCl (PTHrP + NaCl) in their drinking water. PTHrP increased PRA from a median control value of 3.68 to 18.4 ng Ang I·ml−1·h−1 (P < 0.05), whereas the median PTHrP + NaCl PRA value was normal (7.82 ng Ang I·ml−1·h−1, P < 0.05 vs. PTHrP). Plasma Ca2+ (median control: 10.2 mg/dl; PTHrP: 13.7 mg/dl; PTHrP + NaCl: 14.1 mg/dl; P < 0.05) and PTHrP (median control: 0.03 ng/ml; PTHrP: 0.12 ng/ml; PTHrP + NaCl: 0.15 ng/ml; P < 0.05) were elevated in PTHrP- and PTHrP + NaCl-treated rats. Body weights and caloric consumption were lower in PTHrP- and PTHrP + NaCl-treated rats. NaCl consumption was lower in PTHrP-treated rats (mean Na+: 28.5 ± 4.1 mg/day; mean Cl−: 47.8 mg/day) compared with controls (Na+: 67.3 ± 2.7 mg/day; Cl−: 112.8 ± 4.6 mg/day; P < 0.05). NaCl consumption was comparable with control in the PTHrP + NaCl group; 0.3% NaCl in the drinking water had no effect on PRA in normal rats. Thus, our data support the hypothesis that PTHrP increases PRA via its anorexic effects, reducing NaCl intake and causing NaCl restriction.
Keywords: calcium, blood pressure, parathyroid hormone receptor, hypercalcemia, hyperparathyroidism
parathyroid hormone-related protein (PTHrP) is a 141-amino acid peptide that binds to and activates the classical parathyroid hormone (PTH) receptor PTH1R. Because of this, PTHrP produces PTH-like effects on bone resorption and calcium (Ca2+) metabolism when its plasma levels become elevated (1, 19, 21, 29, 32, 47). Under normal conditions, PTHrP levels in the plasma are undetectable (7, 15, 37, 38, 41). However, certain tumors secrete PTHrP, leading to hypercalcemia of malignancy with associated elevated plasma PTHrP levels as high as 70 pmol/l (7, 15, 37, 38, 41). Elevated plasma PTHrPs are the most common cause of hypercalcemia of malignancy, which is characterized by enhanced bone resorption, elevated plasma calcium, polyuria, renal failure, and anorexia (30).
Renin is the rate-limiting enzyme of the renin-angiotensin system, which is integral for the maintenance of normal blood pressure and volume homeostasis (13, 22, 45). Renin secretion is elevated by decreased sodium chloride (NaCl) intake (5, 10, 24), decreased blood pressure or renal perfusion (36, 41), and increased sympathetic nervous activity (43). In vivo, the enzymatic activity of renin is typically quantified as plasma renin activity (PRA) or its ability to generate angiotensin I (Ang I).
The effects of PTHrP on renin are poorly understood. PTH, acting on the common PTH/PTHrP receptor, has been reported to stimulate renin release directly from juxtaglomerular (JG) cells (34). Similarly, it has been reported that PTHrP can acutely stimulate renin release from the isolated perfused kidney at pharmacological concentrations, suggesting an effect of PTHrP on the endothelium or JG cells to directly stimulate renin release (35). PTHrP is also expressed in the macula densa (27, 46), and it is speculated that it may be released there to stimulate renin secretion in response to changes in tubular flow (12). Thus, it is currently thought that PTHrP may act as an autacoid to regulate renin secretion. However, whether PTHrP can stimulate PRA chronically in vivo is unknown. Additionally, since PTHrP exerts a myriad of different effects in vivo, it could conceivably stimulate PRA through one of its many metabolic effects instead of a direct effect on renin secretion. Thus, we wanted to provide a mechanistic explanation of how chronically elevated PTHrP could increase PRA. Anorexia is a common associated symptom of elevated PTHrP, and anti-PTHrP antibodies reverse the anorexic effects of PTHrP-secreting tumors (18, 19, 37). Because PRA is sensitive to changes in dietary NaCl consumption (5, 10, 24), we anticipated that the mechanism by which elevated PTHrP and the associated anorexia would increase PRA would be via impaired NaCl consumption. Thus, we hypothesized that chronic PTHrP-induced hypercalcemia would increase PRA not by a direct effect but due to its anorexic effects resulting in reduced NaCl intake and causing NaCl restriction, which is an established and potent stimulus for renin secretion.
METHODS
Treatment Protocols
Protocol 1: the effect of chronic PTHrP infusions and NaCl repletion on PRA, plasma and urinary parameters, and body weight.
Rats were housed singly in static caging with individual sipper bottles and maintained on normal chow (Harlan Teklad, Madison, WI) containing 0.4% sodium, 0.67% chlorine, and a metabolizable caloric content of 3.11 kcal/g for the duration of the study ad libitum. Model 2001 osmotic minipumps (Model 2001; Alzet, Cupertino, CA) were loaded with vehicle or drugs listed below and primed overnight according to the manufacturer's instructions. Rat PTHrP 1–34 (Bachem, Torrance, CA) was dissolved in sterile 2% cysteine HCl and 0.9% NaCl (pH = 1.4). The cysteine HCl and low pH are necessary to prevent the loss of PTHrP potency over sustained infusions (20). The next day, male Sprague-Dawley rats weighing between 200 and 250 g were anesthetized with 50 mg/kg body wt ip Nembutal (pentobarbital sodium; Ovation Pharmaceuticals, Deerfield, IL). The surgical procedure was performed using aseptic techniques on a heating pad to maintain constant body temperature. An incision was made between the scapulae, and the osmotic minipumps were implanted subcutaneously. The incisions were stapled close, and the rats were allowed to recover on the hot pad before being returned to their cages.
We had three different groups in this protocol. Rats were subcutaneously infused via osmotic minipump with 2% cysteine HCl and 0.9% NaCl (control; n = 14), 125 μg/day PTHrP (PTHrP; n = 15), whereas the last group was infused with 125 μg/day PTHrP while receiving 0.3% NaCl in the drinking water (PTHrP + NaCl; n = 10). The dose and length of the PTHrP was determined empirically based on preliminary studies, since longer infusions or higher doses exerted significant toxicity. Both the control and PTHrP groups received regular tap water for drinking. The day of minipump implantation was considered day 1. Body weights were measured daily. Rats were placed in metabolic caging on day 3, and urine was collected over a 24-h period. Urinary volume, creatinine, and Ca2+ and Na+ excretion were all quantified from the collected urine. Urine was spun twice at 16,100 g for 10 min at 4°C to remove any contaminants. The supernatant was collected each time, passed through a 0.22-μm syringe drive filter unit (Millipore) after the final centrifugation, and stored at −20°C until being analyzed. Systolic blood pressure was measured via tail cuff plesmography on day 4. Rats were euthanized on day 5 by decapitation for the collection of blood for analyses unaffected by anesthesia. Plasma was separated from the blood by centrifugation at 1,164 g for 15 min at 4°C. The plasma was aspirated and stored at −20°C until it was used for the quantification of PRA, Ca2+, and Na+. Additionally, after the collection of plasma, the peritoneal cavity was quickly opened and the left kidney quickly excised, decapsulated, weighed and then split longitudinally, and photographed, and the sections were removed and fixed in 3.8% formalin overnight for making histological slides. All methods are described in greater detail in their respective sections.
All procedures were approved by the Henry Ford Health System Institutional Animal Care and Use Committee and adhered to the guiding principles in the care and use of experimental animals in accordance with the National Institutes of Health (NIH) guidelines. Henry Ford Hospital operates an Association for Assessment and Accreditation of Laboratory Animal Care-certified animal care facility.
Protocol 2: the effect of 0.3% NaCl in the drinking water on PRA under normal conditions.
To determine whether NaCl ingestion alone would influence PRA, rats were housed singly in static caging with sipper bottles and maintained on normal chow, as described in protocol 1, for the duration of the study. Rats in this protocol were not implanted with osmotic minipumps, nor were they treated with PTHrP. Rats received either normal tap water (control; n = 5) or water containing 0.3% NaCl (control + NaCl; n = 5) for 4 days. Rats were weighed daily, and food and water consumption were calculated as described in the analyses section. Rats were euthanized on day 4 as described in protocol 1, and blood for PRA, plasma Ca2+, and plasma Na+ were collected.
Analyses
PRA.
Only plasma collected within the first 3 s after decapitation was used for the determination of PRA to ensure that our results were not contaminated by renal baroreceptor-stimulated renin secretion. Ethylenediaminetetraacetic acid (3.8%) was used as the anticoagulant. Plasma renin activity was analyzed by generation of Ang I·h−1·min−1 using a Gamma Coat RIA kit (DiaSorin, Stillwater, MN) as described previously and according to the manufacturer's instructions (3).
Plasma PTHrP and PTH quantification.
Plasma PTHrP-(1–34) was determined using an enzyme-linked immunoassay kit (Peninsula Laboratories, San Carlos, CA) according to the manufacturer's instructions. Plasma PTH-(1–84) was quantified using an enzyme-linked immunoassay (Alpco Diagnostics, Salem, NH) according to the manufacturer's instructions, as described previously (3).
Plasma and urinary Ca2+ quantification.
Plasma and urinary Ca2+ were measured with a colorimetric (Biovision, Mountain View, CA) assay kit according to the manufacturer's instructions using a colorimetric plate reader (Titertek, Huntsville, AL). Absorbance was measured at 570 nm, and values were analyzed with Multiskan Ascent.
Plasma and urinary Na+ quantification.
Plasma and urinary Na+ were measured with a NOVA-1 electrolyte analyzer (NOVA Biomedical, Waltham, MA) according to the manufacturer's instructions.
Plasma and urinary creatinine quantification and creatinine clearance calculation.
Plasma and urinary creatinine were determined using a colorimetric assay (BioAssay Systems, Hayward, CA). Creatinine clearance was calculated by multiplying the concentration of urinary creatinine by the 24-h urinary volume, dividing by the plasma creatinine concentration, and then correcting the units of time for clearance to milliliters per minute. Finally, clearance values per gram of kidney weight were normalized.
Urine osmolality quantification.
Urine osmolality was measured using a model 3300 Advanced Micro Osmometer (Advanced Instruments, Norwood, MA).
Tail cuff plesmography.
Systolic blood pressure was measured noninvasively using a computerized tail cuff system (Model 1231; IITC, Woodland Hills, CA). Rats were trained over 3 days before systolic blood pressure measurement. Three systolic blood pressure measurements were taken from each rat, and a mean value was calculated for statistical analyses.
Food, H2O, NaCl, and caloric consumption.
Food consumption was determined by weighing the initial food provided as well as measuring the remaining food in each rat's cage to the nearest gram daily. Water consumption was determined gravimetrically to the nearest milliliter. Na+ and Cl− consumption was determined by multiplying the food consumed by its Na+ (0.4%) and Cl− (0.67%) content, as well as by adding any additional NaCl consumed from the H2O in the PTHrP + NaCl group. Caloric consumption was determined by multiplying the food consumed by its metabolizable caloric content (3.11 kcal/g).
Von Kossa staining.
Kidneys from euthanized animals were immediately placed in 3.8% formalin at 4°C overnight before embedding in paraffin for processing. Renal cortical slices were then deparrifinized with xylene and dehydrated with an ethanol gradient before staining for tissue calcification with von Kossa's stain, as described previously (23).
Statistics
All data were tested for normality of their distribution and equality of variances using the Kolmogrov-Smirnov and Levene median tests, respectively. Since many of the data had nonnormal distributions, Kruskal-Wallis one-way ANOVA on ranks with Dunn's post hoc test was employed in most cases. Data analyzed with this test are displayed as the 25th, 50th, and 75th percentiles (Table 1). When multiple comparisons on normally distributed data with equal variances were performed, one-way ANOVA with Student-Newman-Keuls post hoc test was performed. Single intragroup comparisons between basal and final values were performed with a paired Student's t-test. Single intergroup comparisons between two groups were performed with a Student's t-test. Each statistical test used is provided in the figures and tables. In these cases, data are presented as means ± SE. In all cases, P < 0.05 was considered statistically significant.
Table 1.
Effect of chronic PTHrP infusions and NaCl replenishment on plasma and urinary parameters
| Parameter (units) | Control | PTHrP | PTHrP + NaCl |
|---|---|---|---|
| Plasma PTHrP, ng/ml | 0, 0.03, 0.04 | 0.08, 0.12, 0.21* | 0.08, 0.15, 0.42* |
| Plasma Ca2+, mg/dl | 9.4, 10.2, 10.5 | 12.3, 13.7, 14.8* | 12.8, 14.1, 17.4* |
| Plasma PTH, pg/ml | 25.5, 40.7, 66.9 | 0, 0, 1.1* | 0, 0, 0* |
| Plasma Na+, mmol/l | 140, 141, 143 | 139, 141, 142 | 143, 144, 146† |
| Systolic blood pressure, mmHg | 120, 133, 138 | 107, 127, 139 | 106, 118, 128 |
| Creatinine clearance, ml·min−1·g kidney wt−1 | 0.65, 0.74, 0.84 | 0.23, 0.40, 0.95 | 0.21, 0.43, 0.69 |
| Urinary volume, ml/24 h | 7.0, 8.3, 11.0 | 5.3, 10.0, 14.8 | 7.0, 11.3, 14.0 |
| Urinary Ca2+, mg/24 h | 0.55, 0.94, 1.21 | 0.68, 1.77, 3.11 | 1.07, 1.63, 2.11 |
| Urinary Na+, mmol/24 h | 1.31, 1.69, 1.95 | 0.21, 0.51, 1.27* | 0.25, 0.38, 0.59* |
| Urine osmolality, mOsm/kg H2O | 1,509, 1,748, 2,063 | 730, 826, 1,071* | 833, 941, 1,023* |
PTHrP, parathyroid hormone-related protein; PTH, parathyroid hormone.
Data represent the 25th, 50th, and 75th percentiles, respectively. Plasma Ca2+ was elevated in both PTHrP- and PTHrP + NaCl-treated rats. Plasma Na+ was elevated in PTHrP + NaCl rats compared with PTHrP-treated rats but did not differ from control. Plasma PTHrP was elevated and plasma PTH depressed in both PTHrP and PTHrP + NaCl rats. Urinary Na+ excretion was lower in PTHrP- and PTHrP + NaCl-treated rats. Urine osmolality was lower in PTHrP- and PTHrP + NaCl-treated rats. Data were analyzed with Kruskal-Wallis 1-way ANOVA on ranks with Dunn's post hoc test.
P < 0.05 vs. control;
P < 0.05 vs. PTHrP.
RESULTS
Protocol 1: The Effect of Chronic PTHrP Infusions and NaCl Repletion on PRA, Plasma, and Urinary Parameters and Body Weight
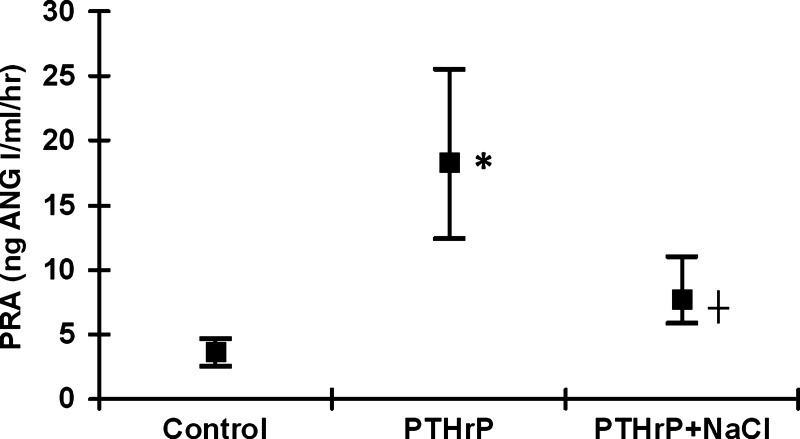
Chronically infusing PTHrP over 5 days increased PRA significantly compared with controls (Fig. 1). However, PRA in the PTHrP + NaCl-treated rats was significantly lower than in PTHrP-treated rats and did not differ from control values (Fig. 1). Plasma PTHrP was significantly elevated in both the PTHrP and PTHrP + NaCl groups, demonstrating that the administration of PTHrP was successful (Table 1). Plasma Ca2+ was significantly elevated in both the PTHrP and PTHrP + NaCl groups (Table 1), demonstrating that PTHrP was bioactive. Additionally, plasma PTH was significantly depressed in both the PTHrP and PTHrP + NaCl groups, demonstrating that the PTHrP administered was bioactive (Table 1). Plasma Na+ was higher in the PTHrP + NaCl group compared with PTHrP alone but did not differ from control values (Table 1). Systolic blood pressure, urine volume, and urinary Ca2+ excretion did not differ between groups (Table 1). Urinary Na+ excretion was markedly depressed in both the PTHrP and PTHrP + NaCl groups (Table 1). Urine osmolality was significantly lower in the PTHrP and PTHrP + NaCl groups, consistent with the effects of a hypercalcemic concentrating defect (Table 1). Additionally, to demonstrate that our infusions of PTHrP were successful, we also took gross anatomic pictures of PTHrP- and PTHrP + NaCl-treated kidneys and examined renal cortical histological mineralization of the different groups using von Kossa's stain. Gross anatomic nephrocalcinosis was visible in both PTHrP- and PTHrP + NaCl-treated kidneys (Fig. 2), and diffuse tissue 3mineralization was also seen in PTHrP and PTHrP + NaCl-treated renal cortices on histological examination as well (Fig. 2). These data are consistent with the effects of elevated plasma PTHrP (20, 23).
Fig. 1.
The effect of chronic parathyroid hormone-related protein (PTHrP) infusions and NaCl repletion on plasma renin activity (PRA). The bottom and top borders of each group's bracket represent the 25th and 75th percentiles, respectively. ■, Median value for each group. PTHrP increased the median PRA value to 18.4 ng angiotensin I (Ang I)·ml−1·h−1 from a control value of 3.7 ng Ang I·ml−1·h−1 (P < 0.05). PRA in the PTHrP + NaCl group was significantly lower than in the PTHrP group (median value: 7.8 ng Ang I·ml−1·h−1, P < 0.05) and did not differ significantly from controls. Statistics determined using Kruskal-Wallis 1-way ANOVA based on ranks with Dunn's post hoc test. *P < 0.05 vs. control; †P < 0.05 vs. PTHrP.
Fig. 2.
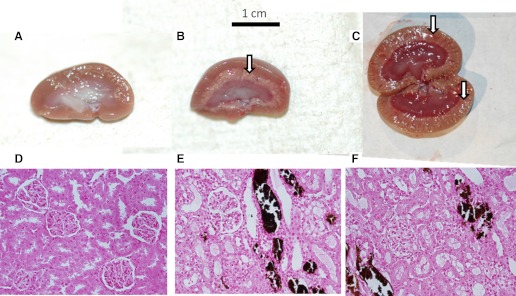
The effects of PTHrP on nephrocalcinosis. From left to right, kidneys came from control (A), PTHrP (B), and PTHrP + NaCl rats (C). Gross calcification is visible in the PTHrP- and PTHrP + NaCl-treated kidneys (denoted by arrows). D–F: von Kossa's stain for tissue mineralization. From left to right, samples came from the renal cortex of control (D), PTHrP-treated (E), and PTHrP + NaCl-treated rats (F). The dark brown-stained tissue represents mineralization.
To determine whether PTHrP was increasing PRA due to its anorexic effects on NaCl intake and causing NaCl restriction, we also measured the effects of PTHrP on body weight, caloric, Na+, Cl−, and H2O consumption. Body weight increased in control rats and decreased similarly in both PTHrP and PTHrP + NaCl rats (Table 2). Caloric intake was similarly depressed in both PTHrP and PTHrP + NaCl rats (Table 2). PTHrP significantly decreased Na+ and Cl− consumption, but this was restored to control levels in the PTHrP + NaCl group (Table 2). H2O consumption did not differ between groups (Table 2).
Table 2.
Effects of PTHrP and NaCl replenishment on body weight, Cl−, Na+, and caloric and H2O consumption
| Parameter (units) | Control | PTHrP | PTHrP + NaCl |
|---|---|---|---|
| Basal body weight, g | 238 ± 9 | 236 ± 7 | 246 ± 3 |
| Final body weight, g | 263 ± 9# | 204 ± 6# | 221 ± 4# |
| Metabolizable calories consumed, kcal/day | 52.3 ± 2.1 | 22.2 ± 3.2* | 20.2 ± 2.8* |
| Na+ consumed, mg/day | 67.3 ± 2.7 | 28.5 ± 4.1* | 72.2 ± 10.3† |
| Cl− consumed, mg/day | 112.8 ± 4.6 | 47.8 ± 6.8* | 116.0 ± 16.6† |
| H2O consumed, ml/day | 36 ± 2 | 29 ± 3 | 40 ± 6 |
Body weight increased in control rats and decreased identically in both PTHrP and PTHrP + NaCl rats. PTHrP decreased Cl− and Na+ consumption significantly, and NaCl replacement in the drinking water attenuated these effects. Calorie consumption was low in both PTHrP and PTHrP + NaCl compared with controls. H2O consumption did not differ between groups. Intragroup analyses were performed with paired Student's t-test. Intergroup analyses were performed using 1-way ANOVA with Student-Newman-Keuls post hoc test.
P < 0.05 vs. basal;
P < 0.05 vs. control;
P < 0.05 vs. PTHrP. Data are presented as means ± SE.
Protocol 2: The Effect of 0.3% NaCl in the Drinking Water on PRA Under Normal Conditions
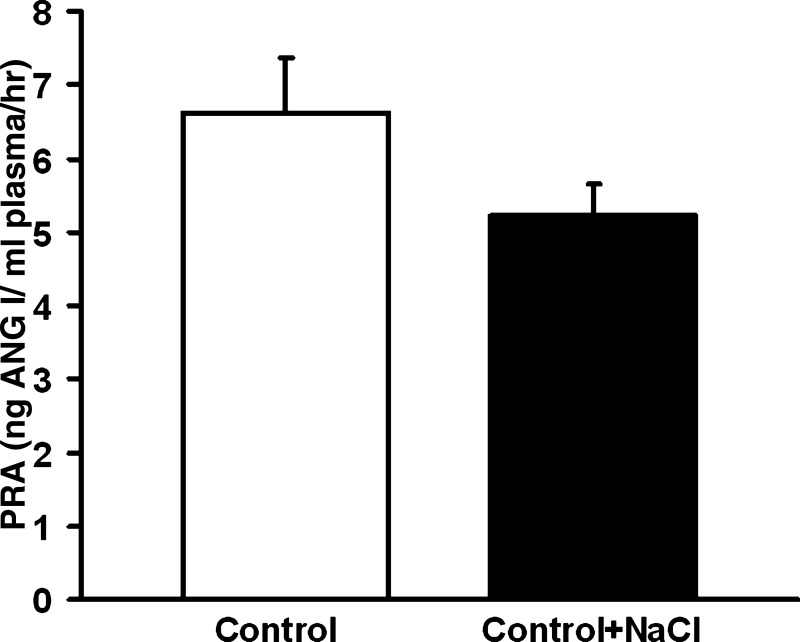
To determine whether the effects of 0.3% NaCl in the drinking water on PRA were specific to our PTHrP-treated rats, we also tested whether the administration of 0.3% NaCl in the drinking water could lower PRA under normal conditions. PRA did not differ significantly between the control and control + NaCl groups (Fig. 3). Plasma Ca2+ and Na+ did not differ significantly between groups (Table 3). Body weights did not differ significantly between groups (Table 3). Caloric intake did not differ between groups (Table 3). Na+ and Cl− consumption were significantly higher in the control + NaCl-treated group compared with control (Table 3). H2O consumption did not differ between groups (Table 3).
Fig. 3.
The effect of 0.3% NaCl on control PRA. Control PRA (6.6 ± 0.7 ng Ang I·ml−1·h−1) did not differ significantly from the control + NaCl PRA (5.2 ± 0.4 ng Ang I·ml−1·h−1). Statistics determined using Student's t-test. Data are presented as means ± SE.
Table 3.
Effects of 0.3% NaCl on control plasma electrolytes, body weight, Cl−, Na+, and caloric and H2O consumption
| Parameter | Control | Control + NaCl |
|---|---|---|
| Plasma Ca2+, mg/dl | 11.5 ± 1.0 | 11.2 ± 0.5 |
| Plasma Na+, mmol/l | 142 ± 1 | 142 ± 1 |
| Basal body weight, g | 223 ± 7 | 232 ± 4 |
| Final body weight, g | 258 ± 4# | 254 ± 5# |
| Metabolizable calories consumed, kcal/day | 46.8 ± 1.4 | 44.3 ± 1.7 |
| Na+ consumed, mg/day | 60.2 ± 1.9 | 113.8 ± 11.1* |
| Cl− consumed, mg/day | 100.8 ± 3.1 | 184.6 ± 17.6* |
| H2O consumed, ml/day | 37 ± 1 | 48 ± 9 |
Plasma Ca2+ and plasma Na+ did not differ significantly between the groups. Body weight increased identically in both groups during the study. Caloric consumption did not differ between groups. Na+ and Cl− consumption were higher in the control + NaCl vs. the control group. H2O consumption did not differ significantly between the groups. Data are presented as means ± SE.
P < 0.05 vs. basal;
P < 0.05 vs. control.
DISCUSSION
We have demonstrated that chronic, subcutaneously infused PTHrP can increase PRA. However, because elevated PTHrP can cause anorexia, and because our PTHrP-treated rats lost weight, we tested whether the PRA-stimulating ability of PTHrP was due indirectly to its anorexic effects. Because PRA is regulated by NaCl balance (5, 10, 24), we tested whether the stimulatory effects of PTHrP on PRA were due to anorexic effects on NaCl consumption. We found that NaCl replenishment during PTHrP administration reversed the stimulatory effects of PTHrP on PRA. Thus, our data support the hypothesis that chronically elevated PTHrP increases PRA in large part due to its anorexic effects by decreasing NaCl intake and resulting in NaCl depletion.
Renin is secreted from the JG cells of the afferent arteriole and is the rate-limiting enzyme of the renin-angiotensin system (13, 22, 45). The renin-angiotensin system acts to homeostatically maintain blood pressure in response to reduced renal perfusion pressure (34, 41), increased renal sympathetic nerve activity (43), or reduced NaCl intake (5, 10, 24). Previous studies have suggested that exogenous PTHrP may stimulate renin secretion in the isolated perfused kidney model (35). However, to our knowledge, no data exist on whether PTHrP could (patho)physiologically stimulate PRA in vivo. Thus, the rationale of our study was to determine mechanistically whether chronically elevated PTHrP could stimulate PRA and examine a possible pathway.
PTHrP mimics the effects of PTH by binding to and stimulating a common receptor, PTH1R (1, 21). Thus, elevated PTHrP in vivo causes hypercalcemia, anorexia, and nephrocalcinosis (18, 19, 23, 37, 47). In adulthood, PTHrP is not normally found circulating in the plasma (38). However, certain tumors stimulate the production and secretion of PTHrP, leading to elevated plasma levels (18, 29). Our model of subcutaneous PTHrP infusions was able to accurately reproduce many of the symptoms of hypercalcemia of maligniancy, specifically, elevated plasma Ca2+, depressed plasma PTH, nephrocalcinosis, and the renal concentrating defect. Plasma PTHrP levels in patients with humoral hypercalcemia of malignancy can exceed 50 pmol/l (7, 15, 37, 41). Our median values were well within this range and corresponded to 25–35 pmol/l. Thus, our data likely reflect what is happening in humoral hypercalcemia of malignancy in patients with elevated plasma PTHrP levels.
PTHrP decreased Na+ and Cl− consumption and Na+ excretion significantly, and the restoration of NaCl consumption significantly attenuated the PTHrP-mediated increase in PRA. This demonstrates that the stimulation of PRA by PTHrP is due at least partially to its anorexic effects reducing NaCl intake and causing NaCl restriction. Previous data suggest that the lack of Cl− is more important for the elevation of PRA than the lack of Na+ (24) and that this effect is mediated by decreased Cl− transport at the macula densa (26). The mechanism by which decreases in NaCl consumption increase PRA is well described; numerous studies have shown that low NaCl consumption increases PRA due to increased cyclooxygenase-2 and neuronal nitric oxide synthase activity at the macula densa (5, 11, 16, 17, 39). We also tested whether NaCl replenishment affected the PTHrP-mediated decline in caloric intake. Caloric intake was significantly impaired in both PTHrP- and PTHrP + NaCl-treated rats, suggesting that the stimulation of PRA by PTHrP is not due just to impaired caloric intake. Additionally, we found that the addition of 0.3% NaCl to the drinking water had no effect on PRA under Na+-replete conditions in the absence of PTHrP. Thus, the amount of NaCl that we supplemented our rats with was sufficient to inhibit PTHrP-stimulated PRA without PRA being affected under normal conditions.
The anorexic effects of PTHrP are well known. The administration of PTHrP-secreting tumors to nude mice causes profound anorexia and cachexia, and these effects are reversed completely by the administration of anti-PTHrP antibodies (30). However, the precise mechanism by which PTHrP exerts its anorexic effects is still under active investigation. Hashimoto et al. (18) suggest that the anorexic effects are not due to modulation of the leptin, hypothalamic anorexogenic, or orexogenic peptides, and their results were replicated by Suzuki et al. (37). However, Asakawa et al. (2) found that PTHrP decreased food intake via impaired gastric emptying and was related to urocortin 2 and 3 expression. While the exact mechanism of PTHrP-mediated anorexia is still being defined, it is patently clear that elevated PTHrP levels have profound anorexic effects.
Prior to our studies, it had been suggested that PTHrP could stimulate renin secretion due to a direct effect on PTH receptors in the isolated perfused kidney (35), perhaps acting as an autacoid released from the macula densa in response to changes in tubular flow (12). Although PTHrP very clearly stimulated renin secretion in those experiments, intricacies with the experimental design make it difficult to extrapolate the ex vivo results to in vivo models. At the pharmacological concentrations used in that study (35), PTHrP can cause renal vasodilation, which itself is a stimulus for renin secretion (44). Thus, it is plausible that PTHrP stimulated renin release solely because of a pharmacological activation of the renal baroreceptor. Additionally, it has been suggested previously that PTH and/or PTHrP may be able to directly stimulate renin release from JG cells (34). However, in that study the authors reveal that PTH actually failed to stimulate renin release from JG cells unless a protease inhibitor, phenylmethylsulfonyl fluoride, was added and that phenylmethylsulfonyl fluoride also stimulated renin release on its own, undermining any direct effect of PTH. Furthermore, emerging data suggest that PTHrP does not directly stimulate renin release from JG cells (4). Thus, it is unlikely that PTHrP increased PRA via a direct effect on JG cells in our experiments. The data we present suggest that PTHrP does not act as an autacoid to directly stimulate PRA but rather stimulates PRA indirectly through its systemic effects, causing elevations in plasma Ca2+ that act through traditional pathways of renin regulation, such as changes in dietary NaCl intake.
Additionally, we would like to contrast our results using chronic, PTHrP-induced hypercalcemia with our previous work describing the effects of acute hypercalcemia on PRA. Acute hypercalcemia decreases PRA via its actions on the JG cell calcium-sensing receptor (3). However, the present data demonstrate that chronic elevations in plasma Ca2+ actually stimulate PRA indirectly via their actions on NaCl consumption and homeostasis. Thus, the effects of Ca2+ on renin are dependent on their site and length of action and the integration of the body's response to these changes in plasma Ca2+.
In conclusion, we tested the hypothesis that chronically elevated PTHrP could indirectly elevate PRA due to its anorexic effects on NaCl consumption. We found that PTHrP increased PRA and that this increase in PRA was associated with hypercalcemia, nephrocalcinosis, decreased plasma PTH, and a urinary concentrating defect. Replenishment of NaCl during PTHrP administration attenuated the increase in PRA. Thus, our data support the notion that PTHrP indirectly increases PRA via its anorexic effects by decreasing NaCl consumption and causing Na restriction.
Perspectives
To our knowledge, these are the first data demonstrating that chronically elevated PTHrP, mimicking the effects of hypercalcemia of malignancy, can stimulate PRA in vivo. The means by which PTHrP increases PRA, namely decreased NaCl consumption, are consistent with both the physiopathology of hypercalcemia and the physiology of renin secretion. Although there are not sufficient studies on renin in hypercalcemia of malignancy with which to compare our results, we can compare our results with those from primary hyperparathyroidism. Patients with primary hyperparathyroidism and hypercalcemia of malignancy present with similar symptoms and biochemical findings. For more than 30 years a controversy over whether PRA values are elevated in patients with primary hyperparathyroidism has existed, with some studies suggesting that they are (8, 9, 14, 25, 31) and others proposing that they are not (6, 33, 40). Since PTH and PTHrP share a similar receptor, our data suggest that PTH may increase PRA in primary hyperparathyroidism. Patients with hyperparathyroidism are much more likely to have cardiorenal disease than the normal population, and elevated PRA might contribute to the increase in cardiorenal morbidity, although this remains an untested question.
GRANTS
This research was supported by funding from NIH with Grants F30-DK-084654-03 and PPG-5PO1-HL-090550-03. D. K. Atchison is a member of the Wayne State University School of Medicine MD/PhD program.
DISCLOSURES
There are no conflicts of interest, financial or otherwise, or disclosures to report.
AUTHOR CONTRIBUTIONS
D.K.A. and W.H.B. did the conception and design of the research; D.K.A., E.P.W., D.L.S., K.L.G., and W.H.B. performed the experiments; D.K.A., E.P.W., D.L.S., K.L.G., and W.H.B. analyzed the data; D.K.A., E.P.W., D.L.S., K.L.G., and W.H.B. interpreted the results of the experiments; D.K.A. prepared the figures; D.K.A., E.P.W., and W.H.B. drafted the manuscript; D.K.A., K.L.G., and W.H.B. edited and revised the manuscript; D.K.A., E.P.W., D.L.S., K.L.G., and W.H.B. approved the final version of the manuscript.
REFERENCES
- 1. Abou-Samra AB, Jüppner H, Force T, Freeman MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV, Potts JT, Jr, Kronenberg HM, Segre GV. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci USA 89: 2732–2736, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asakawa A, Fujimiya M, Niijima A, Fujino K, Kodama N, Sato Y, Kato I, Nanba H, Laviano A, Meguid MM, Inui A. Parathyroid hormone-related protein has an anorexigenic activity via activation of hypothalamic urocortins 2 and 3. Psychoneuroendocrinology 35: 1178–1186, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Atchison DK, Harding P, Beierwaltes WH. Hypercalcemia reduces plasma renin via parathyroid hormone, renal interstitial calcium, and the calcium-sensing receptor. Hypertension 58: 604–610, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atchison DK, Harding P, Beierwaltes WH. Parathyroid Hormone Related Protein Dissociates Increased cAMP from Renin Secretion in Mouse Juxtaglomerular Cells (Abstract No. 24782). Proceedings of the American Society of Nephrology, Renal Week, Philadelphia, PA, 2011 [Google Scholar]
- 5. Beierwaltes WH. Macula densa stimulation of renin is reversed by selective inhibition of neuronal nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 272: R1359–R1364, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Bernini G, Moretti A, Lonzi S, Bendinelli C, Miccoli P, Salvetti A. Renin-angiotensin-aldosterone system in primary hyperparathyroidism before and after surgery. Metabolism 48: 298–300, 1999 [DOI] [PubMed] [Google Scholar]
- 7. Body JJ, Dumon JC, Thirion M, Cleeren A. Circulating PTHrP concentrations in tumor-induced hypercalcemia: influence on the response to bisphosphonate and changes after therapy. J Bone Miner Res 8: 701–706, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Brinton GS, Jubiz W, Lagerquist LD. Hypertension in primary hyperparathyroidism: the role of the renin-angiotensin system. J Clin Endocrinol Metab 41: 1025–1029, 1975 [DOI] [PubMed] [Google Scholar]
- 9. Broulik PD, Horký K, Pacovský V. Blood pressure in patients with primary hyperparathyroidism before and after parathyroidectomy. Exp Clin Endocrinol 86: 346–352, 1985 [DOI] [PubMed] [Google Scholar]
- 10. Brown JJ, Davies DL, Lever AF, Robertson JI. Influence of sodium deprivation and loading on the plasma renin in man. J Physiol 173: 408–419, 1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castrop H, Schweda F, Schumacher K, Wolf K, Kurtz A. Role of renocortical cyclooxygenase-2 for renal vascular resistance and macula densa control of renin secretion. J Am Soc Nephrol 12: 867–874, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Clemens TL, Cormier S, Eichinger A, Endlich K, Fiaschi-Taesch N, Fischer E, Friedman PA, Karaplis AC, Massfelder T, Rossert J, Schlüter KD, Silve C, Stewart AF, Takane K, Helwig JJ. Parathyroid hormone-related protein and its receptors: nuclear functions and roles in the renal and cardiovascular systems, the placental trophoblasts and the pancreatic islets. Br J Pharmacol 134: 1113–1136, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fukamizu A, Sugimura K, Takimoto E, Sugiyama F, Seo MS, Takahashi S, Hatae T, Kajiwara N, Yagami K, Murakami K. Chimeric renin-angiotensin system demonstrates sustained increase in blood pressure of transgenic mice carrying both human renin and human angiotensinogen genes. J Biol Chem 268: 11617–11621, 1993 [PubMed] [Google Scholar]
- 14. Gennari C, Nami R, Gonnelli S. Hypertension and primary hyperparathyroidism: the role of adrenergic and renin-angiotensin-aldosterone systems. Miner Electrolyte Metab 21: 77–81, 1995 [PubMed] [Google Scholar]
- 15. Gurney H, Grill V, Martin TJ. Parathyroid hormone-related protein and response to pamidronate in tumour-induced hypercalcaemia. Lancet 341: 1611–1613, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Harding P, Carretero OA, Beierwaltes WH. Chronic cyclooxygenase-2 inhibition blunts low sodium-stimulated renin without changing renal haemodynamics. J Hypertens 18: 1107–1113, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Harding P, Sigmon DH, Alfie ME, Huang PL, Fishman MC, Beierwaltes WH, Carretero OA. Cyclooxygenase-2 mediates increased renal renin content induced by low-sodium diet. Hypertension 29: 297–302, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Hashimoto H, Azuma Y, Kawasaki M, Fujihara H, Onuma E, Yamada-Okabe H, Takuwa Y, Ogata E, Ueta Y. Parathyroid hormone-related protein induces cachectic syndromes without directly modulating the expression of hypothalamic feeding-regulating peptides. Clin Cancer Res 13: 292–298, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Iguchi H, Onuma E, Sato K, Sato K, Ogata E. Involvement of parathyroid hormone-related protein in experimental cachexia induced by a human lung cancer-derived cell line established from a bone metastasis specimen. Int J Cancer 94: 24–27, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Jaeger P, Jones W, Kashgarian M, Baron R, Clemens TL, Segre GV, Hayslett JP. Animal model of primary hyperparathyroidism. Am J Physiol Endocrinol Metab 252: E790–E798, 1987 [DOI] [PubMed] [Google Scholar]
- 21. Jüppner H, Abou-Samra AB, Freeman M, Kong XF, Schipani E, Richards J, Kolakowski LF, Jr, Hock J, Potts JT, Jr, Kronenberg HM, Segre GV. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science 254: 1024–1026, 1991 [DOI] [PubMed] [Google Scholar]
- 22. Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci USA 92: 2735–2739, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Komatsu Y, Imai Y, Itoh F, Kojima M, Isaji M, Shibata N. Rat model of the hypercalcaemia induced by parathyroid hormone-related protein: characteristics of three bisphosphonates. Eur J Pharmacol 507: 317–324, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Kotchen TA, Galla JH, Luke RG. Contribution of chloride to the inhibition of plasma renin by sodium chloride in the rat. Kidney Int 13: 201–207, 1978 [DOI] [PubMed] [Google Scholar]
- 25. Kovács L, Góth MI, Szabolcs I, Dohán O, Ferencz A, Szilágyi G. The effect of surgical treatment on secondary hyperaldosteronism and relative hyperinsulinemia in primary hyperparathyroidism. Eur J Endocrinol 138: 543–547, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Lorenz JN, Weihprecht H, Schnermann J, Skøtt O, Briggs JP. Renin release from isolated juxtaglomerular apparatus depends on macula densa chloride transport. Am J Physiol Renal Fluid Electrolyte Physiol 260: F486–F493, 1991 [DOI] [PubMed] [Google Scholar]
- 27. Massfelder T, Stewart AF, Endlich K, Soifer N, Judes C, Helwig JJ. Parathyroid hormone-related protein detection and interaction with NO and cyclic AMP in the renovascular system. Kidney Int 50: 1591–1603, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Meng X, Dai X, Liao TD, D'Ambrosio M, Wang F, Yang JJ, Yang XP. Dose-dependent toxic effects of high-dose estrogen on renal and cardiac injury in surgically postmenopausal mice. Life Sci 88: 178–186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moseley JM, Kubota M, Diefenbach-Jagger H, Wettenhall RE, Kemp BE, Suva LJ, Rodda CP, Ebeling PR, Hudson PJ, Zajac JD, Martin TJ. Parathyroid hormone-related protein purified from a human lung cancer cell line. Proc Natl Acad Sci USA 84: 5048–5052, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Onuma E, Tsunenari T, Saito H, Sato K, Yamada-Okabe H, Ogata E. Parathyroid hormone-related protein (PTHrP) as a causative factor of cancer-associated wasting: possible involvement of PTHrP in the repression of locomotor activity in rats bearing human tumor xenografts. Int J Cancer 116: 471–478, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Richards AM, Espiner EA, Nicholls MG, Ikram H, Hamilton EJ, Maslowski AH. Hormone, calcium and blood pressure relationships in primary hyperparathyroidism. J Hypertens 6: 747–752, 1988 [DOI] [PubMed] [Google Scholar]
- 32. Rodan SB, Noda M, Wesolowski G, Rosenblatt M, Rodan GA. Comparison of postreceptor effects of 1–34 human hypercalcemia factor and 1–34 human parathyroid hormone in rat osteosarcoma cells. J Clin Invest 81: 924–927, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salahudeen AK, Thomas TH, Sellars L, Tapster S, Keavey P, Farndon JR, Johnston ID, Wilkinson R. Hypertension and renal dysfunction in primary hyperparathyroidism: effect of parathyroidectomy. Clin Sci (Lond) 76: 289–296, 1989 [DOI] [PubMed] [Google Scholar]
- 34. Saussine C, Judes C, Massfelder T, Musso MJ, Simeoni U, Hannedouche T, Helwig JJ. Stimulatory action of parathyroid hormone on renin secretion in vitro: a study using isolated rat kidney, isolated rabbit glomeruli and superfused dispersed rat juxtaglomerular cells. Clin Sci (Lond) 84: 11–19, 1993 [DOI] [PubMed] [Google Scholar]
- 35. Saussine C, Massfelder T, Parnin F, Judes C, Simeoni U, Helwig JJ. Renin stimulating properties of parathyroid hormone-related peptide in the isolated perfused rat kidney. Kidney Int 44: 764–773, 1993 [DOI] [PubMed] [Google Scholar]
- 36. Skinner SL, McCubbin JW, Page IH. Renal baroreceptor control of acute renin release in normotensive, nephrogenic and neurogenic hypertensive dogs. Circ Res 15: 522–531, 1964 [DOI] [PubMed] [Google Scholar]
- 37. Suzuki H, Hashimoto H, Kawasaki M, Watanabe M, Otsubo H, Ishikura T, Fujihara H, Ohnishi H, Onuma E, Yamada-Okabe H, Takuwa Y, Ogata E, Nakamura T, Ueta Y. Similar changes of hypothalamic feeding-regulating peptides mRNAs and plasma leptin levels in PTHrP-, LIF-secreting tumors-induced cachectic rats and adjuvant arthritic rats. Int J Cancer 128: 2215–2223, 2011 [DOI] [PubMed] [Google Scholar]
- 38. Takahashi S, Hakuta M, Aiba K, Ito Y, Horikoshi N, Miura M, Hatake K, Ogata E. Elevation of circulating plasma cytokines in cancer patients with high plasma parathyroid hormone-related protein levels. Endocr Relat Cancer 10: 403–407, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Traynor TR, Smart A, Briggs JP, Schnermann J. Inhibition of macula densa-stimulated renin secretion by pharmacological blockade of cyclooxygenase-2. Am J Physiol Renal Physiol 277: F706–F710, 1999 [DOI] [PubMed] [Google Scholar]
- 40. Valvo E, Bedogna V, Gammaro L, Casagrande P, Ortalda V, Maschio G. Systemic hemodynamic pattern in primary hyperparathyroidism and its changes after parathyroidectomy. Miner Electrolyte Metab 17: 147–152, 1991 [PubMed] [Google Scholar]
- 41. Vander AJ, Miller R. Control of renin secretion in the anesthetized dog. Am J Physiol 207: 537–546, 1964 [DOI] [PubMed] [Google Scholar]
- 42. Walls J, Ratcliffe WA, Howell A, Bundred NJ. Response to intravenous bisphosphonate therapy in hypercalcaemic patients with and without bone metastases: the role of parathyroid hormone-related protein. Br J Cancer 70: 169–172, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Winer N, Chokshi DS, Walkenhorst WG. Effects of cyclic AMP, sympathomimetic amines, and adrenergic receptor antagonists on renin secretion. Circ Res 29: 239–248, 1971 [DOI] [PubMed] [Google Scholar]
- 44. Wolzt M, Schmetterer L, Dorner G, Zelger G, Entlicher J, Kapiotis S, Eichler HG. Hemodynamic effects of parathyroid hormone-related peptide-(1–34) in humans. J Clin Endocrinol Metab 82: 2548–2551, 1997 [DOI] [PubMed] [Google Scholar]
- 45. Xu D, Borges GR, Grobe JL, Pelham CJ, Yang B, Sigmund CD. Preservation of intracellular renin expression is insufficient to compensate for genetic loss of secreted renin. Hypertension 54: 1240–1247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang T, Hassan S, Huang YG, Smart AM, Briggs JP, Schnermann JB. Expression of PTHrP, PTH/PTHrP receptor, and Ca(2+)-sensing receptor mRNAs along the rat nephron. Am J Physiol Renal Physiol 272: F751–F758, 1997 [DOI] [PubMed] [Google Scholar]
- 47. Yates AJ, Gutierrez GE, Smolens P, Travis PS, Katz MS, Aufdemorte TB, Boyce BF, Hymer TK, Poser JW, Mundy GR. Effects of a synthetic peptide of a parathyroid hormone-related protein on calcium homeostasis, renal tubular calcium reabsorption, and bone metabolism in vivo and in vitro in rodents. J Clin Invest 81: 932–938, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]