Abstract
The present study was designed to examine the effects of short-term diet and exercise on markers of metabolic health, serum-stimulated production of inflammatory biomarkers from cultured monocytes and adipocytes, and serum lipomics. Twenty-one overweight/obese children (9 boys and 12 girls, age 13.0 ± 0.5 yr, BMI 33.0 ± 1.8 kg/m2) were placed on a 2-wk ad libitum, high-fiber, low-fat diet and daily exercise regimen. Fasting serum samples were taken pre- and postintervention for determination of cytokines, metabolic risk markers, and lipomics. Monocytes and adipocytes were incubated with pre- and postintervention serum to investigate changes in cytokine secretion. Correlative associations were calculated, followed by hierarchical clustering to determine relationships between fatty acid (FA) species and clinical biomarkers. Despite remaining overweight/obese, interleukin (IL)-6, IL-8, TNFα, PAI-1, resistin, amylin, leptin, insulin, and IL-1ra decreased and adiponectin increased. Culture studies indicated decreases in monocyte secretion of IL-6, TNFα, and IL-1β and adipocyte secretion of IL-6. Lipomic analysis revealed a decrease in total lipids and decreases in saturated FAs and an increase in 18:1/18:0. In general, Pearson's correlations revealed that inflammatory markers are negatively associated with a cluster of polyunsaturated FAs and positively correlated with several saturated FAs. These results indicate significant modification of multiple indices of metabolic health with short-term rigorous lifestyle modification in overweight/obese children prior to obesity reversal.
Keywords: cytokines, physical activity, adipocyte secretion, monocytes, lipomics
the obesity epidemic in children and adolescents is well recognized and poses a major threat to the health and longevity of American children. Just as obesity in adults is associated with chronic diseases such as cardiovascular disease, type 2 diabetes, metabolic syndrome, and certain forms of cancer (27, 39), some have suggested that the children of today may be the first generation not to outlive their parents due to the premature development of these obesity-related diseases (26). The role that childhood obesity plays in the development of chronic diseases has been well documented. For example, the Pathobiological Determinants of Atherosclerosis in Youth study demonstrated that the extent of coronary artery disease in young males is positively correlated to body mass index (BMI) (21). Weiss et al. (45) reported that, in a large multiethnic group of children, as severity of obesity increased so did metabolic syndrome incidence, reaching 50% in the most obese group. In addition, studies have indicated that obese children tend to remain obese as adults (20). The causes of the obesity epidemic in youth today are multifactorial, and a Westernized high-fat, refined-carbohydrate diet and decreased physical activity play significant roles. One of the links between obesity and the aforementioned comorbidities is chronic low-grade systemic inflammation.
In a pair of seminal studies, we reported that a short-term diet and exercise intervention resulted in significant reductions in serum lipids, several markers of atherosclerosis, and the chronic low-grade inflammatory marker high-sensitivity C-reactive protein (CRP). These differences occur despite small changes in weight, and the subjects remained overweight or obese after the 2-wk intervention (6, 35). Based on the previous observation of a 40% reduction in high-sensitivity CRP, indicating a reduction in chronic low-grade inflammation, the present study was designed to further investigate the effects of daily exercise and an ad libitum plant-based diet on inflammatory cytokines, serum lipomics, and other serum markers of metabolic health. We hypothesized that changes in serum factors, especially a decrease in saturated fatty acids (FAs), could decrease the production of inflammatory cytokines in cultured adipocytes and monocytes. To test these hypotheses, we first performed serum analyses pre- and postintervention. In addition, adipocytes and monocytes were cultured separately and stimulated with serum obtained before and after the intervention, and levels of cytokines were measured postculture. Finally, given that numerous FA species may influence inflammatory status, we investigated the effects of this intervention on lipomic profiling.
METHODS
Subjects.
Twenty-one children, classified as overweight/obese by the Centers for Disease Control (CDC) sex-specific BMI-for-age percentiles, ages 8–17 (mean 13.0 ± 0.5 yr), participated voluntarily in a 2-wk residential lifestyle modification program at the Pritikin Longevity Center in Florida. Other than being overweight/obese, and eight of 21 having the metabolic syndrome, the children did not have any other known medical problems. Pre- and postintervention data were obtained from nine males and 12 females participating in the 2-wk program. None of the subjects were using drugs or therapies for obesity, and none had prior histories of disease or injury that would prevent daily exercise. Consent to participate in a research program was obtained from the parents, all subjects agreed to provide data for the study, and the project was approved by the University of California Los Angeles (UCLA) Human Subjects Protection Committee.
Diet and exercise intervention.
Participants in the program received a complete physical examination and underwent a 14-day diet and exercise intervention, as described previously (6, 35). Briefly, prepared meals, which were well tolerated by the subjects, contained 12–15% of calories from fat (polyunsaturated/saturated FA ratio = 2.4:1), 15–20% of calories from protein, and 65–70% of calories from primarily unrefined carbohydrate high in dietary fiber (>40 g/day). All foods except for animal-derived protein sources were served ad libitum. The exercise intervention consisted of 2–2.5 h/day of supervised activity. Blood samples were drawn after a 12-h overnight fast on days 1 and 12 of the intervention. The blood was separated by centrifugation, and serum was shipped on dry ice to UCLA, where it was stored at −80°C until analysis. Frozen serum was shipped on dry ice to the University of Michigan for lipomics analysis. Anthropometric data were collected as described previously (6).
Determination of serum lipids, glucose, insulin, homeostatic model assessment for insulin resistance, and quantitative insulin sensitivity check index.
Total cholesterol, triglyceride (TG), HDL, and glucose levels were measured at a national commercial laboratory (Quest Diagnostics, Miami, FL) using standardized techniques, as described previously (44). LDL was calculated as described by the Friedewald formula (9). Insulin was quantified in duplicate using Luminex xMAP Multiplex (Millipore, Billerica, MA). The degree of insulin resistance was estimated with the use of the homeostatic model assessment for insulin resistance (HOMA-IR) and calculated as the product of the fasting plasma insulin (μU/ml) and the fasting plasma glucose (mmol/l) divided by 22.5. Insulin sensitivity was also estimated by the quantitative insulin sensitivity check index (QUICKI), as defined by 1/{log[fasting insulin (μU/ml)] + log[fasting glucose (mg/dl)]}.
Determination of serum interleukins, TNFα, adiponectin, plasminogen activator inhibitor-1, resisitin, amylin, and leptin.
Serum IL-8, IL-10, IL-1 receptor antagonist (IL-1ra), IL-6, TNFα, plasminogen activator inhibitor-1 (PAI-1), resistin, amylin, and leptin were measured in duplicate using specific Luminex xMAP Multiplex kits (Millipore) according to the manufacturer's instructions. IL-1β was measured using an enzyme-linked immunosorbent assay (ELISA) kit (minimum detectable dose is 1 pg/ml) but was not detectable in the serum samples (R & D Systems, Minneapolis, MN). Serum adiponectin was also measured using an ELISA kit (R & D Systems).
Adipocyte cell culture in vitro studies.
Human preadipocytes, isolated from the subcutaneous thigh regions of an obese female, were plated at a density of ∼40,000 cells/cm2 on a 24-well plate by a commercial adipocyte culture supplier (Zen-Bio, Research Triangle Park, NC) (7). The cells differentiated into spindle-shaped primary adipocytes in ∼2 wk, and the plate was vacuum-sealed and shipped to UCLA in FBS- and insulin-free DMEM-Ham's F-12 culture medium supplemented with HEPES, biotin, pantothenate, dexamethasone, penicillin, streptomycin, and amphotericin B. Upon arrival, excess medium was removed, and adipocytes were incubated at 37°C in a humidified 5% CO2-95% air incubator.
After 1 wk of incubation for stabilization, the cultured adipocytes were washed three times in basal medium, which was comprised of DMEM-Ham's F-12 medium, HEPES, biotin, and pantothenate (Zen-Bio). Addition of pre- and postintervention serum (10%) in the culture medium was used to investigate adipocyte secretion of IL-6 and monocyte chemoattractant protein-1 (MCP-1) as a result of lifestyle modification. Serum from five subjects (n = 5: 3 males and 2 females) was used. Two-hundred microliters of subject serum was added to each culture well to achieve a total concentration of 10% serum in basal medium. Pre- and postintervention serum samples from each subject were added to wells in duplicate, and four wells remained serum free. After serum addition, cells were incubated for 72 h at 37°C with 5% CO2-95% air.
Cell culture supernatants were collected from the wells by gentle suction and stored at −20°C until analysis of IL-6 and MCP-1 by ELISA (R & D Systems). The immunoassays were performed according to the manufacturer's instructions for analyzing cell culture supernatants. To calculate adipocyte secretion of IL-6 and MCP-1, the serum concentrations measured previously were subtracted from the measured supernatant levels and adjusted for the 10% dilution.
Monocyte cell culture in vitro studies.
Peripheral blood was obtained from healthy human subjects, and monocytes were isolated by Ficoll/Hypaque separation and adherence to culture dishes in the presence of 5% (vol/vol) human type AB serum and 15% heat-inactivated fetal calf serum. After the adhered monolayers were washed successively with PBS, a test well was analyzed for monocyte purity (CD14+) via flow cytometry and found to be on average >97% pure. Adhered monocytes were removed from the petri dishes with 4 ml of Versene and resuspended in Iscove's modified Dulbecco's medium (DMEM; Irvine Scientific) supplemented with 15% fetal calf serum, 5% human AB serum, 1% glutamine, and antibiotics (penicillin-streptomycin, each at 100 U/ml final volume). The monocytes were plated in a flat-bottomed 96-well plate at 2 × 105 cells/well to provide an even monolayer of monocytes in each well. The 96-well plate was then incubated at 37°C with 5% CO2-95% air for 24 h to allow the cells to attach to the wells.
After 24 h, the medium was carefully removed from each well and replaced with 200 μl of Iscove's DMEM with 20% subject serum, 1% glutamine, and antibiotics (penicillin-streptomycin, each at 100 U/ml final volume). The pre- and postintervention serum were used to determine the effect of the intervention on monocyte secretion of TNFα, IL-6, and IL-1β and the effect on the proinflammatory JNK pathway in the monocytes. The same subset of subjects from the adipocyte cultures (n = 5: 3 males and 2 females) was used. Pre- and postintervention serum samples from each subject were added to wells in duplicate. After serum addition, cells were incubated for 96 h at 37°C with 5% CO2-95% air.
After incubation, cell culture supernatants were collected from the wells by gentle suction and stored at −80°C until analysis of TNFα, IL-6, and IL-1β by ELISA (R & D Systems). The immunoassays were performed according to the manufacturer's instructions for analyzing cell culture supernatants. To calculate monocyte secretion of TNFα, IL-6, and IL-1β, the serum concentrations measured previously were subtracted from the measured supernatant levels. Immediately after the supernatants were removed from the wells, the Cellular Activation of Signaling ELISA kit for JNK T183/Y185 (SuperArray, Frederick, MD) was used on the monocytes in the 96-well plate to measure activity of JNK (phosphorylated JNK/total JNK).
Serum lipomics.
Total lipids were extracted from the serum of 16 of the 21 subjects according to the method of Bligh and Dyer (2). Heptadecanoic acid (C17) was added as an internal standard for the quantification of FAs. Lipids were methylated using BF3-methanol (14% solution from Sigma) and analyzed by gas chromatography on an Omega Wax 250 capillary column (Supelco). Relative abundance of 22 different FA species was done by comparison of retention times with known standards.
Statistical analysis.
Statistical analyses were performed with Graph Pad Prism (GraphPad, San Diego, CA). Preintervention and postintervention values were compared using paired t-tests. Cell culture pre- and postintervention values (n = 5) were compared using matched paired Wilcoxon signed-rank tests for nonparametric data. Associations between lipomics and other measurements were calculated using Pearson's correlations. Heat maps of correlation matrices, including hierarchal clustering, were generated using the package “g plots” in R (version 2.14.0). All data are expressed as means ± SE unless otherwise noted. A P value of ≤0.05 was considered statistically significant.
RESULTS
Physical characteristics, blood pressure, serum lipids, glucose, and insulin.
Anthropometric and metabolic data are summarized in Table 1. The mean BMI before the intervention was 33.0 ± 1.8 kg/m2. All subjects had a BMI greater than the 75th percentile, and 18 of the 21 subjects were overweight (>85th percentile) or obese (>95th percentile) according to CDC BMI standards.
Table 1.
Anthropometric and lipid measurements in 21 children undergoing a 14-day diet and exercise intervention
| Parameter | Preintervention | Postintervention | %Change |
|---|---|---|---|
| Body weight, kg | 91.5 ± 6.8 | 87.9 ± 6.6 | −3.9‡ |
| BMI, kg/m2 | 33.0 ± 1.8 | 31.7 ± 1.7 | −3.8‡ |
| BMI percentile | 93.8 ± 1.5 | 90.6 ± 2.5 | −3.4* |
| Systolic blood pressure, mmHg | 125 ± 4 | 115 ± 2 | −7.8‡ |
| Diastolic blood pressure, mmHg | 72 ± 3 | 68 ± 1 | −6.0* |
| Blood glucose, mg/dl | 82.2 ± 1.9 | 87.1 ± 1.5 | 5.9* |
| Insulin, μU/ml | 21.4 ± 3.3 | 15.3 ± 3.6 | −28.8‡ |
| HOMA-IR | 4.9 ± 0.8 | 3.5 ± 0.7 | −28.7‡ |
| QUICKI | 2.71 ± 0.03 | 2.85 ± 0.04 | 5.1‡ |
| Triglycerides, mg/dl | 146.5 ± 15.1 | 89.9 ± 7.7 | −38.7‡ |
| Total cholesterol, mg/dl | 167.7 ± 5.7 | 131.8 ± 5.3 | −20.9‡ |
| LDL cholesterol, mg/dl | 94.3 ± 5.8 | 71.5 ± 4.6 | −24.1‡ |
| HDL cholesterol, mg/dl | 43.2 ± 2.1 | 42.3 ± 2.5 | −2.0 |
| Total cholesterol/HDL cholesterol | 4.09 ± 0.28 | 3.33 ± 0.38 | −18.5‡ |
| LDL cholesterol/HDL cholesterol | 2.32 ± 0.21 | 1.85 ± 0.18 | −20.4‡ |
| Subjects with metabolic syndrome | 8 | 0 |
All data are expressed as means ± SE. BMI, body mass index; HOMA-IR, homeostatic model assessment for insulin resistance; QUICKI, quantitative insulin sensitivity check index.
P < 0.05;
P < 0.01.
Following the intervention, all serum lipids improved significantly, with the exception of HDL, which did not change significantly (Table 1). In addition, both the total cholesterol/HDL and LDL/HDL ratios decreased. Fasting insulin decreased following the program (Table 1). Although blood glucose increased postintervention, both HOMA-IR and QUICKI improved postintervention, driven by the decrease in insulin (Table 1). Prior to the intervention, 8 of the 21 children were classified as having the metabolic syndrome, and at departure, none were classified as having the metabolic syndrome (Table 1).
Effect of the intervention on serum cytokines, adipokines, and endocrine markers.
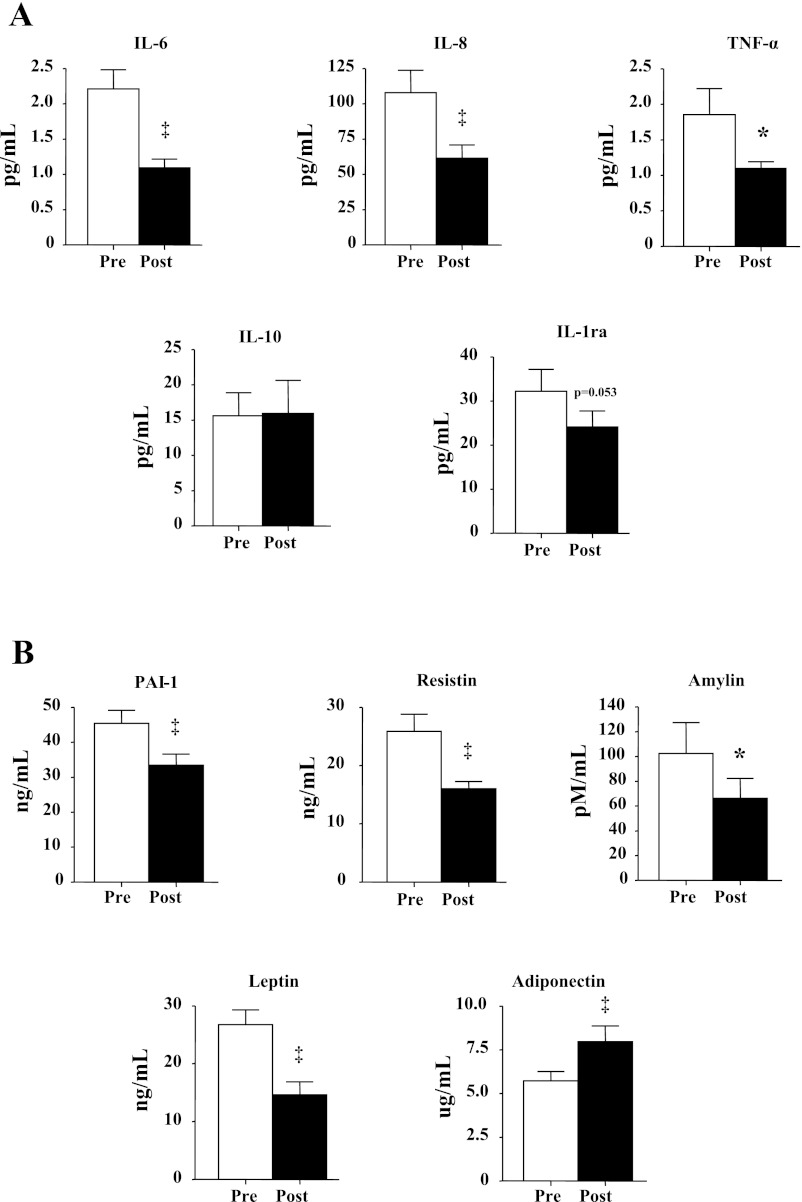
After the diet and exercise intervention, we noted significant decreases in IL-6 (51% decrease), IL-8 (43%), TNFα (40%), and IL-1ra (25%), and the anti-inflammatory cytokine IL-10 did not change (Fig. 1A). Serum PAI-1 (26% decrease), resistin (38%), amylin (36%), and leptin (45%) all decreased, whereas adiponectin increased (39%) (Fig. 1B).
Fig. 1.
Effect of intervention on 21 subjects on serum concentrations of IL-6, IL-8, TNFα, IL-1 receptor antagonist (IL-1ra), and IL-10 (A) and adiponectin, plasminogen activator inhibitor-1 (PAI-1), resistin, amylin, and leptin (B). All data are expressed as means ± SE. *P < 0.05 and ‡P < 0.01, postintervention (pre) vs. preintervention (post).
In vitro adipocyte cytokine secretion.
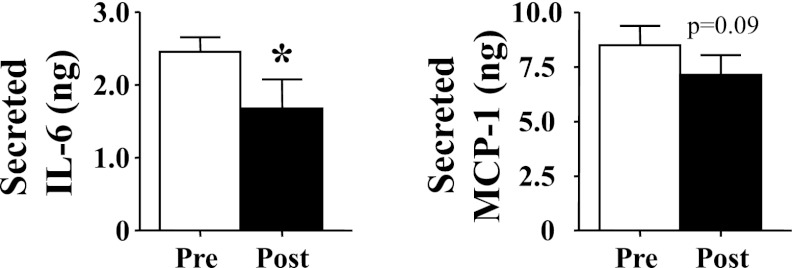
The mean level of IL-6 in the supernate was ∼4,000 times greater than that in added serum, whereas the supernate MCP-1 was ∼100 times the level in added serum. When the calculated amounts of these cytokines were compared between cells treated with preintervention vs. cells exposed to postintervention serum, it was found that secreted IL-6 decreased 32% (Fig. 2). Secreted MCP-1 also decreased nonsignificantly (P = 0.09; Fig. 2).
Fig. 2.
Effect of intervention on a subset of 5 subjects on serum-stimulated production of IL-6 (left) and monocyte chemoattractant protein-1 (MCP-1; right) from cultured adipocytes. All data are expressed as means ± SE. *P < 0.05, post vs. pre.
In vitro monocyte cytokine secretion and JNK activity.
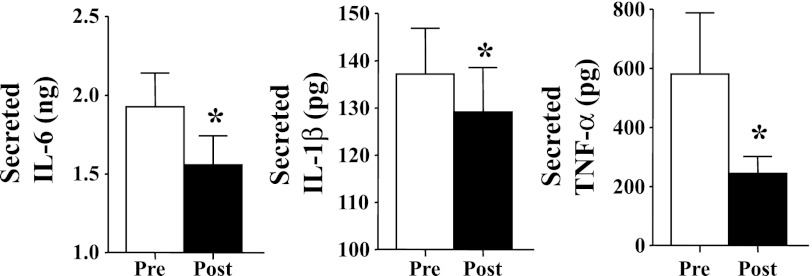
The calculation for the secretion of cytokines was performed in the same manner as described for the adipocytes. Secretions of IL-6 (19% decrease), TNFα (58%), and IL-1β (6%) were all reduced in monocytes incubated with postintervention serum compared with monocytes incubated with preintervention serum (Fig. 3). Activation of the JNK pathway, calculated by the ratio of phosphorylated JNK to total JNK, also trended toward reduction in the monocytes with postintervention serum (data not shown), but it was not significant (P = 0.16).
Fig. 3.
Effect of intervention on a subset of 5 subjects on serum-stimulated production of IL-6, TNFα, and IL-1β from cultured monocytes. All data are expressed as means ± SE. *P < 0.05, post vs. pre.
Serum FA profiles.
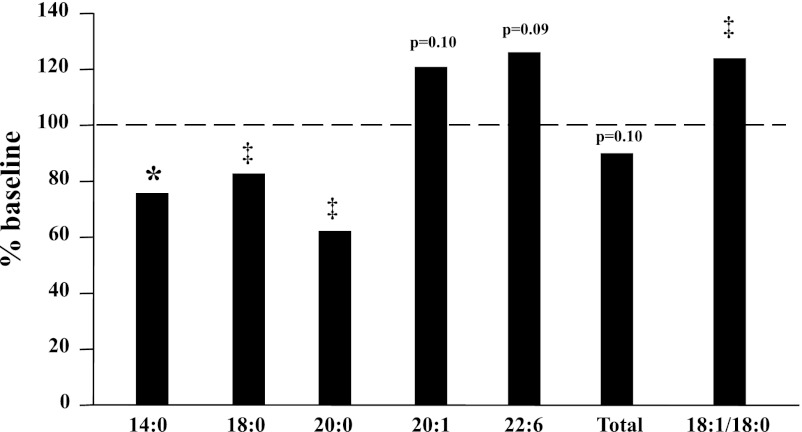
Lipomic analysis of the serum distinguished the types and amounts of FAs in the serum. Generally, the saturated FAs in serum decreased postintervention, and the unsaturated FAs tended to increase from their baseline (BL) levels preintervention. Significant reductions were noted for 14:0 (76% of BL), 18:0 (83% of BL), and 20:0 (62% of BL) (Fig. 4), whereas the 18:1/18:0 ratio (124% of BL), an index of the ratio of unsaturated to saturated FAs, increased significantly (Fig. 4), consistent with an increase in consumption of a lower-fat diet. Other unsaturated FAs, including 20:1 (121% of BL, P = 0.10) and 22:6 (126% of BL, P = 0.09), also increased, but not significantly (Fig. 4).
Fig. 4.
Effect of intervention on serum lipomic species (16 subjects). Data are expressed as %baseline values. *P < 0.05 and ‡P < 0.01, post vs. pre.
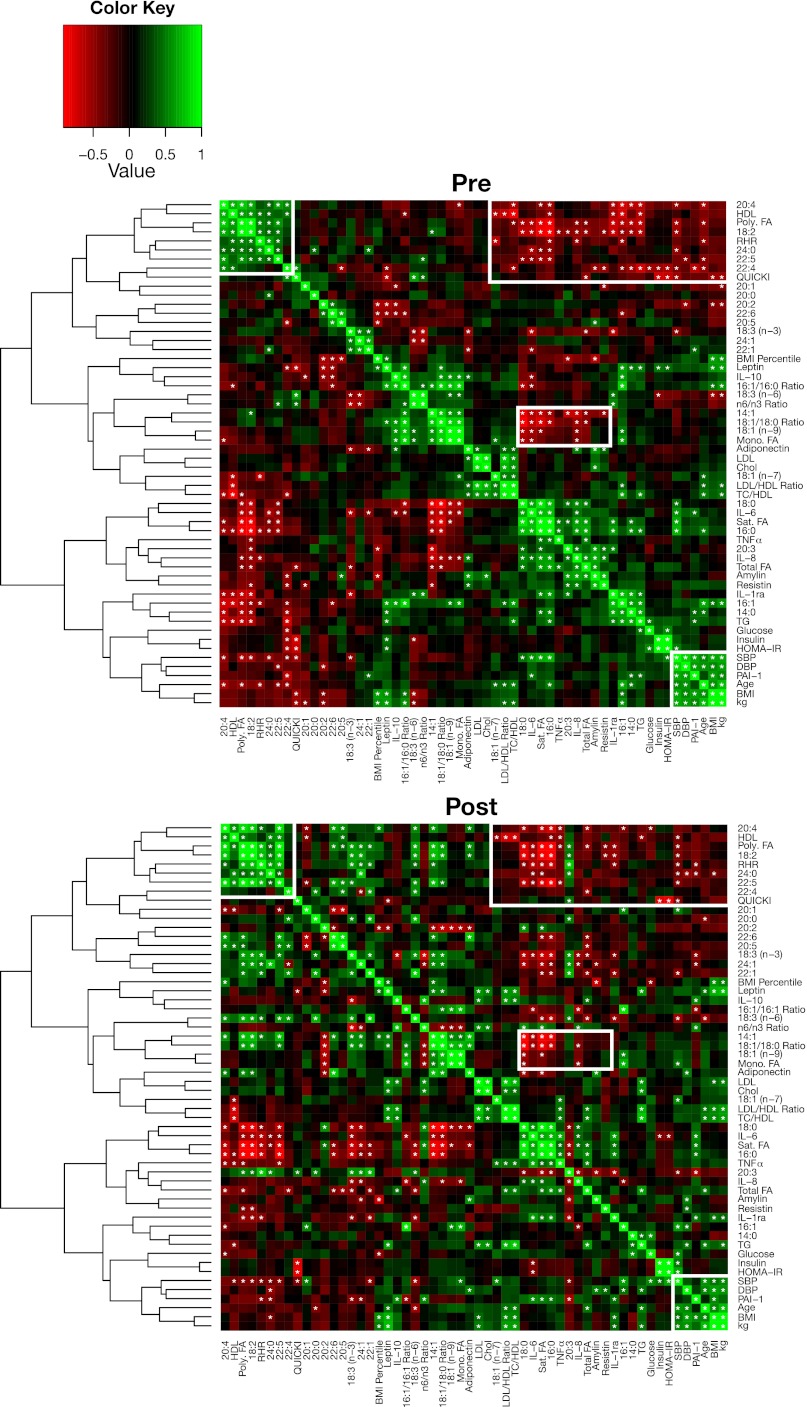
To assess relationships between changes in clinical and serum parameters, we performed hierarchal clustering of the Pearson's correlation coefficients among these values at BL and examined these same relationships following the intervention (Fig. 5). At BL, we found a cluster of longer-chain saturated and unsaturated FAs (20:4, 18:2, 24:0, 22:5, 22:4, and total polyunsaturated FAs) with positive correlation with each other and with HDL cholesterol and resting heart rate. This same cluster was negatively correlated with inflammatory markers (IL-6, TNFα, PAI-1, resistin, and amylin), total FA and TG levels, and shorter-chain FAs. In general, these relationships persisted following the intervention, suggesting that these polyunsaturated FAs may suppress inflammation. This cluster of FAs was also negatively correlated with systolic and diastolic blood pressure. Again, this negative correlation was present both before and after intervention. Likely reflecting the change in diet, following the intervention, additional longer-chain polyunsaturated FAs, including α-linolenic acid [18:3 (n-3)], showed a positive correlation with the aforementioned FA cluster.
Fig. 5.
Pearson's correlation matrices for associativity between measurements pre- and postintervention (16 subjects). Measurements with strong positive correlations are colored in green, and items with negative correlations are colored in red. Measurements from the preintervention set were clustered along the y-axis using hierarchical clustering, and that ordering was induced for the postintervention set. Correlative relationships are considered significant at α < 0.05 and are indicated with an asterisk within the corresponding cell. The white boxes highlight correlative associations of interest in the discussion.
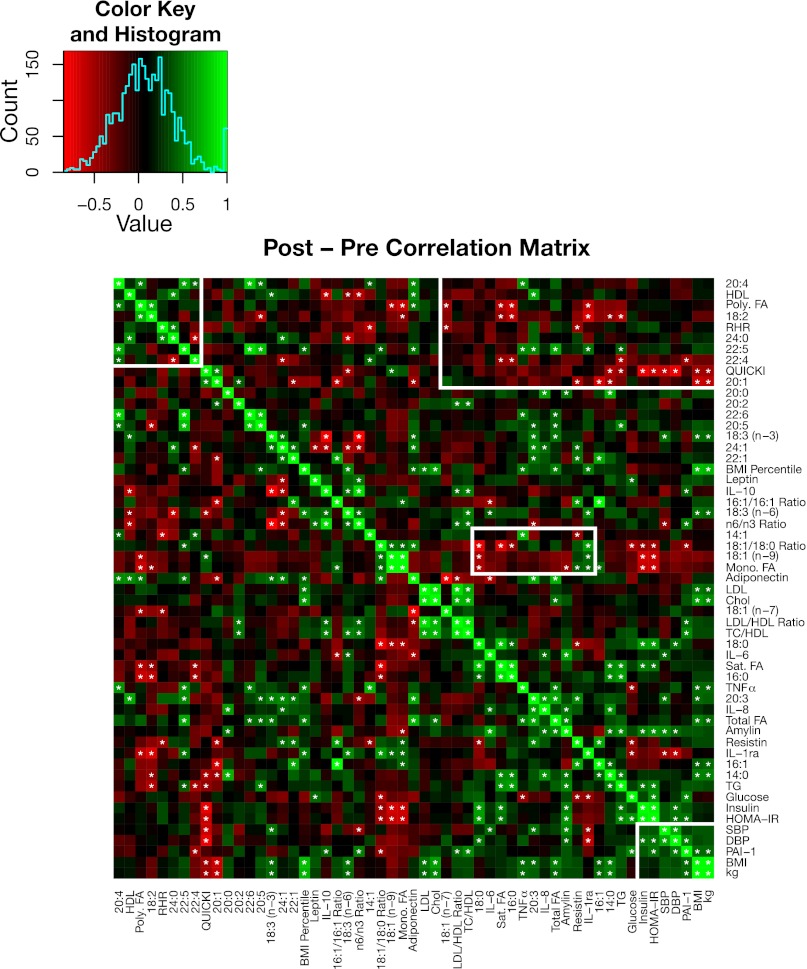
A series of monounsaturated FAs and the 18:1/18:0 ratio were also negatively correlated with the cluster of saturated FAs and inflammatory markers both before and after the intervention. When we examined the correlation of changes with the various parameters following the intervention (Fig. 6), we noted a significant correlation between the reduction in insulin and HOMA-IR and the increase in 18:1 and 18:1/18:0 ratio, suggesting a relationship between the change in diet and the improvement in a surrogate of insulin sensitivity.
Fig. 6.
Visual representation of a Pearson's correlation matrix of the changes in key metabolic measurements before and after the intervention (16 subjects). Changes were calculated by subtracting the preintervention measurements from the postintervention measurements. The changes were then used to calculate pairwise Pearson's correlations. Green cells indicate positive correlations, and red cells indicate negative correlations. The rows and columns are ordered to match the hierarchical clustering scheme shown in Fig. 5. Significant correlations are indicated with an asterisk in the corresponding cell. The white boxes highlight correlative associations of interest in the discussion.
Furthermore, total saturated FAs were positively correlated with proinflammatory markers (IL-6, IL-8, IL-1ra, and TNFα), and total polyunsaturated FAs had negative associations with inflammation (IL-6 and IL-1ra) (all P < 0.05; Fig. 5). In terms of other metabolic markers, the change in amylin was positively correlated with the changes seen in total FAs, IL-6, IL-8, 14:0, 20:0, insulin, HOMA-IR, and TG (all P < 0.05; Fig. 6). Additionally, other than PAI-1, TNFα, and leptin being correlated with BMI, we did not detect any significant correlations between body weight or BMI and serum cytokines, adipokines, or endocrine markers.
DISCUSSION
Previous studies in adults (1, 36, 44) and in children (6, 35), using similar diet and exercise interventions as presented here, resulted in significant reductions in serum lipids, insulin, inflammatory markers, oxidative stress, adhesion molecule expression, and small but significant reductions in body weight and BMI. In the present study, we sought to determine the effects of this short-term intensive diet and exercise program in youth on serum inflammatory cytokines and how changes in serum following the intervention affected the ex vivo production of cytokines by cultured adipocytes and monocytes. We also assessed changes in the FA composition of serum lipids and the correlations with changes in anthropometric and serum markers using hierarchal clustering.
The primary findings demonstrate that 1) even a short-term lifestyle modification program with minimal weight loss may ameliorate serum inflammatory cytokines and other serum metabolic risk factors, 2) incubation of adipocytes and monocytes with postintervention serum led to reductions in levels of secreted cytokines, and 3) the lifestyle intervention modified serum levels of several lipomic species, including saturated fatty acids thought to be related to inflammation.
The present study involved overweight and obese insulin-resistant youth with elevated levels of proinflammatory cytokines. Previous studies have documented associations between these risk factors (12, 15, 37). Short-term lifestyle intervention resulted in reductions in proinflammatory cytokines and metabolic risk factors, including IL-6, IL-8, TNFα, PAI-1, resistin, amylin, and leptin, and an increase in adiponectin. The reductions in proinflammatory cytokines are possibly the result of the low saturated fat content in the diet, since saturated fatty acids are potent inducers of inflammation by activating the NF-κB pathway (23, 40). Indeed, before and after the intervention, we noted strong correlations between saturated FAs and IL-6, IL-8, and TNFα (Fig. 5). Additionally, an increased intake of ω-3 FA from fish and plant sources, which blocks the NF-κB pathway and stimulates anti-inflammatory pathways (17, 25), may also have contributed to the reduced inflammation. Not surprisingly, before and after the intervention, we noted a strong negative correlation between total polyunsaturated FAs and both IL-6 and IL-1ra. Other possible mediators of the reduced inflammation could be the reduced dietary glycemic load and increase in dietary fiber, which have been associated with increases in adiponectin and decreases in inflammatory markers (19, 33), and the increased physical activity that results in the release of cytokines from the muscle that upregulate anti-inflammatory cytokines (29, 30). We did not look at diet and exercise independently, and therefore, we cannot attribute the changes directly to either aspect of the intervention.
Although we predicted that IL-1ra, an anti-inflammatory cytokine, would increase with the intervention, there was a 25% decrease in IL-1ra, and this discrepancy has been shown in animal studies as well (43), but to our knowledge the current study is the first to report this finding in humans. In fact, IL-1ra has been noted to have an alternative, possibly proinflammatory, role in regulating inflammation (11, 38, 41).
In vivo, adipose tissue, especially visceral adiposity, is a major contributor to systemic inflammation, due largely to resident macrophages (24), in addition to adipocyte production of cytokines (4, 8). However, the cultured cells used in this study (free of resident macrophages) secreted high amounts of both IL-6 and MCP-1. IL-6 in the supernatant treated with subject serum decreased significantly postintervention, demonstrating that proinflammatory cytokine secretion by the adipocyte itself may be responsive to serum factors that change as a result of lifestyle modification. One candidate could be decreased insulin levels. Insulin has been reported to stimulate IL-6 gene expression in subcutaneous adipose tissue (16). The subjects of the present study had a significant decrease in fasting insulin and improved surrogates of insulin resistance/sensitivity postintervention. In obese men, decreases in plasma IL-6 after weight loss were significantly correlated with improvement in insulin sensitivity (5). Another possible contribution to the drop in IL-6 in the supernatant could be decreased serum resistin, which induces the expression of TNFα and IL-6 in white adipose tissue and in peripheral blood mononuclear cells (3). In the current study, MCP-1 from the cultured cells was lower following treatment with postintervention serum than with preintervention serum, although this finding was not statistically significant. Previously, no significant changes in MCP-1 serum levels in the study subjects were found, although addition of postintervention serum to human aortic endothelial cells resulted in a decrease in MCP-1 production (35).
Obesity is also associated with an increase in macrophage infiltration of adipose tissue (10, 22). Itoh et al. (14), using an in vitro coculture of adipocytes and macrophages, showed that ω-3 fatty acids reversed the coculture-induced decrease in adiponectin secretion at least in part through downregulation of TNFα in macrophages. Recent work by Oh et al. (25) revealed a mechanistic link between ω-3 fatty acids and inhibition of inflammatory pathways in the macrophage as well as reduction of inflammatory macrophages in adipose tissue. This suggests an additional mechanism by which this intervention reduced inflammation. Indeed, lipomic analysis of FA composition in the serum reflects the reduction in saturated FAs and an increase in docosahexaenoic acid in the diet, and the polyunsaturated FAs were inversely correlated with proinflammatory cytokines IL-6 and IL-1ra.
One of the key findings of the lipomic analyses showed an increase in the 18:1/18:0 ratio, which is commonly used as an index of hepatic stearoyl-CoA desaturase 1 activity (42). Increased stearoyl-CoA desaturase 1 activity has been associated with a decrease in liver fat and improvement in insulin sensitivity (42). Also, the change in the 18:1/18:0 ratio was found to be negatively correlated with insulin and HOMA-IR. In addition, we noted a 24% drop in 14:0 FA levels, and shorter-chain saturated FA levels, especially 14:0, in TG have been associated with an increased risk of type 2 diabetes (34). The decrease in amylin further illustrates the improvement in metabolic health, since hyperamylinemia occurs in type 2 diabetes, and amylin has been shown to cause insulin resistance in animal models (18, 46). The decrease in amylin was positively correlated with decreases seen in total FAs, IL-6, IL-8, insulin, HOMA-IR, and TG, which is in agreement with previous studies (13, 32). Furthermore, the change in amylin was positively correlated to the reductions in 14:0 and 20:0 FAs, which to our knowledge has not been shown previously.
In addition to changes in diet, the exercise intervention is also believed to be a mediator of anti-inflammatory effects, since increases in physical activity correlate with reductions in inflammation. Platat et al. (31) found negative correlations between exercise and inflammation in children independent of adiposity and fat localization. Although our subjects did lose a statistically significant amount of weight, body weight was reduced by ∼4%, whereas inflammatory cytokines were reduced 40–50%, suggesting that there may be a direct effect of physical activity independent of adiposity, mediating the reduction in inflammation. Pedersen and Fischer (29) and Petersen and Pedersen (30) demonstrated that IL-6 released by skeletal muscle acts in an anti-inflammatory manner to inhibit proinflammatory pathways and stimulate anti-inflammatory pathways. Although this theory conflicts with the traditional proinflammatory role of IL-6 and a better understanding of myokines is needed, the difference in IL-6 action may be related to chronic vs. acute production and tissue specificity. Nevertheless, IL-6 acting as a myokine has been associated with an upregulation in IL-1ra and IL-10 and a decrease in production of TNFα and IL-1β (28–30). The overall effect also decreases insulin resistance induced by these inflammatory pathways (28). This mechanism may in part explain the significant changes in inflammation and the metabolic profile that we observed while the subjects remained overweight/obese.
In summary, a major strength of the present study is that the subjects had all of their food provided and physical activity monitored to ensure adherence to the intervention. Additionally, the diet was ad libitum, a major advantage in cases where overeating is an issue, and thus it is a more realistic program to implement into the daily lives of children rather than induced caloric restriction. Overall, the results provide evidence that a short-term diet and exercise intervention can ameliorate the increase in serum metabolic risk factors and inflammation seen in obesity associated with an unhealthy diet and physical inactivity.
GRANTS
C. K. Roberts was supported by a grant from the American Heart Association (BGIA no. 0765139Y) during this study. C. F. Burant was supported by the Michigan Nutrition and Obesity Center (DK089503) and the Dr. Robert C. Atkins Foundation. R. J. Barnard was supported by a grant from the L-B Research/Education Foundation.
DISCLOSURES
R. J. Barnard received consulting honorarium from the Pritikin Longevity Center.
AUTHOR CONTRIBUTIONS
A.I., R.J.B., A.J.E.A., G.C.B., and C.K.R. did the conception and design of the research; A.I., A.J.E.A., G.C.B., and S.A.B. performed the experiments; A.I., A.J.E.A., S.A.B., E.R.S., and C.F.B. analyzed the data; A.I., R.J.B., A.J.E.A., E.R.S., C.F.B., and C.K.R. interpreted the results of the experiments; A.I., E.R.S., and C.F.B. prepared the figures; A.I. and C.K.R. drafted the manuscript; A.I., R.J.B., A.J.E.A., G.C.B., S.A.B., E.R.S., C.F.B., and C.K.R. edited and revised the manuscript; A.I., R.J.B., A.J.E.A., G.C.B., S.A.B., E.R.S., C.F.B., and C.K.R. approved the final version of the manuscript.
REFERENCES
- 1. Barnard RJ. Effects of life-style modification on serum lipids. Arch Intern Med 151: 1389–1394, 1991 [PubMed] [Google Scholar]
- 2. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959 [DOI] [PubMed] [Google Scholar]
- 3. Bokarewa M, Nagaev I, Dahlberg L, Smith U, Tarkowski A. Resistin, an adipokine with potent proinflammatory properties. J Immunol 174: 5789–5795, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bruun JM, Lihn AS, Madan AK, Pedersen SB, Schiøtt KM, Fain JN, Richelsen B. Higher production of IL-8 in visceral vs. subcutaneous adipose tissue. Implication of nonadipose cells in adipose tissue. Am J Physiol Endocrinol Metab 286: E8–E13, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Bruun JM, Verdich C, Toubro S, Astrup A, Richelsen B. Association between measures of insulin sensitivity and circulating levels of interleukin-8, interleukin-6 and tumor necrosis factor-alpha. Effect of weight loss in obese men. Eur J Endocrinol 148: 535–542, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Chen AK, Roberts CK, Barnard RJ. Effect of a short-term diet and exercise intervention on metabolic syndrome in overweight children. Metabolism 55: 871–878, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Frederiksen L, Nielsen TL, Wraae K, Hagen C, Frystyk J, Flyvbjerg A, Brixen K, Andersen M. Subcutaneous rather than visceral adipose tissue is associated with adiponectin levels and insulin resistance in young men. J Clin Endocrinol Metab 94: 4010–4015, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 83: 847–850, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499–502, 1972 [PubMed] [Google Scholar]
- 10. Hajer GR, van Haeften TW, Visseren FL. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29: 2959–2971, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Herder C, Brunner EJ, Rathmann W, Strassburger K, Tabak AG, Schloot NC, Witte DR. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: the Whitehall II study. Diabetes Care 32: 421–423, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993 [DOI] [PubMed] [Google Scholar]
- 13. Hou X, Sun L, Li Z, Mou H, Yu Z, Li H, Jiang P, Yu D, Wu H, Ye X, Lin X, Le Y. Associations of amylin with inflammatory markers and metabolic syndrome in apparently healthy Chinese. PLoS One 6: e24815, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, Tanaka M, Kawano H, Yano T, Aoe S, Takeya M, Shimatsu A, Kuzuya H, Kamei Y, Ogawa Y. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol 27: 1918–1925, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Kim J, Bhattacharjee R, Kheirandish-Gozal L, Khalyfa A, Sans Capdevila O, Tauman R, Gozal D. Insulin sensitivity, serum lipids, and systemic inflammatory markers in school-aged obese and nonobese children. Int J Pediatr 2010: 846098, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krogh-Madsen R, Plomgaard P, Keller P, Keller C, Pedersen BK. Insulin stimulates interleukin-6 and tumor necrosis factor-α gene expression in human subcutaneous adipose tissue. Am J Physiol Endocrinol Metab 286: E234–E238, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem 276: 16683–16689, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Leighton B, Cooper GJ. Pancreatic amylin and calcitonin gene-related peptide cause resistance to insulin in skeletal muscle in vitro. Nature 335: 632–635, 1988 [DOI] [PubMed] [Google Scholar]
- 19. Levitan EB, Cook NR, Stampfer MJ, Ridker PM, Rexrode KM, Buring JE, Manson JE, Liu S. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism 57: 437–443, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Magarey AM, Daniels LA, Boulton TJ, Cockington RA. Predicting obesity in early adulthood from childhood and parental obesity. Int J Obes Relat Metab Disord 27: 505–513, 2003 [DOI] [PubMed] [Google Scholar]
- 21. McGill HC, Jr, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, Strong JP. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation 105: 2712–2718, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest 116: 33–35, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282: 35279–35292, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Nicklas BJ, You T, Pahor M. Behavioural treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ 172: 1199–1209, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142: 687–698, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med 352: 1138–1145, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Owen CG, Whincup PH, Orfei L, Chou QA, Rudnicka AR, Wathern AK, Kaye SJ, Eriksson JG, Osmond C, Cook DG. Is body mass index before middle age related to coronary heart disease risk in later life? Evidence from observational studies. Int J Obes 33: 866–877, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pedersen BK. The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays Biochem 42: 105–117, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Pedersen BK, Fischer CP. Beneficial health effects of exercise—the role of IL-6 as a myokine. Trends Pharmacol Sci 28: 152–156, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol 98: 1154–1162, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Platat C, Wagner A, Klumpp T, Schweitzer B, Simon C. Relationships of physical activity with metabolic syndrome features and low-grade inflammation in adolescents. Diabetologia 49: 2078–2085, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Qi D, Cai K, Wang O, Li Z, Chen J, Deng B, Qian L, Le Y. Fatty acids induce amylin expression and secretion by pancreatic β-cells. Am J Physiol Endocrinol Metab 298: E99–E107, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Qi L, Rimm E, Liu S, Rifai N, Hu FB. Dietary glycemic index, glycemic load, cereal fiber, and plasma adiponectin concentration in diabetic men. Diabetes Care 28: 1022–1028, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Rhee EP, Cheng S, Larson MG, Walford GA, Lewis GD, McCabe E, Yang E, Farrell L, Fox CS, O'Donnell CJ, Carr SA, Vasan RS, Florez JC, Clish CB, Wang TJ, Gerszten RE. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest 121: 1402–1411, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Roberts CK, Chen AK, Barnard RJ. Effect of a short-term diet and exercise intervention in youth on atherosclerotic risk factors. Atherosclerosis 191: 98–106, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Roberts CK, Won D, Pruthi S, Kurtovic S, Sindhu RK, Vaziri ND, Barnard RJ. Effect of a short-term diet and exercise intervention on oxidative stress, inflammation, MMP-9, and monocyte chemotactic activity in men with metabolic syndrome factors. J Appl Physiol 100: 1657–1665, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Roth CL, Kratz M, Ralston MM, Reinehr T. Changes in adipose-derived inflammatory cytokines and chemokines after successful lifestyle intervention in obese children. Metabolism 60: 445–452, 2011 [DOI] [PubMed] [Google Scholar]
- 38. Saltevo J, Laakso M, Jokelainen J, Keinänen-Kiukaanniemi S, Kumpusalo E, Vanhala M. Levels of adiponectin, C-reactive protein and interleukin-1 receptor antagonist are associated with insulin sensitivity: a population-based study. Diabetes Metab Res Rev 24: 378–383, 2008 [DOI] [PubMed] [Google Scholar]
- 39. Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature. Prev Med 22: 167–177, 1993 [DOI] [PubMed] [Google Scholar]
- 40. Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, Karin M. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab 6: 386–397, 2007 [DOI] [PubMed] [Google Scholar]
- 41. Somm E, Cettour-Rose P, Asensio C, Charollais A, Klein M, Theander-Carrillo C, Juge-Aubry CE, Dayer JM, Nicklin MJ, Meda P, Rohner-Jeanrenaud F, Meier CA. Interleukin-1 receptor antagonist is upregulated during diet-induced obesity and regulates insulin sensitivity in rodents. Diabetologia 49: 387–393, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Stefan N, Peter A, Cegan A, Staiger H, Machann J, Schick F, Claussen CD, Fritsche A, Haring HU, Schleicher E. Low hepatic stearoyl-CoA desaturase 1 activity is associated with fatty liver and insulin resistance in obese humans. Diabetologia 51: 648–656, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Vieira VJ, Valentine RJ, Wilund KR, Woods JA. Effects of diet and exercise on metabolic disturbances in high-fat diet-fed mice. Cytokine 46: 339–345, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wegge JK, Roberts CK, Ngo TH, Barnard RJ. Effect of diet and exercise intervention on inflammatory and adhesion molecules in postmenopausal women on hormone replacement therapy and at risk for coronary artery disease. Metabolism 53: 377–381, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, Allen K, Lopes M, Savoye M, Morrison J, Sherwin RS, Caprio S. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350: 2362–2374, 2004 [DOI] [PubMed] [Google Scholar]
- 46. Ye JM, Lim-Fraser M, Cooney GJ, Cooper GJ, Iglesias MA, Watson DG, Choong B, Kraegen EW. Evidence that amylin stimulates lipolysis in vivo: a possible mediator of induced insulin resistance. Am J Physiol Endocrinol Metab 280: E562–E569, 2001 [DOI] [PubMed] [Google Scholar]